Solvolysis Artifacts: Leucettazoles as Cryptic Macrocyclic Alkaloid Dimers from a Southern Australian Marine Sponge, Leucetta sp.
Abstract
:1. Introduction
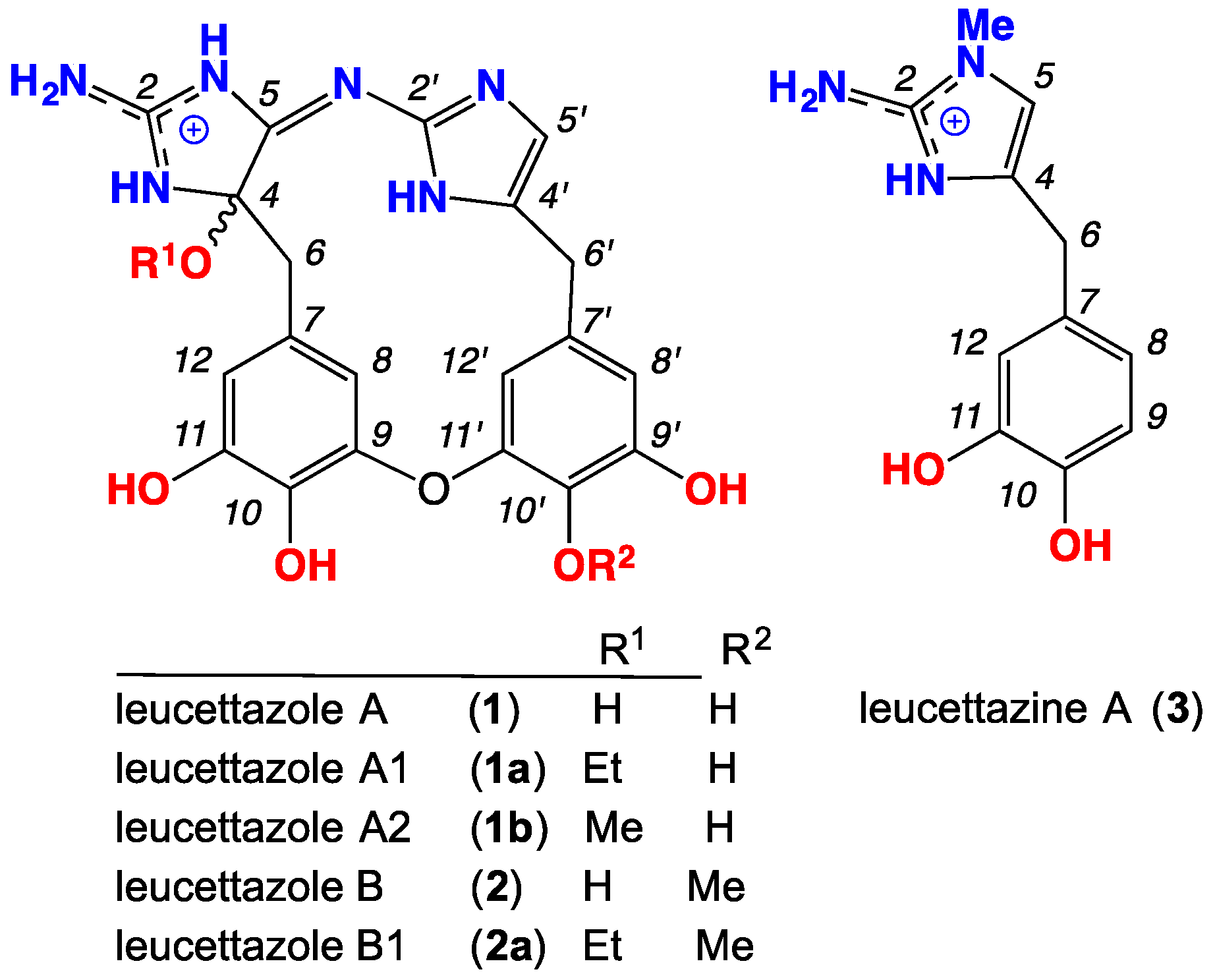
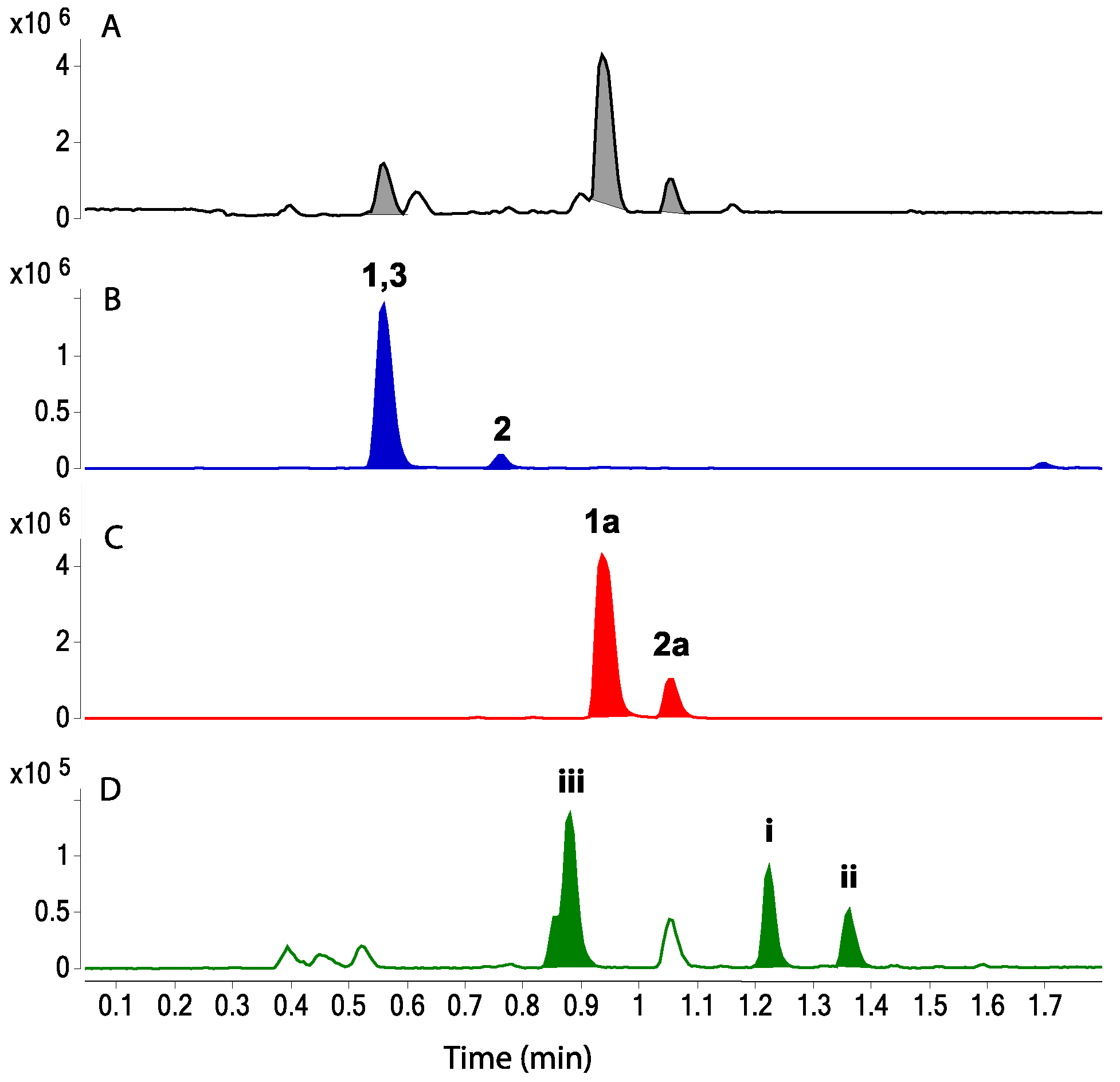
2. Results and Discussion
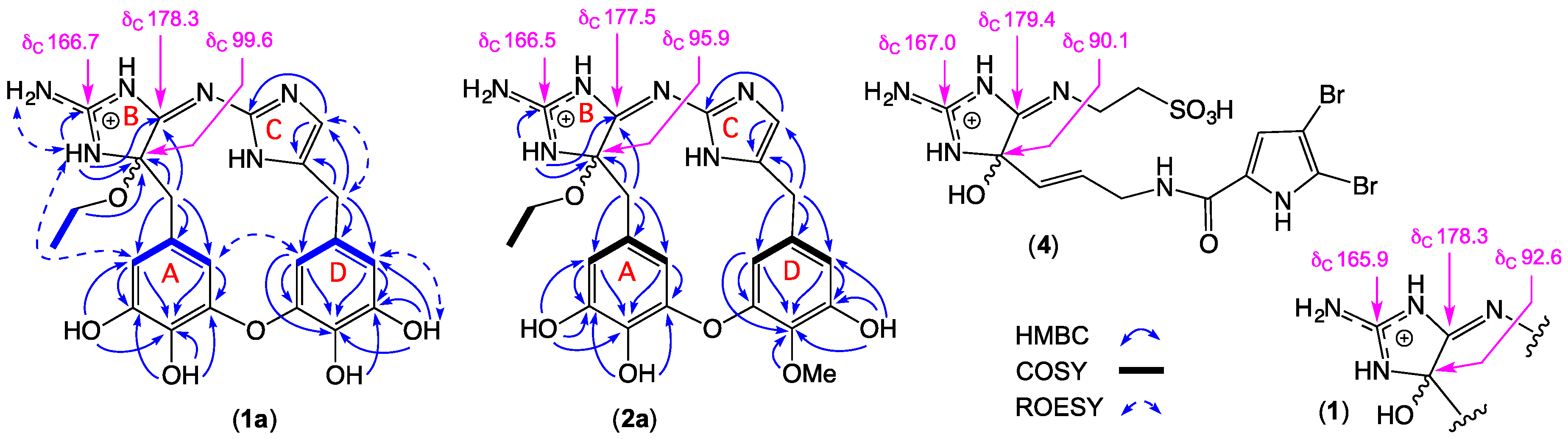
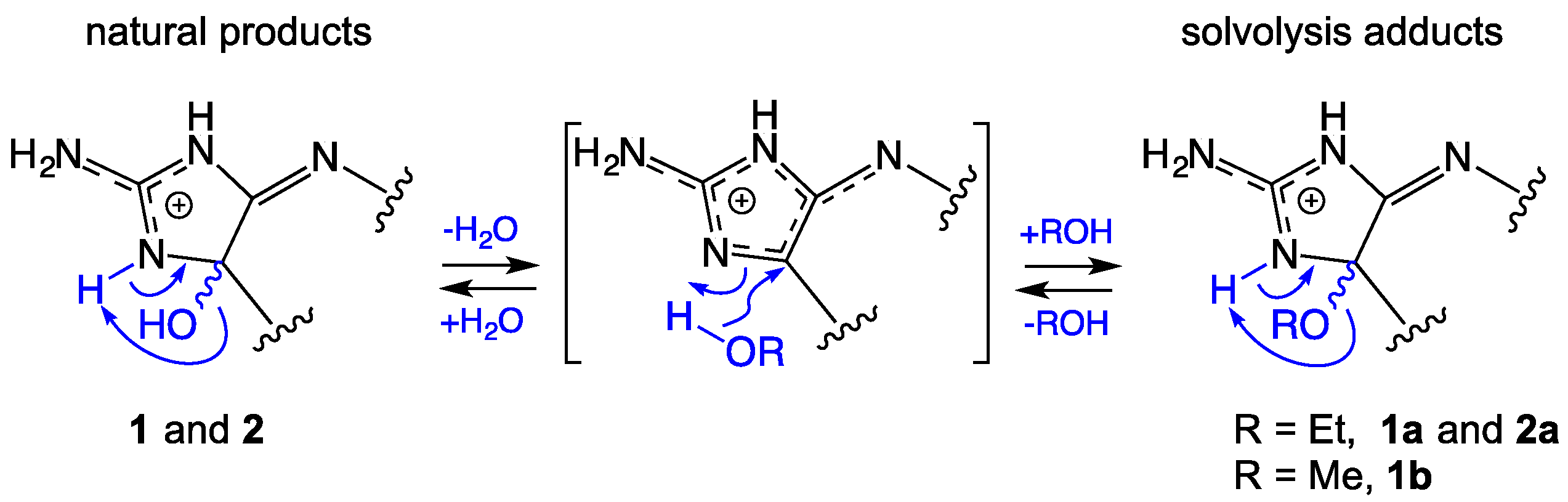
2.1. Leucettazole A1 (1a)
2.2. Leucettazole B1 (2a)
2.3. Leucettazine A (3)
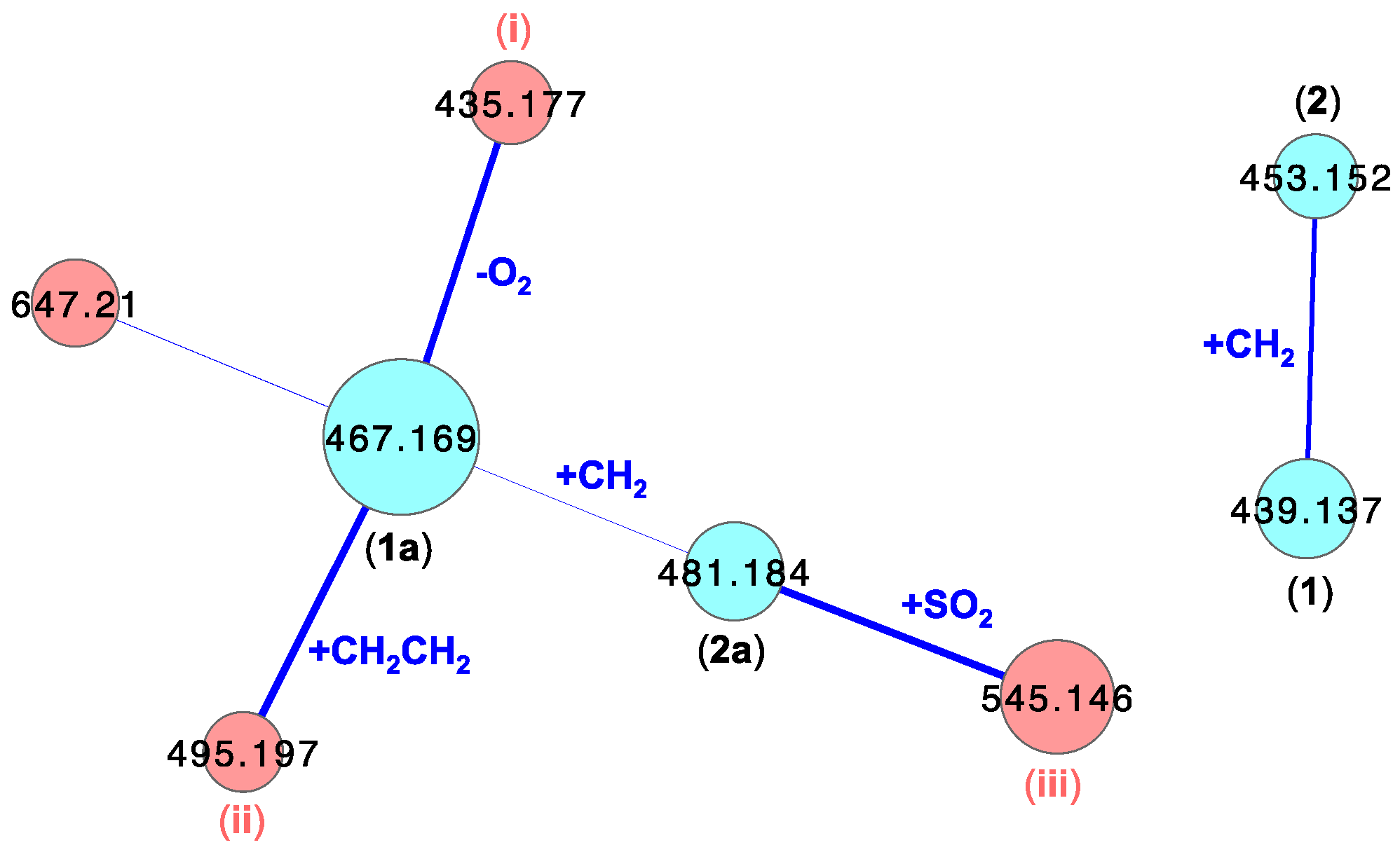
2.4. Visualization of Leucettazoles A (1) and B (2)
2.5. Leucettazole A (1)
2.6. Leucettazole Biology
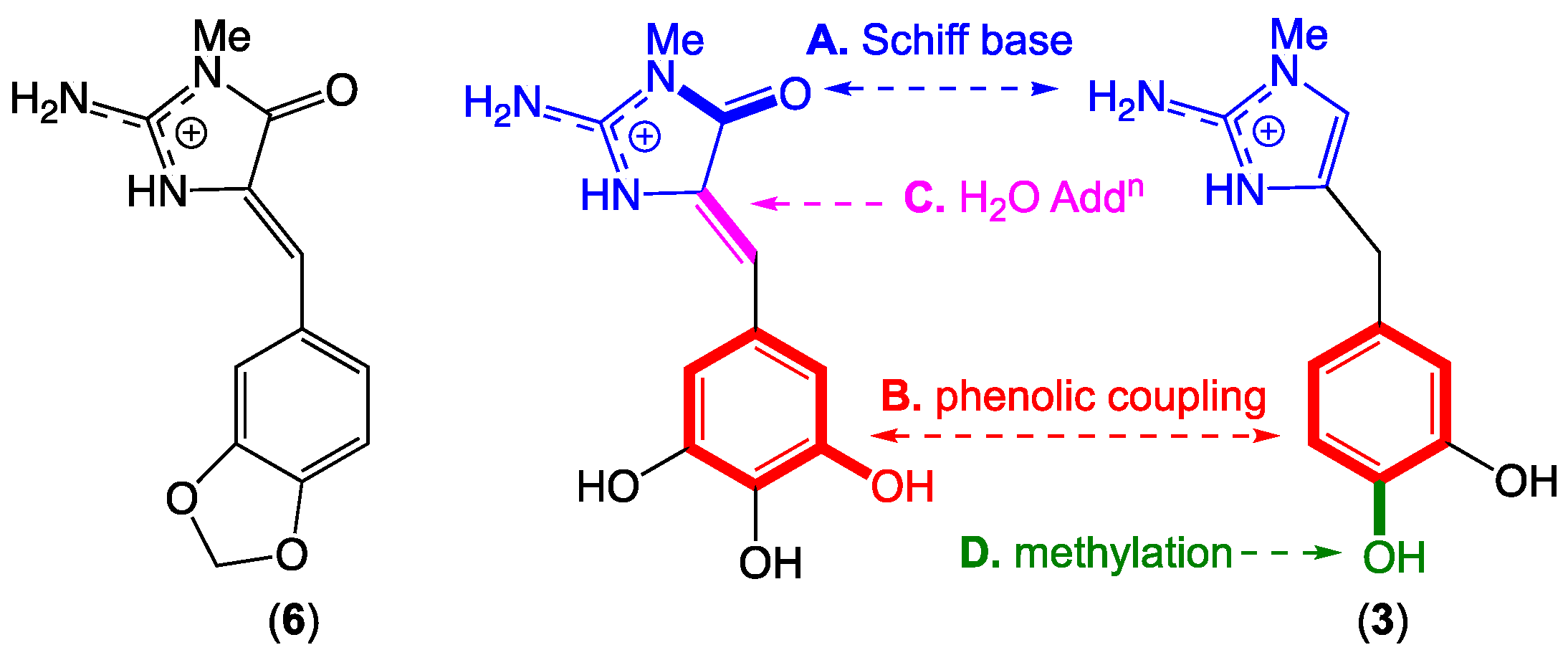
2.7. Leucettazole Biosynthesis
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Collection and Taxonomy
3.3. Extraction and Isolation
3.4. Crude EtOH Extract Solvolysis Studies
3.5. Leucettazole A1 (1a) Solvolysis Studies
3.6. UHPLC-QTOF (SIE) Analysis of Crude Extracts
3.7. GNPS Analyses
3.8. Antibacterial Assays
3.9. Antifungal Assay
3.10. Cytotoxicity Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Butler, M.S.; Capon, R.J. The luffarins (A–Z), novel terpenes from an Australian marine sponge, Luffariella geometrica. Aust. J. Chem. 1992, 45, 1705–1743. [Google Scholar] [CrossRef]
- Capon, R.J.; MacLeod, J.K.; Willis, A.C. Trunculins A and B, norsesterterpene cyclic peroxides from a marine sponge, Latrunculia brevis. J. Org. Chem. 1987, 52, 339–342. [Google Scholar] [CrossRef]
- Butler, M.S.; Capon, R.J. Trunculin-F and contrunculin-A and -B: Novel oxygenated norterpenes from a southern Australian marine sponge, Latrunculia conulosa. Aust. J. Chem. 1993, 43, 1363–1374. [Google Scholar] [CrossRef]
