Isolation, Optimization of Fermentation Conditions, and Characterization of an Exopolysaccharide from Pseudoalteromonas agarivorans Hao 2018
Abstract
1. Introduction
2. Results
2.1. Extraction and Purification of EPS
2.2. Optimization of Fermentation Conditions
2.2.1. Single Factor Experimental Results
2.2.2. Plackett-Burman Results
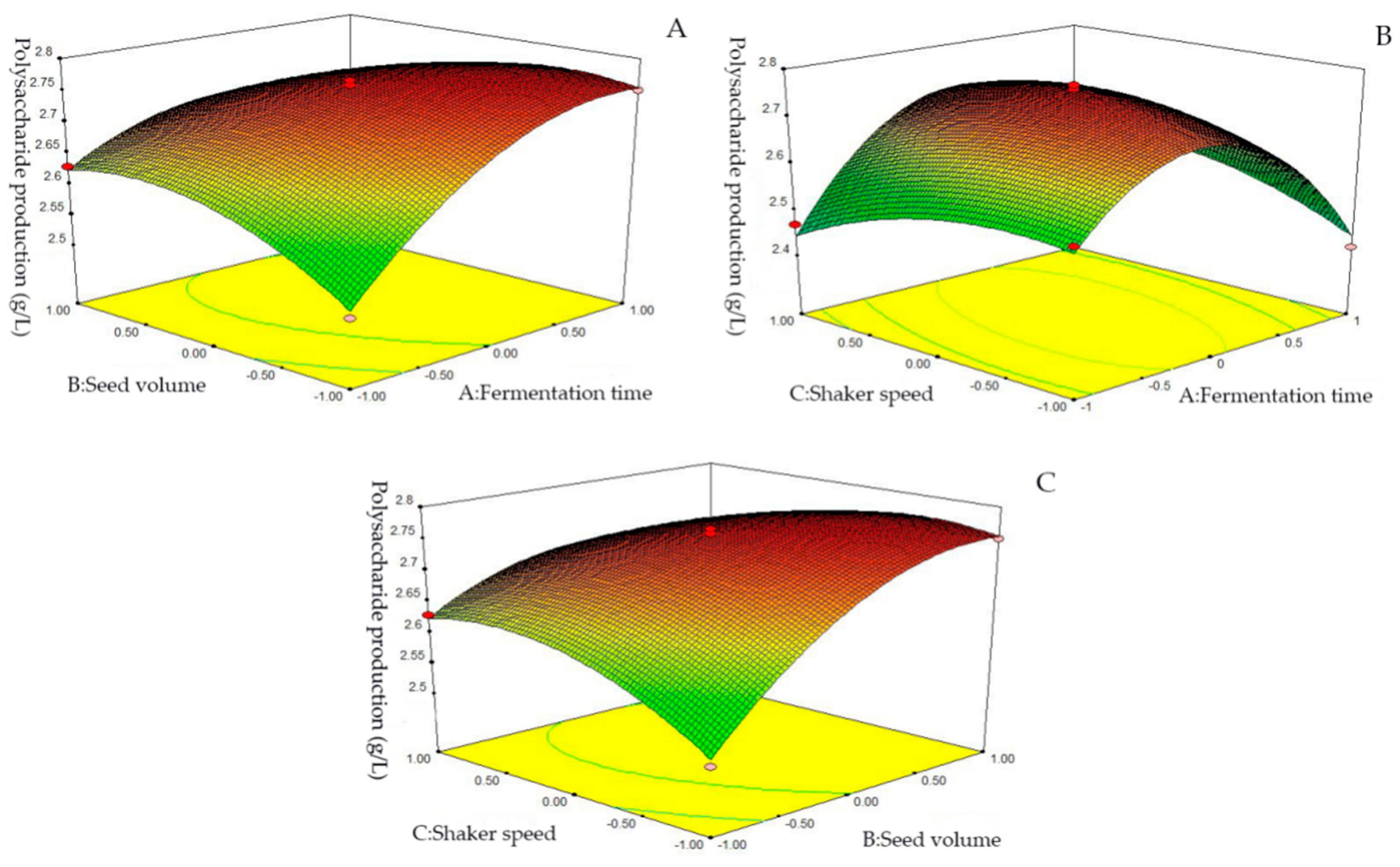
2.2.3. Box-Behnken Results
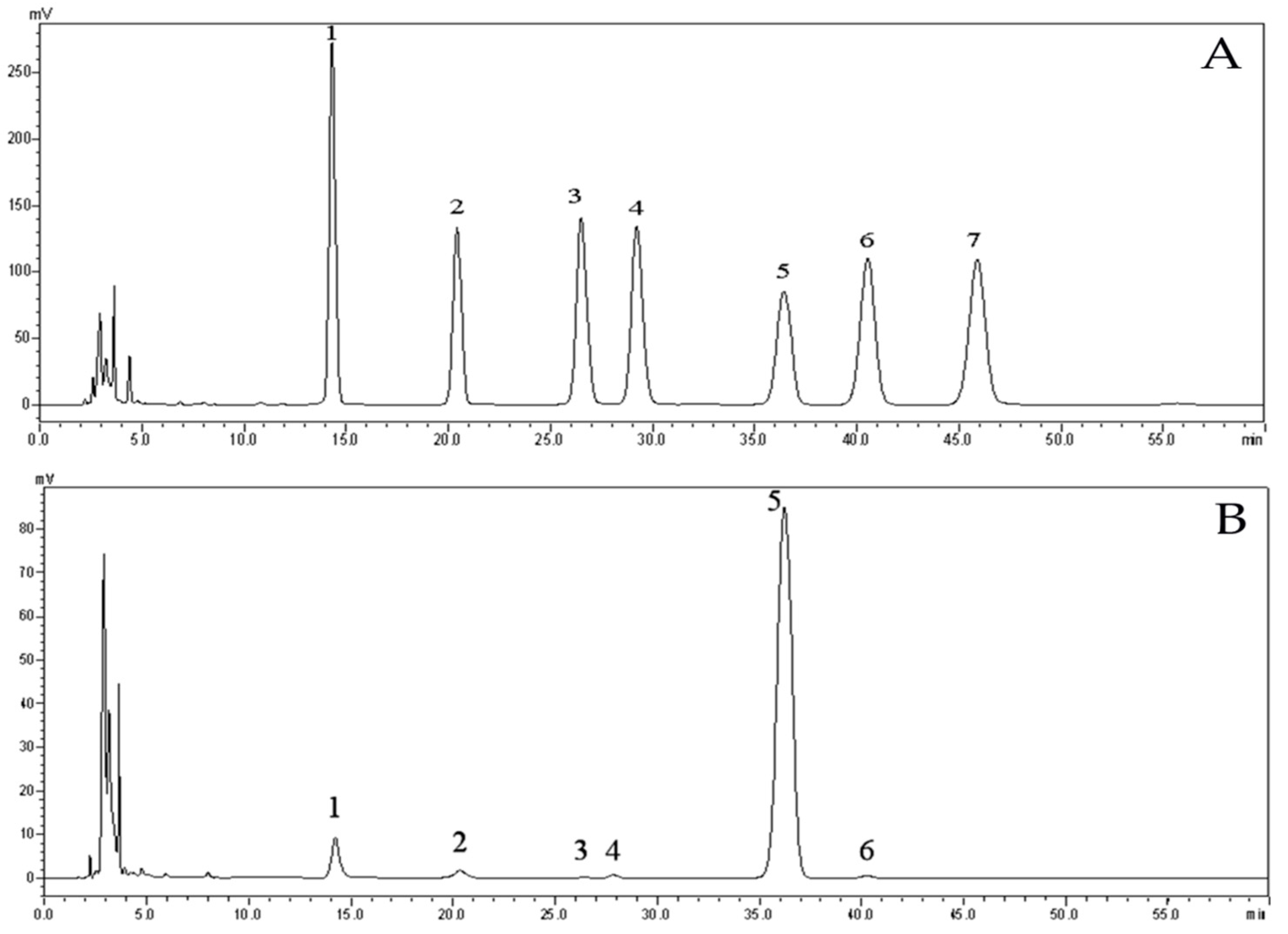
2.3. Analysis of Monosaccharide Composition of EPS
2.4. Fourier Transform Infrared (FT-IR) Analysis
2.5. Biological Activity
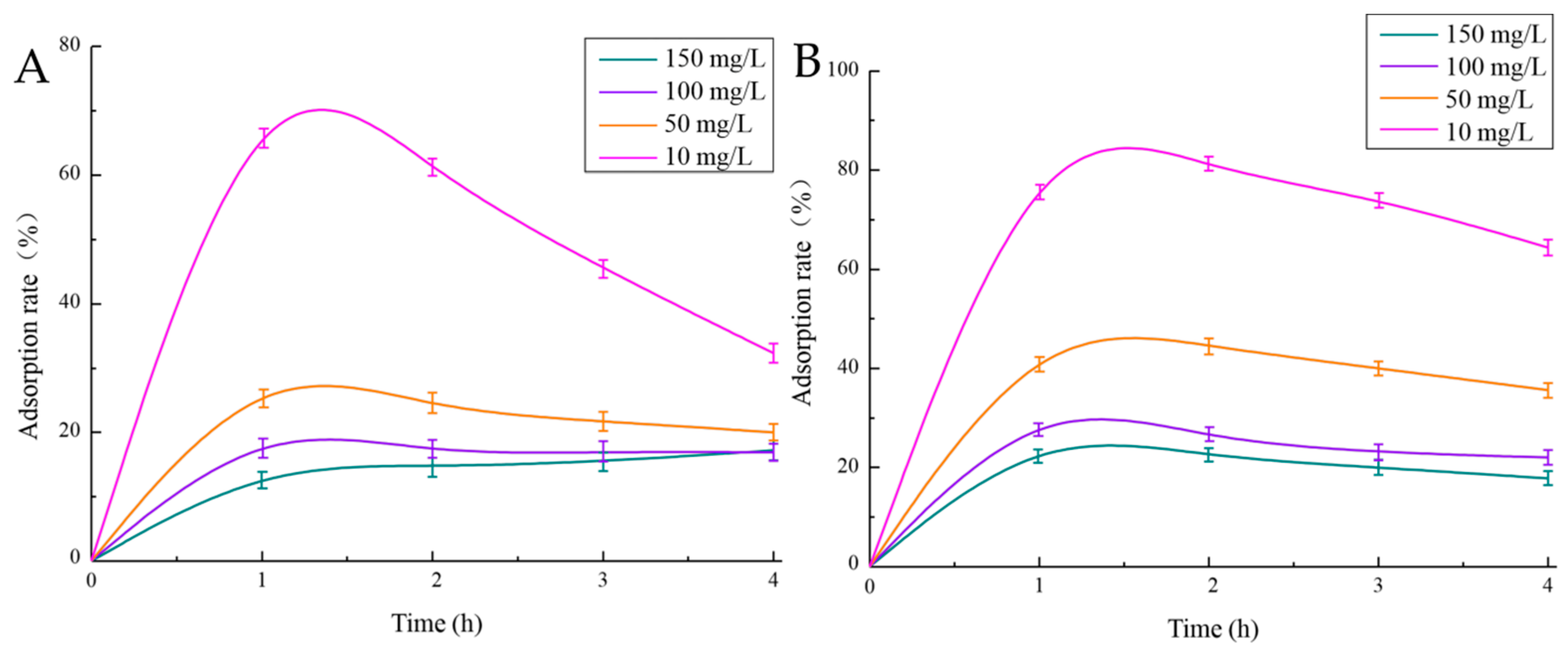
2.5.1. Adsorption of Cu2+ and Pb2+ by EPS
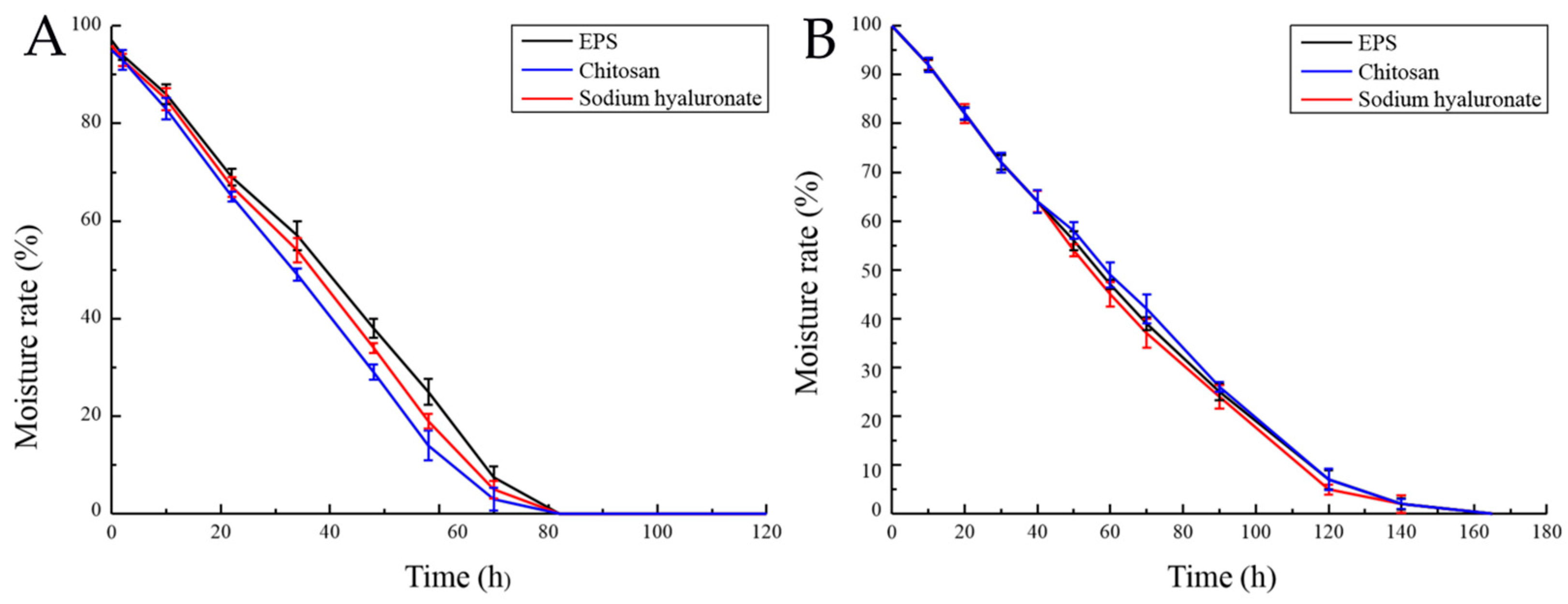
2.5.2. Hygroscopicity and Moisture Retention of EPS
2.5.3. Free Radical Scavenging
3. Discussion
4. Materials and Methods
4.1. Separation and Purification of EPS
4.2. Optimization of Fermentation Conditions
4.2.1. Single Factor Experiment Method
4.2.2. Plackett-Burman Design
4.2.3. Box-Behnken Design
4.2.4. Data Statistical Analysis
4.3. Determination of Polysaccharide Content
4.4. Determination of the Molecular Weight of EPS
4.4.1. Hydrolysis and Derivatization of EPS
4.4.2. Analysis of Monosaccharide Composition by High Performance Liquid Chromatography (HPLC)
4.4.3. Ultraviolet (UV) Visible Light and FT-IR Spectrum
4.5. Biological Activity
4.5.1. Adsorption of Cu 2+ and Pb 2+ by EPS
4.5.2. EPS Hygroscopic Activity
4.5.3. Determination of Moisture Retention Rate of EPS
4.5.4. OH Free Radical Scavenging Activity
4.5.5. O2− Free Radical Scavenging Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Holmström, C.; Kjelleberg, S. Marine Pseudoalteromonas species are associated with higher organisms and produce biologically active extracellular agents. FEMS Microbiol. Ecol. 1999, 30, 285–293. [Google Scholar] [CrossRef]
- Wang, C.; Fan, Q.; Zhang, X. Isolation, characterization, and pharmaceutical applications of an exopolysaccharide from Aerococcus uriaeequi. Mar. Drugs 2018, 16, 337. [Google Scholar] [CrossRef] [PubMed]
