The α9α10 Nicotinic Acetylcholine Receptor Antagonist αO-Conotoxin GeXIVA[1,2] Alleviates and Reverses Chemotherapy-Induced Neuropathic Pain
Abstract
1. Introduction
2. Results
2.1. Development of Neuropathic Pain in Oxaliplatin-Treated Rats
2.2. Analgesic Effects of GeXIVA[1,2] on Oxaliplatin-Induced Mechanical Allodynia
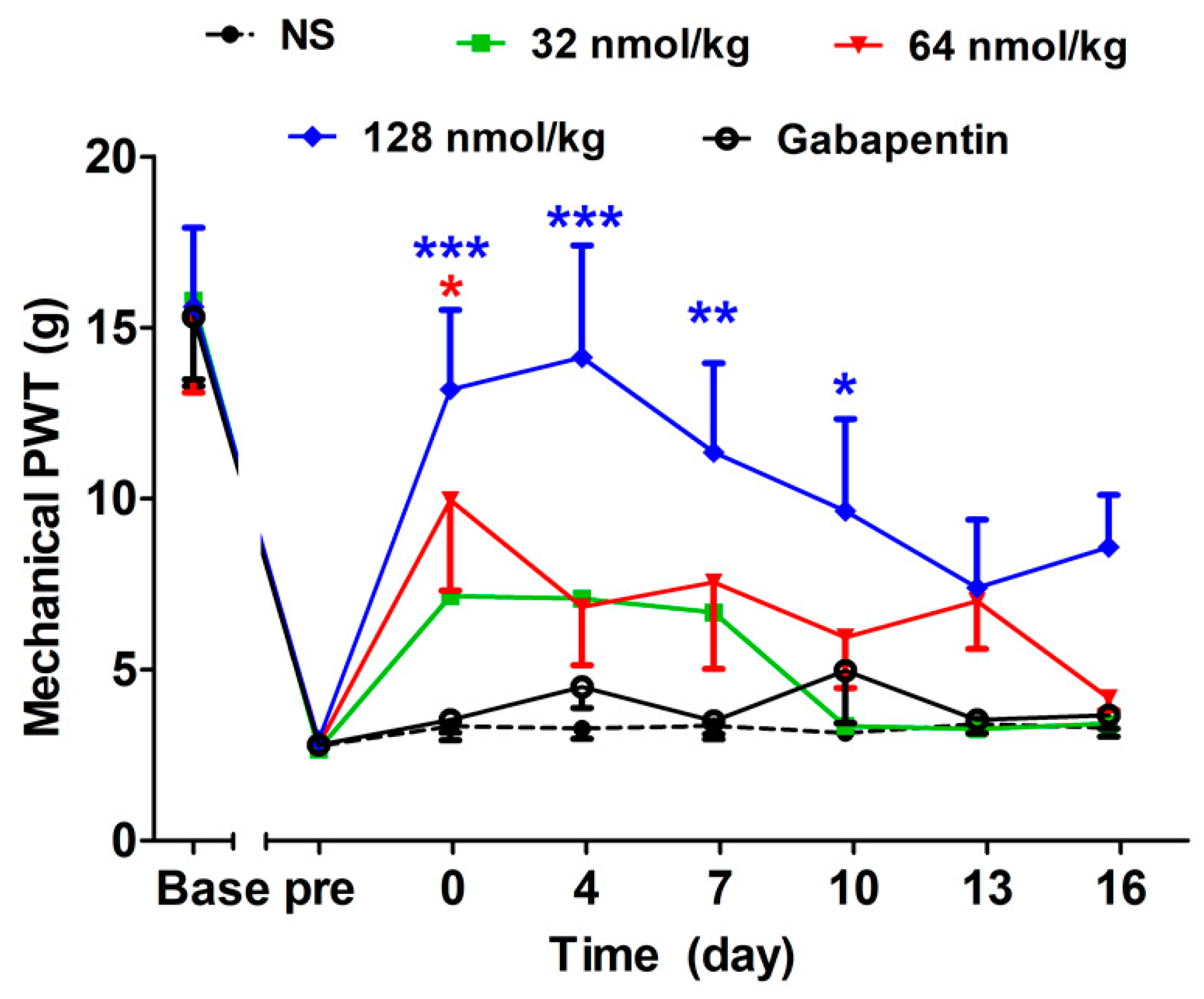
2.2.1. Analgesic Effect of GeXIVA[1,2] by Single Administration
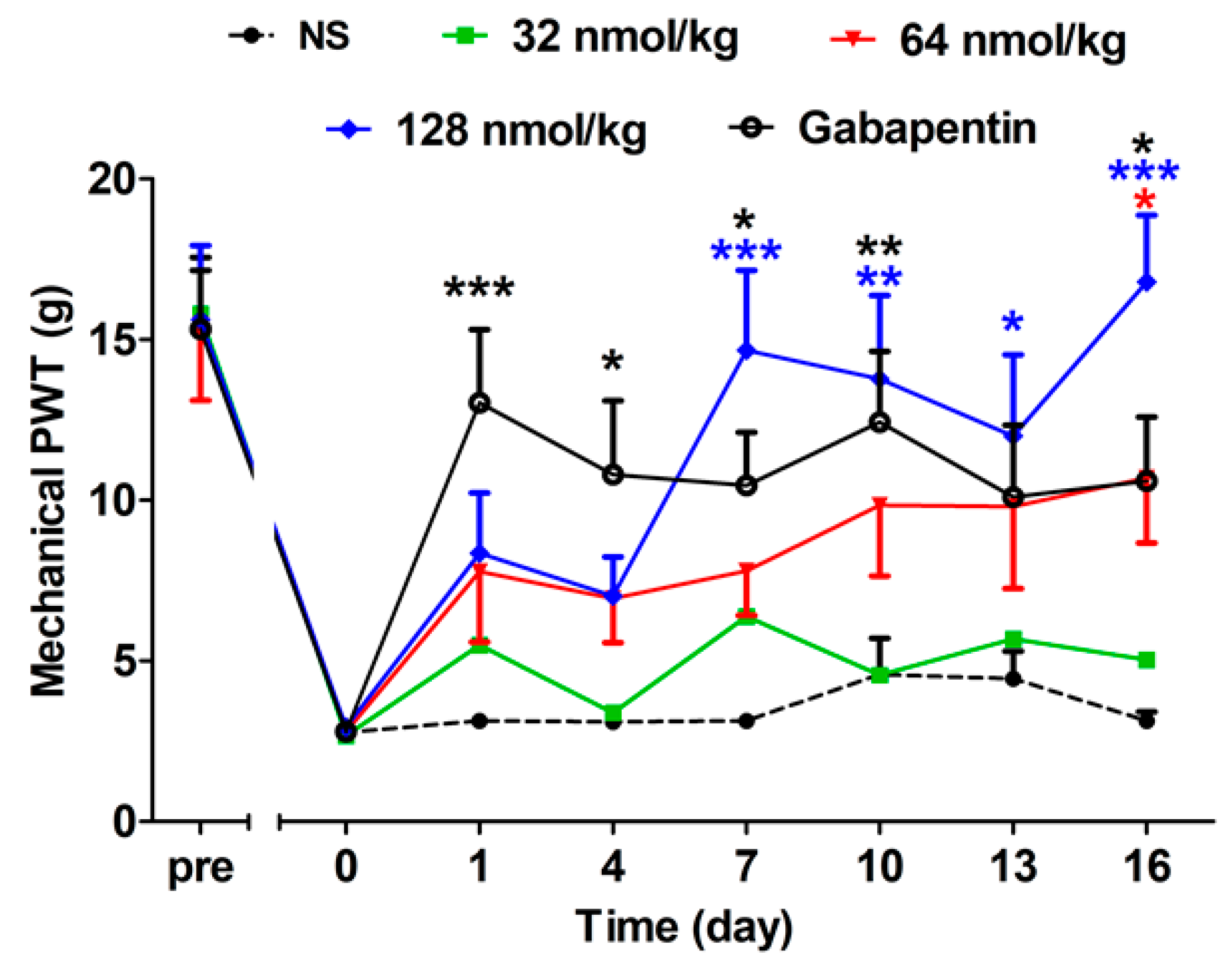
2.2.2. Acute Analgesic Effect of GeXIVA[1,2] Following Repeated Injections
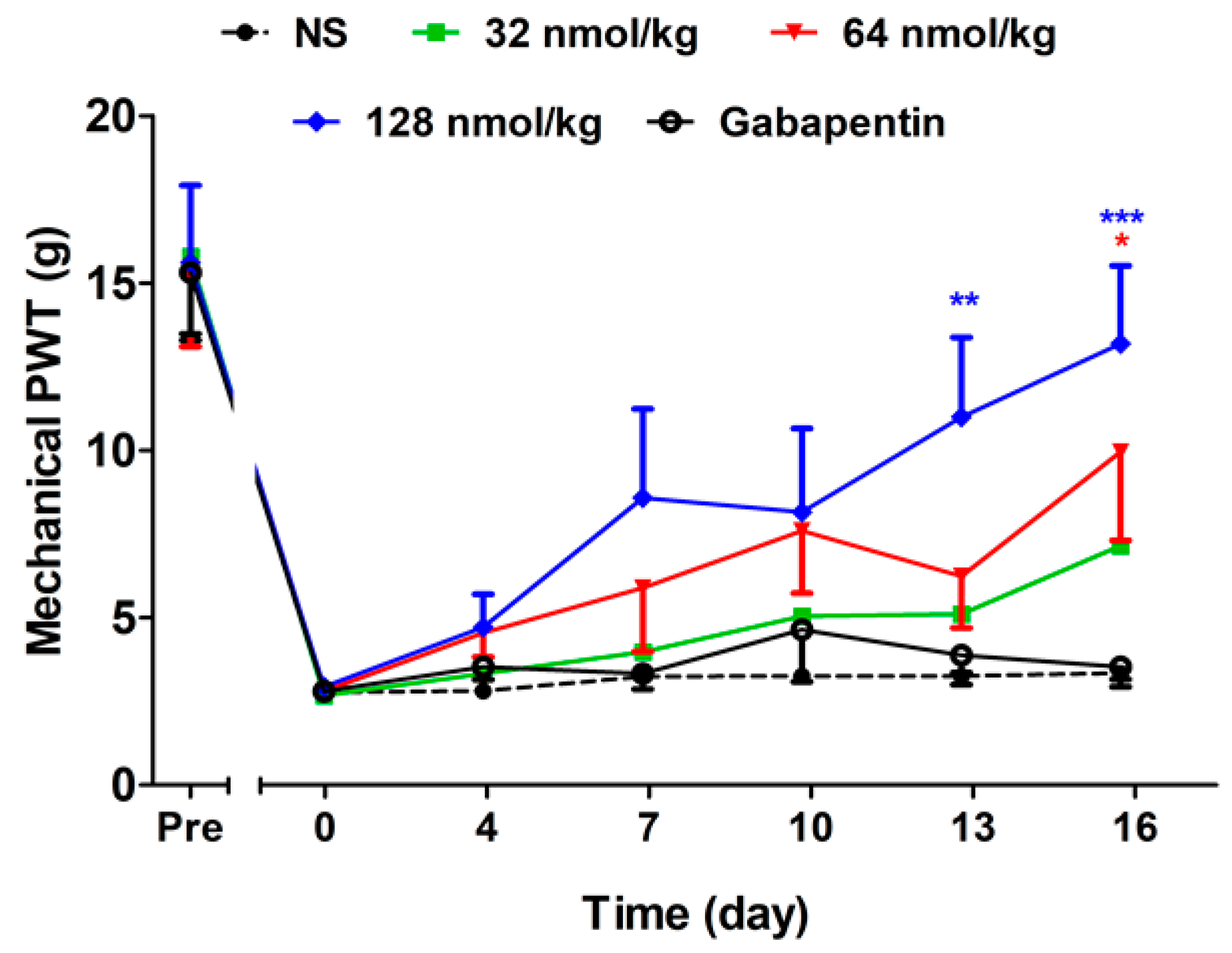
2.2.3. Short-Term Analgesic Effect of GeXIVA[1,2] by Repeated Treatments
2.2.4. Long-Term Analgesic Effect of GeXIVA[1,2] by Repeated Treatments
2.3. Analgesic Fffects of GeXIVA[1,2] on Oxaliplatin-Induced Cold Allodynia
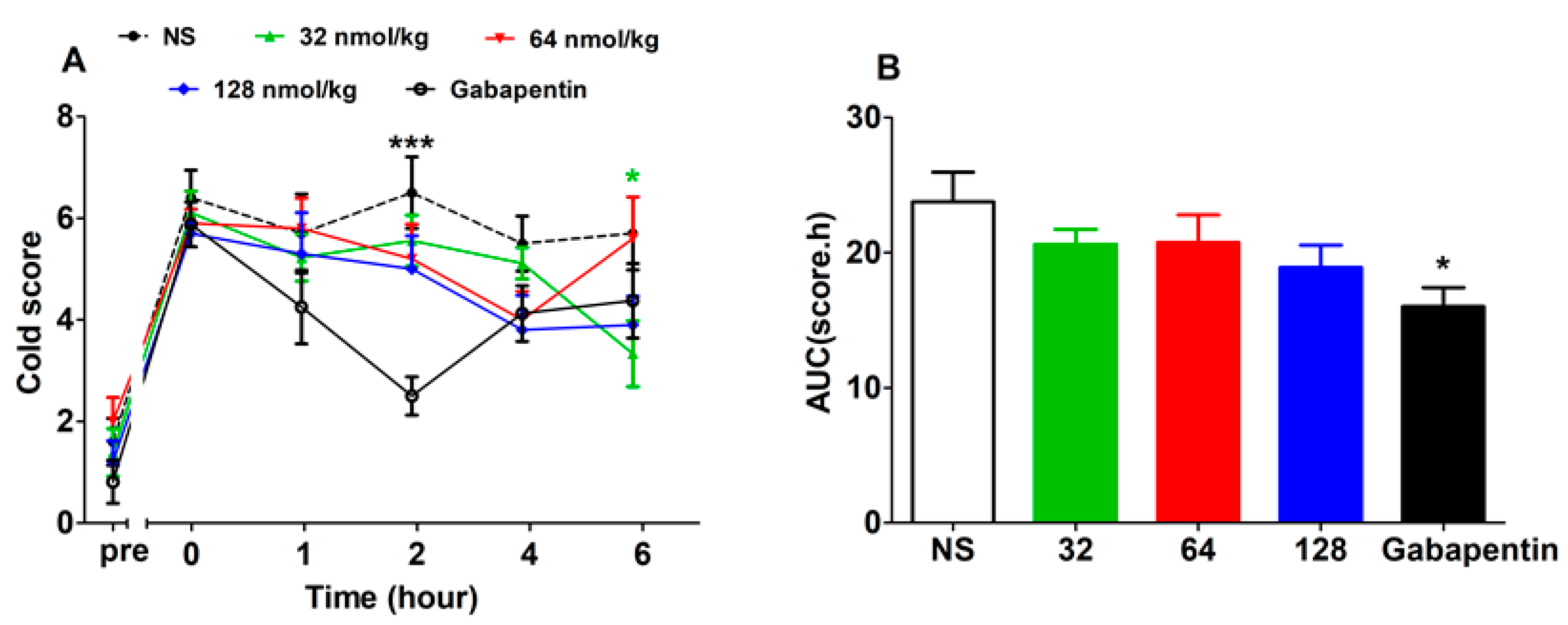
2.3.1. Analgesic Effect of GeXIVA[1,2] by Single Administration
2.3.2. Short-Term Analgesic Effect of GeXIVA[1,2] by Repeated Treatments
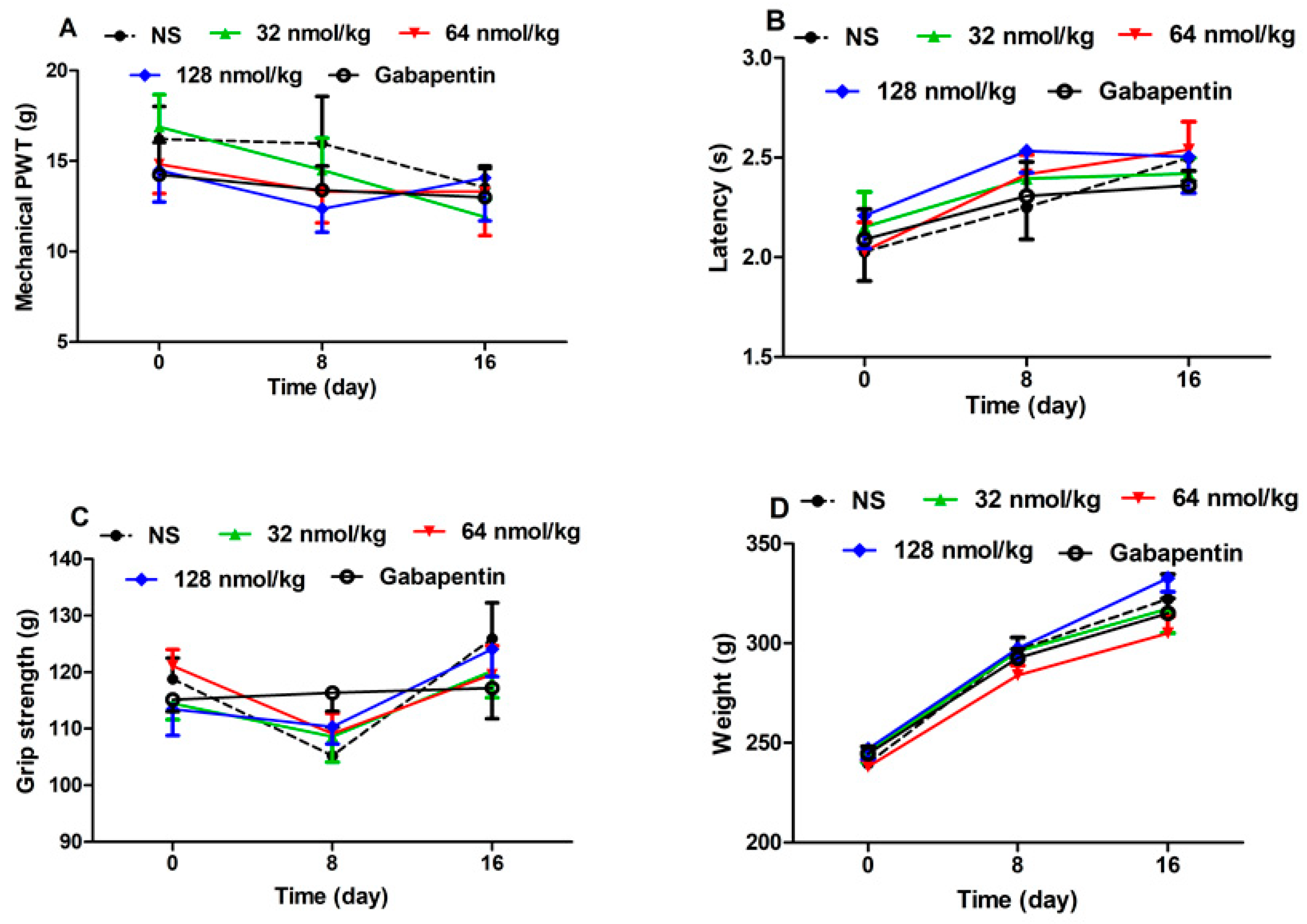
2.3.3. Effect of Repeated Treatment of GeXIVA[1,2] in Normal Rats
3. Discussion
4. Material and Methods
4.1. Animals
4.2. Compounds
4.3. Oxaliplatin-Induced Neuropathic Pain
4.4. Measurement of Mechanical Allodynia
4.5. Measurement of Cold Allodynia
4.6. Measurement of Tail-Flick Latency
4.7. Measurement of Grip Strength
4.8. Drug Administration Procedure
4.9. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Chamberlain, M.C. Neurotoxicity of cancer treatment. Curr. Oncol. Rep. 2010, 12, 60–67. [Google Scholar] [CrossRef]
- Han, Y.; Smith, M.T. Pathobiology of cancer chemotherapy-induced peripheral neuropathy (CIPN). Front. Pharmacol. 2013, 4, 156. [Google Scholar] [CrossRef] [PubMed]
