Fucoidan from Undaria pinnatifida Ameliorates Epidermal Barrier Disruption via Keratinocyte Differentiation and CaSR Level Regulation
Abstract
1. Introduction
2. Results
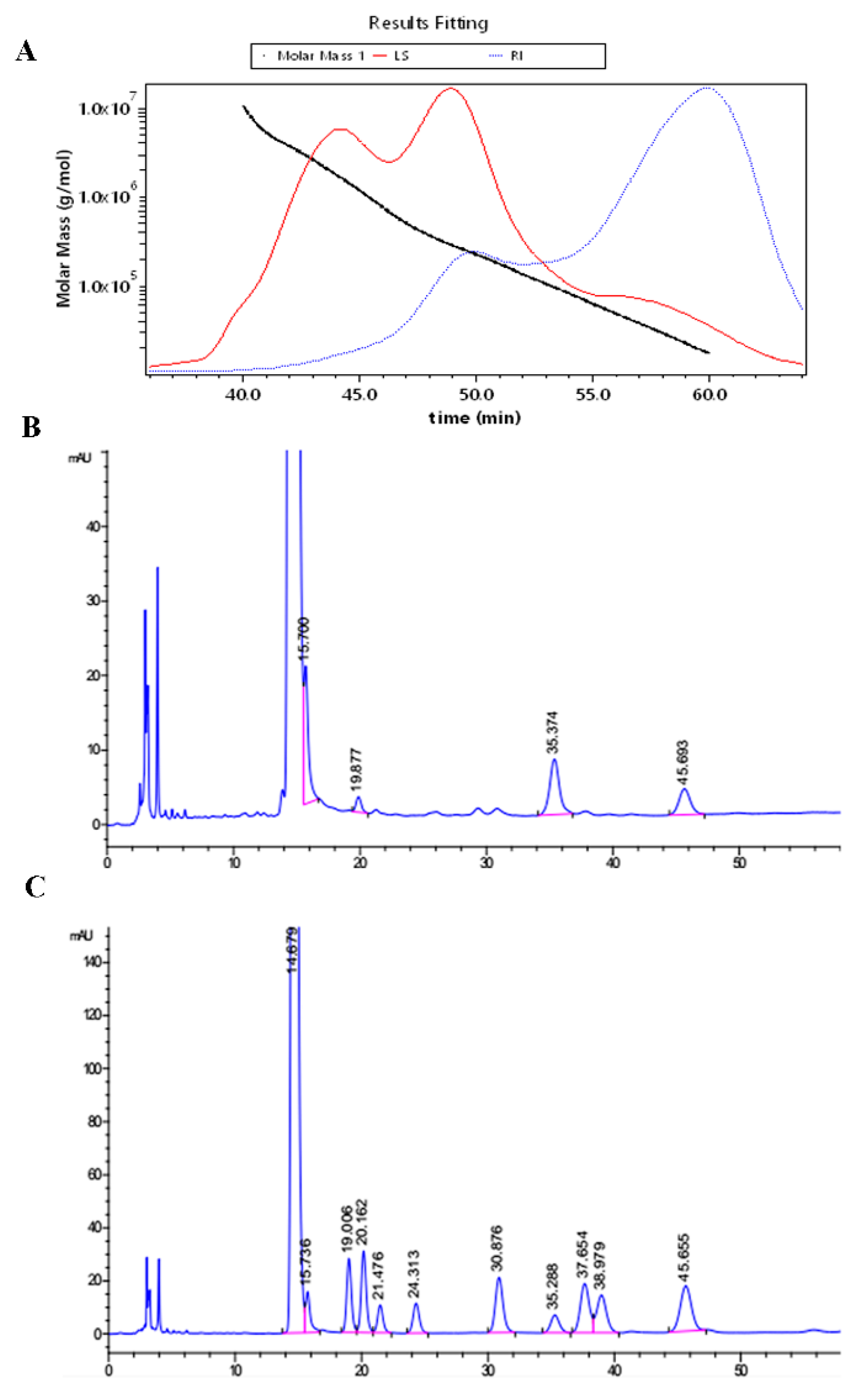
2.1. The Molecular Weight and Monosaccharide Composition
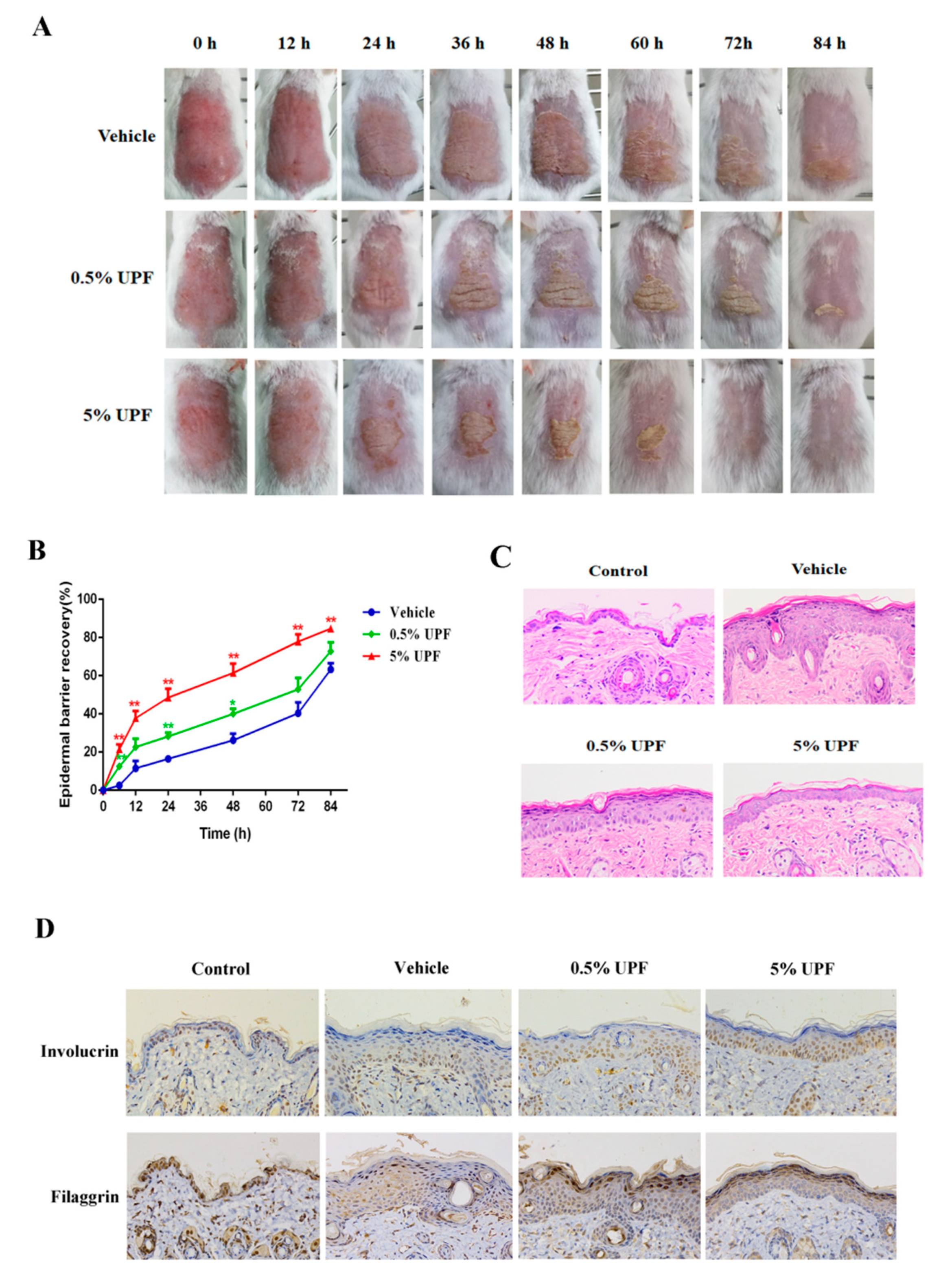
2.2. UPF Could Promote the Epidermal Barrier Recovery
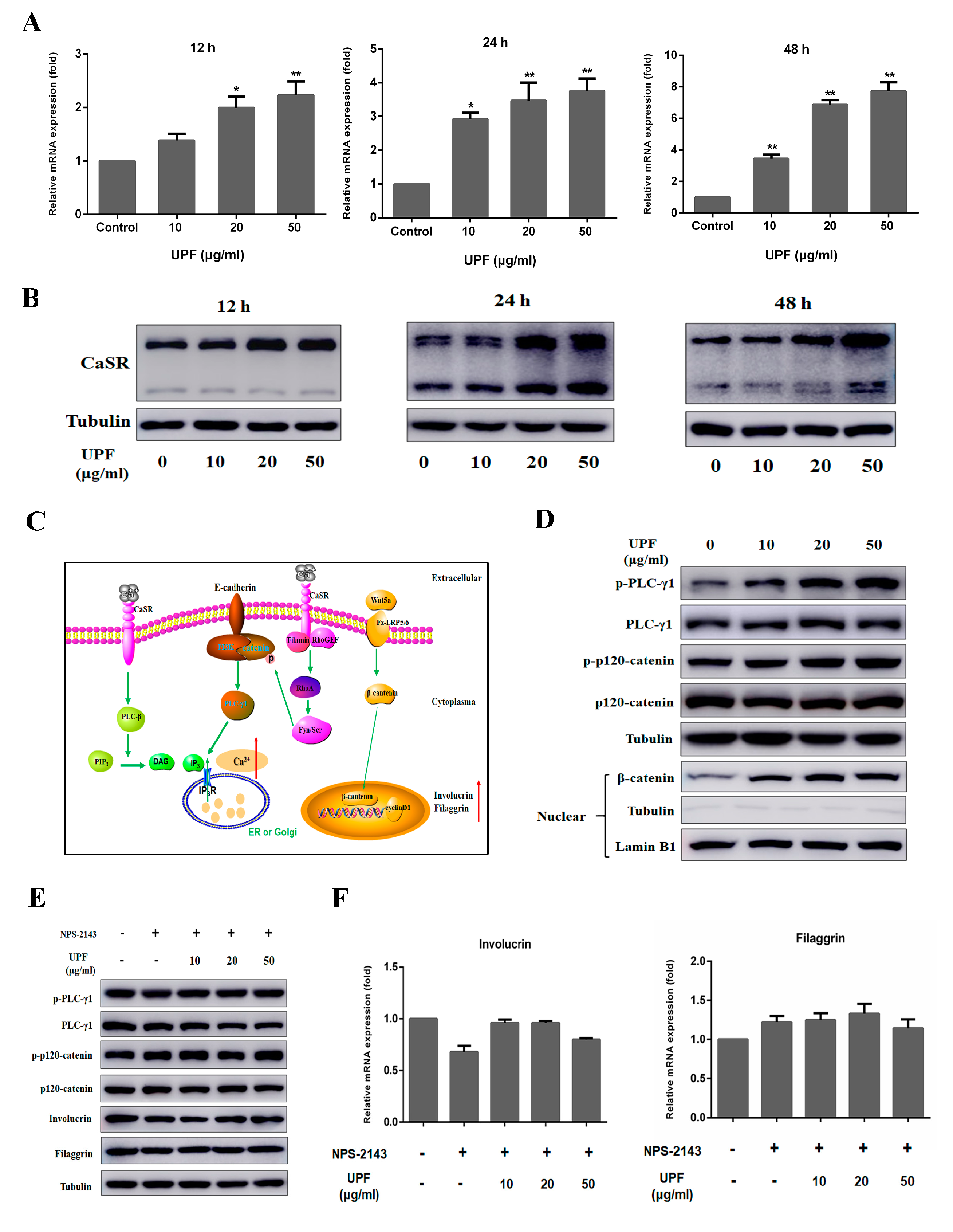
2.3. Keratinocytes Differentiation and Sustained Ca2+ Concentration by UPF in HaCaT Cells
2.4. UPF Could Boost the Expression of CaSR and Activate CaSR Mediated Signaling Pathway
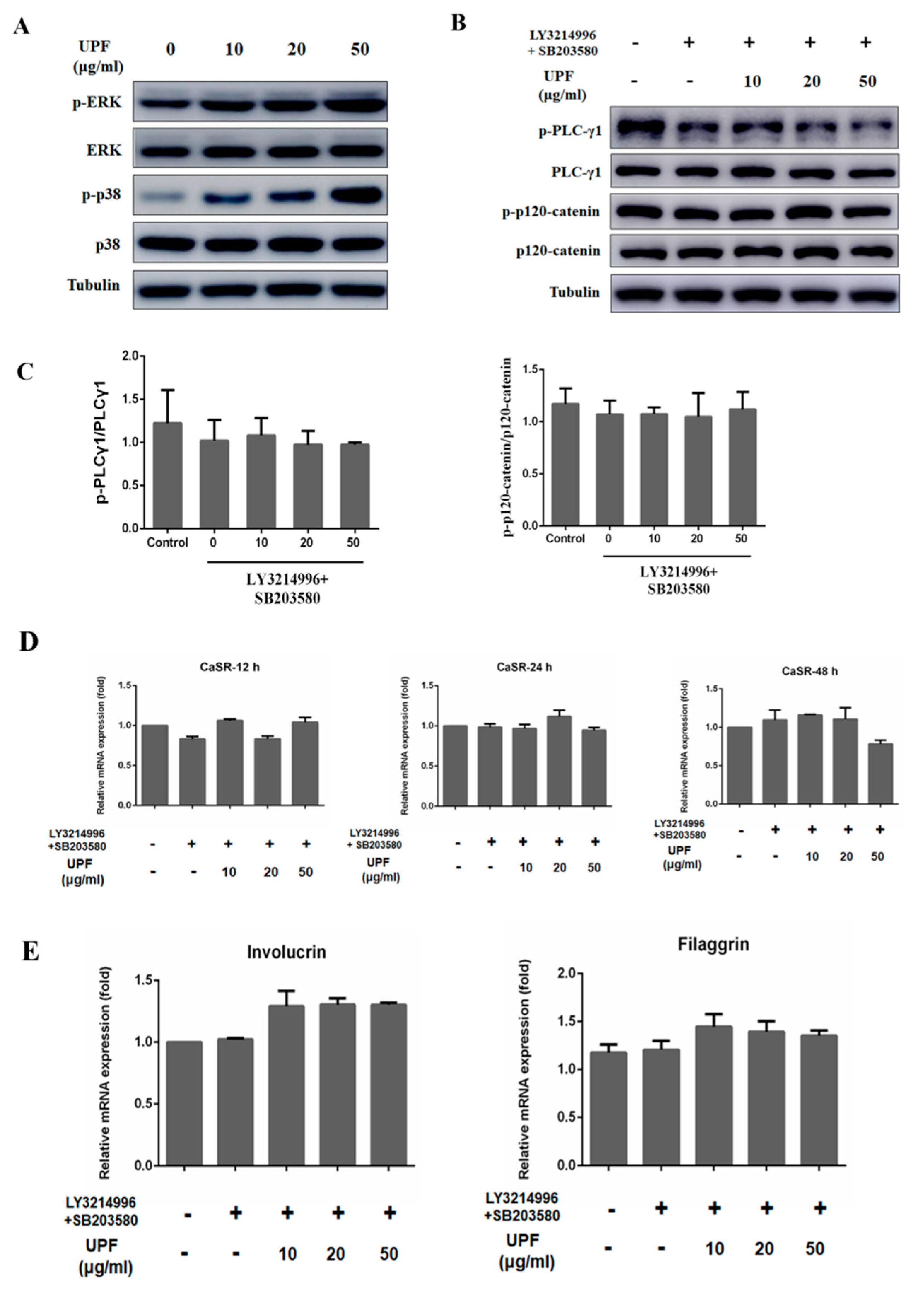
2.5. UPF Activated ERK and p38 Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Mice
4.3. Determination of Molecular Weight
4.4. Analysis of Monosaccharides
4.5. Epidermal Barrier Disruption
4.6. Histological Examination
4.7. Cell Culture
4.8. BrdU Assay
4.9. RNA Isolation and Quantitative Real-time PCR (qRT-PCR) Analysis
4.10. Measurement of Sustained Intracellular Calcium
4.11. Western Blotting
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baroni, A.; Buommino, E.; De Gregorio, V.; Ruocco, E.; Ruocco, V.; Wolf, R. Structure and function of the epidermis related to barrier properties. Clin. Dermatol. 2012, 30, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Eckhart, L.; Zeeuwen, P. The skin barrier: Epidermis vs Environment. Exp. Dermatol. 2018, 27, 805–806. [Google Scholar] [CrossRef] [PubMed]
- Wikramanayake, T.C.; Stojadinovic, O.; Tomic-Canic, M. Epidermal Differentiation in Barrier Maintenance and Wound Healing. Adv. Wound Care 2014, 3, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.L.; Patel, D.M.; Green, K.J. Deconstructing the skin: Cytoarchitectural determinants of epidermal morphogenesis. Nat. Rev. Mol. Cell Biol. 2011, 12, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Steven, A.C.; Steinert, P.M. Protein composition of cornified cell envelopes of epidermal keratinocytes. J. Cell Sci. 1994, 107 Pt 2, 693–700. [Google Scholar]
