Investigating the Mechanism for the Enhanced Oxidation Stability of Microencapsulated Omega-3 Concentrates
Abstract
1. Introduction
2. Results
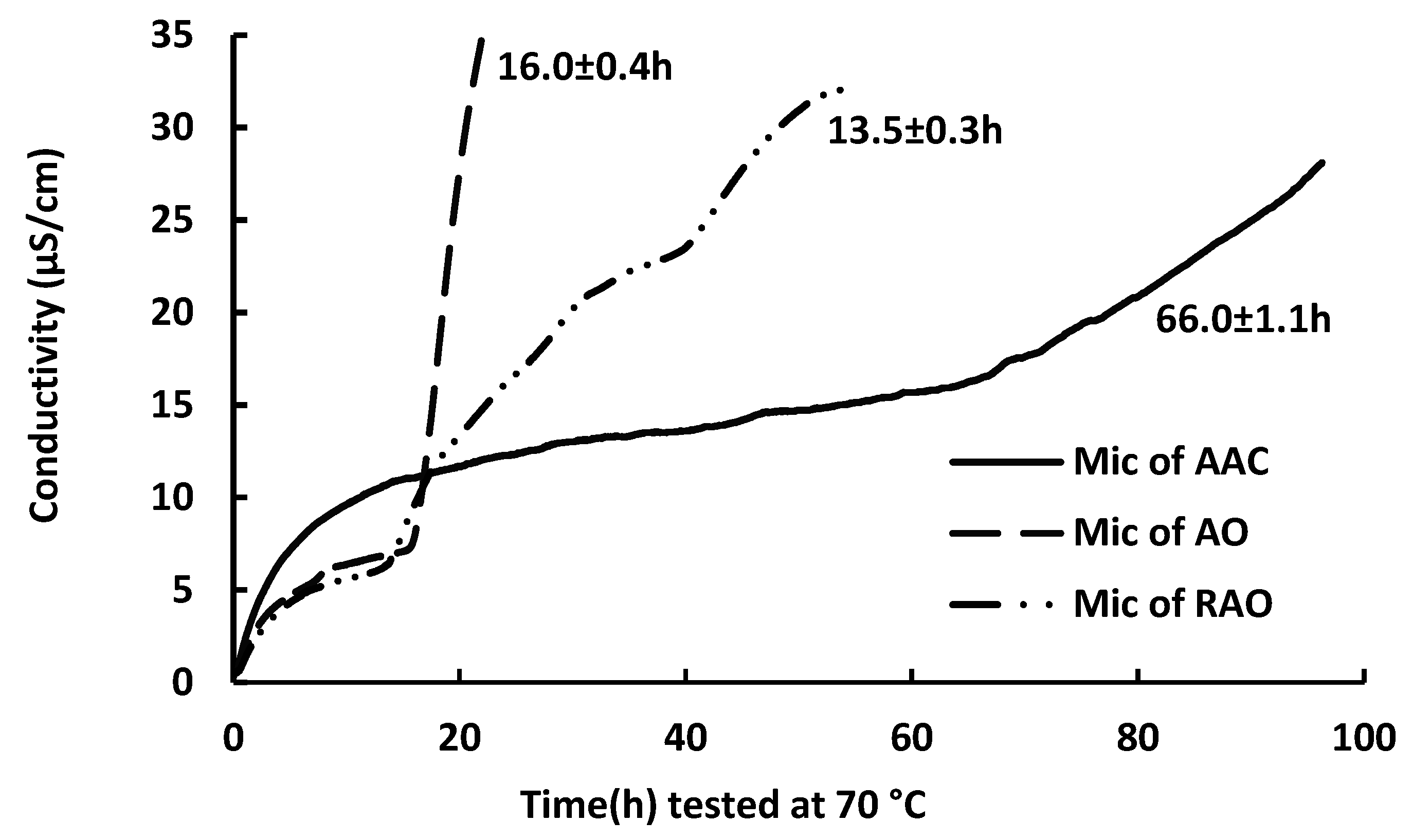
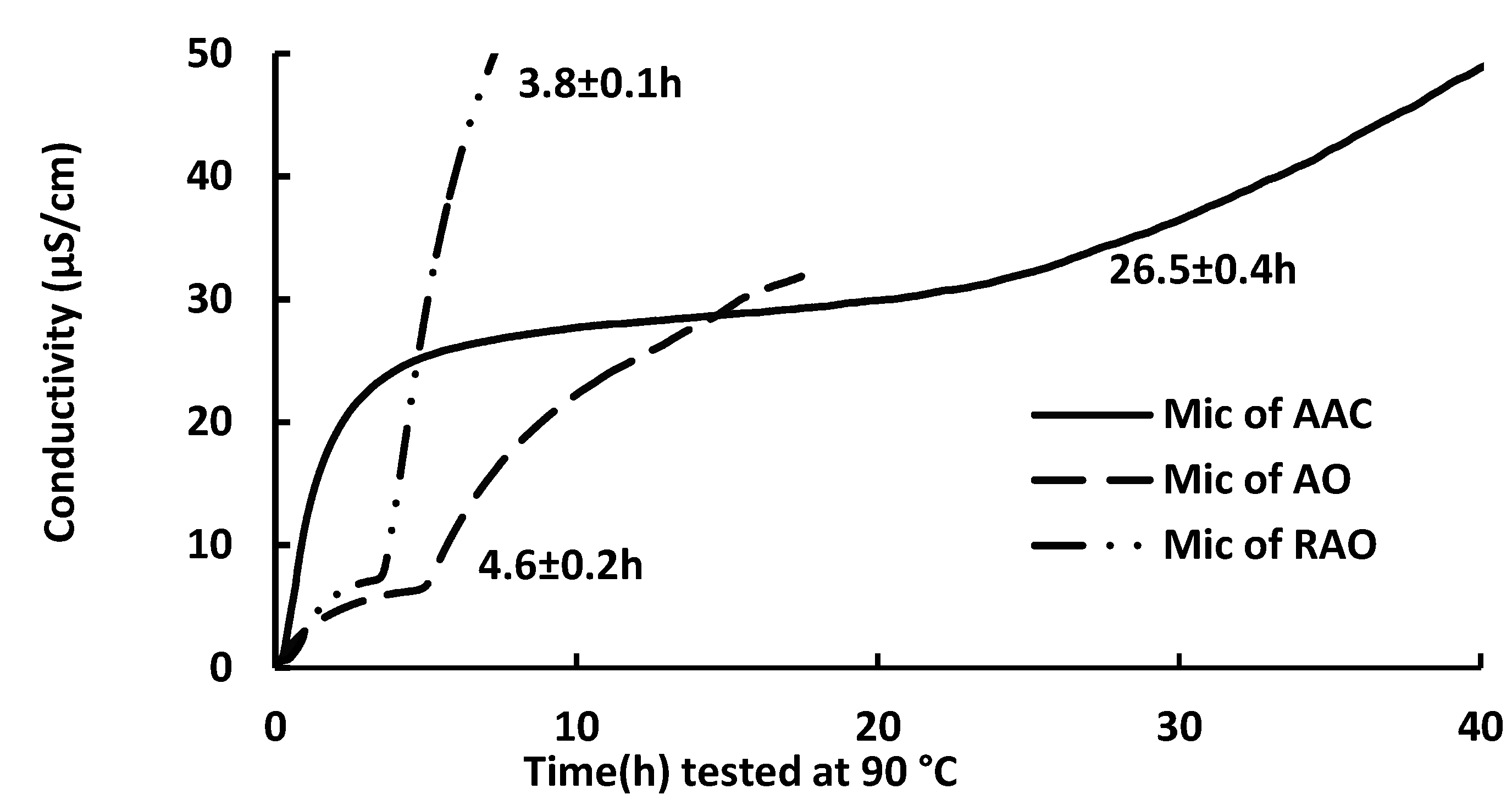
2.1. Oxidative Stability of Anchovy Oil, Anchovy Oil Acylglycerol Concentrate, and Refined Anchovy Oil Microcapsule
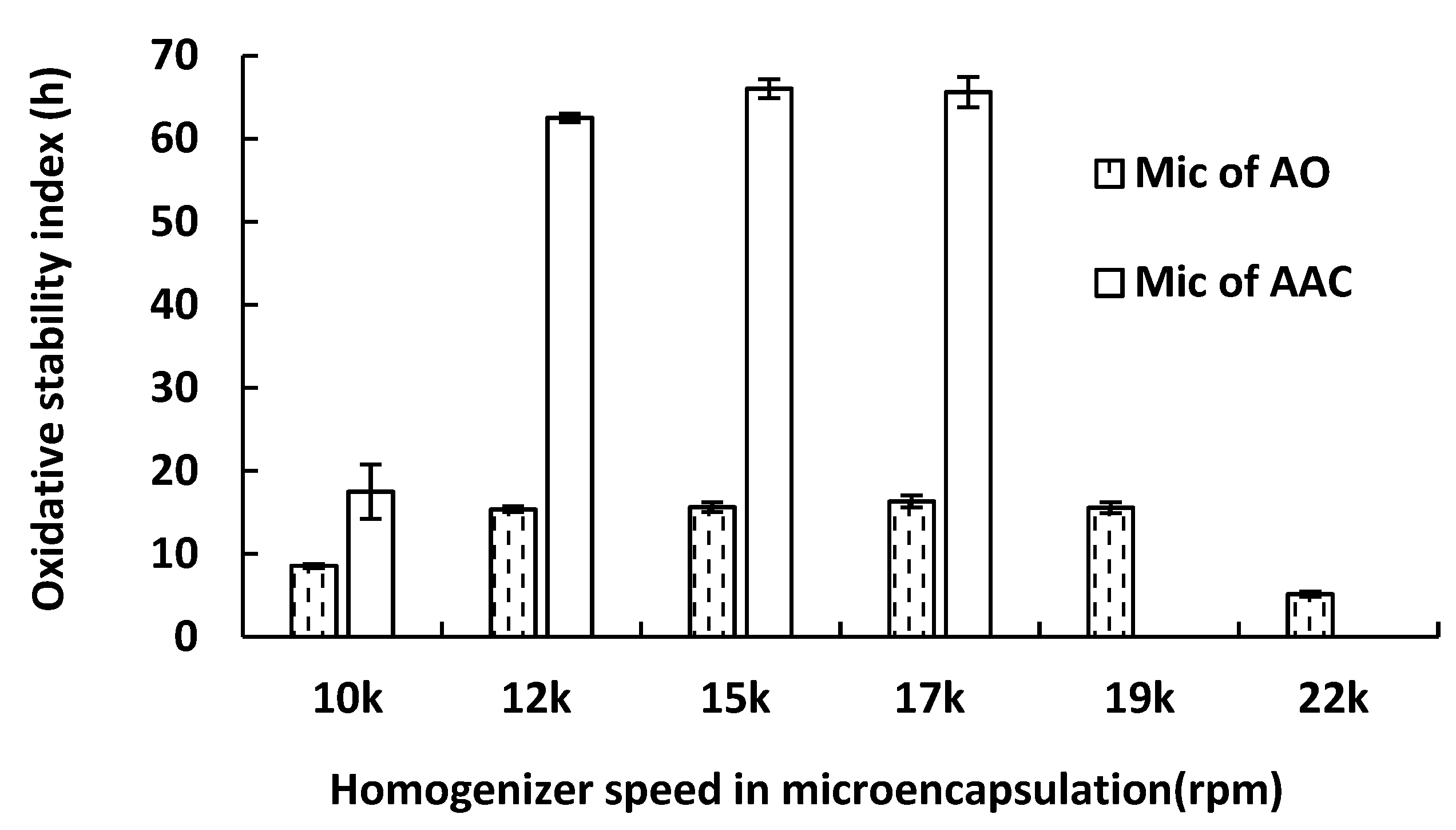
2.2. Effect of Homogenizer Conditions on the OSI of Microcapsule
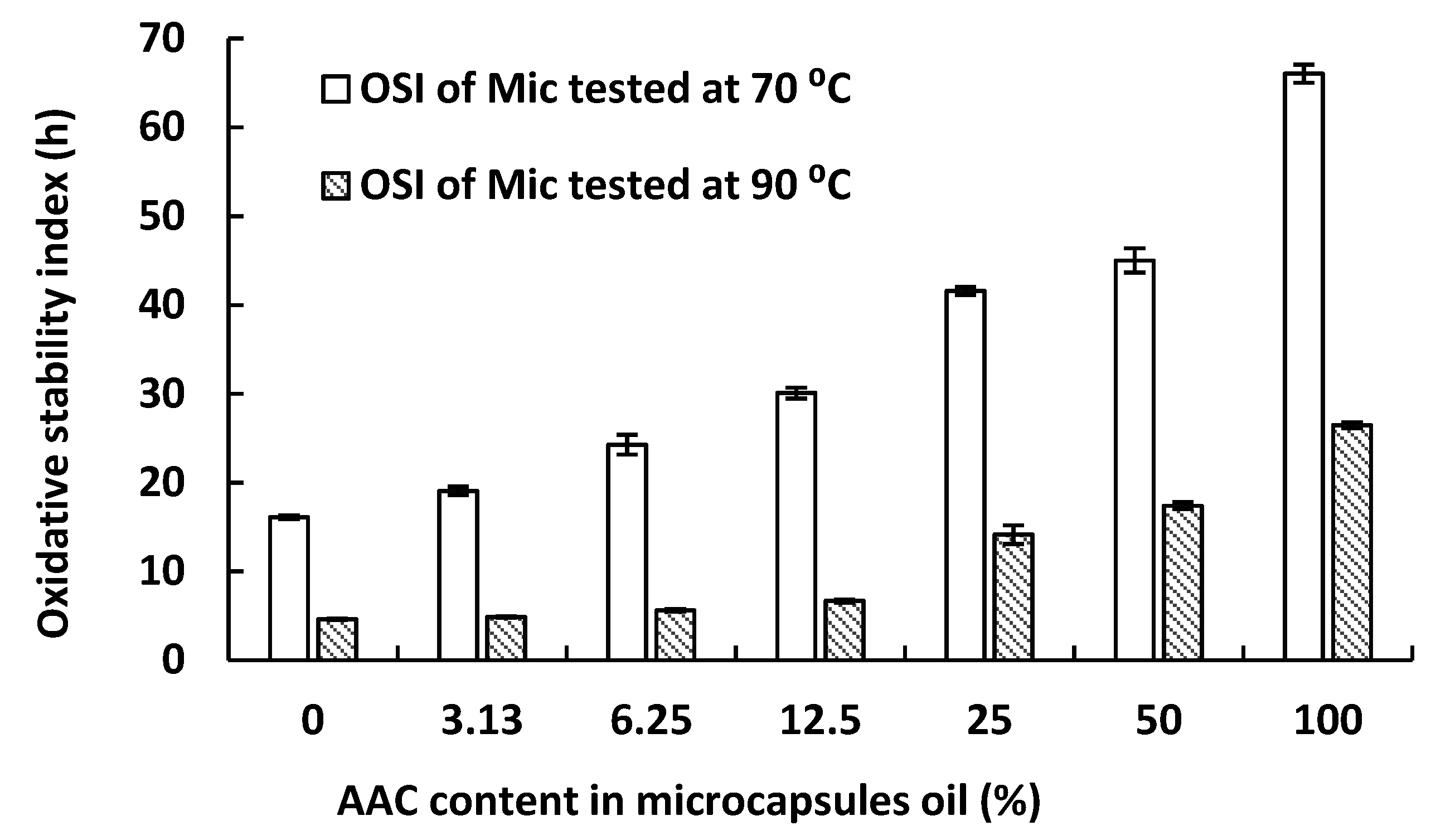
2.3. Oxidative Stability of Anchovy Oil Microcapsules with a Range of Concentrate Content
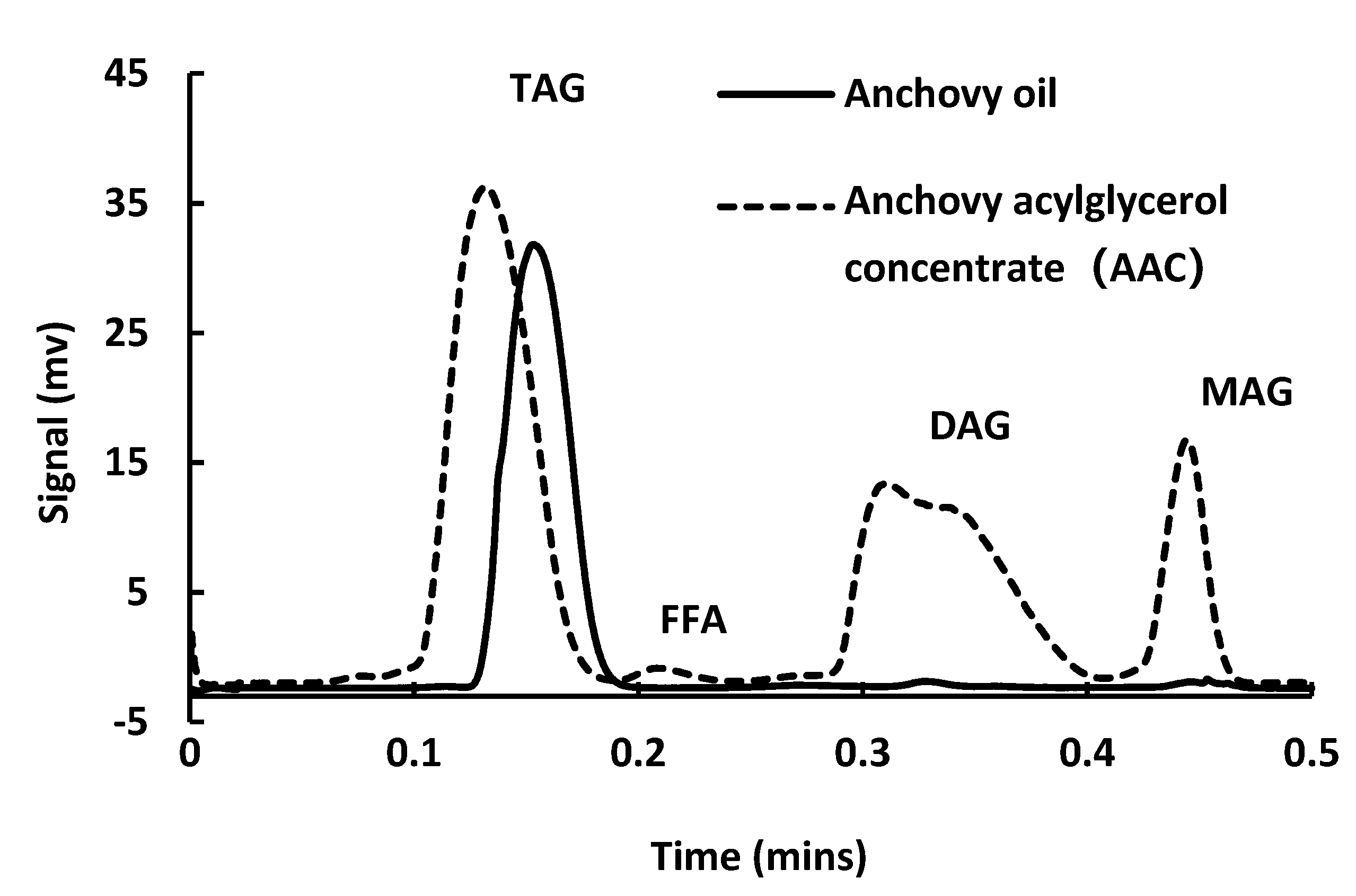
2.4. Lipid Class Analysis of the Acylglycerol
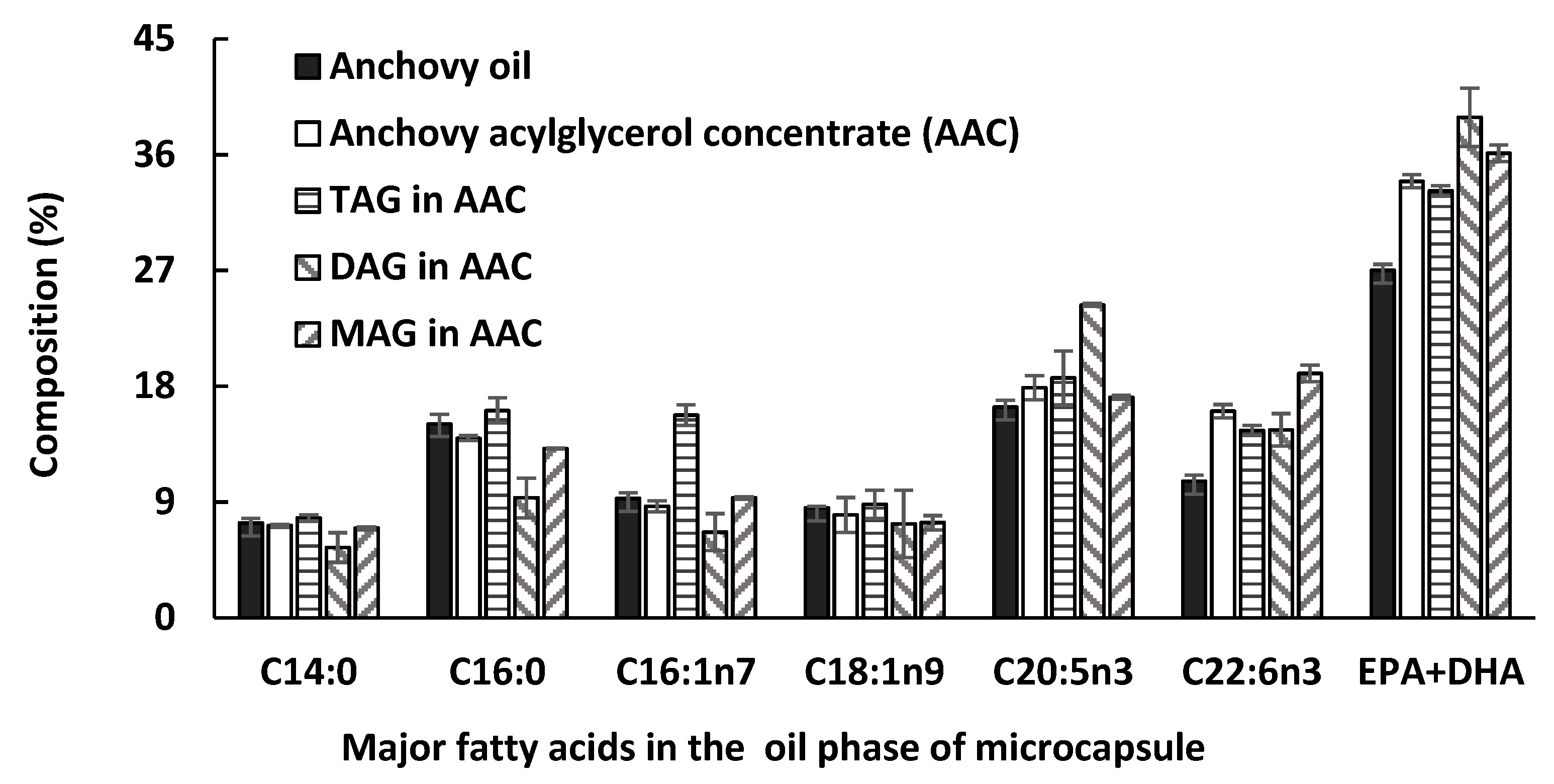
2.5. Fatty Acid Composition Analysis of the Acylglycerol
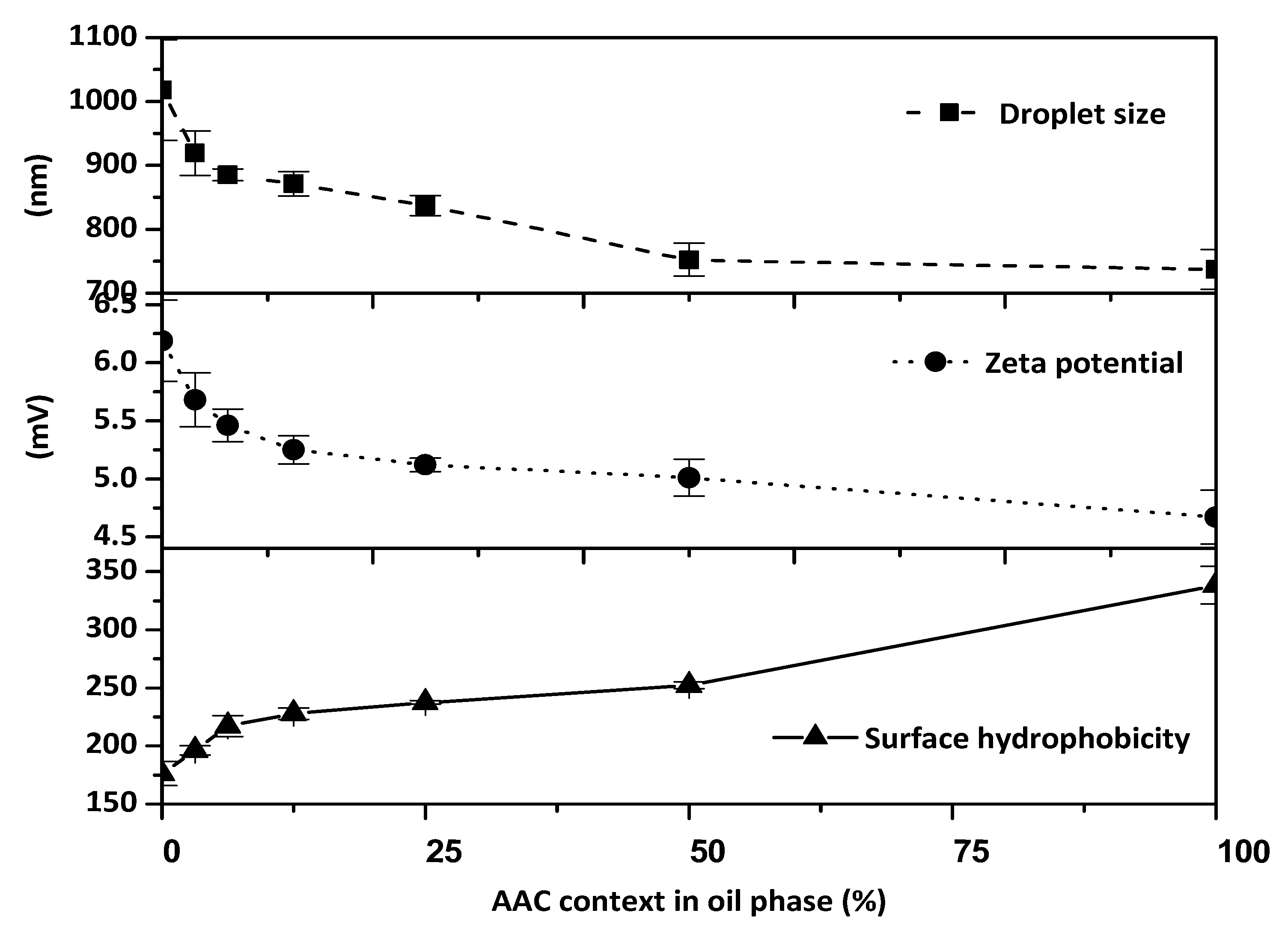
2.6. Effect of the Concentrate Incorporation on the Interfacial and Emulsion Characteristics of Gelatin-Stabilised Oil-in-Water Emulsions
2.7. Physicochemical Properties of Various Microcapsules Using Anchovy Oil Incorporated with Various Amounts of Concentrate
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Anchovy Oil Acylglycerol Concentrate
4.3. Preparation of Oil-in-Water Emulsion
4.4. Microencapsulation of Oil Phases Using Complex Coacervation
4.5. Accelerated Oxidative Stability Test of Microcapsules
4.6. Separation of Lipid Class by TLC
4.7. Analysis of Lipid Class and Fatty Acid Profile
4.8. Measurement of Emulsion Average Droplet Size and Zeta Potential
4.9. Measurement of Surface Hydrophobicity
