Preparation, Mechanical Properties, and Biocompatibility of Graphene Oxide-Reinforced Chitin Monofilament Absorbable Surgical Sutures
Abstract
1. Introduction
2. Results
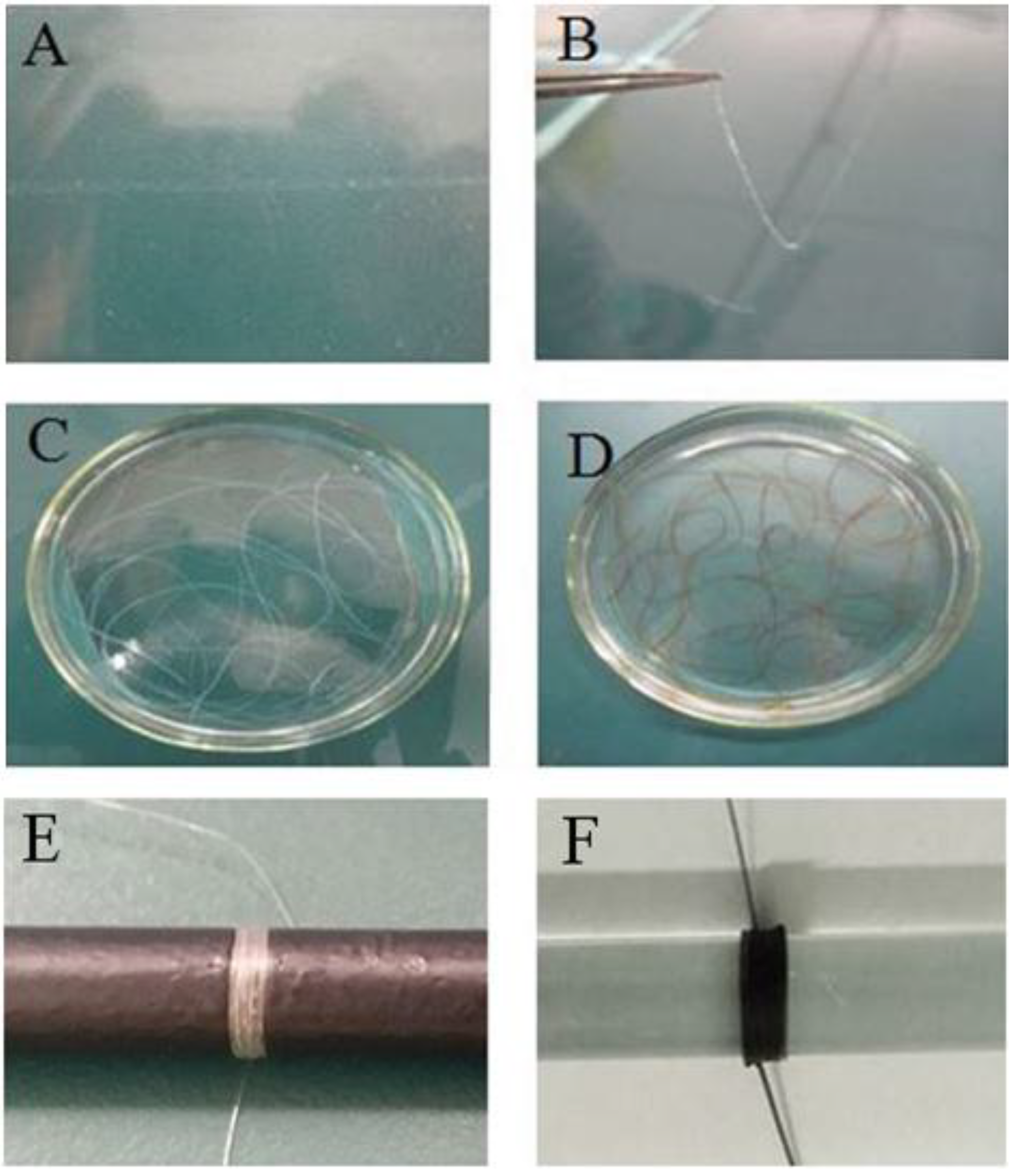
2.1. Preparation of GO-CT Sutures
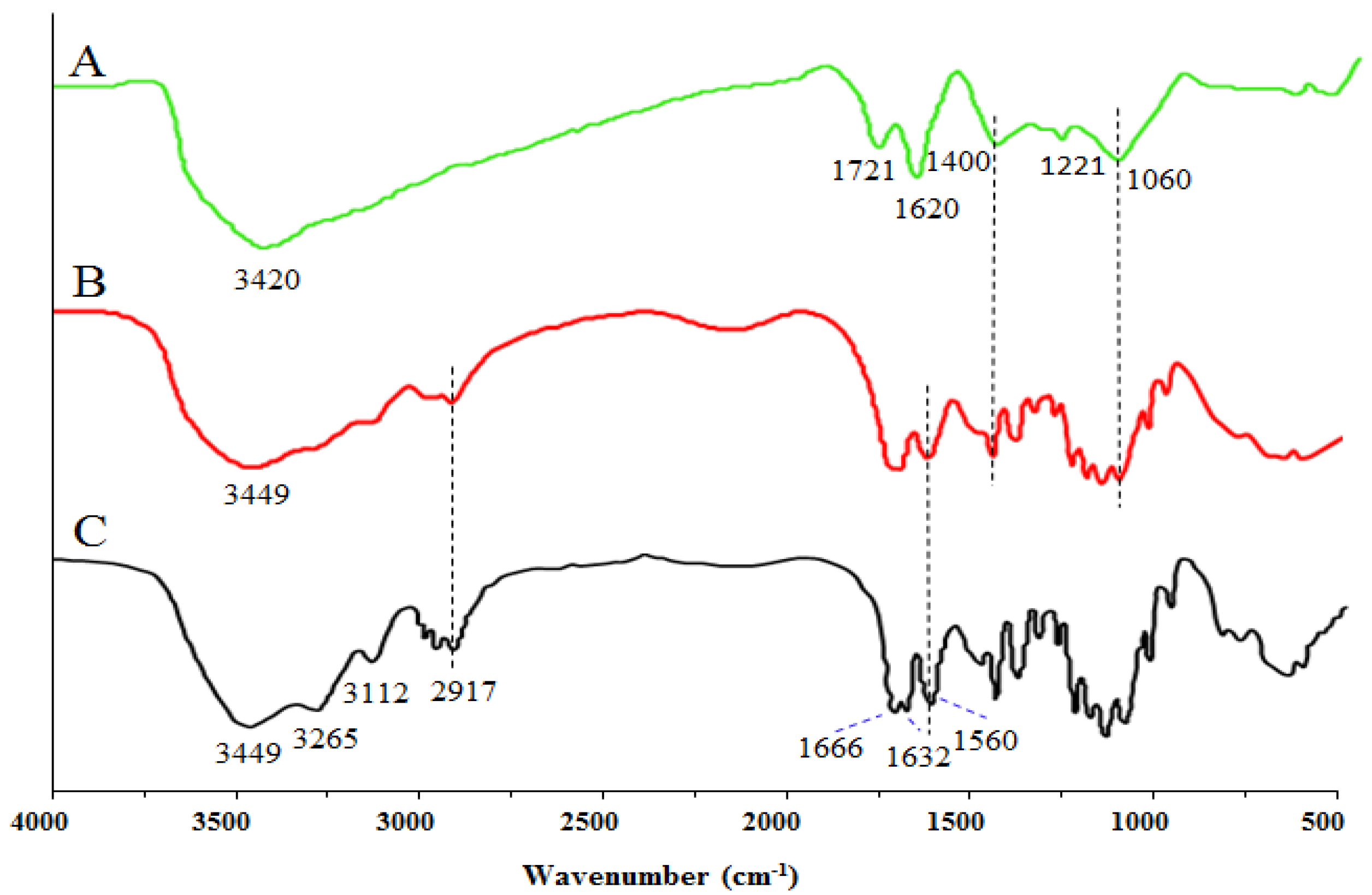
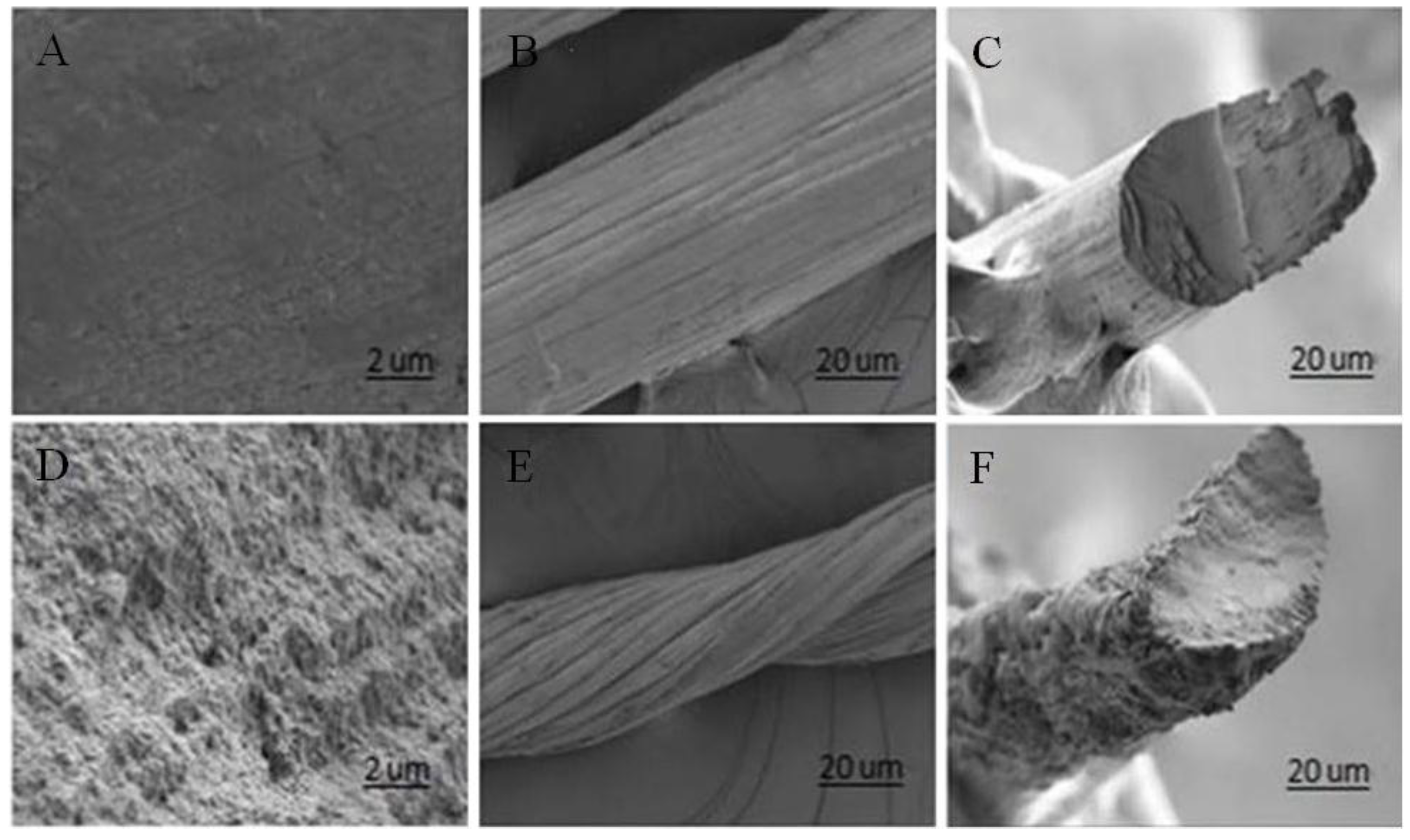
2.2. Structure of GO-CT Sutures
2.3. Physicochemical Properties of GO-CT Sutures
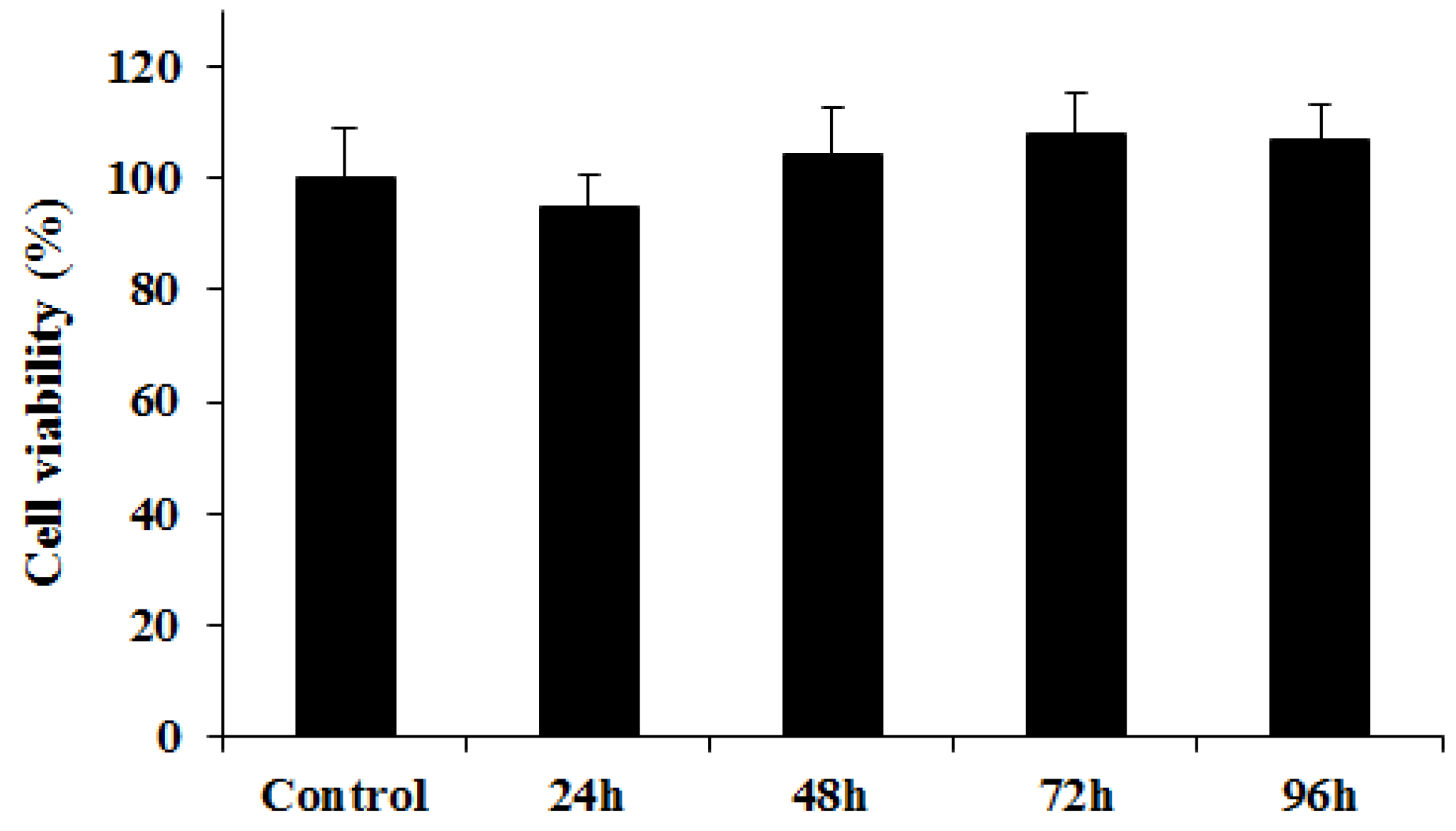
2.4. Cytotoxicity Assay
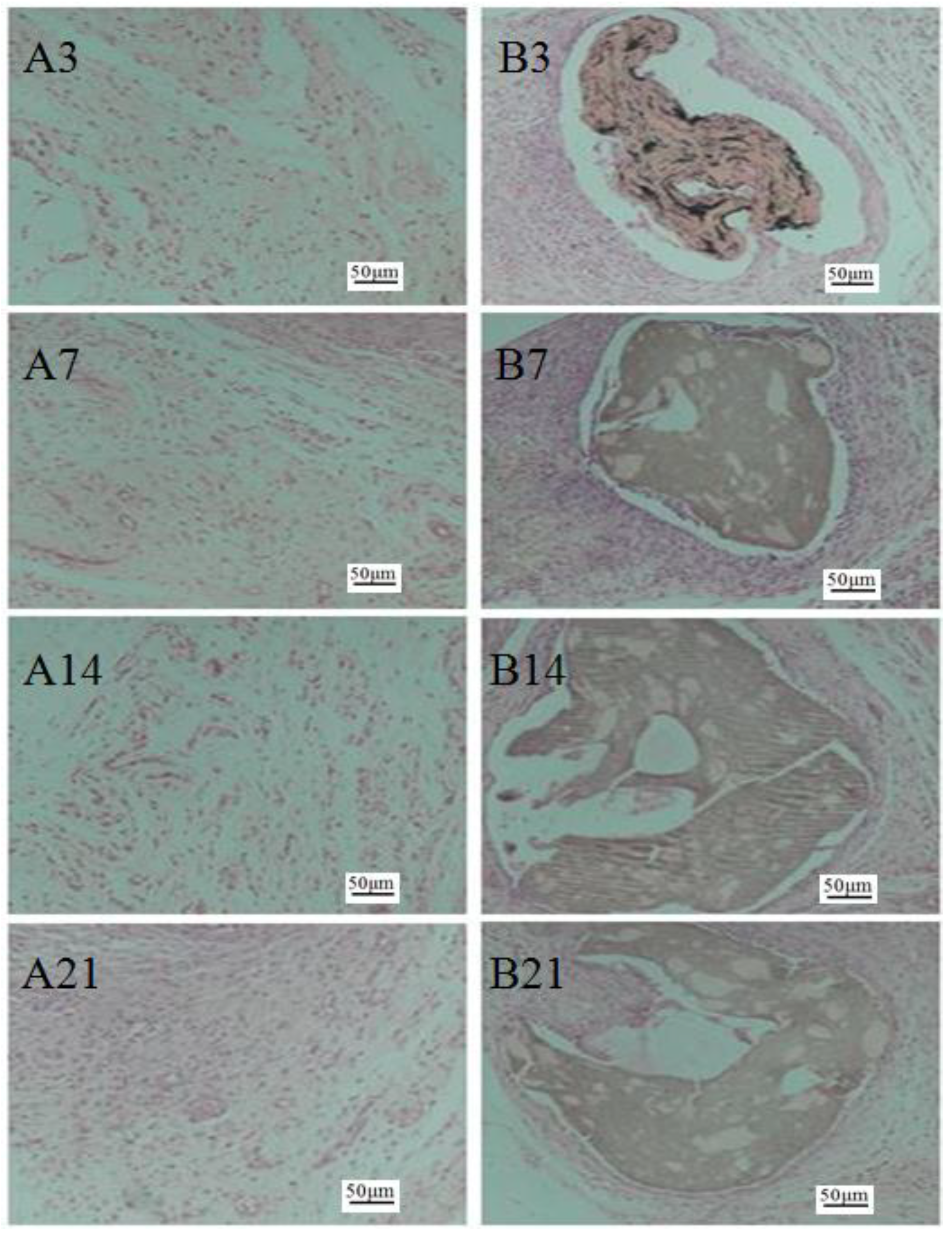
2.5. Histological Observation
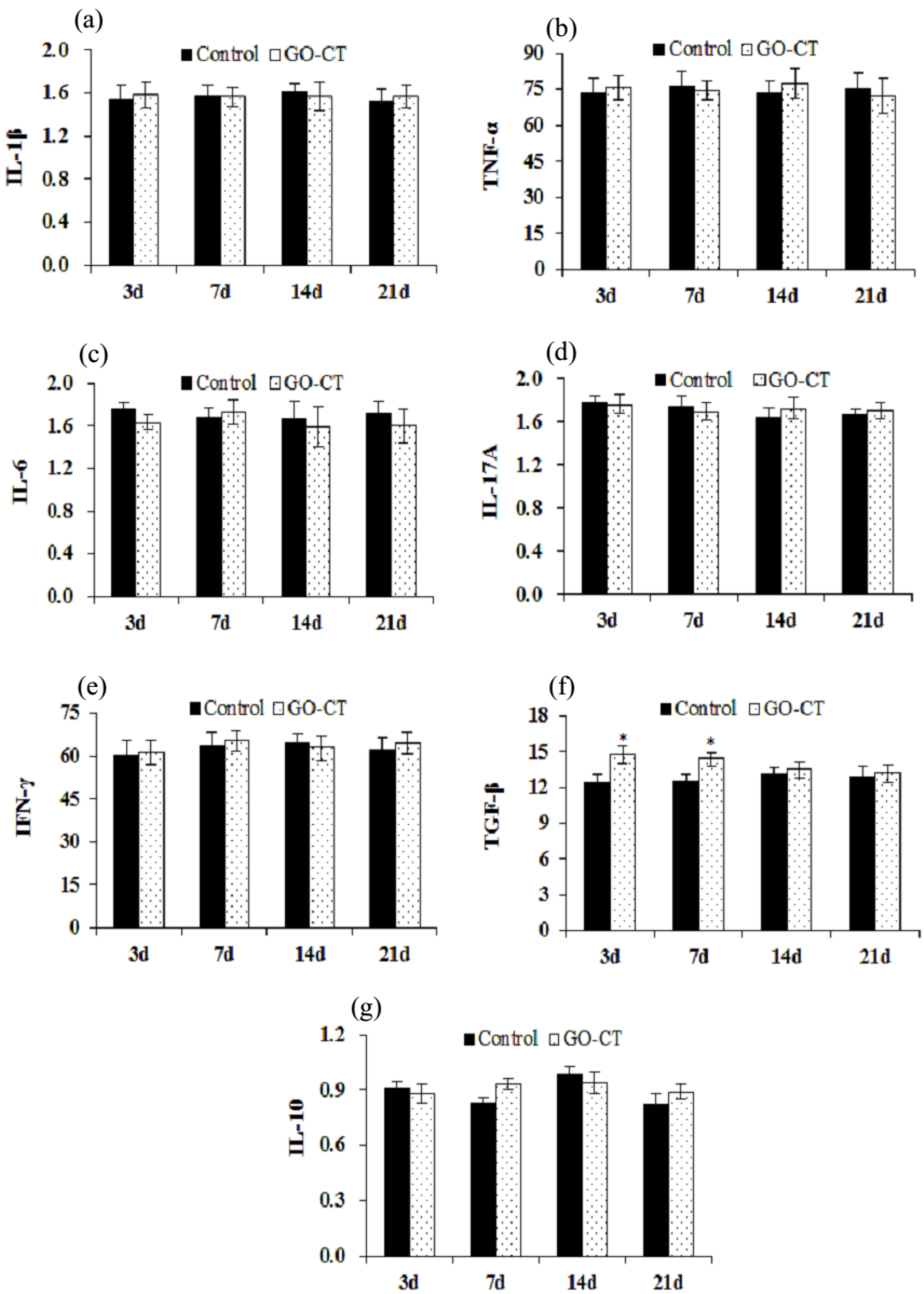
2.6. Gene Expression of Inflammation-Related Factors
3. Discussion
4. Materials and Methods
4.1. Materials and Regents
4.2. Preparation of CT and GO-CT Sutures
4.3. Fourier-Transformed Infrared Spectroscopy and Scanning Electron Microscopy
4.4. Mechanical Properties
4.5. Cytotoxicity Assay
4.6. Histological Observation
4.7. Sample Collection
4.8. Quantitative Real-Time PCR
4.9. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pillai, C.K.S.; Sharma, C.P. Review paper: Absorbable polymeric surgical sutures: Chemistry, production, properties, biodegradability, and performance. J. Biomater. Appl. 2010, 25, 291–366. [Google Scholar] [CrossRef] [PubMed]
- Shao, K.; Han, B.Q.; Gao, J.N.; Jiang, Z.W.; Liu, W.Z.; Liu, W.S.; Liang, Y. Fabrication and feasibility study of an absorbable diacetyl chitin surgical suture for wounding healing. J. Biomed. Mater. Res. B 2016, 104, 116–125. [Google Scholar] [CrossRef]
- Moy, R.L.; Waldman, B.; Hein, D.W. A review of sutures and suturing techniques. Dermatol. Surg. 1992, 18, 785–795. [Google Scholar] [CrossRef]
- Shao, K. Biological Function and Biosafety Research of the Absorbable Chitin Surgical Suture. Ph.D. Thesis, Ocean University of China, Qingdao, China, 2013. [Google Scholar]