- Elias, P.M.; Ahn, S.K.; Denda, M.; Brown, B.E.; Crumrine, D.; Kimutai, L.K.; Komuves, L.; Lee, S.H.; Feingold, K.R. Modulations in epidermal calcium regulate the expression of differentiation-specific markers. J. Investig. Dermatol. 2002, 119, 1128–1136. [Google Scholar] [CrossRef]
- Brettmann, E.A.; de Guzman Strong, C. Recent evolution of the human skin barrier. Exp. Dermatol. 2018, 27, 859–866. [Google Scholar] [CrossRef]
- Wolf, R.; Orion, E.; Ruocco, E.; Ruocco, V. Abnormal epidermal barrier in the pathogenesis of psoriasis. Clin. Dermatol. 2012, 30, 323–328. [Google Scholar] [CrossRef]
- Deters, A.M.; Schroder, K.R.; Hensel, A. Kiwi fruit (Actinidia chinensis L.) polysaccharides exert stimulating effects on cell proliferation via enhanced growth factor receptors, energy production, and collagen synthesis of human keratinocytes, fibroblasts, and skin equivalents. J. Cell. Physiol. 2005, 202, 717–722. [Google Scholar] [CrossRef]
- Gescher, K.; Deters, A.M. Typha latifolia L. fruit polysaccharides induce the differentiation and stimulate the proliferation of human keratinocytes in vitro. J. Ethnopharmacol. 2011, 137, 352–358. [Google Scholar] [CrossRef]
- Deters, A.M.; Lengsfeld, C.; Hensel, A. Oligo- and polysaccharides exhibit a structure-dependent bioactivity on human keratinocytes in vitro. J. Ethnopharmacol. 2005, 102, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, K.; Manivasagan, P.; Venkatesan, J.; Kim, S.K. Brown seaweed fucoidan: Biological activity and apoptosis, growth signaling mechanism in cancer. Int. J. Biol. Macromol. 