4.10. Physicochemical Properties of the Microcapsules
4.11. Particle Size Distribution of Microcapsules
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jacobsen, C.; Let, M.B.; Nielsen, N.S.; Meyer, A.S. Antioxidant strategies for preventing oxidative flavour deterioration of foods enriched with n-3 polyunsaturated lipids: A comparative evaluation. Trends Food Sci. Technol. 2008, 19, 76–93. [Google Scholar] [CrossRef]
- Arab-Tehrany, E.; Jacquot, M.; Gaiani, C.; Imran, M.; Desobry, S.; Linder, M. Beneficial effects and oxidative stability of omega-3 long-chain polyunsaturated fatty acids. Trends Food Sci. Technol. 2012, 25, 24–33. [Google Scholar] [CrossRef]
- Calder, P.C.; Yaqoob, P. Omega-3 polyunsaturated fatty acids and human health outcomes. Biofactors 2009, 35, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Drusch, S.; Serfert, Y.; Scampicchio, M.; Schmidt-Hansberg, B.; Schwarz, K. Impact of physicochemical characteristics on the oxidative stability of fish oil microencapsulated by spray-drying. J. Agric. Food Chem. 2007, 55, 11044–11051. [Google Scholar] [CrossRef] [PubMed]
- Barrow, C.J.; Nolan, C.; Jin, Y. Stabilization of highly unsaturated fatty acids and delivery into foods. Lipid Technol. 2007, 19, 108–111. [Google Scholar] [CrossRef]
- Halvorsen, B.L.; Blomhoff, R. Determination of lipid oxidation products in vegetable oils and marine omega-3 supplements. Food Nutr. Res. 2011, 55, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Hogan, S.A.; O’Riordan, E.D.; O’Sullivan, M. Microencapsulation and oxidative stability of spray-dried fish oil emulsions. J. Microencapsul. 2003, 20, 675–688. [Google Scholar] [CrossRef] [PubMed]