- Komandrova, N.A.; Isakov, V.V.; Tomshich, S.V.; Romanenko, L.A.; Perepelov, A.V.; Shashkov, A.S. Structure of an acidic O-specific polysaccharide of the marine bacterium Pseudoalteromonas agarivorans KMM 232 (R-form). Biochemistry (Mosc) 2010, 75, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Komandrova, N.A.; Kokoulin, M.S.; Kalinovskiy, A.I.; Tomshich, S.V.; Romanenko, L.A.; Vaskovsky, V.E. The O-specific polysaccharide from the marine bacterium Pseudoalteromonas agarivorans KMM 255(T). Carbohydr. Res. 2015, 414, 60–64. [Google Scholar] [CrossRef]
- Sengupta, S.; Banerjee, A.; Halder, U.; Gupta, P.; Banerjee, C.; Bandopadhyay, R. Comparative study on structure of exopolysaccharide and capsular polysaccharide produced by Southern ocean origin Pseudoalteromonas sp. MB-16. In Proceedings of the National Academy of Sciences, India Section B: Biological Sciences; Springer: Allahabad, India, March 2019; Volume 89, pp. 283–290. [Google Scholar]
- Gram, L.; Melchiorsen, J.; Bruhn, J.B. Antibacterial activity of marine culturable bacteria collected from a global sampling of ocean surface waters and surface swabs of marine organisms. Mar. Biotechnol. (NY) 2010, 12, 439–451. [Google Scholar] [CrossRef]
- Vynne, N.G.; Mansson, M.; Nielsen, K.F.; Gram, L. Bioactivity, chemical profiling, and 16S rRNA-based phylogeny of Pseudoalteromonas strains collected on a global research cruise. Mar. Biotechnol. (NY) 2011, 13, 1062–1073. [Google Scholar] [CrossRef]
- Szewzyk, U.; Holmström, C.; Wrangstadh, M.; Samuelsson, M.-O.; Maki, J.S.; Kjelleberg, S. Relevance of the exopolysaccharide of marine Pseudomonas sp. strain S9 for the attachment of Ciona intestinalis larvae. Mar. Ecol. Prog. Ser. 1991, 75, 259–265. [Google Scholar] [CrossRef]
- Wrangstadh, M.; Szewzyk, U.; Ostling, J.; Kjelleberg, S. Starvation-specific formation of a peripheral exopolysaccharide by a marine Pseudomonas sp., strain S9. Appl. Environ. Microbiol. 1990, 56, 2065–2072. [Google Scholar]
- Wrangstadh, M.; Conway, P.L.; Kjelleberg, S. The production and release of an extracellular polysaccharide during starvation of a marine Pseudomonas sp. and the effect thereof on adhesion. Arch. Microbiol. 1986, 145, 220–227. [Google Scholar] [CrossRef]