2013, 60, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Joo, H.G. Immunostimulatory effects of fucoidan on bone marrow-derived dendritic cells. Immunol. Lett. 2008, 115, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Chandia, N.P.; Matsuhiro, B. Characterization of a fucoidan from Lessonia vadosa (Phaeophyta) and its anticoagulant and elicitor properties. Int. J. Biol. Macromol. 2008, 42, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.S.; Li, H.; Balcos, M.C.; Yun, H.Y.; Baek, K.J.; Kwon, N.S.; Choi, H.R.; Park, K.C.; Kim, D.S. Fucoidan promotes the reconstruction of skin equivalents. Korean J. Physiol. Pharmacol. 2014, 18, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.J.; Park, K.S.; Ku, M.J.; Lee, M.S.; Jeong, S.H.; Imbs, T.I.; Zvyagintseva, T.N.; Ermakova, S.P.; Lee, Y.H. Effect of Costaria costata fucoidan on expression of matrix metalloproteinase-1 promoter, mRNA, and protein. J. Nat. Prod. 2009, 72, 1731–1734. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Choi, S.H.; Park, S.J.; Lee, Y.J.; Park, J.H.; Song, P.H.; Cho, C.M.; Ku, S.K.; Song, C.H. Promoting Wound Healing Using Low Molecular Weight Fucoidan in a Full-Thickness Dermal Excision Rat Model. Mar. Drugs 2017, 15, 112. [Google Scholar] [CrossRef]
- Tu, C.L.; Oda, Y.; Bikle, D.D. Effects of a calcium receptor activator on the cellular response to calcium in human keratinocytes. J. Investig. Dermatol. 1999, 113, 340–345. [Google Scholar] [CrossRef]
- Bikle, D.D.; Xie, Z.; Tu, C.L. Calcium regulation of keratinocyte differentiation. Expert Rev. Endocrinol. Metab. 2012, 7, 461–472. [Google Scholar] [CrossRef]
- Komuves, L.; Oda, Y.; Tu, C.L.; Chang, W.H.; Ho-Pao, C.L.; Mauro, T.; Bikle, D.D. Epidermal expression of the full-length extracellular calcium-sensing receptor is required for normal keratinocyte differentiation. J. Cell. Physiol. 2002, 192, 45–54. [Google Scholar] [CrossRef]