- Kagami, Y.; Sugimura, S.; Fujishima, N.; Matsuda, K.; Kometani, T.; Matsumura, Y. Oxidative Stability, Structure, and Physical Characteristics of Microcapsules Formed by Spray Drying of Fish Oil with Protein and Dextrin Wall Materials. J. Food Sci. 2003, 68, 2248–2255. [Google Scholar] [CrossRef]
- Kim, Y.D.; Morr, C.V. Microencapsulation properties of gum arabic and several food proteins: Spray-dried orange oil emulsion particles. J. Agric. Food Chem. 1996, 44, 1314–1320. [Google Scholar] [CrossRef]
- Wang, B.; Vongsvivut, J.; Adhikari, B.; Barrow, C.J. Microencapsulation of tuna oil fortified with the multiple lipophilic ingredients vitamins A, D3, E, K2, curcumin and coenzyme Q10. J. Funct. Foods 2015, 19 Pt B, 893–901. [Google Scholar] [CrossRef]
- Eratte, D.; Dowling, K.; Barrow, C.J.; Adhikari, B. Recent advances in the microencapsulation of omega-3 oil and probiotic bacteria through complex coacervation: A review. Trends Food Sci. Technol. 2018, 71, 121–131. [Google Scholar] [CrossRef]
- Wang, B.; Adhikari, B.; Barrow, C.J. Optimisation of the microencapsulation of tuna oil in gelatin–sodium hexametaphosphate using complex coacervation. Food Chem. 2014, 158, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Kralovec, J.A.; Zhang, S.; Zhang, W.; Barrow, C.J. A review of the progress in enzymatic concentration and microencapsulation of omega-3 rich oil from fish and microbial sources. Food Chem. 2012, 131, 639–644. [Google Scholar] [CrossRef]