- Zhou, D.; Yang, R.; Yang, T.; Xing, M.; Luo, G. Preparation of chitin-amphipathic anion/quaternary ammonium salt ecofriendly dressing and its effect on wound healing in mice [Corrigendum]. Int. J. Nanomed. 2018, 13, 5255–5256. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Ifuku, S.; Osaki, T.; Okamoto, Y.; Minami, S. Preparation and biomedical applications of chitin and chitosan nanofibers. J. Biomed. Nanotechnol. 2014, 10, 2891–2920. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Yamada, H.; Tanaka, I.; Kaba, N.; Matsuura, M.; Okumura, M.; Kadosawa, T.; Fujinaga, T. Accelerating effects of chitosan for healing at early phase of experimental open wound in dogs. Biomaterials 1999, 20, 1407–1414. [Google Scholar] [CrossRef]
- Izumi, R.; Komada, S.; Ochi, K.; Karasawa, L.; Osaki, T.; Murahata, Y.; Tsuka, T.; Imagawa, T.; Itoh, N.; Okamoto, Y.; et al. Favorable effects of superficially deacetylated chitin nanofibrils on the wound healing process. Carbohydr. Polym. 2015, 123, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Prabaharan, M.; Kumar, P.T.S.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Tabuchi, R.; Azuma, K.; Izumi, R.; Tanou, T.; Okamoto, Y.; Nagae, T.; Iohara, D.; Uekama, K.; Otagiri, M.; Hirayama, F.; et al. Biomaterials based on freeze dried surface-deacetylated chitin nanofibers reinforced with sulfobutyl ether β-cyclodextrin gel in wound dressing applications. Int. J. Pharm. 2016, 511, 1080–1087. [Google Scholar] [CrossRef]
- King, C.A.; Shamshina, J.L.; Zavgorodnya, O.; Cutfield, T.; Rogers, R.D. Porous chitin microbeads for more sustainable cosmetics. ACS Sustain. Chem. Eng. 2017, 5, 11660–11667. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Zavgorodnya, O.; Rogers, R.D. Advances in processing chitin as a promising biomaterial from ionic liquids. Adv. Biochem. Eng. Biotechnol. 2018, 63, 1–22. [Google Scholar]
- Jayakumar, R.; Prabaharan, M.; Nair, S.V.; Tokura, S.; Selvamurugan, N. Novel carboxymethyl derivatives of chitin and chitosan materials and their biomedical applications. Prog. Mater. Sci. 2010, 55, 675–709. [Google Scholar] [CrossRef]