- Tu, C.L.; Chang, W.; Bikle, D.D. The role of the calcium sensing receptor in regulating intracellular calcium handling in human epidermal keratinocytes. J. Investig. Dermatol. 2007, 127, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.L.; Chang, W.; Bikle, D.D. The calcium-sensing receptor-dependent regulation of cell-cell adhesion and keratinocyte differentiation requires Rho and filamin A. J. Investig. Dermatol. 2011, 131, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. Cell signalling. A tale of two messengers. Nature 1993, 365, 388–389. [Google Scholar] [CrossRef] [PubMed]
- Pece, S.; Gutkind, J.S. Signaling from E-cadherins to the MAPK pathway by the recruitment and activation of epidermal growth factor receptors upon cell-cell contact formation. J. Biol. Chem. 2000, 275, 41227–41233. [Google Scholar] [CrossRef]
- Xie, Z.; Bikle, D.D. The recruitment of phosphatidylinositol 3-kinase to the E-cadherin-catenin complex at the plasma membrane is required for calcium-induced phospholipase C-gamma1 activation and human keratinocyte differentiation. J. Biol. Chem. 2007, 282, 8695–8703. [Google Scholar] [CrossRef]
- Elsholz, F.; Harteneck, C.; Muller, W.; Friedland, K. Calcium—A central regulator of keratinocyte differentiation in health and disease. Eur. J. Dermatol. 2014, 24, 650–661. [Google Scholar] [CrossRef]
- Popp, T.; Steinritz, D.; Breit, A.; Deppe, J.; Egea, V.; Schmidt, A.; Gudermann, T.; Weber, C.; Ries, C. Wnt5a/beta-catenin signaling drives calcium-induced differentiation of human primary keratinocytes. J. Investig. Dermatol. 2014, 134, 2183–2191. [Google Scholar] [CrossRef]
