Preparation of Antioxidant Peptide by Microwave- Assisted Hydrolysis of Collagen and Its Protective Effect Against H2O2-Induced Damage of RAW264.7 Cells
Abstract
:1. Introduction
2. Results and Discussion
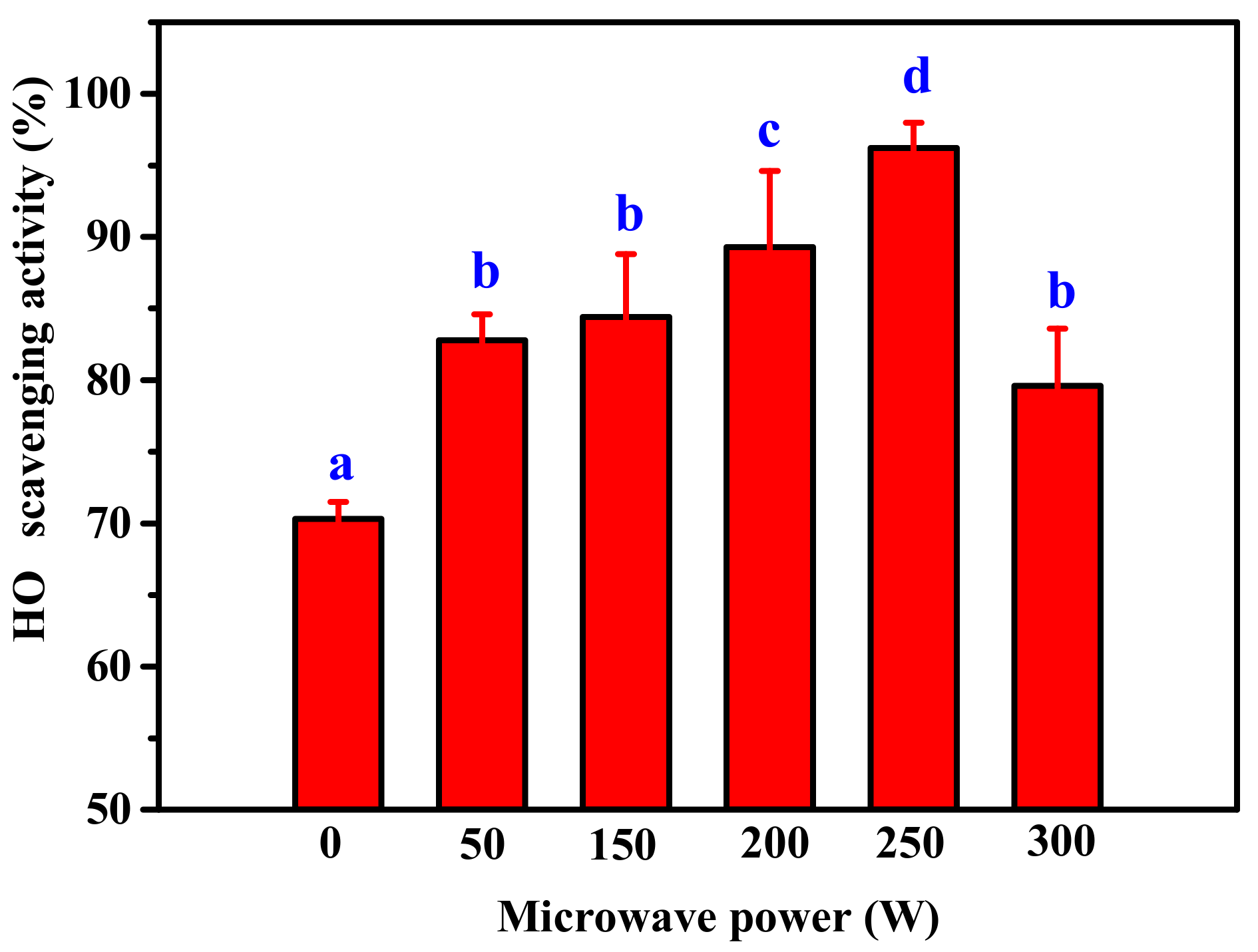
2.1. Effect of Microwave Assisted on Alkaline Protease Hydrolysis of ASC-Am
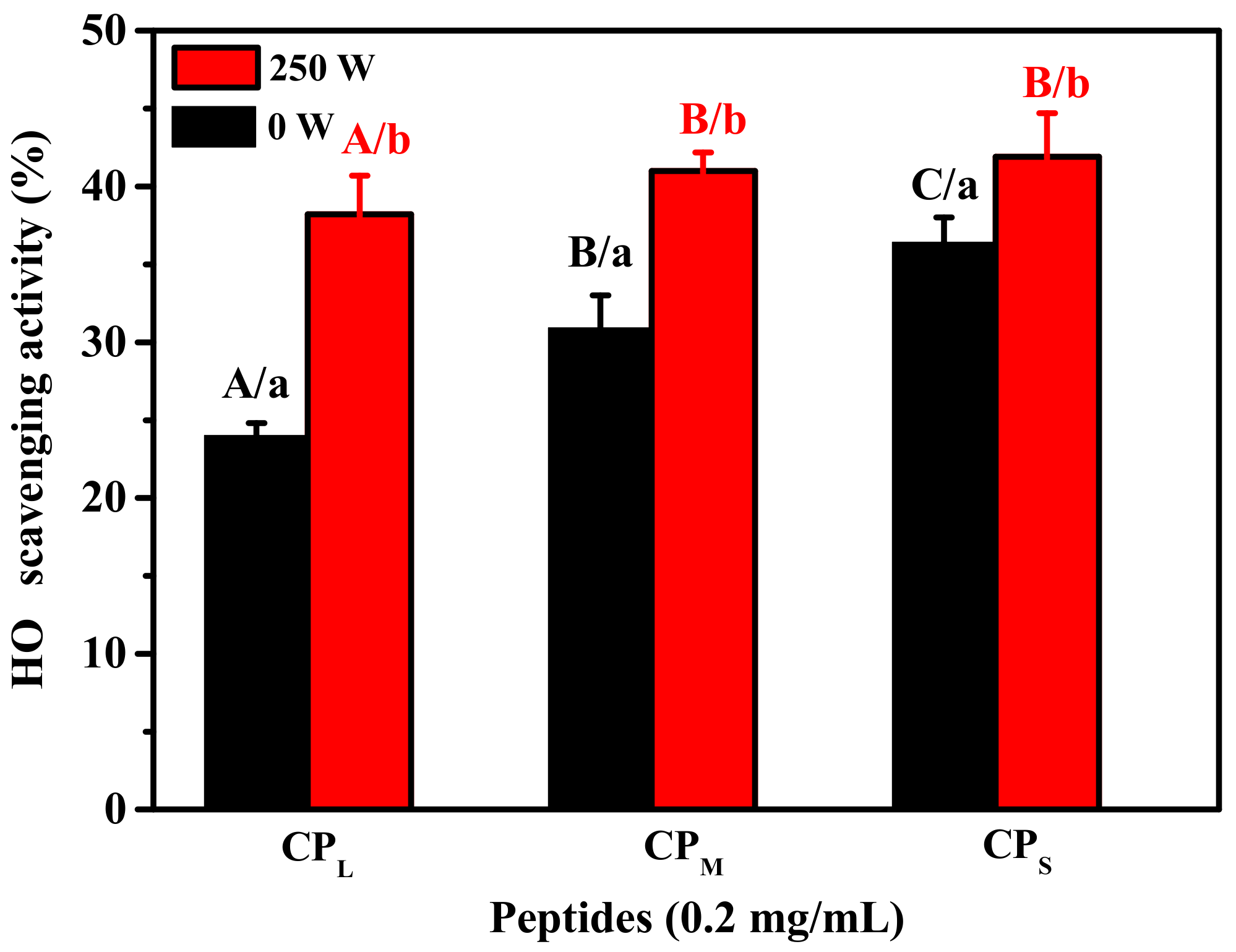
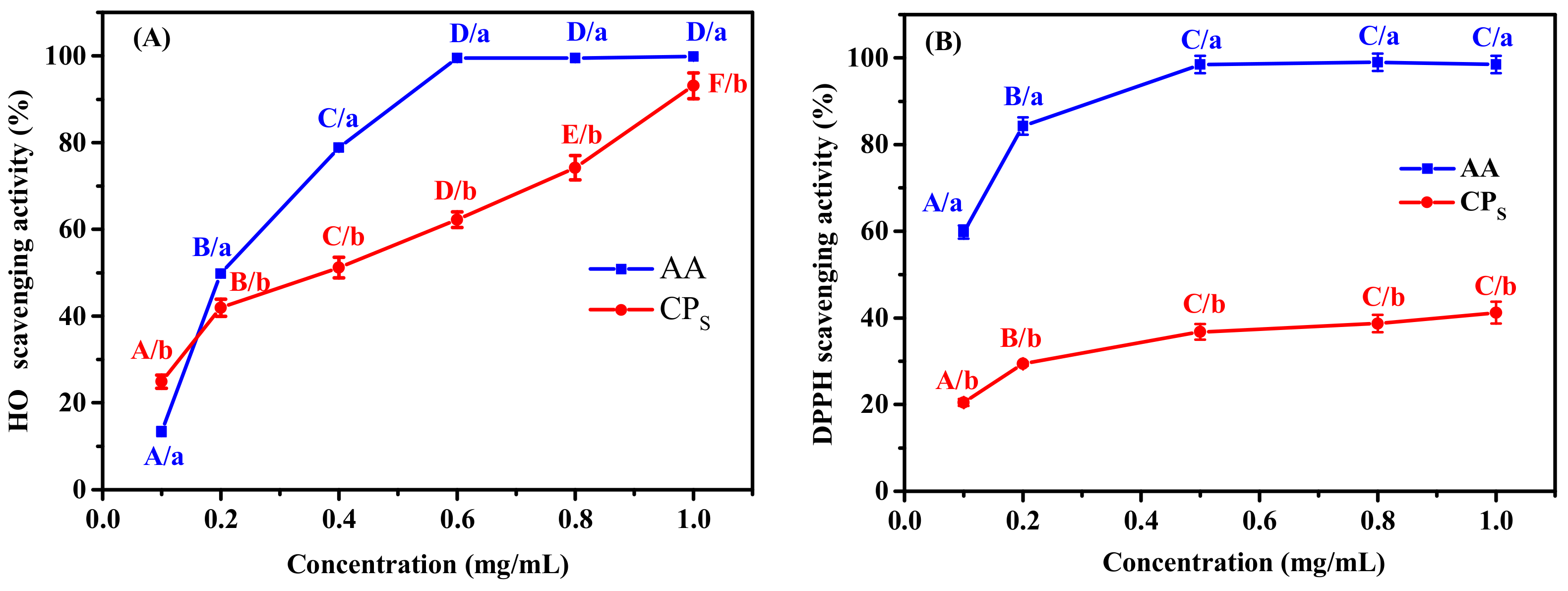
2.2. Antioxidant Activity of CPS
2.3. Antioxidant Activity Evaluated by H2O2-Induced Injury Cell Model
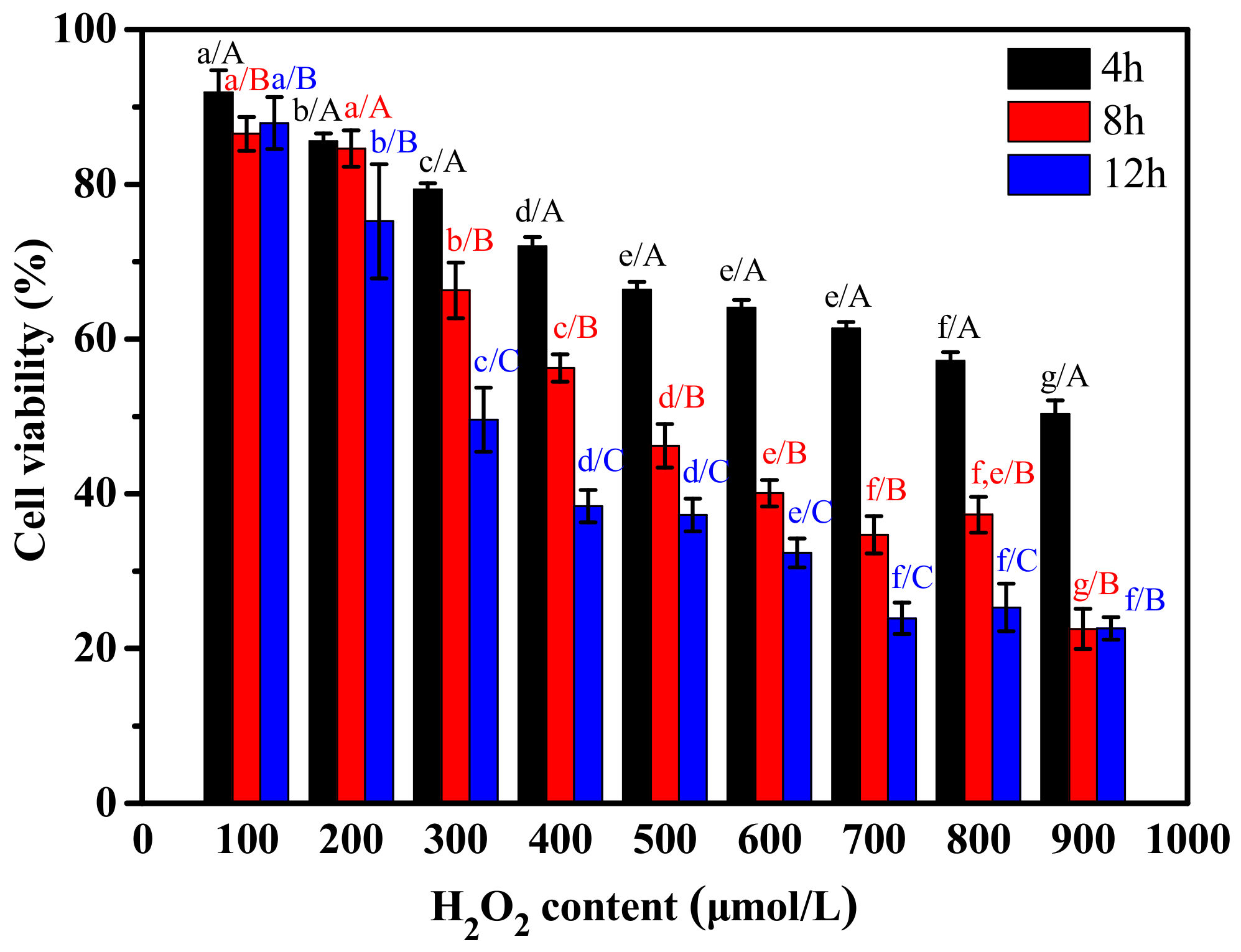
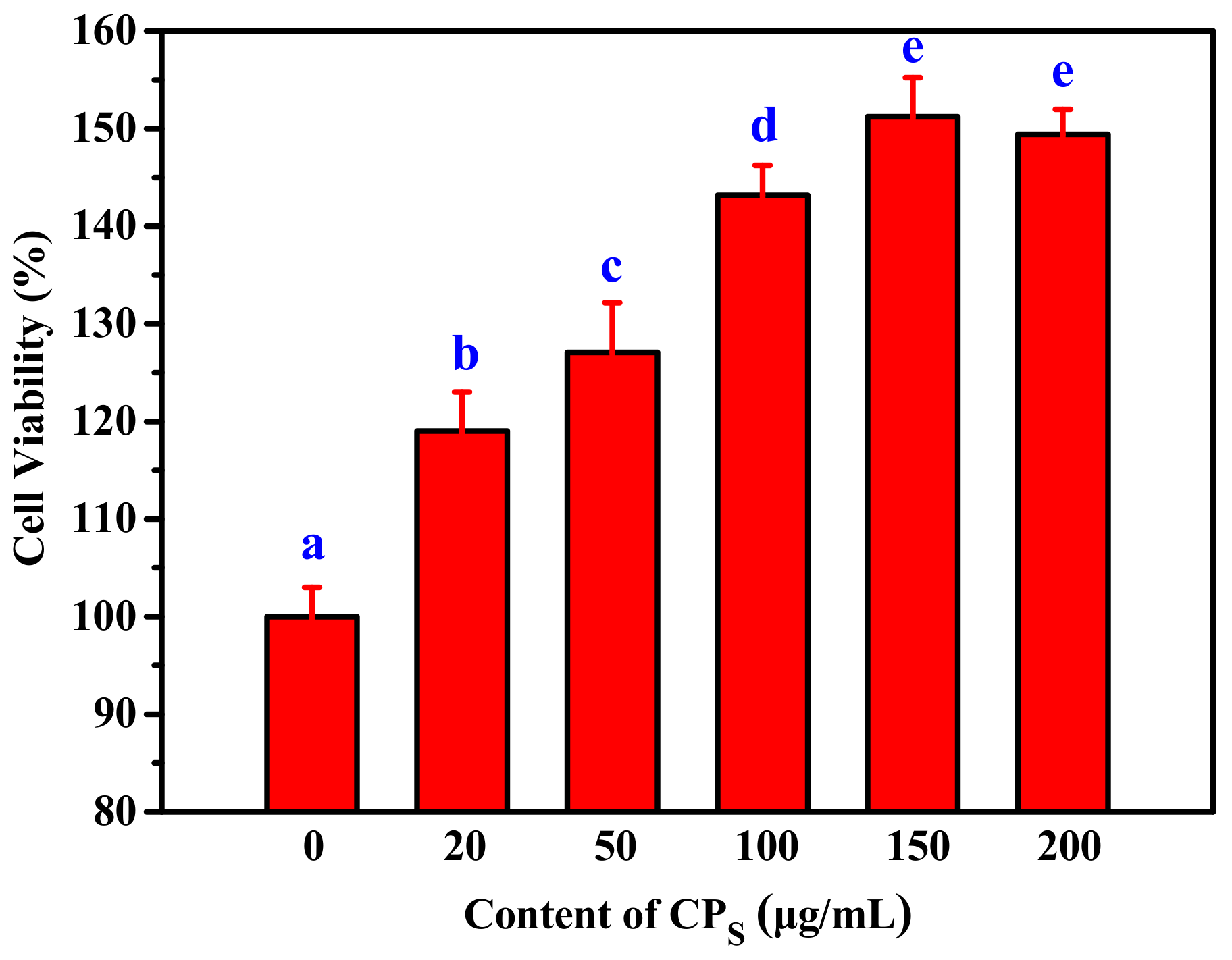
2.3.1. The Effect of H2O2 and CPs on the Proliferation of RAW264.7 Cells
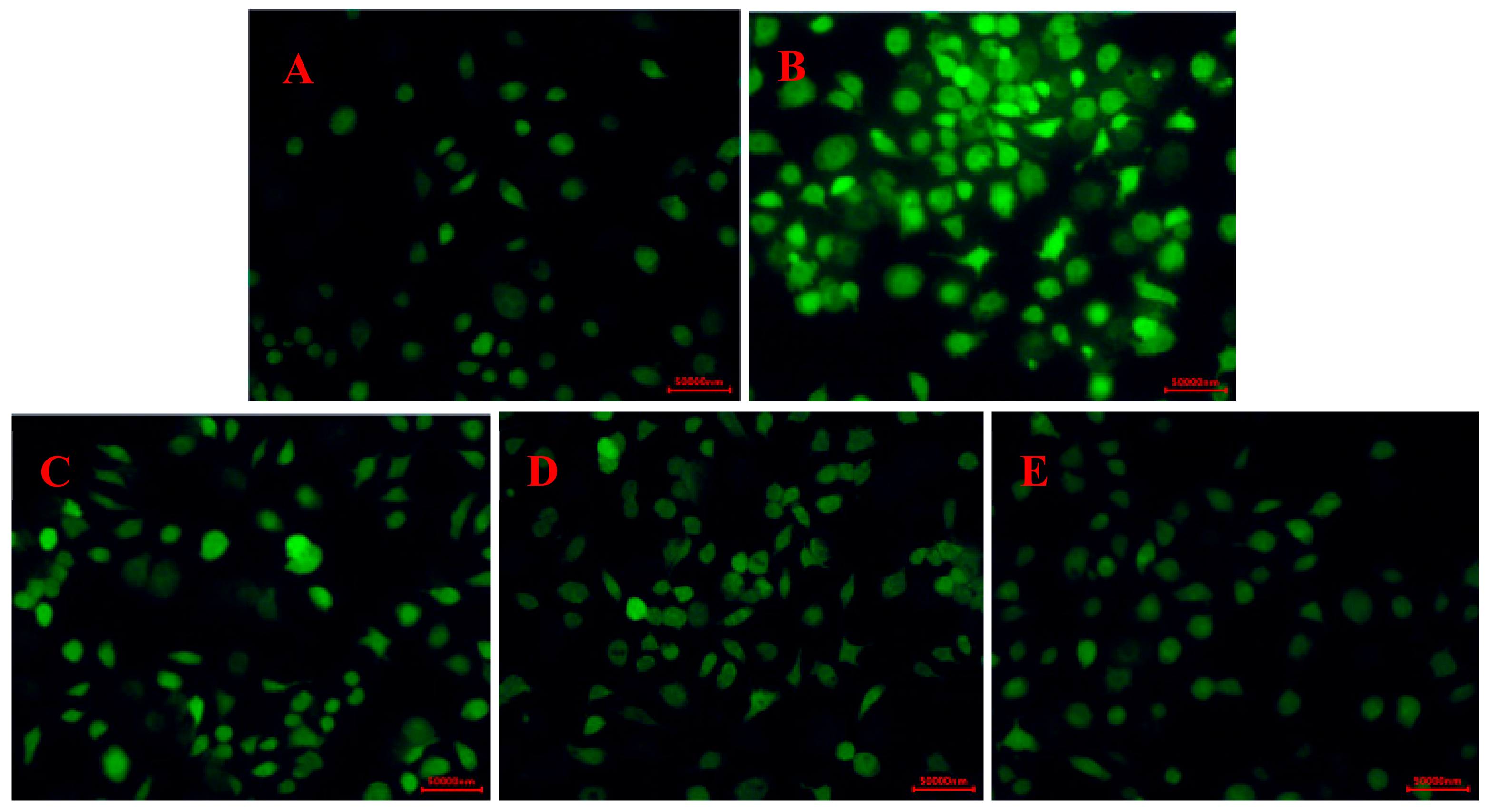
2.3.2. Effects of CPS on the ROS Levels in RAW264.7 Cells
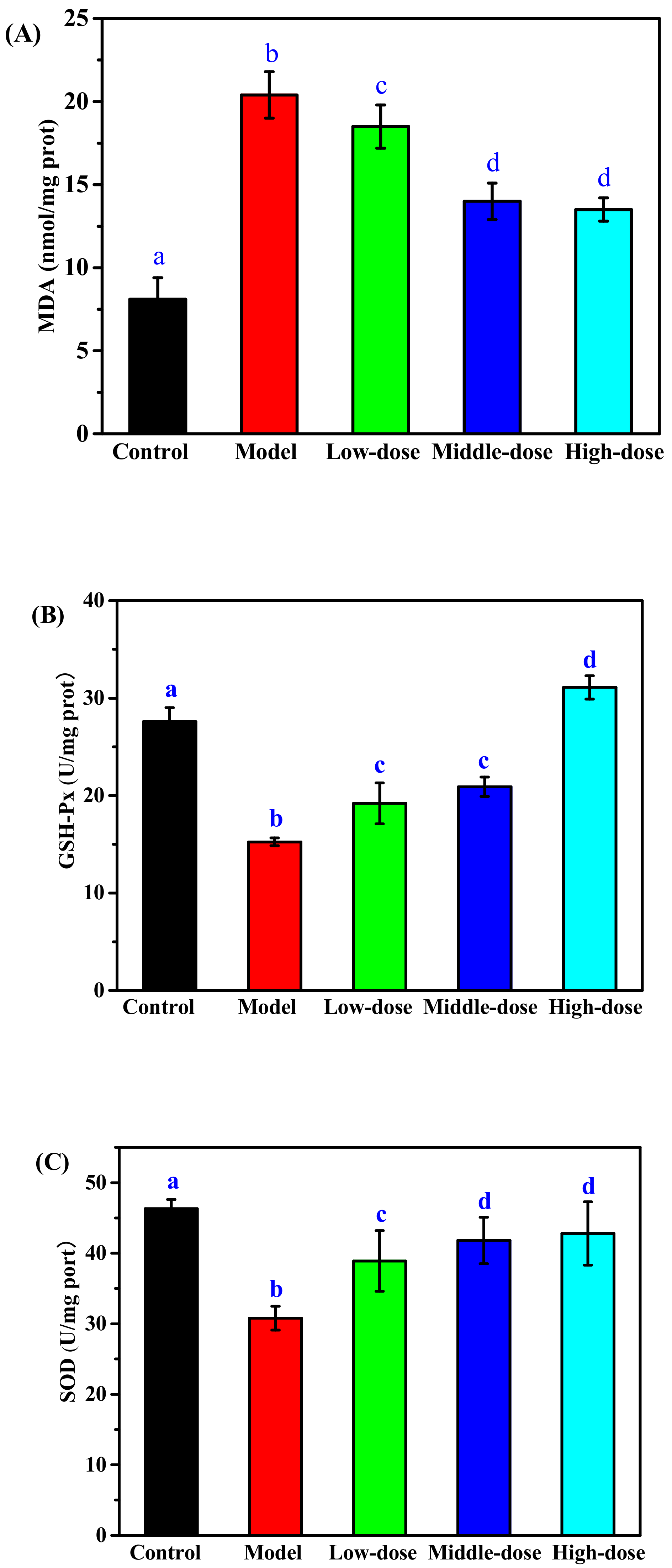
2.3.3. Effect of CPS on the Level of MDA, SOD, and GSH-Px in Cells
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation of Collagen Peptides
3.3. Antioxidant Activity Measurements
3.3.1. Scavenging Activity of Hydroxyl Radical
3.3.2. Scavenging Activity of DPPH Radical
3.4. The Effect of H2O2 and Peptides on the Proliferation of RAW264.7 Cells
3.5. Reactive Oxygen Species (ROS) Assay
3.6. Assays for Antioxidant Enzyme Activity
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, R.; Zheng, W.; Li, J.; Wang, L.; Wu, H.; Wang, X.; Shi, L. Rapid identification of bioactive peptides with antioxidant activity from the enzymatic hydrolysate of Mactra veneriformis by UHPLC-Q-TOF mass spectrometry. Food Chem. 2015, 167, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Reyes Jara, A.M.; Liggieri, C.S.; Bruno, M.A. Preparation of soy protein hydrolysates with antioxidant activity by using peptidases from latex of Maclura pomifera fruits. Food Chem. 2018, 264, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Dinstel, R.R.; Cascio, J.; Koukel, S. The antioxidant level of Alaska’s wild berries: High, higher and highest. Int. J. Circumpolar Health 2013, 72. [Google Scholar] [CrossRef]
- Lin, X.; Bai, D.; Wei, Z.; Zhang, Y.; Huang, Y.; Deng, H.; Huang, X. Curcumin attenuates oxidative stress in RAW264.7 cells by increasing the activity of antioxidant enzymes and activating the Nrf2-Keap1 pathway. PLoS ONE 2019, 14, e0216711. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Song, Z.; Liu, T.; Liang, J.; Yuan, J.; Xu, Z.; Sun, Z.; Lai, X.; Xiong, Q.; Zhang, D. Polysaccharide from Ostrea rivularis attenuates reproductive oxidative stress damage via activating Keap1-Nrf2/ARE pathway. Carbohydr. Polym. 2018, 186, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Liua, J.; Zhu, X.; Hua, Y.; Wang, C.; Zhang, Y. Protective effects of protopine on hydrogen peroxide-induced oxidative injury of PC12 cells via Ca antagonism and antioxidant mechanisms. Eur. J. Pharmacol. 2008, 591, 21–27. [Google Scholar] [CrossRef]
- Duranti, G.; Ceci, R.; Sgrò, P.; Sabatini, S.; Luigi, L.D. Influence of the PDE5 inhibitor tadalafil on redox status and antioxidant defense system in C2C12 skeletal muscle cells. Cell Stress Chaperones 2017, 22, 389–396. [Google Scholar] [CrossRef]