- Shen, X.P.; Shamshina, J.L.; Berton, P.; Gurau, G.; Rogers, R.D. Hydrogels based on cellulose and chitin: Fabrication, properties, and applications. Green Chem. 2015, 18. [Google Scholar] [CrossRef]
- Chang, C.; Chen, S.; Zhang, L. Novel hydrogels prepared via direct dissolution of chitin at low temperature: Structure and biocompatibility. J. Mater. Chem. 2011, 21, 3865–3871. [Google Scholar] [CrossRef]
- Terbojevich, M.; Carraro, C.; Cosani, A. Solution studies of the chitin-lithium chloride-N,N-di-methylacetamide system. Carbohydr. Res. 1988, 180, 73–86. [Google Scholar] [CrossRef]
- Tamura, H.; Nagahama, H.; Tokura, S. Preparation of chitin hydrogel under mild conditions. Cellulose 2006, 13, 357–364. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R.; Muzzarelli, R.A.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef]
- Barber, P.S.; Griggs, C.S.; Bonner, J.R.; Rogers, R.D. Electrospinning of chitin nanofibers directly from an ionic liquid extract of shrimp shells. Green Chem. 2013, 15, 601–607. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of cellulose with ionic liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhong, Z.; Duan, B.; Wang, Y.; Zhang, L. Novel fibers fabricated directly from chitin solution and their application as wound dressing. J. Mater. Chem. B 2014, 2, 3427–3432. [Google Scholar] [CrossRef]
- Guo, Y.; Duan, B.; Zhou, J.; Zhu, P. Chitin/graphene oxide composite films with enhanced mechanical properties prepared in NaOH/urea aqueous solution. Cellulose 2014, 21, 1781–1791. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Oleksandra, Z.; Paula, B.; Chhotaray, P.K.; Hemant, C.; Rogers, R.D. An ionic liquid platform for spinning composite chitin-poly(lactic acid) fibers. ACS Sustain. Chem. Eng. 2018. [Google Scholar] [CrossRef]
- Guo, Y.; Duan, B.; Cui, L.; Zhu, P. Construction of chitin/graphene oxide hybrid hydrogels. Cellulose 2015, 22, 2035–2043. [Google Scholar] [CrossRef]
