Evaluation of Marine Diindolinonepyrane in Vitro and in Vivo: Permeability Characterization in Caco-2 Cells Monolayer and Pharmacokinetic Properties in Beagle Dogs
Abstract
:1. Introduction
2. Results
2.1. Establishment and Evaluation of Caco-2 Cell Model
2.2. Analysis of Absorption and Transport Characteristics of 2,5-BHPA
2.3. Linearity of Standard Curve
2.4. Detection of 2,5-BHPA in Plasma System
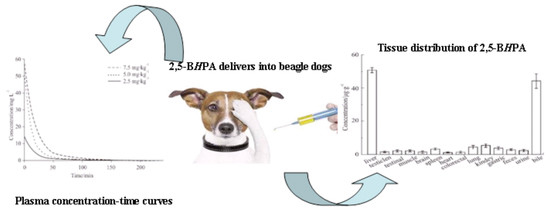
2.5. Pharmacokinetics
2.6. Tissue Distribution
3. Discussion
4. Materials and Methods
4.1. Reagents and Instruments
4.2. Analysis Conditions
4.3. Animals and Ethical Statement
4.4. In Vitro Experiments
4.4.1. Preparation of Samples
4.4.2. Establishment of Caco-2 Cells Monolayer Model
4.4.3. Absorption and Bidirectional Transport Experiment
4.4.4. Calibration Curve for 2,5-BHPA
4.4.5. Detection of 2,5-BHPA in Plasma System in Vitro
4.5. In Vivo Experiments
4.6. Data Processing
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heit, J.A. Venous thromboembolism: Disease burden, outcomes and risk factors. J. Thromb. Haemost. 2005, 3, 1611–1617. [Google Scholar] [CrossRef] [PubMed]
- Naess, I.A.; Christiansen, S.C.; Romundstad, P.; Cannegieter, S.C.; Rosendaal, F.R.; Hammerstrøm, J. Incidence and mortality of venous thrombosis: A population-based study. J. Thromb. Haemost. 2007, 5, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.W.; Gao, R.L.; Liu, L.S. China Cardiovascular Disease Report 2013 Summary. J. Chin. Circ. 2014, 29, 487–491. [Google Scholar]
- Volpe, D.A. Drug-permeability and transporter assays in Caco-2 and MDCK cell lines. Future Med. Chem. 2011, 3, 2063–2077. [Google Scholar] [CrossRef]
- Gan, L.S.; Arthur, M.M.; Bobbie, K.; Patrick, A.; Timothy, B.; Wayne, H.; Dhiren, T. CYP3A-like cytochrome P-450-mediated metabolism and polarized efflux of cyclosporin A in Caco-2 cells. Drug Metab. Dispos. 1996, 24, 344–349. [Google Scholar]
- Hu, M.; Zheng, L.; Chen, J.; Liu, L.J.; Zhu, Y.P.; Dantzig, A.H.; Stratford, R.E., Jr. Mechanisms of Transport of Quinapril in Caco-2 Cell Monolayers: Comparison with Cephalexin. Pharm. Res. 1995, 12, 1120–1125. [Google Scholar] [CrossRef]
- Narumi, S.; Kumiko, T.; Tastuaki, O.; Masaaki, K.; Kazumi, T.; Koji, F. Effects of benzo(e)pyrene and benzo(a)pyrene on P-glycoprotein-mediated transport in Caco-2 cell monolayer: A comparative approach. Toxicol. In Vitro 2007, 21, 827–834. [Google Scholar]
- Rouquayrol, M.; Gaucher, B.; Roche, D.; Greiner, J.; Vierling, P. Transepithelial transport of prodrugs of the HIV protcase inhibitors saquinavir indinavir and nelfinavir across Caco-2 cell monolayers. Pharm. Res. 2002, 19, 1704–1712. [Google Scholar] [CrossRef]
- Xing, W.; Wen-Hui, W.; Li-Chun, S.; Zhi-Hua, C.; Jie, Z.; Bin, B. Isolation of Fibrinolytic Active Compound from Marine Fungi and Initial Identification of the Strain. Nat. Prod. Res. Dev. 2012, 24, 57–61. [Google Scholar]
- Zhang, Y.; Wu, W.H.; Zhou, P.G.; Bao, B. Screening and isolation of fibrinolytic active compound from marine microorganism. Chin. J. Mar. Drugs 2008, 27, 39–43. [Google Scholar]
- Guo, R.H.; Zhang, Y.T.; Duan, D.; Fu, Q.; Yu, X.W.; Wang, S.J.; Bao, B.; Wu, W.H. Fibrinolytic Evaluation of Compounds Isolated from a Marine Fungus Stachybotrys longispora FG216. Chin. J. Chem. 2016, 34, 1194–1198. [Google Scholar] [CrossRef]