- Akanbi, T.O.; Adcock, J.L.; Barrow, C.J. Selective concentration of EPA and DHA using Thermomyces lanuginosus lipase is due to fatty acid selectivity and not regioselectivity. Food Chem. 2013, 138, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Wang, B.; Akanbi, T.O.; Li, R.; Yang, W.; Adhikari, B.; Barrow, C.J. Microencapsulation of lipase produced omega-3 concentrates resulted in complex coacervates with unexpectedly high oxidative stability. J. Funct. Foods 2017, 35, 499–506. [Google Scholar] [CrossRef]
- Farhoosh, R. The effect of operational parameters of the Rancimat method on the determination of the oxidative stability measures and shelf-life prediction of soybean oil. JAOCS J. Am. Oil Chem. Soc. 2007, 84, 205–209. [Google Scholar] [CrossRef]
- Kaushik, P.; Dowling, K.; Barrow, C.J.; Adhikari, B. Microencapsulation of omega-3 fatty acids: A review of microencapsulation and characterization methods. J. Funct. Foods 2015, 19, 868–881. [Google Scholar] [CrossRef]
- Bornscheuer, U.T. Lipase-catalyzed syntheses of monoacylglycerols. Enzym. Microb. Technol. 1995, 17, 578–586. [Google Scholar] [CrossRef]
- Feltes, M.M.C.; de Oliveira, D.; Block, J.M.; Ninow, J.L. The production, benefits, and applications of monoacylglycerols and diacylglycerols of nutritional interest. Food Bioprocess Technol. 2013, 6, 17–35. [Google Scholar] [CrossRef]