- Solìs, Y.M.; Panella, G.; Fioravanti, G.; Perrozzi, F.; Passacantando, M.; Giansanti, F.; Ardini, M.; Ottaviano, L.; Cimini, A.; Peniche, C.; et al. Biocompatibility of composites based on chitosan, apatite and graphene oxide for tissue applications. J. Biomed. Mater. Res. A 2018, 106, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xiong, P.; Yan, F.; Li, S.J.; Ren, C.H.; Yin, Z.C.; Li, A.; Li, H.F.; Ji, X.M.; Zheng, Y.F.; et al. An overview of graphene-based hydroxyapatite composites for orthopedic applications. Bioact. Mater. 2018, 3, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Rana, V.K.; Choi, M.C.; Kong, J.Y.; Kim, G.Y. Synthesis and drug-delivery behavior of chitosan-functionalized graphene oxide hybrid nanosheets. Macromol. Mater. Eng. 2011, 296, 131–140. [Google Scholar] [CrossRef]
- Chung, C.; Kim, Y.K.; Shin, D.; Ryoo, S.R.; Hong, B.H.; Min, D.H. Biomedical applications of graphene and graphene oxide. Acc. Chem. Res. 2013, 46, 2211–2224. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Huang, X.; Zhou, X.; Wu, H.; Guo, S. Assembly of graphene oxide-enzyme conjugates through hydrophobic interaction. Small 2012, 8, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef] [PubMed]
- Peruzynska, M.; Cendrowski, K.; Barylak, M.; Tkacz, M.; Piotrowska, K.; Kurzawski, M.; Mijowska, E.; Drozdzik, M. Comparative in vitro study of single and four layer graphene oxide nanoflakes-cytotoxicity and cellular uptake. Toxicol. In Vitro 2017, 41, 205–213. [Google Scholar] [CrossRef]
- Chen, Y.; Qi, Y.; Tai, Z.; Yan, X.; Xue, Q. Preparation, mechanical properties and biocompatibility of graphene oxide/ultrahigh molecular weight polyethylene composites. Eur. Polym. J. 2012, 48, 1026–1033. [Google Scholar] [CrossRef]
- Yang, K.; Wan, J.M.; Zhang, S.; Zhang, Y.J.; Lee, S.T.; Liu, Z. In vivo pharmacokinetics, long-term biodistribution, and toxicology of PEGylated graphene in mice. ACS Nano 2011, 5, 516–522. [Google Scholar] [CrossRef]