- Hurst, R.; Rollema, H.; Bertrand, D. Nicotinic acetylcholine receptors: from basic science to therapeutics. Pharmacol. Ther. 2013, 137, 22–54. [Google Scholar] [CrossRef]
- Bertrand, D.; Terry, A.V., Jr. The wonderland of neuronal nicotinic acetylcholine receptors. Biochem. Pharmacol. 2018, 151, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Cardinale, A.; Margaritora, S.; Cesario, A. Nicotinic receptor and tobacco-related cancer. Life Sci. 2012, 91, 1087–1092. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Weyn, A.; Mikkelsen, J.D. Hippocampal alpha7 nicotinic acetylcholine receptor levels in patients with schizophrenia, bipolar disorder, or major depressive disorder. Bipolar. Disord. 2011, 13, 701–707. [Google Scholar] [CrossRef]
- Rahman, S.; Engleman, E.A.; Bell, R.L. Nicotinic receptor modulation to treat alcohol and drug dependence. Front. Neurosci. 2014, 8, 426. [Google Scholar] [CrossRef] [PubMed]
- Leib, C.; Goser, S.; Luthje, D.; Ottl, R.; Tretter, T.; Lasitschka, F.; Zittrich, S.; Pfitzer, G.; Katus, H.A.; Kaya, Z. Role of the cholinergic antiinflammatory pathway in murine autoimmune myocarditis. Circ. Res. 2011, 109, 130–140. [Google Scholar] [CrossRef]
- Umana, I.C.; Daniele, C.A.; Miller, B.A.; Abburi, C.; Gallagher, K.; Brown, M.A.; Mason, P.; McGehee, D.S. Nicotinic modulation of descending pain control circuitry. Pain 2017, 158, 1938–1950. [Google Scholar] [CrossRef]
- Hone, A.J.; Servent, D.; McIntosh, J.M. alpha9-containing nicotinic acetylcholine receptors and the modulation of pain. Br. J. Pharmacol. 2018, 175, 1915–1927. [Google Scholar] [CrossRef]
- Hone, A.J.; McIntosh, J.M. Nicotinic acetylcholine receptors in neuropathic and inflammatory pain. FEBS Lett. 2018, 592, 1045–1062. [Google Scholar] [CrossRef]