- Damstrup, M.L.; Jensen, T.; Sparsø, F.V.; Kiil, S.Z.; Jensen, A.D.; Xu, X. Solvent optimization for efficient enzymatic monoacylglycerol production based on a glycerolysis reaction. J. Am. Oil Chem. Soc. 2005, 82, 559–564. [Google Scholar] [CrossRef]
- Waraho, T.; Cardenia, V.; Nishino, Y.; Seneviratne, K.N.; Rodriguez-Estrada, M.T.; McClements, D.J.; Decker, E.A. Antioxidant effects of mono- and diacylglycerols in non-stripped and stripped soybean oil-in-water emulsions. Food Res. Int. 2012, 48, 353–358. [Google Scholar] [CrossRef]
- Da Pieve, S.; Calligaris, S.; Nicoli, M.C.; Marangoni, A.G. Shear nanostructuring of monoglyceride organogels. Food Biophys. 2010, 5, 211–217. [Google Scholar] [CrossRef]
- Miyashita, K.; Hirano, S.; Itabashi, Y.; Ota, T.; Nishikawa, M.; Nakayama, S. Oxidative Stability of Polyunsaturated Monoacylglycerol and Triacylglycerol in Aqueous Micelles. J. Oleo Sci. 1997, 46, 205–208. [Google Scholar] [CrossRef]
- Solaesa, Á.G.; Sanz, M.T.; Falkeborg, M.; Beltrán, S.; Guo, Z. Production and concentration of monoacylglycerols rich in omega-3 polyunsaturated fatty acids by enzymatic glycerolysis and molecular distillation. Food Chem. 2016, 190, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Cho, Y.I.; Lorsch, H.G. Effects of emulsifier on particle size of a phase change material in a mixture with water. Int. Commun. Heat Mass Transf. 1991, 18, 759–766. [Google Scholar] [CrossRef]