- Wang, G.; Wu, W.H.; Zhu, Q.G.; Fu, S.Q.; Wang, X.Y.; Hong, S.T.; Guo, R.H.; Bao, B. Identification and Fibrinolytic Evaluation of an Isoindolone Derivative Isolated from a Rare Marine Fungus Stachybotrys longispora FG216. Chin. J. Chem. 2015, 33, 1089–1095. [Google Scholar] [CrossRef]
- Yan, T.; Wu, W.H.; Su, T.W.; Chen, J.J.; Zhu, Q.G.; Zhang, C.Y.; Wang, X.Y.; Bao, B. Effects of a novel marine natural product: Pyrano indolone alkaloid fibrinolytic compound on thrombolysis and hemorrhagic activities In Vitro and In Vivo. Arch. Pharm. Res. 2015, 38, 1530–1540. [Google Scholar] [CrossRef] [PubMed]
- Su, T.W.; Wu, W.H.; Yan, T.; Zhang, C.Y.; Zhu, Q.G.; Bao, B. Pharmacokinetics and tissue distribution of a novel marine fibrinolytic compound in Wistar rat following intravenous administrations. J. Chromatogr. B 2013, 942–943, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Yan, L.Q.; Yao, G.C.; Guo, X.J. Estimation of p-coumaric acid as metabolite of E-6-O-p-coumaroyl scandoside methyl ester in rat plasma by HPLC and its application to a pharmacokinetic study. J. Chromatogr. B 2006, 831, 303–306. [Google Scholar] [CrossRef]
- Petra, K.; Jiří, K.; Martin, Š.; Olga, P.; Vladimír, G.; Přemysl, P. HPLC determination of a novel aroylhydrazone iron chelator (o-108) in rabbit plasma and its application to a pilot pharmacokinetic study. J. Chromatogr. B 2006, 838, 107–112. [Google Scholar]
- Li, M.; Cui, J.; Ngadi, M.O.; Ma, Y. Absorption mechanism of whey-protein-delivered curcumin using Caco-2 cell monolayers. Food Chem. 2015, 180, 48–54. [Google Scholar] [CrossRef]
- Walle, U.K.; Galijatovic, A.; Walle, T. Transport of the Flavonoid Chrysin and Its Conjugated Metabolites by the Human Intestinal Cell Line Caco-2. Biochem. Pharmacol. 1999, 58, 431–438. [Google Scholar] [CrossRef]
- Artursson, P.; Palm, K.; Luthman, K. Caco-2 monolayer in experimental and theoretical predictions of drug transport. Adv. Drug Deliv. Rev. 2001, 46, 27–43. [Google Scholar] [CrossRef]
- Haber, B.A.; Mohn, K.L.; Diamond, R.H.; Taub, R. Induction patterns of 70 genes during nine days after hepatectomy define the temporal course of liver regeneration. J. Clin. Investig. 1993, 91, 1319–1326. [Google Scholar] [CrossRef]
- Patil, K.R.; Tripathi, A.D.; Pathak, G.; Katti, S.S. Thermodynamic properties of aqueous electrolyte solutions. 1. Vapor-pressure of aqueous-solutions of lithium chloride, lithium bromide, and lithium iodide. J. Chem. Eng. Data 1990, 35, 166–168. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.R.; Zhou, J.P.; Xie, S.F. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Programs Biomed. 2010, 99, 306–314. [Google Scholar] [CrossRef] [PubMed]







| Compounds | Concentration/ μM | Mean Papp/10−6 cm·s−1 | Efflux Ratio | Recoveries/% | Total Recoveries/% | |||
|---|---|---|---|---|---|---|---|---|
| AP to BL | BL to AP | AP to BL | BL to AP | AP to BL | BL to AP | |||
| Fenoterol | 2 | 0.055 ± 0.008 | / | / | 87.67 ± 1.24 | / | 90.92 ± 4.23 | / |
| Propranolol | 2 | 26.406 ± 0.854 | / | / | 92.15 ± 2.42 | / | 110.01 ± 5.21 | / |
| Digoxin | 2 | 0.033 ± 0.003 | 9.05 ± 0.62 | 260 | 83.66 ± 3.14 | 82.87 ± 4.38 | 86.44 ± 3.51 | 83.31 ± 2.40 |