- Wrangstadh, M.; Conway, P.L.; Kjelleberg, S. The role of an extracellular polysaccharide produced by the marine Pseudomonas sp. S9 in cellular detachment during starvation. Can. J. Microbiol. 1989, 35, 309–312. [Google Scholar] [CrossRef]
- Nichols, C.M.; Bowman, J.P.; Guezennec, J. Effects of incubation temperature on growth and production of exopolysaccharides by an antarctic sea ice bacterium grown in batch culture. Appl. Environ. Microbiol. 2005, 71, 3519–3523. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.S.; Li, D.S.; Li, H.M.; Tang, Y.J. Response surface modeling the significance of nitrogen source on the cell growth and Tuber polysaccharides production by submerged cultivation of Chinese truffle Tuber sinense. Process. Biochem. 2008, 43, 868–876. [Google Scholar] [CrossRef]
- Matsuda, M.; Shigeta, S.; Okutani, K. Antiviral activities of marine Pseudomonas polysaccharides and their oversulfated derivative. Mar. Biotechnol. 1999, 1, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wang, J.-W.; Lei, F.; Gao, X.-D.; Tan, R.-X. Free radical scavenging and antioxidant activities of EPS2, an exopolysaccharide produced by a marine filamentous fungus Keissleriella sp. YS 4108. Life Sci. 2004, 75, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, K.-S.; Li, Y.-Y.; Liu, S.-F.; Li, G.-Y. Phylogenetic analysis of antarctic bacterium Pseudoalteromonas sp. S-15-13 and the effect of its extracellular polysaccharide on proliferation of murine lymphocytes. Chin. J. Mar. Drugs 1994, 25, 1–5. [Google Scholar]
- Liang, Z.; Yi, Y.; Guo, Y.; Wang, R.; Hu, Q.; Xiong, X. Chemical Characterization and Antitumor Activities of Polysaccharide Extracted from Ganoderma lucidum. Int. J. Mol. Sci. 2014, 15, 9103–9116. [Google Scholar] [CrossRef]
- Verhoef, R.; Schols, H.A.; Blanco, A.; Siika-Aho, M.; Rättö, M.; Buchert, J.; Lenon, G.; Voragen, A.G.J. Sugar composition and FT-IR analysis of exopolysaccharides produced by microbial isolates from paper millslime deposits. Biotechnol. Bioeng. 2005, 91, 91–105. [Google Scholar] [CrossRef]
- Pereira, L. Identification of phycocolloids by vibrational spectroscopy. In World Seaweed Resources–An Authoritative Reference System; Alan, T., Critchley, M.O., Danilo, B.L., Eds.; UNESCO: Paris, France, 2006. [Google Scholar]