- Chung, C.; Sher, A.; Rousset, P.; Decker, E.A.; McClements, D.J. Formulation of food emulsions using natural emulsifiers: Utilization of quillaja saponin and soy lecithin to fabricate liquid coffee whiteners. J. Food Eng. 2017, 209, 1–11. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, Y.; Wang, S.; Jiang, C.; Zhang, J. Relationship between the zeta potential and the chemical agglomeration efficiency of fine particles in flue gas during coal combustion. Fuel 2018, 215, 756–765. [Google Scholar] [CrossRef]
- Kirby, B.J. Micro- and Nanoscale Fluid Mechanics: Transport in Microfluidic Devices; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Hanaor, D.; Michelazzi, M.; Leonelli, C.; Sorrell, C.C. The effects of carboxylic acids on the aqueous dispersion and electrophoretic deposition of ZrO2. J. Eur. Ceram. Soc. 2012, 32, 235–244. [Google Scholar] [CrossRef]
- Wu, Z.; Wu, J.; Zhang, R.; Yuan, S.; Lu, Q.; Yu, Y. Colloid properties of hydrophobic modified alginate: Surface tension, ζ-potential, viscosity and emulsification. Carbohydr. Polym. 2018, 181, 56–62. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, R.W.; Midmore, B.R.; Lamb, A.; Hunter, R.J. Electroacoustic studies of moderately concentrated colloidal suspensions. Faraday Discuss. Chem. Soc. 1990, 90, 301–312. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, Q.; Shan, L.; Liu, Y.; Shen, W.; Wang, X. The effect of ultrasound on lipase-catalyzed hydrolysis of soy oil in solvent-free system. Ultrason. Sonochem. 2008, 15, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Akanbi, T.O.; Barrow, C.J.; Byrne, N. Increased hydrolysis by Thermomyces lanuginosus lipase for omega-3 fatty acids in the presence of a protic ionic liquid. Catal. Sci. Technol. 2012, 2, 1839–1841. [Google Scholar] [CrossRef]