- Di Cesare Mannelli, L.; Cinci, L.; Micheli, L.; Zanardelli, M.; Pacini, A.; McIntosh, J.M.; Ghelardini, C. alpha-conotoxin RgIA protects against the development of nerve injury-induced chronic pain and prevents both neuronal and glial derangement. Pain 2014, 155, 1986–1995. [Google Scholar] [CrossRef]
- McIntosh, J.M.; Absalom, N.; Chebib, M.; Elgoyhen, A.B.; Vincler, M. Alpha9 nicotinic acetylcholine receptors and the treatment of pain. Biochem. Pharmacol. 2009, 78, 693–702. [Google Scholar] [CrossRef]
- Holtman, J.R.; Dwoskin, L.P.; Dowell, C.; Wala, E.P.; Zhang, Z.; Crooks, P.A.; McIntosh, J.M. The novel small molecule alpha9alpha10 nicotinic acetylcholine receptor antagonist ZZ-204G is analgesic. Eur. J. Pharmacol. 2011, 670, 500–508. [Google Scholar] [CrossRef]
- Satkunanathan, N.; Livett, B.; Gayler, K.; Sandall, D.; Down, J.; Khalil, Z. Alpha-conotoxin Vc1.1 alleviates neuropathic pain and accelerates functional recovery of injured neurones. Brain Res. 2005, 1059, 149–158. [Google Scholar] [CrossRef]
- Pacini, A.; Micheli, L.; Maresca, M.; Branca, J.J.; McIntosh, J.M.; Ghelardini, C.; Di Cesare Mannelli, L. The alpha9alpha10 nicotinic receptor antagonist alpha-conotoxin RgIA prevents neuropathic pain induced by oxaliplatin treatment. Exp. Neurol. 2016, 282, 37–48. [Google Scholar] [CrossRef]
- Christensen, S.B.; Hone, A.J.; Roux, I.; Kniazeff, J.; Pin, J.P.; Upert, G.; Servent, D.; Glowatzki, E.; McIntosh, J.M. RgIA4 Potently Blocks Mouse alpha9alpha10 nAChRs and Provides Long Lasting Protection against Oxaliplatin-Induced Cold Allodynia. Front. Cell Neurosci. 2017, 11, 219. [Google Scholar] [CrossRef] [PubMed]