- Pereira, L.; Gheda, F.S.; Ribeiro-Claro, P.J.A. Analysis by vibrational spectroscopy of seaweed polysaccharides with potential use in food, pharmaceutical and cosmetic industries. Int. J. Carbohydr. Chem. 2013, 2013, 537202. [Google Scholar] [CrossRef]
- Muthukumar, A.; Baskaralingam, V.; Mani, D.; Sekar, V.; Marimuthu, G.; Naiyf, S.A.; Jamal, M.K.; Mohammed, N.A.; Giovanni, B. Structural characterization of Bacillus licheniformis Dahb1exopolysaccharide—Antimicrobial potential and larvicidal activity on malaria and Zika virus mosquito vectors. Environ. Sci. Pollut. Res. 2018, 25, 18604–18619. [Google Scholar]
- Koening, J.L. Vibrational Spectroscopy of Carbohydrates. In Infrared and Raman Spectroscopy of Biological Molecules; Springer: Cham, Switzerland, 1979; pp. 125–137. [Google Scholar]
- Mathlouthi, M.; Koening, J.L. Vibrational spectra of carbohydrates. Adv. Carbohydr. Chem. Biochem. 1987, 44, 7–89. [Google Scholar]
- Helm, R.F.; Huang, Z.; Edwards, D.; Leeson, H.; Peery, W.; Potts, M. Structural characterization of the released polysaccharide of desiccation-tolerant Nostoc commune DRH-1. J. Bacteriol. 2000, 182, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Freitas, F.; Alves, V.D.; Reis, M.A. Advances in bacterial exopolysaccharides: From production to biotechnological applications. Trends Biotechnol. 2011, 29, 388–398. [Google Scholar] [CrossRef] [PubMed]
- El-Zaher, E.H.A.; Mostafa, A.A.; El-Souod, S.M.A.; Enas, M. Optimization and characterization of exopolysaccharides from Pleurotus salmoneo-stramineus and its possible application. Egypt J. Exp. Biol. (Bot.) 2015, 11, 181–188. [Google Scholar]
- Kodali, V.P.; Sen, R. Antioxidant and free radical scavenging activities of an exopolysaccharide from a probiotic bacterium. Biotechnol. J. 2008, 3, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, C.; Shi, Q.; Ma, C. Preparation of different molecular weight polysaccharides from Porphyridium cruentum and their antioxidant activities. Int. J. Biol. Macromol. 2009, 45, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Asker, M.M.S.; Shawky, B.T. Structural characterization and antioxidant activity of an extracellular polysaccharide isolated from Brevibacterium otitidis BTS 44. Food Chem. 2010, 123, 315–320. [Google Scholar] [CrossRef]