- Huang, Y.; Ruan, G.; Qin, Z.; Li, H.; Zheng, Y. Antioxidant activity measurement and potential antioxidant peptides exploration from hydrolysates of novel continuous microwave-assisted enzymolysis of the Scomberomorus niphonius protein. Food Chem. 2017, 223, 89–95. [Google Scholar] [CrossRef]
- Zhong, M.; Chen, T.; Hu, C.; Ren, C. Isolation and characterization of collagen from the body wall of sea cucumber Stichopus monotuberculatus. J. Food Sci. 2015, 80, C671–C679. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, S.; Zhang, Y.; Lu, Y.; Wang, M.; Wang, G.; Liu, X. Purification and antioxidant ability of peptide from egg in sea cucumber Apostichopus japonicus. Int. J. Food Prop. 2016, 20, 306–317. [Google Scholar] [CrossRef]
- Ferrario, C.; Leggio, L.; Leone, R.; Di Benedetto, C.; Guidetti, L.; Cocce, V.; Ascagni, M.; Bonasoro, F.; La Porta, C.A.M.; Candia Carnevali, M.D.; et al. Marine-derived collagen biomaterials from echinoderm connective tissues. Mar. Environ. Res. 2017, 128, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Fujioka, M.; Sugimoto, K.; Mu, G.; Ishimi, Y. Assessment of effectiveness of oral administration of collagen peptide on bone metabolism in growing and mature rats. J. Bone Miner. Metab. 2004, 22, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Ohara, H.; Iida, H.; Ito, K.; Takeuchi, Y.; Nomura, Y. Effects of Pro-Hyp, a collagen hydrolysate-derived peptide, on hyaluronic acid synthesis using in vitro cultured synovium cells and oral ingestion of collagen hydrolysates in a guinea pig model of osteoarthritis. Biosci. Biotechnol. Biochem. 2010, 74, 2096–2099. [Google Scholar] [CrossRef] [PubMed]