- Kurapati, R.; Russier, J.; Squillaci, M.A.; Treossi, E.; Ménard-Moyon, C.; Del Rio-Castillo, A.E.; Vazquez, E.; Samorì, P.; Palermo, V.; Bianco, A. Dispersibility-dependent biodegradation of graphene oxide by myeloperoxidase. Small 2015, 11, 3985–3994. [Google Scholar] [CrossRef]
- Liang, S.; Xu, S.; Zhang, D.; He, J.; Chu, M. Reproductive toxicity of nanoscale graphene oxide in male mice. Nanotoxicology 2015, 9, 92–105. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Liu, Q.; Li, Q.; Cheng, Y.; Zheng, Y.; Xi, T.; Wei, S. In situ synthesis and biocompatibility of nano hydroxyapatite on pristine and chitosan functionalized graphene oxide. J. Mater. Chem. B 2013, 1, 475–484. [Google Scholar] [CrossRef]
- Shin, S.R.; Aghaei-Ghareh-Bolagh, B.; Dang, T.; Topkaya, S.N.; Gao, X.; Yang, S.; Jung, S.; Oh, J.; Dokmeci, M.R.; Tang, X.; et al. Cell-laden microengineered and mechanically tunable hybrid hydrogels of gelatin and graphene oxide. Adv. Mater. 2013, 25, 6385–6639. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wu, Q.; Sun, Y.; Bai, H.; Shi, G. There-dimensional self-assembly of graphene oxide and DNA into multifunctional hydrogels. ACS Nano 2010, 4, 7358–7362. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, L.; Yang, J.; Zhang, X.; Xu, M. Structure and properties of cellulose films reinforced by chitin whiskers. Macromol. Mater. Eng. 2013, 298, 303–310. [Google Scholar] [CrossRef]
- Park, S.; Lee, K.; Bozoklu, G.; Cai, W.; Nguyen, S.T.; Ruoff, R.S. Graphene oxide papers modified by divalent ions enhancing mechanical properties via chemical crosslinking. ACS Nano 2008, 2, 572–578. [Google Scholar] [CrossRef]
- Ifuku, S.; Nogi, M.; Abe, K.; Yoshioka, M.; Morimoto, M.; Saimoto, H.; Yano, H. Preparation of chitin nanofibers with a uniform width as α-chitin from crab shells. Biomacromolecule 2009, 10, 1584–1588. [Google Scholar] [CrossRef]
- Koziol, K.; Vilatela, J.; Moisala, A.; Motta, M.; Cunniff, P.; Sennett, M.; Windle, A. High-performance carbon nanotube fiber. Science 2007, 318, 1892–1895. [Google Scholar] [CrossRef]
- Han Gi, C.; Satish, K. Making strong fibers. Science 2008, 319, 908–909. [Google Scholar]