- Seo, H.R.; Kwan, Y.W.; Cho, C.K.; Bae, S.; Lee, S.J.; Soh, J.W.; Chung, H.Y.; Lee, Y.S. PKCalpha induces differentiation through ERK1/2 phosphorylation in mouse keratinocytes. Exp. Mol. Med. 2004, 36, 292–299. [Google Scholar] [CrossRef]
- Iversen, L.; Johansen, C.; Kragballe, K. Signal transduction pathways in human epidermis. Eur. J. Dermatol. 2005, 15, 4–12. [Google Scholar]
- Cursons, J.; Gao, J.; Hurley, D.G.; Print, C.G.; Dunbar, P.R.; Jacobs, M.D.; Crampin, E.J. Regulation of ERK-MAPK signaling in human epidermis. BMC Syst. Biol. 2015, 9, 41. [Google Scholar] [CrossRef]
- Efimova, T.; Broome, A.M.; Eckert, R.L. A regulatory role for p38 delta MAPK in keratinocyte differentiation. Evidence for p38 delta-ERK1/2 complex formation. J. Biol. Chem. 2003, 278, 34277–34285. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.; Li, A.; Kaur, P. Location and phenotype of human adult keratinocyte stem cells of the skin. Differ. Res. Biol. Divers. 2004, 72, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Pullmann, H.; Lennartz, K.J.; Steigleder, G.K. Disturbance of DNA-Synthesis in early psoriasis. Arch. Dermatol. Res. 1977, 258, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.; Geyer, S.; Weninger, W.; Guimberteau, J.C.; Wong, J.K. The dynamic anatomy and patterning of skin. Exp. Dermatol. 2016, 25, 92–98. [Google Scholar] [CrossRef]
- Alameda, J.P.; Navarro, M.; Ramirez, A.; Page, A.; Suarez-Cabrera, C.; Moreno-Maldonado, R.; Paramio, J.M.; del Carmen Farina, M.R.; Del Rio, M.; Fernandez-Acenero, M.J.; et al. IKKalpha regulates the stratification and differentiation of the epidermis: Implications for skin cancer development. Oncotarget 2016, 7, 76779–76792. [Google Scholar] [CrossRef]