- Midori, T.; Yoh-Ichi, K.; Yoshihiro, N.J.B.B. Biochemistry, effects of collagen peptide ingestion on UV-B-induced skin damage. Biosci. Biotechnol. Biochem. 2009, 73, 930–932. [Google Scholar]
- Izquierdo, F.J.; Peñas, E.; Baeza, M.L.; Gomez, R. Effects of combined microwave and enzymatic treatments on the hydrolysis and immunoreactivity of dairy whey proteins. Int. Dairy J. 2008, 18, 918–922. [Google Scholar] [CrossRef]
- Xu, Z.; Fang, Y.; Chen, Y.; Yang, W.; Ma, N.; Pei, F.; Kimatu, B.M.; Hu, Q.; Qiu, W. Protective effects of Se-containing protein hydrolysates from Se-enriched rice against Pb(2+)-induced cytotoxicity in PC12 and RAW264.7 cells. Food Chem. 2016, 202, 396–403. [Google Scholar] [CrossRef]
- Lin, Y.J.; Le, G.W.; Wang, J.Y.; Li, Y.X.; Shi, Y.H.; Sun, J. Antioxidative peptides derived from enzyme hydrolysis of bone collagen after microwave assisted acid pre-treatment and nitrogen protection. Int. J. Mol. Sci. 2010, 11, 4297–4308. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, L.; Yang, Q.; Sun, J.; Bi, J.; Liu, S.; Zhang, C.; Tang, L. Optimization of a microwave-coupled enzymatic digestion process to prepare peanut peptides. Molecules 2012, 17, 5661–5674. [Google Scholar] [CrossRef]
- Abedin, M.Z.; Karim, A.A.; Ahmed, F.; Latiff, A.A.; Gan, C.Y.; Che, G.F.; Islam Sarker, M.Z. Agriculture, isolation and characterization of pepsin-solubilized collagen from the integument of sea cucumber (Stichopus vastus). J. Sci. Food Agric. 2013, 93, 1083–1088. [Google Scholar] [CrossRef]