- Xu, Z.; Sun, H.; Zhao, X.; Gao, C. Ultrastrong fibers assembled from giant graphene oxide sheets. Adv. Mater. 2013, 25, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Chusak, R.B.; Dibbell, D.G. Clinical experience with polydioxanone monofilament absorbable sutures in plastic surgery. Plast. Reconstr. Surg. 1983, 72, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Benicewicz, B.C.; Hopper, P.K. Polymers for absorbable surgical sutures. J. Bioact. Compat. Pol. 1990, 5, 453–472. [Google Scholar] [CrossRef]
- Benicewicz, B.C.; Hopper, P.K. Review: Polymers for Absorbable Surgical Sutures-Part II. J. Bioact. Compat. Pol. 1991, 6, 64–94. [Google Scholar] [CrossRef]
- Alt, V.; Bechert, T.; Steinrucke, P.; Wagener, M.; Seidel, P.; Dingeldein, E.; Domann, E.; Schnettler, R. An in vitro assessment of the antibacterial properties and cytotoxicity of nanoparticulate silver bone cement. Biomaterials 2004, 25, 4383–4391. [Google Scholar] [CrossRef]
- Yang, Z. Characterization of Macromolecular Carboxymethyl Chitosan and the Study of Its Hemostatic and Woundhealing Funcitons. Master’s Thesis, China Ocean University, Qingdao, Shandong, China, June 2012. [Google Scholar]
- Wang, K.; Ruan, J.; Song, H.; Zhang, J.; Wo, Y.; Guo, S.; Cui, D. Biocompatibility of graphene oxide. Nanoscale Res. Lett. 2011, 6, 1–8. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, X.M.; Wang, C.S. Research progress of graphene-based materials in the application to biomedicine. Acta Pharm. Sin. 2012, 47, 291–298. [Google Scholar]
- Muzzarelli, R.A.A. Chitins and chitosans for the repair of wounded skin, nerve, cartilage and bone. Carbohydr. Polym. 2009, 76, 167–182. [Google Scholar] [CrossRef]
- Glazer, S.; Kruse, B. Effects of low-level He–Ne laser irradiation on the gene expression of IL-1β, TNF-α, IFN-γ, TGF-β, bFGF, and PDGF in rat’s gingiva. Lasers Med. Sci. 2008, 23, 331–335. [Google Scholar]