- Patel, A.; Prajapat, J. Food and health applications of exopolysaccharides produced by lactic acid bacteria. Adv. Dairy Res. 2013, 1–8. [Google Scholar] [CrossRef]
- Sun, M.L.; Zhao, F.; Shi, M.; Zhang, X.Y.; Zhou, B.C.; Zhang, Y.Z.; Chen, X.L. Characterization and biotechnological potential analysis of a new exopolysaccharide from the arctic marine bacterium Polaribacter sp. SM1127. Sci. Rep. 2015, 5, 18435. [Google Scholar] [CrossRef]
- Chen, L.Y.; Du, Y.M.; Wu, H.Q.; Xiao, L. Relationship Between Molecular Structure and Moisture-Retention Ability of Carboxymethyl Chitin and Chitosan. J. Appl. Polym. Sci. 2002, 83, 1233–1241. [Google Scholar] [CrossRef]
- More, T.T.; Yan, S.; John, R.P.; Tyagi, R.D.; Surampalli, R.Y. Biochemical diversity of the bacterial strains and their biopolymer producing capabilities in wastewater sludge. Bioresour. Technol. 2012, 121, 304–311. [Google Scholar] [CrossRef]
- Sutherland, I.W. Novel and established applications of microbial polysac-charides. Trends Biotechnol. 1998, 16, 41–46. [Google Scholar] [CrossRef]
- Boyle, C.D.; Reade, A.E. Characterization of two extracellular polysaccharides from marine bacteria. Appl. Environ. Microbiol. 1983, 46, 392–399. [Google Scholar] [PubMed]
- Park, S.; Kelley, K.A.; Vinogradov, E.; Solinga, R.; Weidenmaier, C.; Misawa, Y.; Lee, J.C. Characterization of the Structure and Biological Functions of a Capsular Polysaccharide Produced by Staphylococcus saprophyticus. J. Bacteriol. 2010, 192, 4618–4626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F. The Screen of Polysaccharide-producing Marine Bacteria and the Study of its Polysaccharide Fermentation. Master’s Thesis, Shandong Polytechnic University, Jinan, China, 2012. [Google Scholar]
- Shang, N.; Xu, R.; Li, P. Structure characterization of an exopolysaccharide produced by Bifidobacterium animalis RH. Carbohydr. Polym. 2013, 91, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Lv, Y.; Yang, X.B.; Zhao, Y.; Ruan, Y.; Yang, Y.; Wang, Z.Z. Separation and quantification of component monosaccharides of the tea polysaccharides from Gynostemma pentaphyllum by HPLC with indirect UV detection. Food Chem. 2009, 112, 742–746. [Google Scholar] [CrossRef]