- Lin, S.J.; Xue, Y.P.; San, E.L.; Keong, T.C.; Chen, L.F.; Zheng, Y.G. Extraction and characterization of pepsin soluble collagen from the body wall of sea cucumber Acaudina leucoprocta. J. Aquat. Food Prod. Technol. 2017, 26, 502–515. [Google Scholar] [CrossRef]
- Liu, Z.; Oliveira, A.C.; Su, Y.C. Purification and characterization of pepsin-solubilized collagen from skin and connective tissue of giant red sea cucumber (Parastichopus californicus). J. Agric. Food Chem. 2010, 58, 1270–1274. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Tuo, F.Y.; Song, L.; Liu, Y.X.; Dong, X.P.; Li, D.M.; Zhou, D.Y.; Shahidi, F. Action of trypsin on structural changes of collagen fibres from sea cucumber (Stichopus japonicus). Food Chem. 2018, 256, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.W.; Dong, X.P.; Zhou, D.Y.; Gao, Y.; Yang, J.F.; Li, D.M.; Zhao, X.K.; Ren, T.T.; Ye, W.X.; Tan, H.; et al. Physicochemical properties and radical scavenging capacities of pepsin-solubilized collagen from sea cucumber Stichopus japonicus. Food Hydrocoll. 2012, 28, 182–188. [Google Scholar] [CrossRef]
- Zhuang, Y.; Sun, L.; Zhao, X.; Wang, J.; Hou, H.; Li, B. Antioxidant and melanogenesis-inhibitory activities of collagen peptide from jellyfish (Rhopilema esculentum). J. Sci. Food Agric. 2009, 89, 1722–1727. [Google Scholar] [CrossRef]
- Zhou, X. Technology, antioxidant peptides isolated from sea cucumber Stichopus Japonicus. Eur. Food Res. Technol. 2012, 234, 441–447. [Google Scholar] [CrossRef]