- Nomura, K.; Yamaguchi, M.; Abiko, Y. Inhibition of interleukin-1β production and gene expression in human gingival fibroblasts by low-energy laser irradiation. Lasers Med. Sci. 2001, 16, 218–223. [Google Scholar] [CrossRef]
- Abbas, A.K.; Lichtman, A.H.; Pober, J.S. Cellular and Molecular Immunology; Chapter 3; W.B. Saunders: Philadelphia, PA, USA, 1999. [Google Scholar]
- Awane, M.; Andres, P.G.; Li, D.J.; Reinecker, H.C. NF-κB-inducing kinase is a common mediator of IL17-, TNF-α-, and IL-1β-induced chemokine promoter activation in intestinal epithelial cells. J. Immunol. 1999, 162, 5337–5344. [Google Scholar] [PubMed]
- Heldin, C.H.; Westermark, B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol. Rev. 1999, 79, 1283–1316. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Wang, L.; Jin, P.; Li, N.; Meng, Y.; Wang, C.; Xu, M.; Zhang, Y.; Bian, J.; Deng, X. Methane alleviates carbon tetrachloride induced liver injury in mice: Anti-inflammatory action demonstrated by increased PI3k/Akt/GSK-3β-mediated IL-10 expression. J. Mol. Histol. 2017, 48, 301–310. [Google Scholar] [CrossRef]
- Yao, Y.; Simard, A.R.; Shi, F.D.; Hao, J. IL-10-producing lymphocytes in inflammatory disease. Int. Rev. Immunol. 2013, 32, 324–336. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, Y.L.; Chen, D.H. Effects of chitin and sepia ink hybrid hemostatic sponge on the blood parameters of mice. Mar. Drugs 2014, 12, 2269–2281. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
| Suture | Water (%) | Dash (%) | Bacterial endotoxin (EU/mg) | Heavy metal (ppm) |
|---|---|---|---|---|
| GO-CT | 0.03 | 0.04 | <0.20 | <6 |
| Index | Suture | |
|---|---|---|
| GO-CT | CT | |
| Breaking strength (MPa) | 183.14 ± 29.87 * | 89.93 ± 9.55 |
| Breaking elongation (%) | 17.34 ± 2.78 | 20.43 ± 3.21 |
| Knot strength (MPa) | 96.92 ± 10.76 * | 43.95 ± 8.07 |
| Knot-pull strength (MPa) | 124.66 ± 11.03 * | 68.35 ± 7.85 |
| Swelling (%) | 108.18 ± 9.88 | 115.65 ± 10.13 |
| USP size | 2–0 | 2–0 |
| Diameter | 0.30–0.35 mm | 0.30–0.35 mm |
| Memory | scare | scare |