| 2,5-BHPA | 0.5 | 0.493 ± 0.016 | 0.555 ± 0.09 | 1.13 | 8.745 ± 0.155 | 25.54 ± 1.55 | 35.77 ± 1.29 | 25.48 ± 1.13 |
| 2 | 0.048 ± 0.006 | 0.065 ± 0.005 | 1.55 | 13.419 ± 0.566 | 47.16 ± 1.32 | 39.67 ± 1.47 | 47.69 ± 2.10 | |
| 5 | 0.044 ± 0.003 | 0.043 ± 0.004 | 1.06 | 26.76 ± 1.02 | 52.42 ± 2.20 | 52.34 ± 4.34 | 53.02 ± 2.01 | |
| Compounds | Concentration/ µM | Mean Papp/10−6 cm·s−1 | Efflux Ratio | Recoveries/% | Total Recoveries/% | |||
|---|---|---|---|---|---|---|---|---|
| AP to BL | BL to AP | AP to BL | BL to AP | AP to BL | BL to AP | |||
| Fenoterol | 2 | 0.091 ± 0.012 | / | / | 94.38 ± 5.14 | / | 96.16 ± 6.72 | / |
| Propranolol | 2 | 18.12 ± 1.02 | / | / | 80.32 ± 3.50 | / | 92.24 ± 3.64 | / |
| Digoxin | 2 | 0.023 ± 0.001 | 10.351 ± 0.911 | 484.58 | 84.14 ± 3.13 | 93.35 ± 3.56 | 85.37 ± 3.52 | 93.43 ± 4.63 |
| 2,5-BHPA | 5 | 0.097 ± 0.005 | 0.153 ± 0.012 | 1.56 | 85.52 ± 2.34 | 96.32 ± 5.15 | 92.83 ± 3.44 | 96.41 ± 3.52 |
| 15 | 0.045 ± 0.002 | 0.132 ± 0.035 | 2.91 | 84.85 ± 3.36 | 96.18 ± 6.56 | 92.83 ± 4.26 | 96.97 ± 4.32 | |
| 25 | 0.146 ± 0.009 | 0.216 ± 0.031 | 1.49 | 82.25 ± 2.28 | 89.52 ± 4.19 | 90.91 ± 4.63 | 89.63 ± 3.26 | |
| Concentration/μg·mL−1 | Recovery/% | RSD/% | Stability/% | RSD/% |
|---|---|---|---|---|
| 1.0 | 98.36 ± 6.38 | 6.49 | 94.69 ± 5.37 | 5.67 |
| 100 | 102.26 ± 4.69 | 4.59 | 98.39 ± 2.25 | 2.29 |
| 400 | 96.58 ± 5.61 | 5.81 | 97.63 ± 5.91 | 6.05 |
| Parameters | 7.5 mg·kg−1 | 5.0 mg·kg−1 | 2.5 mg·kg−1 |
|---|---|---|---|
| K10 (min−1) | 0.051 ± 0.015 | 0.075 ± 0.012 | 0.055 ± 0.021 |
| K12 (min−1) | 0.007 ± 0.004 | 0.017 ± 0.002 | 0.009 ± 0.004 |
| K21 (min−1) | 0.017 ± 0.003 | 0.018 ± 0.001 | 0.018 ± 0.001 |
| T1/2 (min) | 49 ± 2 | 48 ± 2 | 49 ± 2 |
| AUC0-t (μg·(mL·min)−1 | 1180.5 ± 49.1 | 717.2 ± 23.6 | 268.6 ± 19.3 |
| AUC0-inf (μg·(mL·min)−1) | 1189.0 ± 58.2 | 723.3 ± 14.78 | 270.8 ± 35.8 |
| Cmax(μg·mL−1) | 56.48 ± 6.23 | 48.63 ± 5.53 | 13.64 ± 2.76 |
| MRT (min) | 28.17 ± 1.16 | 26.23 ± 0.35 | 28.66 ± 0.84 |
| CL (L·min−1·kg−1) | 0.0062 ± 0.0004 | 0.0071 ± 0.0008 | 0.0092 ± 0.0006 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Z.; Guo, R.; Elango, J.; Bao, B.; Wu, W. Evaluation of Marine Diindolinonepyrane in Vitro and in Vivo: Permeability Characterization in Caco-2 Cells Monolayer and Pharmacokinetic Properties in Beagle Dogs. Mar. Drugs 2019, 17, 651. https://doi.org/10.3390/md17120651
Ma Z, Guo R, Elango J, Bao B, Wu W. Evaluation of Marine Diindolinonepyrane in Vitro and in Vivo: Permeability Characterization in Caco-2 Cells Monolayer and Pharmacokinetic Properties in Beagle Dogs. Marine Drugs. 2019; 17(12):651. https://doi.org/10.3390/md17120651
Chicago/Turabian StyleMa, Zibin, Ruihua Guo, Jeevithan Elango, Bin Bao, and Wenhui Wu. 2019. "Evaluation of Marine Diindolinonepyrane in Vitro and in Vivo: Permeability Characterization in Caco-2 Cells Monolayer and Pharmacokinetic Properties in Beagle Dogs" Marine Drugs 17, no. 12: 651. https://doi.org/10.3390/md17120651
APA StyleMa, Z., Guo, R., Elango, J., Bao, B., & Wu, W. (2019). Evaluation of Marine Diindolinonepyrane in Vitro and in Vivo: Permeability Characterization in Caco-2 Cells Monolayer and Pharmacokinetic Properties in Beagle Dogs. Marine Drugs, 17(12), 651. https://doi.org/10.3390/md17120651