- Ovenden, S.P.B.; Capon, R.J. Trunculins G–I: New norsesterterpene cyclic peroxides from a southern Australian marine sponge, Latrunculia sp. Aust. J. Chem. 1998, 51, 573–579. [Google Scholar] [CrossRef]
- Capon, R.J.; Peng, C.; Dooms, C. Trachycladindoles A–G: Cytotoxic heterocycles from an Australian marine sponge, Trachycladus laevispirulifer. Org. Biomol. Chem. 2008, 6, 2765–2771. [Google Scholar] [CrossRef] [PubMed]
- Capon, R.J.; Rooney, F.; Murray, L.M.; Collins, E. Dragmacidins: New protein phosphatase inhibitors from a southern australian deep-water marine sponge, Spongosorites sp. J. Nat. Prod. 1998, 61, 660–662. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Conte, M.M.; Capon, R.J. Franklinolides A–C from an Australian marine sponge complex: Phosphodiesters strongly enhance polyketide cytotoxicity. Angew. Chemie Int. Ed. 2010, 49, 9904–9906. [Google Scholar] [CrossRef]
- Ovenden, S.P.B.; Capon, R.J.; Lacey, E.; Friedel, T.; Wadsworth, D. Amphilactams A–D: Novel nematocides from southern Australian marine sponges of the genus Amphimedon. J. Org. Chem. 1999, 64, 1140–1144. [Google Scholar] [CrossRef]
- Salim, A.A.; Rae, J.; Fontaine, F.; Conte, M.M.; Khalil, Z.; Martin, S.; Parton, R.G.; Capon, R.J. Heterofibrins: Inhibitors of lipid droplet formation from a deep-water southern Australian marine sponge, Spongia (Heterofibria) sp. Org. Biomol. Chem. 2010, 8, 3188–3194. [Google Scholar] [CrossRef]
