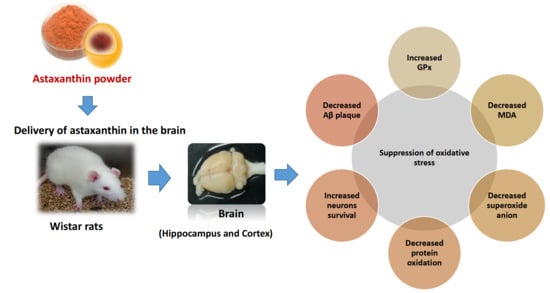
Effects of Astaxanthin from Shrimp Shell on Oxidative Stress and Behavior in Animal Model of Alzheimer’s Disease
Abstract
1. Introduction
2. Results
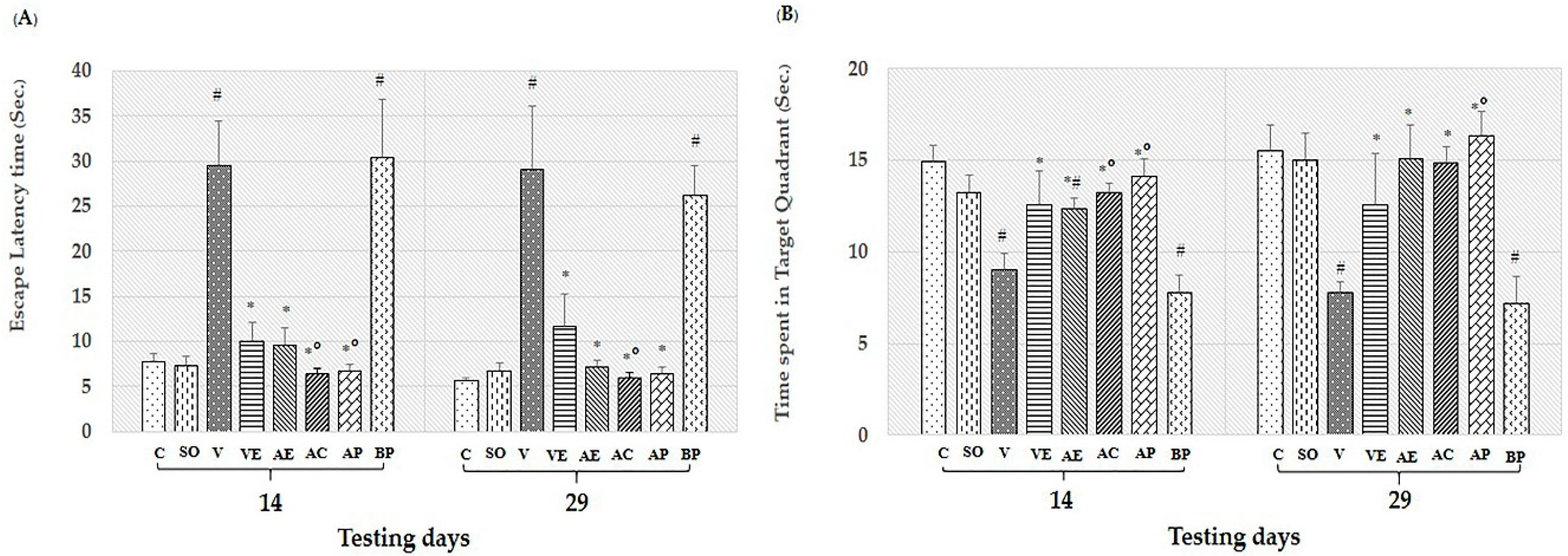