- Xie, J.Z.; Zou, L.H.; Luo, X.; Qiu, L.; Wei, Q.; Luo, D.; Wu, Y.Q.; Jiao, Y. Structural characterization and immunomodulating activities of a novel polysaccharide from Nervilia fordii. Int. J. Biol. Macromol. 2018, 114, 520–528. [Google Scholar] [CrossRef]
- Fan, G.; Tang, C.; Li, Y.; Yang, Y.D.; Zhang, Y. Analysis of Monosaccharide Compositions of Polysaccharides in Coptidis Rhizoma by Pre-column Derivatization HPLC Method. Chin. J. Exp. Tradit. Med. Formulae 2014, 20, 74–78. [Google Scholar]
- Papageorgiou, S.K.; Kouvelos, E.P.; Favvas, E.P.; Sapalidis, A.A.; Romanos, G.E.; Katsaros, F.K. Metal-carboxylate int eractions in metal-alginate complexes studied with FTIR spectroscopy. Carbohydr. Res. 2010, 345, 469–473. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, J.; Du, H.; Li, Q.; Chen, J.; Zhang, G.; Liu, H.; Wang, J. Structural characterization and antioxidant activities of polysaccharides extracted from the pulp of Elaeagnus angustifolia L. Int. J. Mol. Sci. 2014, 15, 11446–11455. [Google Scholar] [CrossRef]
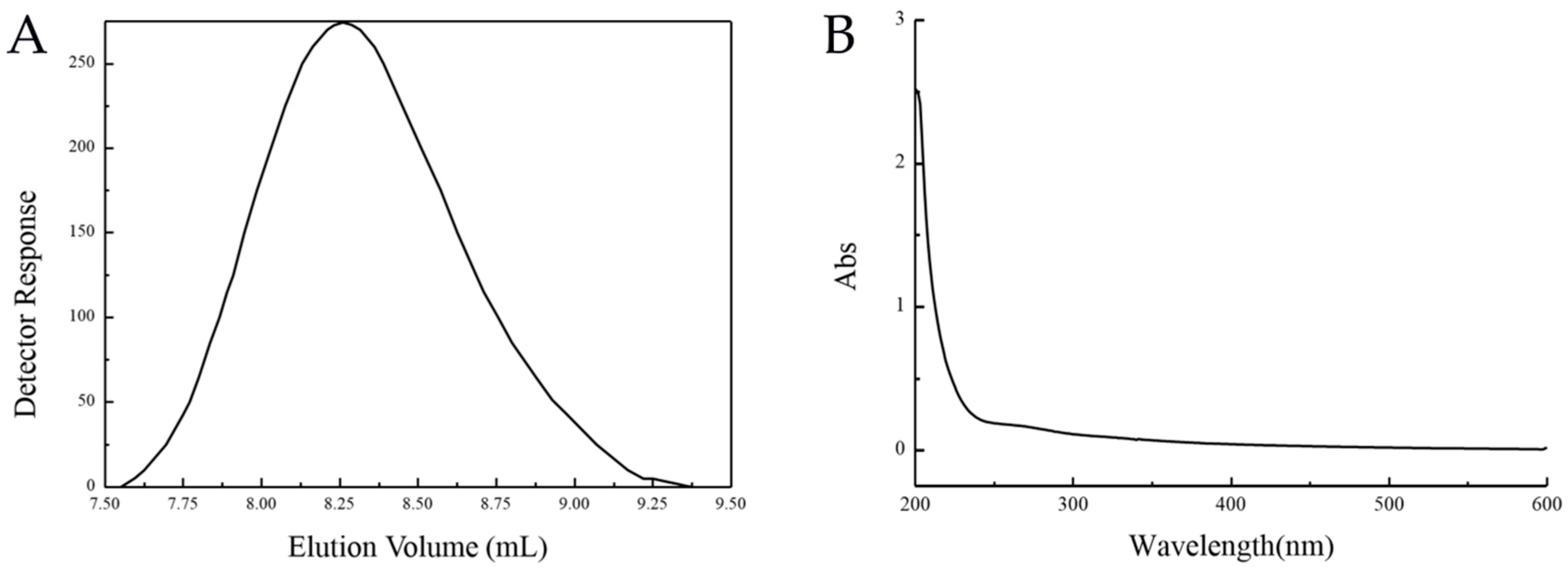
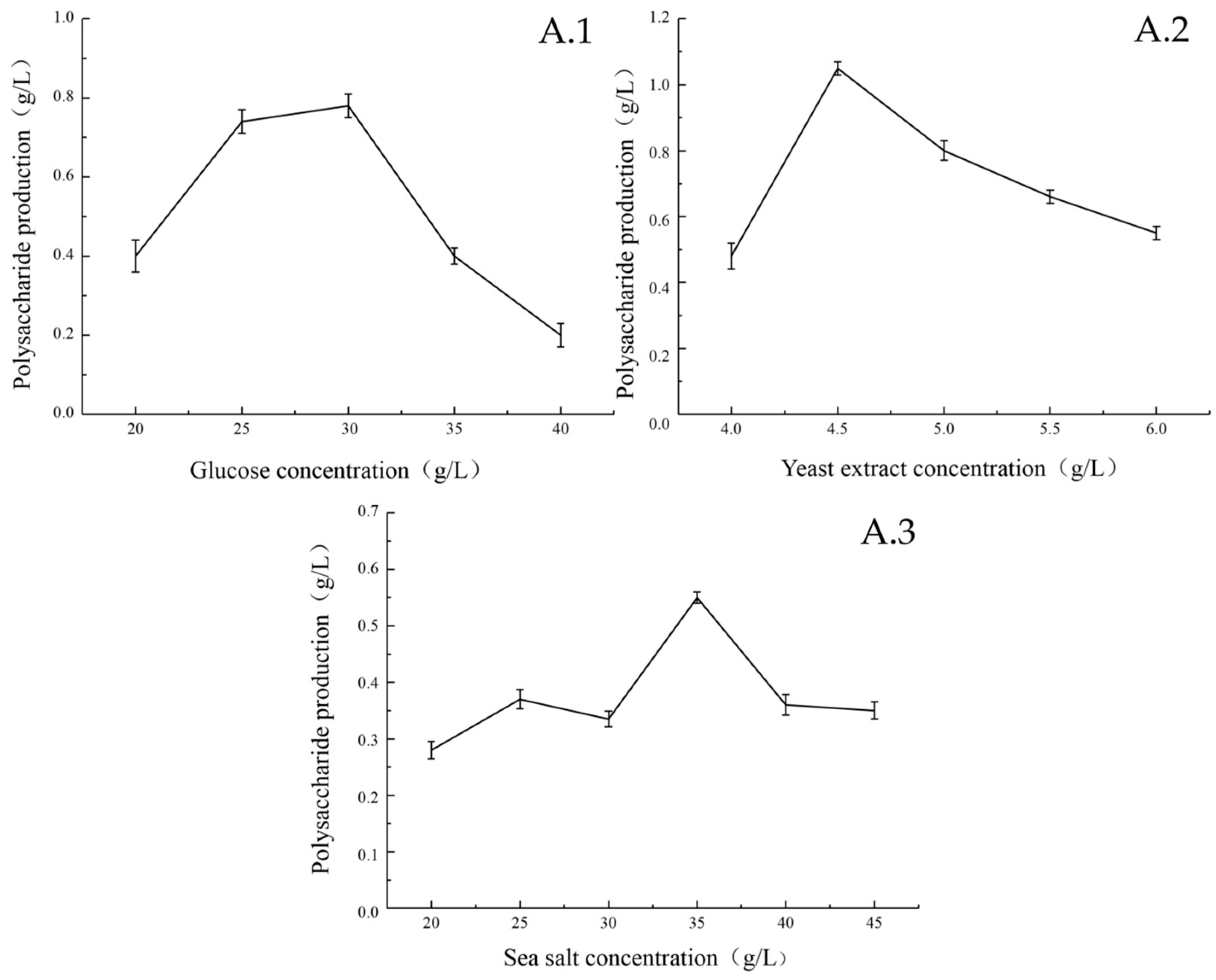
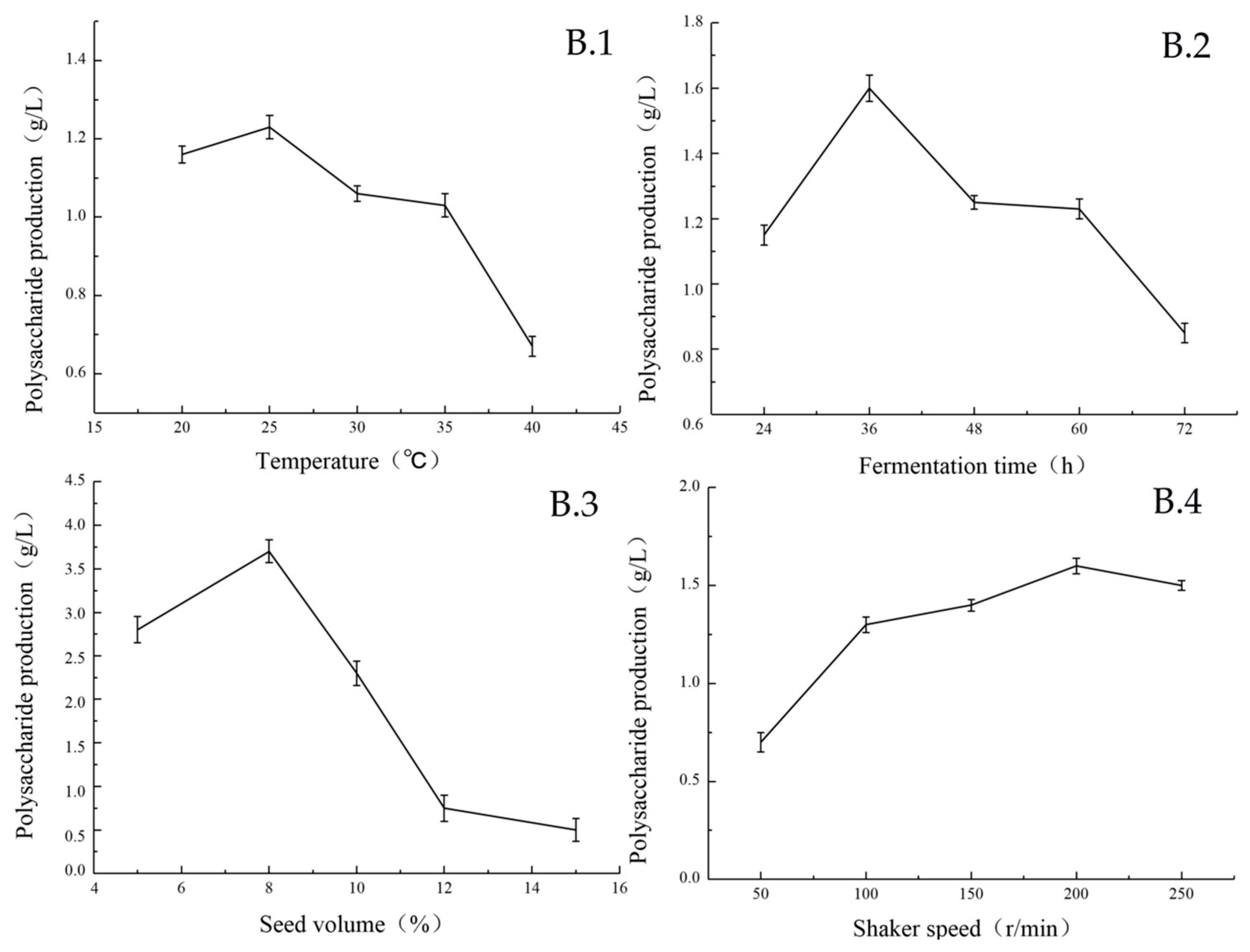
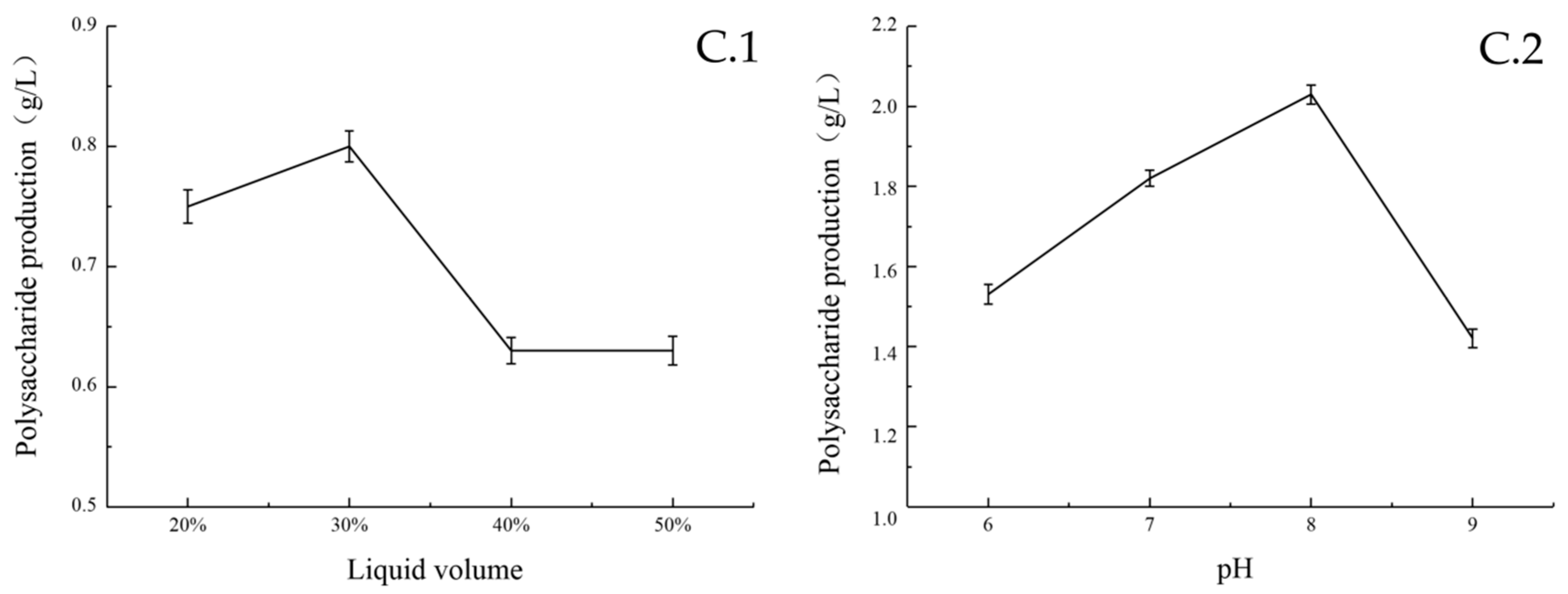


| Serial Number | A | B | C | D | E | F | G | H | J | K | L | Polysaccharide Production (g/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | −1 | −1 | −1 | 1 | −1 | 1 | 1 | −1 | 1 | 2.315 |
| 2 | 1 | −1 | 1 | 1 | 1 | −1 | −1 | −1 | 1 | −1 | 1 | 2.629 |
| 3 | −1 | 1 | 1 | −1 | 1 | 1 | 1 | −1 | −1 | −1 | 1 | 2.573 |
| 4 | −1 | −1 | −1 | 1 | −1 | 1 | 1 | −1 | 1 | 1 | 1 | 2.444 |
| 5 | −1 | −1 | 1 | −1 | 1 | 1 | −1 | 1 | 1 | 1 | −1 | 2.621 |
| 6 | 1 | 1 | −1 | 1 | 1 | 1 | −1 | −1 | −1 | 1 | −1 | 2.694 |
| 7 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | 2.653 |
| 8 | 1 | −1 | 1 | 1 | −1 | 1 | 1 | 1 | −1 | −1 | −1 | 2.726 |
| 9 | −1 | 1 | −1 | 1 | 1 | −1 | 1 | 1 | 1 | −1 | −1 | 2.637 |
| 10 | 1 | 1 | 1 | −1 | −1 | −1 | 1 | −1 | 1 | 1 | −1 | 2.879 |
| 11 | −1 | 1 | 1 | 1 | −1 | −1 | −1 | 1 | −1 | 1 | 1 | 2.935 |