- Capon, R.J.; Skene, C.; Liu, E.H.; Lacey, E.; Gill, J.H.; Heiland, K.; Friedel, T. The isolation and synthesis of novel nematocidal dithiocyanates from an Australian marine sponge, Oceanapia sp. J. Org. Chem. 2001, 66, 7765–7769. [Google Scholar] [CrossRef]
- Capon, R.J.; Skene, C.; Liu, E.H.-T.; Lacey, E.; Gill, J.H.; Heiland, K.; Friedel, T. Nematocidal thiocyanatins from a southern Australian marine sponge Oceanapia sp. J. Nat. Prod. 2004, 67, 1277–1282. [Google Scholar] [CrossRef] [PubMed]
- Capon, R.J.; MacLeod, J.K. 5-Thio-d-mannose from the marine sponge Clathria pyramida (Lendenfeld). The first example of a naturally occurring 5-thiosugar. J. Chem. Soc. Chem. Commun. 1987, 1200–1201. [Google Scholar] [CrossRef]
- Khalil, Z.G.; Huang, X.-C.; Raju, R.; Piggott, A.M.; Capon, R.J. Shornephine A: structure, chemical stability, and P-glycoprotein inhibitory properties of a rare diketomorpholine from an Australian marine-derived Aspergillus sp. J. Org. Chem. 2014, 79, 8700–8705. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, O.G.; Mohamed, O.G.; Khalil, Z.G.; Capon, R.J. Prolinimines: N-amino-l-Pro-methyl ester (hydrazine) Schiff bases from a fish gastrointestinal tract-derived fungus, Trichoderma sp. CMB-F563. Org. Lett. 2018, 20, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Shang, Z.; Raju, R.; Salim, A.A.; Khalil, Z.G.; Capon, R.J. Cytochalasins from an Australian marine sediment-derived Phomopsis sp. (CMB-M0042F): Acid-mediated intramolecular cycloadditions enhance chemical diversity. J. Org. Chem. 2017, 82, 9704–9709. [Google Scholar] [CrossRef] [PubMed]