2.1. Effects of ASX on Learning and Memory Deficit in Aβ1-42-Induced AD Rats
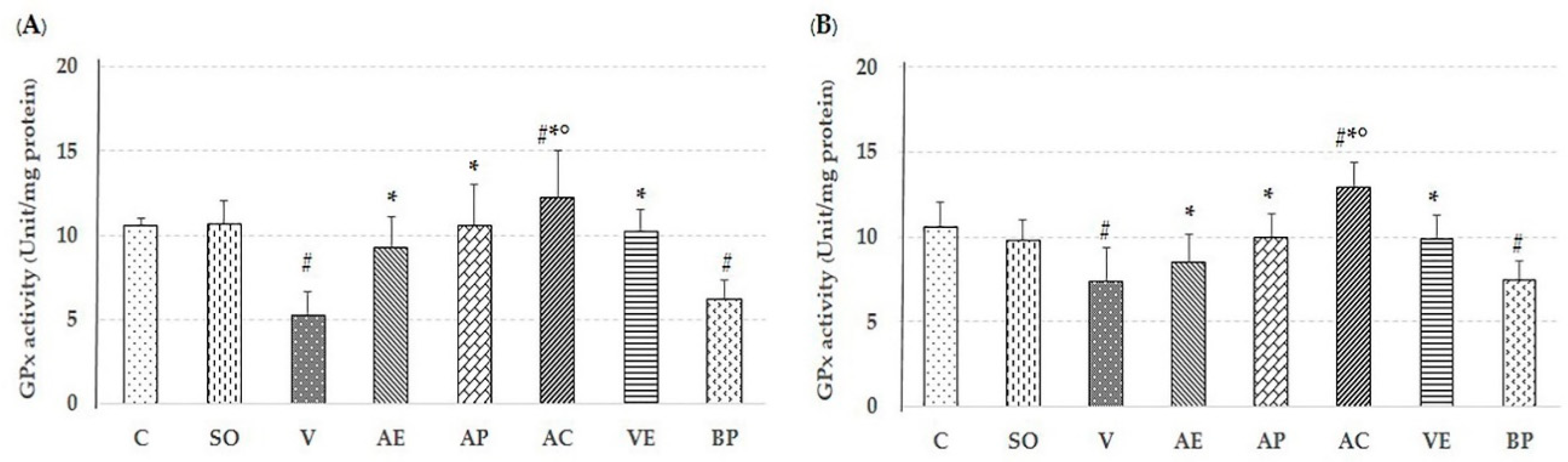