| Formation | monofilament | monofilament |
| Length | 50 cm | 50 cm |
| Gene | Primer | Sequence (5′-3′) | Amplicon Size (bp) |
|---|---|---|---|
| IL-1β | F | ACAGCAGCACATCAACAAGAG | 563 |
| R | CTTTCATCACACAGGACAGG | ||
| TNF-α | F | TACTGAACTTCGGGGTGATCGGTCC | 412 |
| R | CAGCCTTGTCCCTTGAAGAGAACC | ||
| IL-6 | F | GAAATGATGGATGCTTCCAAACTGG | 548 |
| R | CACTAGGTTTGCCGAGTAGATCTC | ||
| IL-17A | F | GCCGAGGCCAATAACTTTCT | 204 |
| R | AGAGTCCAGGGTGAAGTGGA | ||
| IFN-γ | F | ATGAGTGCTACACGCCGCGTCTTGG | 420 |
| R | GAGTTCATTGACAGCTTTGTGCTGG | ||
| TGF-β | F | GTAGCTCTTGCCATCGGG | 218 |
| R | GAACGTCCCGTCAACTCG | ||
| IL-10 | F | CAATAACTGCACCCACTTCC | 352 |
| R | ATTCTTCACCTGCTCCACTGC | ||
| β-actin | F | GTGGGGCGCCCCAGGCACCA | 416 |
| R | GTCCTTAATGTCACGCACGATTTC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Yin, B.; Xin, Y.; Li, L.; Ye, G.; Wang, J.; Shen, J.; Cui, X.; Yang, Q. Preparation, Mechanical Properties, and Biocompatibility of Graphene Oxide-Reinforced Chitin Monofilament Absorbable Surgical Sutures. Mar. Drugs 2019, 17, 210. https://doi.org/10.3390/md17040210
Zhang W, Yin B, Xin Y, Li L, Ye G, Wang J, Shen J, Cui X, Yang Q. Preparation, Mechanical Properties, and Biocompatibility of Graphene Oxide-Reinforced Chitin Monofilament Absorbable Surgical Sutures. Marine Drugs. 2019; 17(4):210. https://doi.org/10.3390/md17040210
Chicago/Turabian StyleZhang, Wei, Bin Yin, Yu Xin, Lei Li, Guanlin Ye, Junxian Wang, Jianfei Shen, Xiao Cui, and Qihui Yang. 2019. "Preparation, Mechanical Properties, and Biocompatibility of Graphene Oxide-Reinforced Chitin Monofilament Absorbable Surgical Sutures" Marine Drugs 17, no. 4: 210. https://doi.org/10.3390/md17040210
APA StyleZhang, W., Yin, B., Xin, Y., Li, L., Ye, G., Wang, J., Shen, J., Cui, X., & Yang, Q. (2019). Preparation, Mechanical Properties, and Biocompatibility of Graphene Oxide-Reinforced Chitin Monofilament Absorbable Surgical Sutures. Marine Drugs, 17(4), 210. https://doi.org/10.3390/md17040210