- Jin, H.X.; Xu, H.P.; Li, Y.; Zhang, Q.W.; Xie, H. Preparation and evaluation of peptides with potential antioxidant activity by microwave assisted enzymatic hydrolysis of collagen from sea cucumber Acaudina molpadioides obtained from Zhejiang Province in China. Mar. Drugs 2019, 17, 169. [Google Scholar] [CrossRef]
- Uluko, H.; Zhang, S.; Liu, L.; Tsakama, M.; Lu, J.; Lv, J. Effects of thermal, microwave, and ultrasound pretreatments on antioxidative capacity of enzymatic milk protein concentrate hydrolysates. J. Funct. Foods 2015, 18, 1138–1146. [Google Scholar] [CrossRef]
- Chi, C.F.; Hu, F.Y.; Wang, B.; Li, Z.R.; Luo, H.Y. Influence of amino acid compositions and peptide profiles on antioxidant capacities of two protein hydrolysates from skipjack tuna (Katsuwonus pelamis) dark muscle. Mar. Drugs 2015, 13, 2580–2601. [Google Scholar] [CrossRef]
- Kong, X.; Zhou, H.; Hua, Y. Preparation and antioxidant activity of wheat gluten hydrolysates (WGHs) using ultrafiltration membranes. J. Sci. Food Agric. 2008, 88, 920–926. [Google Scholar] [CrossRef]
- Chi, C.-F.; Wang, B.; Hu, F.-Y.; Wang, Y.-M.; Zhang, B.; Deng, S.-G.; Wu, C.-W. Purification and identification of three novel antioxidant peptides from protein hydrolysate of bluefin leatherjacket (Navodon septentrionalis) skin. Food Res. Int. 2015, 73, 124–129. [Google Scholar] [CrossRef]