- Fremlin, L.; Farrugia, M.; Piggott, A.M.; Khalil, Z.; Lacey, E.; Capon, R.J. Reveromycins revealed: New polyketide spiroketals from Australian marine-derived and terrestrial Streptomyces spp. A case of natural products vs. artifacts. Org. Biomol. Chem. 2011, 9, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Raju, R.; Piggott, A.M.; Conte, M.M.; Capon, R.J. Heronamides A–C, new polyketide macrolactams from an Australian marine-derived Streptomyces sp. A biosynthetic case for synchronized tandem electrocyclization. Org. Biomol. Chem. 2010, 8, 4682–4689. [Google Scholar] [CrossRef]
- Booth, T.J.; Booth, T.J.; Alt, S.; Alt, S.; Capon, R.J.; Wilkinson, B.; Wilkinson, B. Synchronous intramolecular cycloadditions of the polyene macrolactam polyketide heronamide C. Chem. Commun. 2016, 52, 6383–6386. [Google Scholar] [CrossRef]
- Kusama, T.; Tanaka, N.; Kashiwada, Y.; Kobayashi, J. Agelamadin F and tauroacidin E, bromopyrrole alkaloids from an Okinawan marine sponge Agelas sp. Tet. Letts. 2015, 56, 4502–4504. [Google Scholar] [CrossRef]
- Alvi, K.A.; Peters, B.M.; Lisa, H.M.; Phillip, C. 2-Aminoimidazoles and their zinc complexes from Indo-Pacific Leucetta sponges and Notodoris nudibranchs. Tetrahedron 1993, 49, 329–336. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.-Z.; Yang, Z.-Z.; Wu, W.; Tang, J.; Sun, F.; Wang, S.-P.; Cheng, C.-W.; Yang, F.; Lin, H.-W. Imidazole alkaloids and their zinc complexes from the calcareous marine sponge Leucetta chagosensis. J. Nat. Prod. 2018, 81, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.W.; Mong, S.; Hemling, M.E.; Freyer, A.J.; Offen, P.H.; DeBrosse, C.W.; Sarau, H.M.; Westley, J.W. New leukotriene B4 receptor antagonist: Leucettamine A and related imidazole alkaloids from the marine sponge Leucetta microraphis. J. Nat. Prod. 1993, 56, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Tahtouh, T.; Elkins, J.M.; Filippakopoulos, P.; Soundararajan, M.; Burgy, G.; Durieu, E.; Cochet, C.; Schmid, R.S.; Lo, D.C.; Delhommel, F.; et al. Selectivity, cocrystal structures, and neuroprotective properties of leucettines, a family of protein kinase inhibitors derived from the marine sponge alkaloid leucettamine B. J. Med. Chem. 2012, 55, 9312–9330. [Google Scholar] [CrossRef] [PubMed]
- Kessner, D.; Chambers, M.; Burke, R.; Agus, D.; Mallick, P. ProteoWizard: open source software for rapid proteomics tools development. Bioinformatics 2008, 24, 2534–2536. [Google Scholar] [CrossRef]
- Frank, A.M.; Bandeira, N.; Shen, Z.; Tanner, S.; Briggs, S.P.; Smith, R.D.; Pevzner, P.A. Clustering millions of tandem mass spectra. J. Prot. Res. 2008, 7, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]

| Position | δC | δH, mult. (J in Hz) | COSY | gHMBC | ROESY |
|---|---|---|---|---|---|
| 2 | 166.7, C | ||||
| 2-NH2 | 8.21, br s | 3-NH | |||
| 8.00, br s | 3-NH | ||||
| 3-NH | 9.16, s | 2, 4, 5, 2′ | 6a, 12, 4-OCH2a, 2-NH2 | ||
| 4 | 96.6, C | ||||
| 4-OCH2CH3 | 59.0, CH2 | a. 3.45, dq (8.8, 7.0) | 4-OCHbCH3 | 4, 4-OCH2CH3 | 3, 4-OCHb, 4-OCH2CH3 |
| b. 3.31, dq (8.8, 7.0) | 4-OCHaCH3 | 4, 4-OCH2CH3 | 4-OCHa, 4-OCH2CH3 | ||
| 4-OCH2CH3 | 15.1, CH3 | 1.16, dd (7.0, 7.0) | 4-OCH2 | 4-OCH2 | 4-OCH2 |
| 5 | 178.3, C | ||||
| 6 | 43.4, CH2 | a. 3.03, d (13.1) | 5, 7, 8, 12 | 3-NH, 12 | |