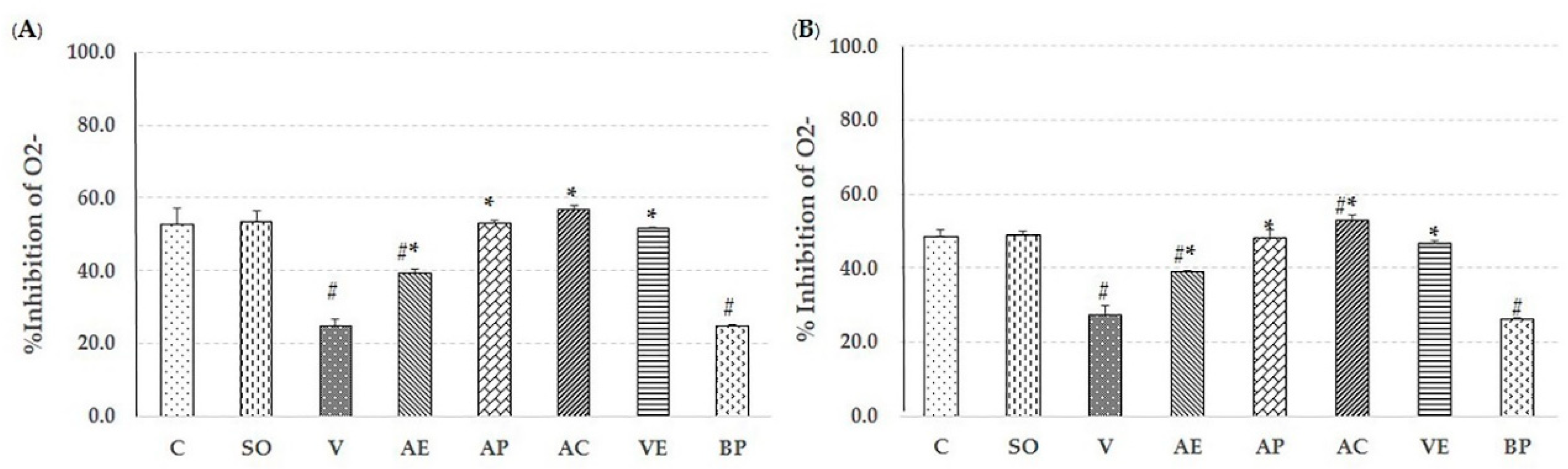
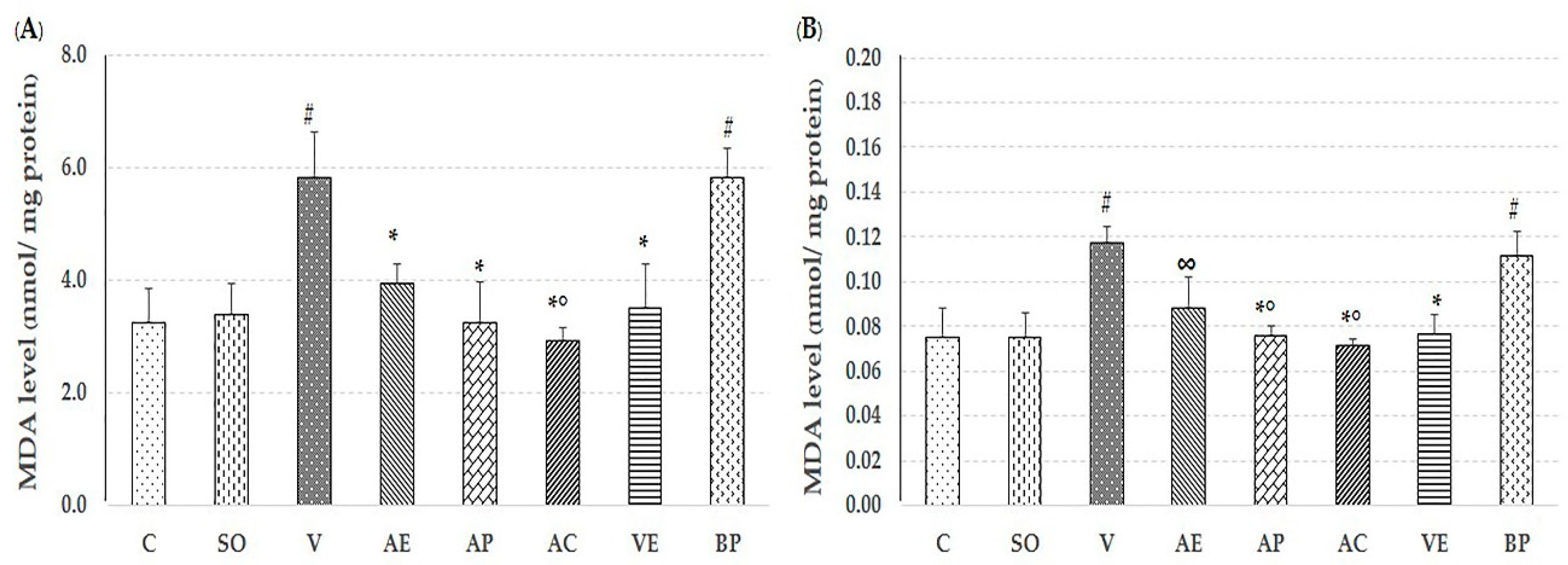
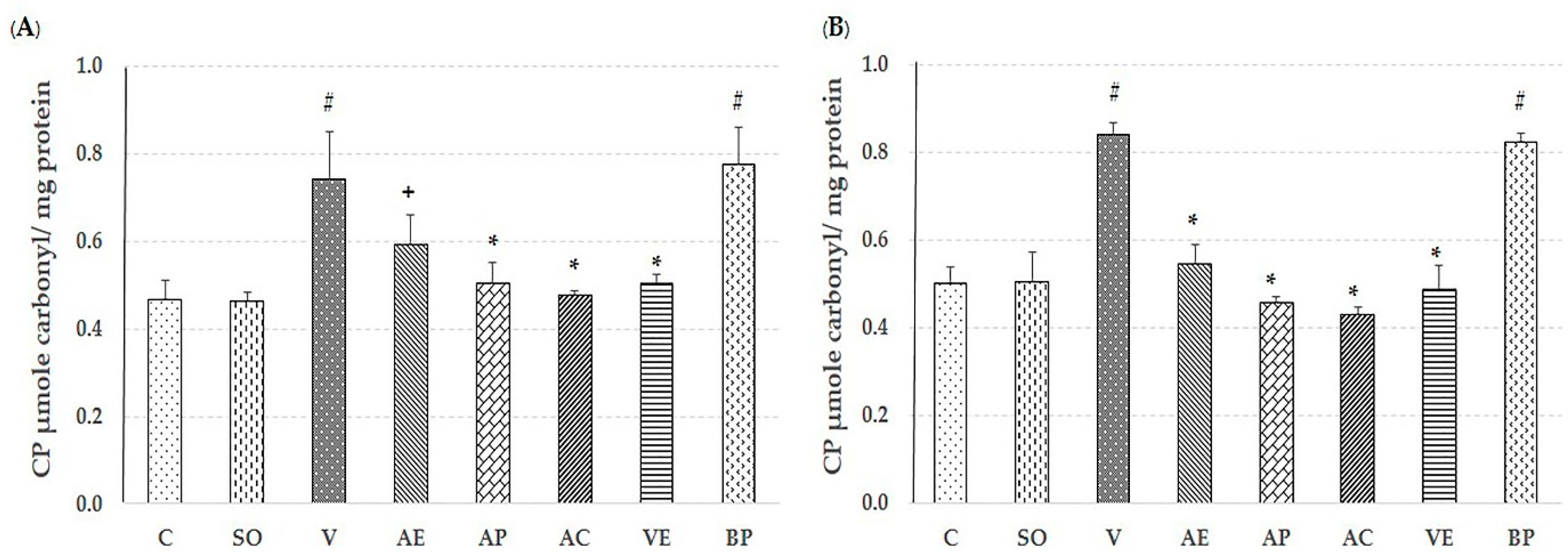