- Ma, Y.L.; Zhu, D.Y.; Thakur, K.; Wang, C.H.; Wang, H.; Ren, Y.F.; Zhang, J.G.; Wei, Z.J. Antioxidant and antibacterial evaluation of polysaccharides sequentially extracted from onion ( Allium cepa L.). Int. J. Biol. Macromol. 2018, 111, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Zhao, M.; Shi, J.; Ning, Y.; Jiang, Y.J.F.C. Effect of ultrasonic treatment on the recovery and DPPH radical scavenging activity of polysaccharides from longan fruit pericarp. Food Chem. 2008, 106, 685–690. [Google Scholar]
- Pan, X.; Zhao, Y.Q.; Hu, F.Y.; Wang, B. Preparation and identification of antioxidant peptides from protein hydrolysate of skate ( Raja porosa ) cartilage. J. Funct. Foods 2016, 25, 220–230. [Google Scholar] [CrossRef]
- Qiu, W.; Chen, X.; Tian, Y.; Wu, D.; Du, M.; Wang, S. Protection against oxidative stress and anti-aging effect in Drosophila of royal jelly-collagen peptide. Food Chem. Toxicol. 2019, 110881. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xie, J.; Gao, H.; Xia, Y.; Chen, X.; Wang, C. Hepatoprotective effect of collagen peptides from cod skin against liver oxidative damage in vitro and in vivo. Cell Biochem. Biophys. 2015, 71, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Li, H.; Li, C.; Chen, B.; Shen, Y. Chemical composition and radical scavenging activity of Amygdalus pedunculata Pall leaves’ essential oil. Food Chem. Toxicol. 2018, 119, 368–374. [Google Scholar] [CrossRef]
- Aissi, O.; Boussaid, M.; Messaoud, C. Essential oil composition in natural populations of Pistacia lentiscus L. from Tunisia: Effect of ecological factors and incidence on antioxidant and antiacetylcholinesterase activities. Ind. Crop. Prod. 2016, 91, 56–65. [Google Scholar] [CrossRef]