- Akanbi, T.O.; Sinclair, A.J.; Barrow, C.J. Pancreatic lipase selectively hydrolyses DPA over EPA and DHA due to location of double bonds in the fatty acid rather than regioselectivity. Food Chem. 2014, 160, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, B.; Barrow, C.J.; Adhikari, B. Relating the variation of secondary structure of gelatin at fish oil-water interface to adsorption kinetics, dynamic interfacial tension and emulsion stability. Food Chem. 2014, 143, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Nakai, S. Hydrophobicity determined by a fluorescence probe method and its correlation with surface properties of proteins. Biochim. Biophys. Acta 1980, 624, 13–20. [Google Scholar] [CrossRef]
- Chaudhuri, T.K.; Das, K.P.; Sinha, N.K. Surface hydrophobicity of a low molecular weight basic trypsin subtilisin inhibitor from marine turtle eggwhite. J. Biochem. 1993, 113, 729–733. [Google Scholar] [CrossRef] [PubMed]

| Concentrate Context in the Mixed Oil Phase | Surface Oil (%) | Total Oil (%) | Encapsulation Efficiency (%) | Microcapsule Droplet Size | ||
|---|---|---|---|---|---|---|
| D [4,3] (µm) | D [3,2] (µm) | Span | ||||
| Unconcentrated anchovy oil | 0.82 ± 0.27a | 54.83 ± 1.17 | 98.51 ± 0.51 | 96.00 ± 8.80d | 63.99 ± 2.28d | 1.78 ± 0.39 |
| 3.13% Concentrate | 1.91 ± 0.22b | 53.02 ± 0.84 | 96.40 ± 0.39 | 69.29 ± 7.25c | 40.20 ± 3.87c | 1.73 ± 0.05 |
| 6.25% Concentrate | 1.64 ± 0.23b | 53.92 ± 0.57 | 96.97 ± 0.41 | 62.83 ± 2.91c | 40.30 ± 6.24c | 1.52 ± 0.01 |