2.2. Effect of Astaxanthin in Reducing Brain Oxidative Stress
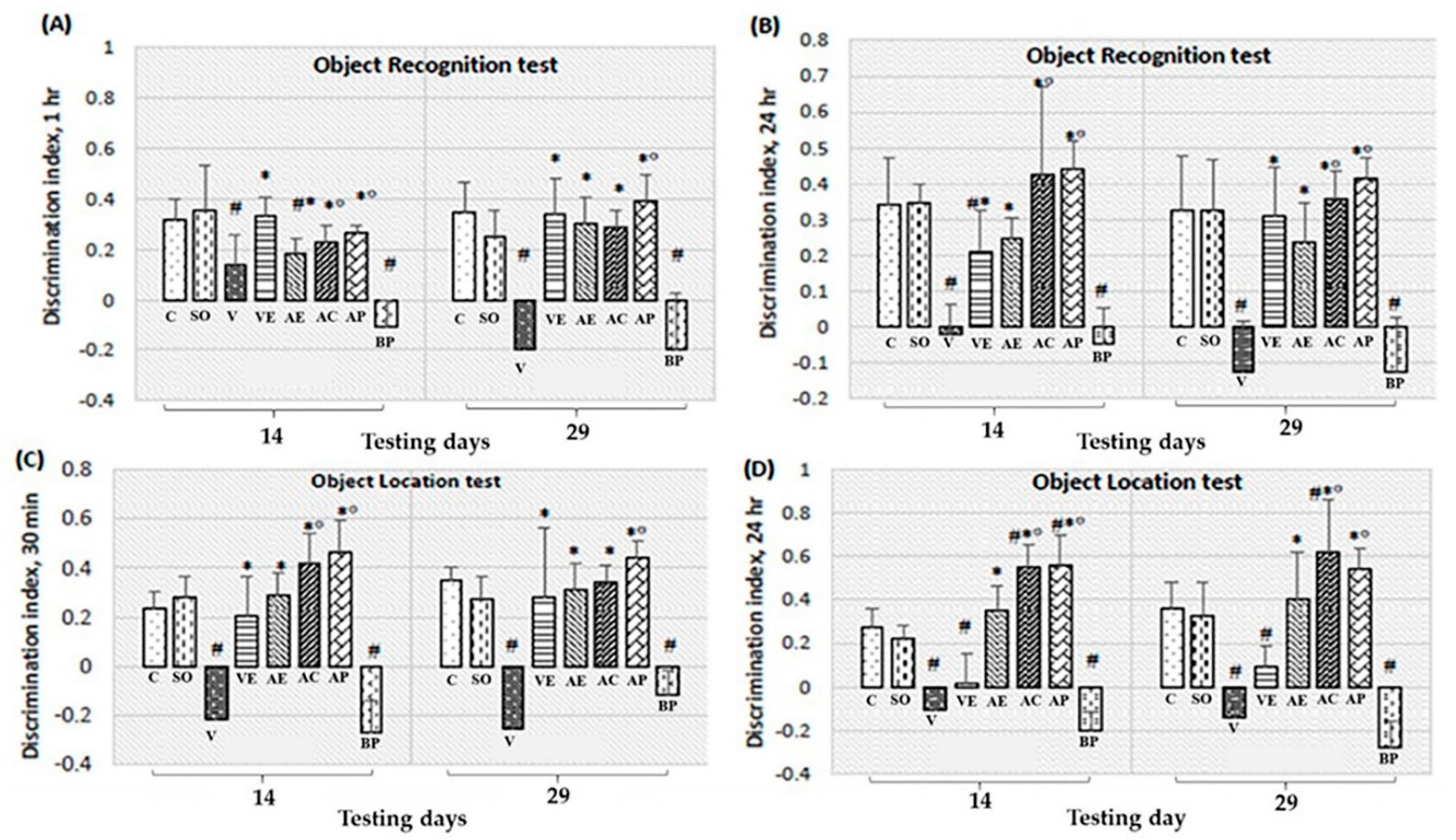