| 12 | 1 | −1 | −1 | −1 | 1 | −1 | 1 | 1 | −1 | 1 | 1 | 3.024 |
| Factor | Regression Coefficients | Impact Level | Contribution |
|---|---|---|---|
| A | 0.034 | 0.067 | 3.13 |
| B | −5.333 × 10−003 | −0.011 | 0.079 |
| C | 0.05 | 0.099 | 6.82 |
| D | 0 | 0 | 0 |
| E | 0.019 | 0.0038 | 0.98 |
| F | −0.12 | −0.23 | 36.76 |
| G | 0.036 | 0.073 | 3.65 |
| H | 0.032 | 0.064 | 2.86 |
| J | −0.09 | −0.18 | 22.38 |
| K | 0.089 | 0.18 | 21.73 |
| L | −0.024 | −0.048 | 1.61 |
| Source of Variation | Sum of Squares | Degree of Freedom | Mean Square | F Value | P Value |
|---|---|---|---|---|---|
| Model | 0.35 | 3 | 0.12 | 11.27 | 0.003 |
| Fermentation time | 0.16 | 1 | 0.16 | 15.37 | 0.0044 |
| Seed volume | 0.097 | 1 | 0.097 | 9.36 | 0.0156 |
| Shaker speed | 0.094 | 1 | 0.094 | 9.09 | 0.0167 |
| Serial Number | A | B | C | Polysaccharide Production (g/L) |
|---|---|---|---|---|
| 1 | −1 | 1 | 0 | 2.637 |
| 2 | 0 | 0 | 0 | 2.766 |
| 3 | 0 | 0 | 0 | 2.758 |
| 4 | −1 | −1 | 0 | 2.298 |
| 5 | −1 | 0 | −1 | 2.573 |
| 6 | 0 | −1 | 1 | 2.629 |
| 7 | 0 | 0 | 0 | 2.742 |
| 8 | 1 | 0 | 1 | 2.548 |
| 9 | 0 | −1 | −1 | 2.508 |
| 10 | 0 | 0 | 0 | 2.766 |
| 11 | 1 | 0 | −1 | 2.419 |
| 12 | 1 | −1 | 0 | 2.556 |
| 13 | 1 | 1 | 0 | 2.460 |
| 14 | 0 | 1 | −1 | 2.750 |
| 15 | −1 | 0 | 1 | 2.468 |
| 16 | 0 | 0 | 0 | 2.742 |
| 17 | 0 | 1 | 1 | 2.661 |
| Source of Variation | Sum of Squares | Degree of Freedom | Mean Square | F Value | p Value |
|---|---|---|---|---|---|
| Model | 0.32 | 9 | 0.036 | 66.81 | <0.0001 |
| A-Fermentation time | 6.125 × 10−6 | 1 | 6.125 × 10−6 | 0.012 | 0.9175 |
| B-Seed volume | 0.033 | 1 | 0.033 | 62.87 | <0.0001 |
| C-Shaker speed | 3.920 × 10−4 | 1 | 3.920 × 10−4 | 0.74 | 0.4189 |
| AB | 0.047 | 1 | 0.047 | 89.02 | <0.0001 |
| AC | 0.014 | 1 | 0.014 | 25.76 | 0.0014 |
| BC | 0.011 | 1 | 0.011 | 20.75 | 0.0026 |
| A2 | 0.17 | 1 | 0.17 | 320.17 | <0.0001 |
| B2 | 0.018 | 1 | 0.018 | 34.54 | 0.0006 |
| C2 | 0.011 | 1 | 00.011 | 21.24 | 0.0025 |
| residual | 4.1184 × 10−3 | 9 | 4.576 × 10−4 | ||
| Lack of fit | 3.529 × 10−3 | 5 | 7.059 × 10−4 | 4.80 | 0.077 |
| Net Errors | 5.888 × 10−4 | 4 | 1.472 × 10−4 | ||
| Total deviation | 0.32 | 16 |