- Micallef, L.; Belaubre, F.; Pinon, A.; Jayat-Vignoles, C.; Delage, C.; Charveron, M.; Simon, A. Effects of extracellular calcium on the growth-differentiation switch in immortalized keratinocyte HaCaT cells compared with normal human keratinocytes. Exp. Dermatol. 2009, 18, 143–151. [Google Scholar] [CrossRef]
- Lee, S.E.; Lee, S.H. Skin Barrier and Calcium. Ann. Dermatol. 2018, 30, 265–275. [Google Scholar] [CrossRef]
- Mascia, F.; Denning, M.; Kopan, R.; Yuspa, S.H. The black box illuminated: Signals and signaling. J. Investig. Dermatol. 2012, 132, 811–819. [Google Scholar] [CrossRef]
- Menon, G.K.; Elias, P.M.; Feingold, K.R. Integrity of the permeability barrier is crucial for maintenance of the epidermal calcium gradient. Br. J. Dermatol. 1994, 130, 139–147. [Google Scholar] [CrossRef]
- Ahn, B.K.; Jeong, S.K.; Kim, H.S.; Choi, K.J.; Seo, J.T.; Choi, E.H.; Ahn, S.K.; Lee, S.H. Rottlerin, a specific inhibitor of protein kinase C-delta, impedes barrier repair response by increasing intracellular free calcium. J. Investig. Dermatol. 2006, 126, 1348–1355. [Google Scholar] [CrossRef]
- Lee, S.H.; Elias, P.M.; Proksch, E.; Menon, G.K.; Mao-Quiang, M.; Feingold, K.R. Calcium and potassium are important regulators of barrier homeostasis in murine epidermis. J. Clin. Investig. 1992, 89, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Menon, G.K.; Price, L.F.; Bommannan, B.; Elias, P.M.; Feingold, K.R. Selective obliteration of the epidermal calcium gradient leads to enhanced lamellar body secretion. J. Investig. Dermatol. 1994, 102, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.L.; Bikle, D.D. Role of the calcium-sensing receptor in calcium regulation of epidermal differentiation and function. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Jiang, Y.; Nguyen, T.; Oda, Y.; Tu, C.L. Disruption of Vitamin D and Calcium Signaling in Keratinocytes Predisposes to Skin Cancer. Front. Physiol. 2016, 7, 296. [Google Scholar] [CrossRef]