| Hydrolysates | Content (%) | Mw Distribution (%) | ||
|---|---|---|---|---|
| 0 W | 250 W | 0 W | 250 W | |
| CPL (Mw > 5 kDa) | 100 | 90.3 ± 4.3 | 21.7 ± 2.1 | 15.7 ± 1.5 |
| CPM (1 kDa < Mw ≤ 5 kDa) | 100 | 148.0 ± 8.5 | 20.3 ± 1.2 | 24.0 ± 1.8 |
| CPS (Mw ≤ 1 kDa) | 100 | 130.2 ± 6.4 | 58.0 ± 3.1 | 60.3 ± 2.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Li, J.; Lin, S.-J.; Yang, Z.-S.; Jin, H.-X. Preparation of Antioxidant Peptide by Microwave- Assisted Hydrolysis of Collagen and Its Protective Effect Against H2O2-Induced Damage of RAW264.7 Cells. Mar. Drugs 2019, 17, 642. https://doi.org/10.3390/md17110642
Li Y, Li J, Lin S-J, Yang Z-S, Jin H-X. Preparation of Antioxidant Peptide by Microwave- Assisted Hydrolysis of Collagen and Its Protective Effect Against H2O2-Induced Damage of RAW264.7 Cells. Marine Drugs. 2019; 17(11):642. https://doi.org/10.3390/md17110642
Chicago/Turabian StyleLi, Yan, Jie Li, Sai-Jun Lin, Zui-Su Yang, and Huo-Xi Jin. 2019. "Preparation of Antioxidant Peptide by Microwave- Assisted Hydrolysis of Collagen and Its Protective Effect Against H2O2-Induced Damage of RAW264.7 Cells" Marine Drugs 17, no. 11: 642. https://doi.org/10.3390/md17110642
APA StyleLi, Y., Li, J., Lin, S.-J., Yang, Z.-S., & Jin, H.-X. (2019). Preparation of Antioxidant Peptide by Microwave- Assisted Hydrolysis of Collagen and Its Protective Effect Against H2O2-Induced Damage of RAW264.7 Cells. Marine Drugs, 17(11), 642. https://doi.org/10.3390/md17110642