- Romero, H.K.; Christensen, S.B.; Di Cesare Mannelli, L.; Gajewiak, J.; Ramachandra, R.; Elmslie, K.S.; Vetter, D.E.; Ghelardini, C.; Iadonato, S.P.; Mercado, J.L.; Olivera, B.M.; et al. Inhibition of alpha9alpha10 nicotinic acetylcholine receptors prevents chemotherapy-induced neuropathic pain. Proc. Natl. Acad. Sci. USA 2017, 114, E1825–E1832. [Google Scholar] [CrossRef]
- Luo, S.; Zhangsun, D.; Harvey, P.J.; Kaas, Q.; Wu, Y.; Zhu, X.; Hu, Y.; Li, X.; Tsetlin, V.I.; Christensen, S.; Romero, H.K.; et al. Cloning, synthesis, and characterization of alphaO-conotoxin GeXIVA, a potent alpha9alpha10 nicotinic acetylcholine receptor antagonist. Proc. Natl. Acad. Sci. USA 2015, 112, E4026–E4035. [Google Scholar] [CrossRef]
- Li, X.; Hu, Y.; Wu, Y.; Huang, Y.; Yu, S.; Ding, Q.; Zhangsun, D.; Luo, S. Anti-hypersensitive effect of intramuscular administration of alphaO-conotoxin GeXIVA[1,2] and GeXIVA[1,4] in rats of neuropathic pain. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 66, 112–119. [Google Scholar] [CrossRef]
- Seretny, M.; Currie, G.L.; Sena, E.S.; Ramnarine, S.; Grant, R.; MacLeod, M.R.; Colvin, L.A.; Fallon, M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 2014, 155, 2461–2470. [Google Scholar] [CrossRef]
- Hershman, D.L.; Lacchetti, C.; Dworkin, R.H.; Lavoie Smith, E.M.; Bleeker, J.; Cavaletti, G.; Chauhan, C.; Gavin, P.; Lavino, A.; Lustberg, M.B.; Paice, J.; et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 2014, 32, 1941–1967. [Google Scholar] [CrossRef]
- Chou, R.; Turner, J.A.; Devine, E.B.; Hansen, R.N.; Sullivan, S.D.; Blazina, I.; Dana, T.; Bougatsos, C.; Deyo, R.A. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann. Intern. Med. 2015, 162, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Devereaux, A.L.; Mercer, S.L.; Cunningham, C.W. DARK Classics in Chemical Neuroscience: Morphine. ACS Chem. Neurosci. 2018, 9, 2395–2407. [Google Scholar] [CrossRef]