| b. 2.81, d (13.1) | 4, 5, 7, 8, 12 | 8 | |||
| 7 | 124.3, C | ||||
| 8 | 112.3, CH | 5.99, d (2.0) | 12 | 6, 9, 10, 12 | 6b, 12′, 12 |
| 9 | 144.8, C | ||||
| 10 | 136.2, C | ||||
| 10-OH | 8.68, s | 9, 10, 11 | |||
| 11 | 146.1, C | ||||
| 11-OH | 8.87 a | 10, 12 | |||
| 12 | 114.6, CH | 6.57, d (2.0) | 8 | 6, 8, 10, 11 | 6a, 8, 3-NH |
| 2′ | 148.5, C | ||||
| 3′-NH | 11.82, s | ||||
| 4′ | 127.0, C | ||||
| 5′ | 112.9, CH | 7.07, s | 2′, 4′ | 6′a/b, 12′ | |
| 6′ | 29.1, CH2 | a. 3.82, d (16.7) | 4′, 5′, 7′, 8′, 12′ | 5′, 8′, 12′ | |
| b. 3.72, d (16.7) | 4′, 5′, 7′, 8′, 12′ | 5′, 8′, 12′ | |||
| 7′ | 130.0, C | ||||
| 8′ | 111.4, CH | 6.51, d (2.0) | 12′ | 6′, 9′, 10′, 12′ | 6′a/b, 12′, 9′-OH |
| 9′ | 146.6, C | ||||
| 9′-OH | 9.29, s | 8′, 9′, 10′ | 8′ | ||
| 10′ | 134.1, C | ||||
| 10′-OH | 8.86 a | 9′ | |||
| 11′ | 145.8, C | ||||
| 12′ | 108.9, CH | 5.46, d (2.0) | 8′ | 6′, 8′, 10′, 11′ | 8, 5′, 6′a/b, 8′ |
| Position | δC | δH, mult. (J in Hz) | COSY | gHMBC |
|---|---|---|---|---|
| 2 | 166.5, C | |||
| 2-NH2 | a. 8.23, s | |||
| b. 8.02, s | ||||
| 3-NH | 9.20, s | 2, 4, 5 | ||
| 4 | 95.9, C | |||
| 4-OCH2CH3 | 58.4, CH2 | a. 3.45, dq (8.8, 7.0) | 4-OCHbCH3 | 4-OCH2CH3 |
| b. 3.28, dq (8.8, 7.0) | 4-OCHaCH3 | |||
| 4-OCH2CH3 | 14.6, CH3 | 1.15, dd (7.0, 7.0) | 4-OCH2 | 4-OCH2 |
| 5 | 177.5, C | |||
| 6 | 42.7, CH2 | a. 3.07, d (13.2) | 4, 5, 7, 8, 12 | |
| b. 2.75, d (13.2) | 4, 5, 7, 8, 12 | |||
| 7 | 123.9, C | |||
| 8 | 111.5, CH | 5.92, d (2.0) | 12 | 6, 9, 10, 12 |
| 9 | 143.1, C | |||
| 10 | 135.7 a, C | |||
| 10-OH | 8.52, s | 9, 11 | ||
| 11 | 145.9, C | |||