| Variable | Factor | Low Level (−) | High Level (+) |
|---|---|---|---|
| A | Glucose content | 30 g/L | 35 g/L |
| B | Yeast extract content | 2.5 g/L | 5 g/L |
| C | Sea salt content | 30 g/L | 35 g/L |
| E | Liquid volume | 30% | 40% |
| F | Cultivation time | 36 | 48 |
| G | pH | 7 | 8 |
| J | Seed volume | 8% | 10% |
| K | Shaker speed | 100 rpm | 200 rpm |
| L | Temperature (°C) | 25 | 30 |
| Factor | Number | Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Culture time | A | 30 h | 36 h | 42 h |
| Seed volume | B | 7% | 8% | 9% |
| Shaker speed | C | 150 rpm | 200 rpm | 250 rpm |
| Standard Samples LOT | MW (g/mol) | Weight (mg) |
|---|---|---|
| PSS929n | 63,900 | 10.0 |
| PSS8065n | 152,000 | 10.2 |
| PSS13092 | 282,000 | 10.1 |
| PSS14052 | 976,000 | 10.0 |
| PSS9304-4 | 2,260,000 | 10.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, L.; Liu, W.; Liu, K.; Shan, K.; Wang, C.; Xi, C.; Liu, J.; Fan, Q.; Zhang, X.; Lu, X.; et al. Isolation, Optimization of Fermentation Conditions, and Characterization of an Exopolysaccharide from Pseudoalteromonas agarivorans Hao 2018. Mar. Drugs 2019, 17, 703. https://doi.org/10.3390/md17120703
Hao L, Liu W, Liu K, Shan K, Wang C, Xi C, Liu J, Fan Q, Zhang X, Lu X, et al. Isolation, Optimization of Fermentation Conditions, and Characterization of an Exopolysaccharide from Pseudoalteromonas agarivorans Hao 2018. Marine Drugs. 2019; 17(12):703. https://doi.org/10.3390/md17120703
Chicago/Turabian StyleHao, Lujiang, Wenlin Liu, Kai Liu, Kai Shan, Chunlei Wang, Chenxiang Xi, Jianbang Liu, Qiuping Fan, Xiaofei Zhang, Xiaoping Lu, and et al. 2019. "Isolation, Optimization of Fermentation Conditions, and Characterization of an Exopolysaccharide from Pseudoalteromonas agarivorans Hao 2018" Marine Drugs 17, no. 12: 703. https://doi.org/10.3390/md17120703
APA StyleHao, L., Liu, W., Liu, K., Shan, K., Wang, C., Xi, C., Liu, J., Fan, Q., Zhang, X., Lu, X., Xu, Y., Cao, R., Ma, Y., Zheng, L., & Cui, B. (2019). Isolation, Optimization of Fermentation Conditions, and Characterization of an Exopolysaccharide from Pseudoalteromonas agarivorans Hao 2018. Marine Drugs, 17(12), 703. https://doi.org/10.3390/md17120703