- Perez de Vega, M.J.; Ferrer-Montiel, A.; Gonzalez-Muniz, R. Recent progress in non-opioid analgesic peptides. Arch. Biochem. Biophys. 2018, 660, 36–52. [Google Scholar] [CrossRef]
- Williams, J.A.; Day, M.; Heavner, J.E. Ziconotide: an update and review. Expert Opin. Pharmacother. 2008, 9, 1575–1583. [Google Scholar] [CrossRef]
- Avan, A.; Postma, T.J.; Ceresa, C.; Avan, A.; Cavaletti, G.; Giovannetti, E.; Peters, G.J. Platinum-induced neurotoxicity and preventive strategies: past, present, and future. Oncologist 2015, 20, 411–432. [Google Scholar] [CrossRef]
- Julius, D. TRP channels and pain. Annu. Rev. Cell Dev. Biol. 2013, 29, 355–384. [Google Scholar] [CrossRef]
- Descoeur, J.; Pereira, V.; Pizzoccaro, A.; Francois, A.; Ling, B.; Maffre, V.; Couette, B.; Busserolles, J.; Courteix, C.; Noel, J.; Lazdunski, M.; et al. Oxaliplatin-induced cold hypersensitivity is due to remodelling of ion channel expression in nociceptors. EMBO Mol. Med. 2011, 3, 266–278. [Google Scholar] [CrossRef]
- Joseph, E.K.; Levine, J.D. Comparison of oxaliplatin- and cisplatin-induced painful peripheral neuropathy in the rat. J. Pain 2009, 10, 534–541. [Google Scholar] [CrossRef]
- Elgoyhen, A.B.; Johnson, D.S.; Boulter, J.; Vetter, D.E.; Heinemann, S. Alpha 9: an acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell 1994, 79, 705–715. [Google Scholar] [CrossRef]
- Elgoyhen, A.B.; Vetter, D.E.; Katz, E.; Rothlin, C.V.; Heinemann, S.F.; Boulter, J. alpha10: a determinant of nicotinic cholinergic receptor function in mammalian vestibular and cochlear mechanosensory hair cells. Proc. Natl. Acad. Sci. USA 2001, 98, 3501–3506. [Google Scholar] [CrossRef]