| 11-OH | 8.91, s | 10, 11, 12 | ||
| 12 | 112.8, CH | 6.53, d (2.0) | 8 | 6, 8, 11 |
| 2′ | 148.0, C | |||
| 3′-NH | 12.1, br s | |||
| 4′ | 126.9, C | |||
| 5′ | 112.3, CH | 6.97, s | 2′, 4′ | |
| 6′ | 29.0, CH2 | a. 3.86, d (16.2) | 4′, 5′, 7′, 8′ | |
| b. 3.63, d (16.2) | 4′, 5′, 7′, 8′, 12′ | |||
| 7′ | 134.6, C | |||
| 8′ | 110.7, CH | 6.52, d (2.0) | 12′ | 6′, 9′, 10′, 12′ |
| 9′ | 150.3, C | |||
| 9′-OH | 9.37, s | 8′, 9′, 10′ | ||
| 10′ | 135.6 a, C | |||
| 10′-OCH3 | 59.9, CH3 | 3.71, s | 10′ | |
| 11′ | 149.9, C | |||
| 12′ | 107.1, CH | 5.43, d (2.0) | 8′ | 6′, 8′, 10′, 11′ |
| Position | δC | δH, mult. (J in Hz) | COSY | gHMBC |
|---|---|---|---|---|
| 1-NMe | 31.8, CH3 | 3.37, s | 2, 5 | |
| 2 | 146.2, C | |||
| 2-NH2 | 6.54, br s | |||
| 3-NH | 12.06, s | |||
| 4 | 125.9, C | |||
| 5 | 113.7, CH | 6.61, s | 2, 4 | |
| 6 | 29.4, CH2 | 3.58, s | 4, 5, 7, 8, 12 | |
| 7 | 128.1, C | |||
| 8 | 119.1, CH | 6.47, dd (8.0, 1.5) | 9,12 | 6, 10, 12 |
| 9 | 115.5, CH | 6.66, d (8.0) | 8 | 7, 11 |
| 10 | 144.0, C | |||
| 10-OH | 7.43, s | |||
| 11 | 145.2, C | |||
| 11-OH | 8.82, s | 10, 11, 12 | ||
| 12 | 115.9, CH | 6.59, d (1.5) | 8 | 6, 8, 10 |
| Position | δC | δH, mult. (J in Hz) | Position | δC | δH, mult. (J in Hz) |
|---|---|---|---|---|---|
| 2 | 165.9, C | 12 | 114.6, CH | 6.55, d (2.0) | |
| 2-NH2 | a. 7.94, br s | 2′ | 148.5, C | ||
| b. 7.83, br s | 3′-NH | 11.73, br s | |||
| 3-NH | 9.17, s | 4′ | 126.8, C | ||
| 4 | 92.1, C | 5′ | 112.9, CH | 7.05, s | |
| 4-OH | 6.86, s | 6′ | 29.1, CH2 | a. 3.81, d (16.7) | |
| 5 | 180.8, C | b. 3.72, d (16.7) | |||