- Dang, N.; Pang, S.; Song, H.; An, L.; Ma, X. Inactivation of mitogen-activated protein kinase signaling pathway reduces caspase-14 expression in impaired keratinocytes. Iran. J. Basic Med Sci. 2016, 19, 28–33. [Google Scholar]
- Xia, Y.G.; Wang, T.L.; Sun, L.M.; Liang, J.; Yang, B.Y.; Kuang, H.X. A New UPLC-MS/MS Method for the Characterization and Discrimination of Polysaccharides from Genus Ephedra Based on Enzymatic Digestions. Molecules 2017, 22, 1992. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Li, X.; Gan, X.; Qi, J.; Che, B.; Tai, M.; Gao, S.; Zhao, W.; Xu, N.; Hu, Z. Fucoidan from Undaria pinnatifida Ameliorates Epidermal Barrier Disruption via Keratinocyte Differentiation and CaSR Level Regulation. Mar. Drugs 2019, 17, 660. https://doi.org/10.3390/md17120660
Chen Y, Li X, Gan X, Qi J, Che B, Tai M, Gao S, Zhao W, Xu N, Hu Z. Fucoidan from Undaria pinnatifida Ameliorates Epidermal Barrier Disruption via Keratinocyte Differentiation and CaSR Level Regulation. Marine Drugs. 2019; 17(12):660. https://doi.org/10.3390/md17120660
Chicago/Turabian StyleChen, Yu, Xuenan Li, Xiaoshuang Gan, Junmei Qi, Biao Che, Meiling Tai, Shuang Gao, Wengang Zhao, Nuo Xu, and Zhenlin Hu. 2019. "Fucoidan from Undaria pinnatifida Ameliorates Epidermal Barrier Disruption via Keratinocyte Differentiation and CaSR Level Regulation" Marine Drugs 17, no. 12: 660. https://doi.org/10.3390/md17120660
APA StyleChen, Y., Li, X., Gan, X., Qi, J., Che, B., Tai, M., Gao, S., Zhao, W., Xu, N., & Hu, Z. (2019). Fucoidan from Undaria pinnatifida Ameliorates Epidermal Barrier Disruption via Keratinocyte Differentiation and CaSR Level Regulation. Marine Drugs, 17(12), 660. https://doi.org/10.3390/md17120660