- Baumann, L.; Kauschke, V.; Vikman, A.; Durselen, L.; Krasteva-Christ, G.; Kampschulte, M.; Heiss, C.; Yee, K.T.; Vetter, D.E.; Lips, K.S. Deletion of nicotinic acetylcholine receptor alpha9 in mice resulted in altered bone structure. Bone 2019, 120, 285–296. [Google Scholar] [CrossRef]
- Lips, K.S.; Pfeil, U.; Kummer, W. Coexpression of alpha 9 and alpha 10 nicotinic acetylcholine receptors in rat dorsal root ganglion neurons. Neuroscience 2002, 115, 1–5. [Google Scholar] [CrossRef]
- Uspenska, K.; Lykhmus, O.; Obolenskaya, M.; Pons, S.; Maskos, U.; Komisarenko, S.; Skok, M. Mitochondrial Nicotinic Acetylcholine Receptors Support Liver Cells Viability After Partial Hepatectomy. Front. Pharmacol. 2018, 9, 626. [Google Scholar] [CrossRef]
- Peng, H.; Ferris, R.L.; Matthews, T.; Hiel, H.; Lopez-Albaitero, A.; Lustig, L.R. Characterization of the human nicotinic acetylcholine receptor subunit alpha (alpha) 9 (CHRNA9) and alpha (alpha) 10 (CHRNA10) in lymphocytes. Life Sci. 2004, 76, 263–280. [Google Scholar] [CrossRef]
- Lykhmus, O.; Voytenko, L.P.; Lips, K.S.; Bergen, I.; Krasteva-Christ, G.; Vetter, D.E.; Kummer, W.; Skok, M. Nicotinic Acetylcholine Receptor alpha9 and alpha10 Subunits Are Expressed in the Brain of Mice. Front. Cell Neurosci. 2017, 11, 282. [Google Scholar] [CrossRef]
- Morley, B.J.; Whiteaker, P.; Elgoyhen, A.B. Commentary: Nicotinic Acetylcholine Receptor alpha9 and alpha10 Subunits Are Expressed in the Brain of Mice. Front. Cell Neurosci. 2018, 12, 104. [Google Scholar] [CrossRef]
- Simard, A.R.; Gan, Y.; St-Pierre, S.; Kousari, A.; Patel, V.; Whiteaker, P.; Morley, B.J.; Lukas, R.J.; Shi, F.D. Differential modulation of EAE by alpha9*- and beta2*-nicotinic acetylcholine receptors. Immunol. Cell Biol. 2013, 91, 195–200. [Google Scholar] [CrossRef]