| 12.50% Concentrate | 2.26 ± 0.19c | 53.21 ± 0.94 | 95.76 ± 0.42 | 57.41 ± 1.85b | 32.25 ± 4.48b | 1.47 ± 0.10 |
| 25.00% Concentrate | 2.16 ± 0.25c | 52.42 ± 0.96 | 95.89 ± 4.14 | 52.58 ± 1.24a | 35.67 ± 3.16b | 1.63 ± 0.01 |
| 50.00% Concentrate | 2.30 ± 0.54c | 53.10 ± 1.16 | 95.67 ± 0.91 | 51.77 ± 3.72a | 30.94 ± 3.24a | 1.55 ± 0.01 |
| Concentrate | 2.59 ± 0.57c | 53.34 ± 0.81 | 95.12 ± 1.09 | 49.34 ± 4.67a | 28.86 ± 1.94a | 1.85 ± 0.06 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Q.; Akanbi, T.O.; Wang, B.; Li, R.; Yang, W.; Barrow, C.J. Investigating the Mechanism for the Enhanced Oxidation Stability of Microencapsulated Omega-3 Concentrates. Mar. Drugs 2019, 17, 143. https://doi.org/10.3390/md17030143
Xia Q, Akanbi TO, Wang B, Li R, Yang W, Barrow CJ. Investigating the Mechanism for the Enhanced Oxidation Stability of Microencapsulated Omega-3 Concentrates. Marine Drugs. 2019; 17(3):143. https://doi.org/10.3390/md17030143
Chicago/Turabian StyleXia, Qiuyu, Taiwo O. Akanbi, Bo Wang, Rui Li, Wenrong Yang, and Colin J. Barrow. 2019. "Investigating the Mechanism for the Enhanced Oxidation Stability of Microencapsulated Omega-3 Concentrates" Marine Drugs 17, no. 3: 143. https://doi.org/10.3390/md17030143
APA StyleXia, Q., Akanbi, T. O., Wang, B., Li, R., Yang, W., & Barrow, C. J. (2019). Investigating the Mechanism for the Enhanced Oxidation Stability of Microencapsulated Omega-3 Concentrates. Marine Drugs, 17(3), 143. https://doi.org/10.3390/md17030143