| 6 | 44.1, CH2 | a. 2.98, d (13.1) | 7′ | 130.0, C | |
| b. 2.80, d (13.1) | 8′ | 111.3, CH | 6.51, d (2.0) | ||
| 7 | 125.1, C | 9′ | 146.5, C | ||
| 8 | 112.3, CH | 5.98, d (2.0) | 9′-OH | 9.29, s | |
| 9 | 144.7, C | 10′ | 134.1, C | ||
| 10 | 136.1, C | 10′-OH | 8.85 | ||
| 10-OH | 8.67, s | 11′ | 145.9, C | ||
| 11 | 146.0, C | 12′ | 109.0, CH | 5.48, d (2.0) | |
| 11-OH | 8.88 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasad, P.; Salim, A.A.; Khushi, S.; Khalil, Z.G.; Quezada, M.; Capon, R.J. Solvolysis Artifacts: Leucettazoles as Cryptic Macrocyclic Alkaloid Dimers from a Southern Australian Marine Sponge, Leucetta sp. Mar. Drugs 2019, 17, 106. https://doi.org/10.3390/md17020106
Prasad P, Salim AA, Khushi S, Khalil ZG, Quezada M, Capon RJ. Solvolysis Artifacts: Leucettazoles as Cryptic Macrocyclic Alkaloid Dimers from a Southern Australian Marine Sponge, Leucetta sp. Marine Drugs. 2019; 17(2):106. https://doi.org/10.3390/md17020106
Chicago/Turabian StylePrasad, Pritesh, Angela A. Salim, Shamsunnahar Khushi, Zeinab G. Khalil, Michelle Quezada, and Robert J. Capon. 2019. "Solvolysis Artifacts: Leucettazoles as Cryptic Macrocyclic Alkaloid Dimers from a Southern Australian Marine Sponge, Leucetta sp." Marine Drugs 17, no. 2: 106. https://doi.org/10.3390/md17020106
APA StylePrasad, P., Salim, A. A., Khushi, S., Khalil, Z. G., Quezada, M., & Capon, R. J. (2019). Solvolysis Artifacts: Leucettazoles as Cryptic Macrocyclic Alkaloid Dimers from a Southern Australian Marine Sponge, Leucetta sp. Marine Drugs, 17(2), 106. https://doi.org/10.3390/md17020106