- Zakrzewicz, A.; Richter, K.; Agne, A.; Wilker, S.; Siebers, K.; Fink, B.; Krasteva-Christ, G.; Althaus, M.; Padberg, W.; Hone, A.J.; McIntosh, J.M.; et al. Canonical and Novel Non-Canonical Cholinergic Agonists Inhibit ATP-Induced Release of Monocytic Interleukin-1beta via Different Combinations of Nicotinic Acetylcholine Receptor Subunits alpha7, alpha9 and alpha10. Front. Cell Neurosci. 2017, 11, 189. [Google Scholar] [CrossRef]
- Wala, E.P.; Crooks, P.A.; McIntosh, J.M.; Holtman, J.R., Jr. The Novel Small Molecule alpha9alpha10 Nicotinic Receptor Antagonist Prevents and Reverses Chemotherapy-Evoked Neuropathic Pain in Rats. Anesth. Analg. 2012, 115, 713. [Google Scholar]
- Livett, B.G.; Sandall, D.W.; Keays, D.; Down, J.; Gayler, K.R.; Satkunanathan, N.; Khalil, Z. Therapeutic applications of conotoxins that target the neuronal nicotinic acetylcholine receptor. Toxicon 2006, 48, 810–829. [Google Scholar] [CrossRef] [PubMed]
- Safavi-Hemami, H.; Brogan, S.E.; Olivera, B.M. Pain therapeutics from cone snail venoms: From Ziconotide to novel non-opioid pathways. J. Proteomics 2019, 190, 12–20. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- National Research Council Committee. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press (US): Washington, DC, USA, 2011. [Google Scholar]
- Duan, Z.; Su, Z.; Wang, H.; Pang, X. Involvement of pro-inflammation signal pathway in inhibitory effects of rapamycin on oxaliplatin-induced neuropathic pain. Mol. Pain 2018, 14, 1744806918769426. [Google Scholar] [CrossRef]
- Deuis, J.R.; Dvorakova, L.S.; Vetter, I. Methods Used to Evaluate Pain Behaviors in Rodents. Front. Mol. Neurosci. 2017, 10, 284. [Google Scholar] [CrossRef]
- Dixon, W.J. Efficient analysis of experimental observations. Annu. Rev. Pharmacol. Toxicol. 1980, 20, 441–462. [Google Scholar] [CrossRef]
- Duggett, N.A.; Flatters, S.J.L. Characterization of a rat model of bortezomib-induced painful neuropathy. Br. J. Pharmacol. 2017, 174, 4812–4825. [Google Scholar] [CrossRef]
- Ali, A.; Ahmad, F.J.; Pillai, K.K.; Vohora, D. Evidence of the antiepileptic potential of amiloride with neuropharmacological benefits in rodent models of epilepsy and behavior. Epilepsy Behav. 2004, 5, 322–328. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Li, X.; Zhangsun, D.; Yu, G.; Su, R.; Luo, S. The α9α10 Nicotinic Acetylcholine Receptor Antagonist αO-Conotoxin GeXIVA[1,2] Alleviates and Reverses Chemotherapy-Induced Neuropathic Pain. Mar. Drugs 2019, 17, 265. https://doi.org/10.3390/md17050265
Wang H, Li X, Zhangsun D, Yu G, Su R, Luo S. The α9α10 Nicotinic Acetylcholine Receptor Antagonist αO-Conotoxin GeXIVA[1,2] Alleviates and Reverses Chemotherapy-Induced Neuropathic Pain. Marine Drugs. 2019; 17(5):265. https://doi.org/10.3390/md17050265
Chicago/Turabian StyleWang, Huanbai, Xiaodan Li, Dongting Zhangsun, Gang Yu, Ruibin Su, and Sulan Luo. 2019. "The α9α10 Nicotinic Acetylcholine Receptor Antagonist αO-Conotoxin GeXIVA[1,2] Alleviates and Reverses Chemotherapy-Induced Neuropathic Pain" Marine Drugs 17, no. 5: 265. https://doi.org/10.3390/md17050265
APA StyleWang, H., Li, X., Zhangsun, D., Yu, G., Su, R., & Luo, S. (2019). The α9α10 Nicotinic Acetylcholine Receptor Antagonist αO-Conotoxin GeXIVA[1,2] Alleviates and Reverses Chemotherapy-Induced Neuropathic Pain. Marine Drugs, 17(5), 265. https://doi.org/10.3390/md17050265