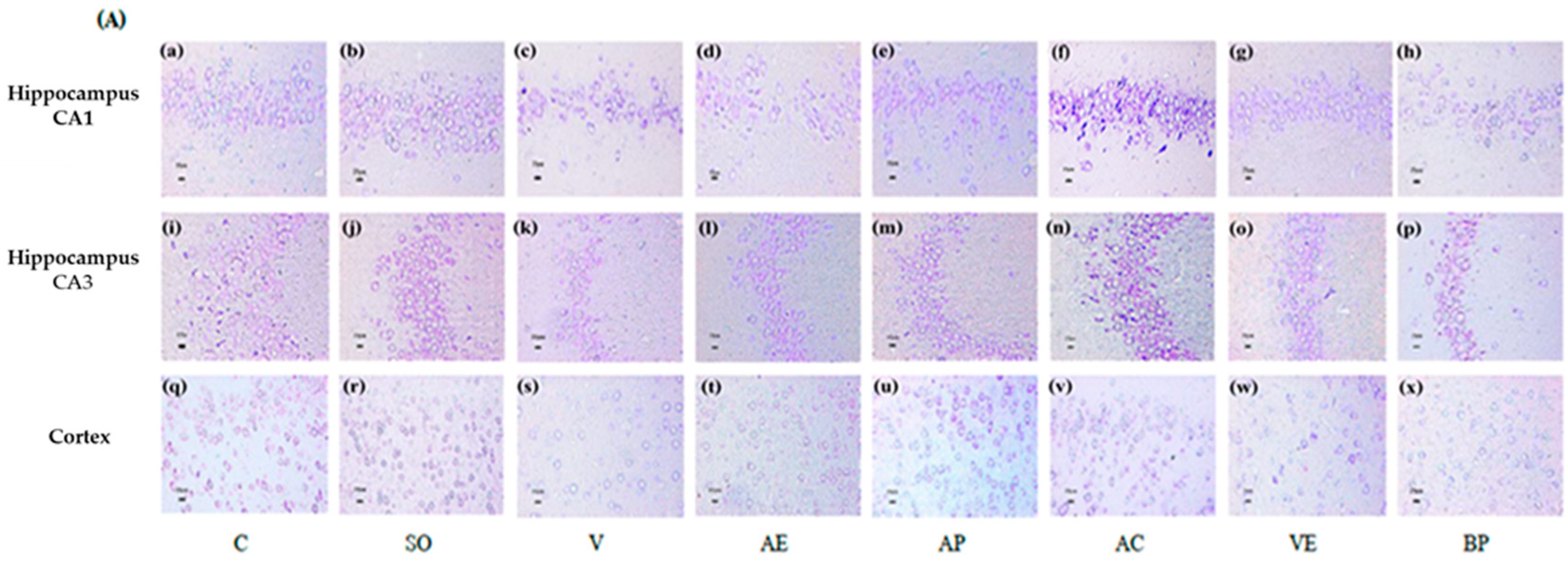
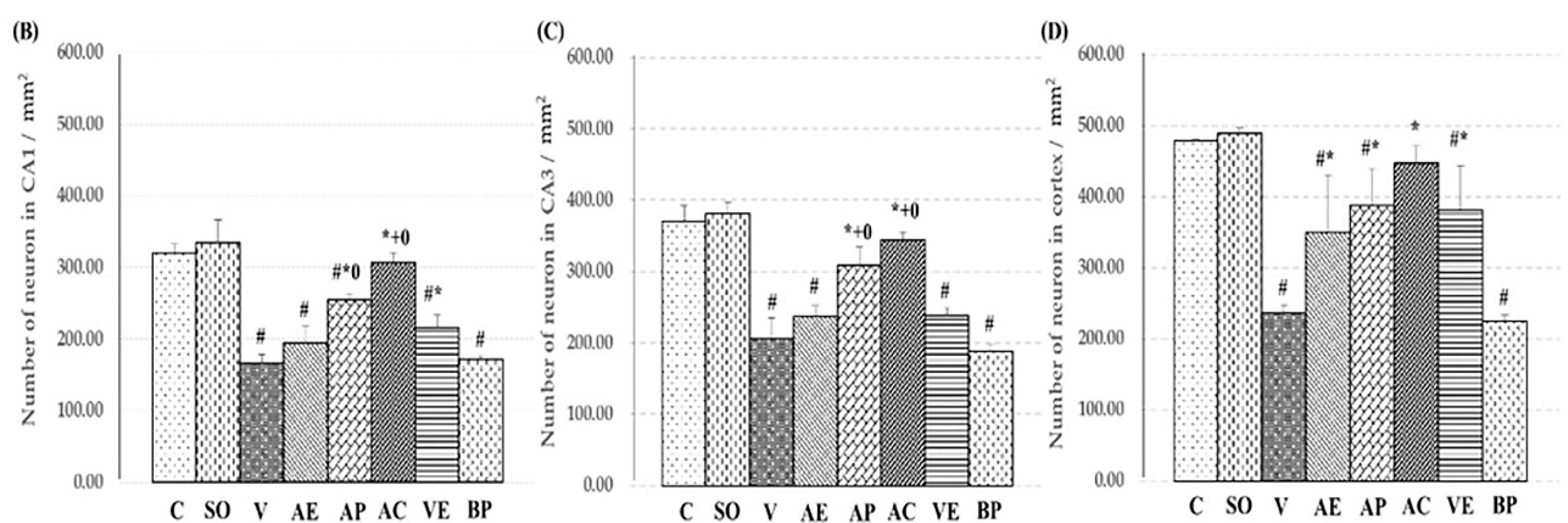
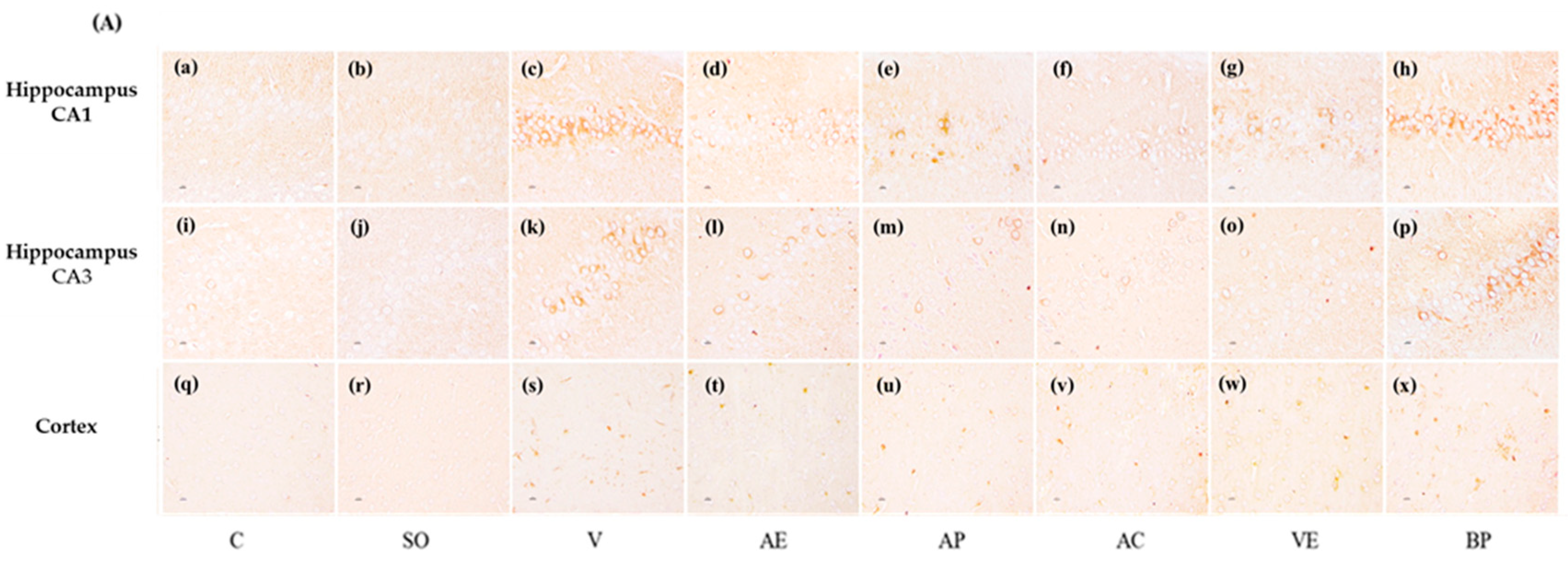
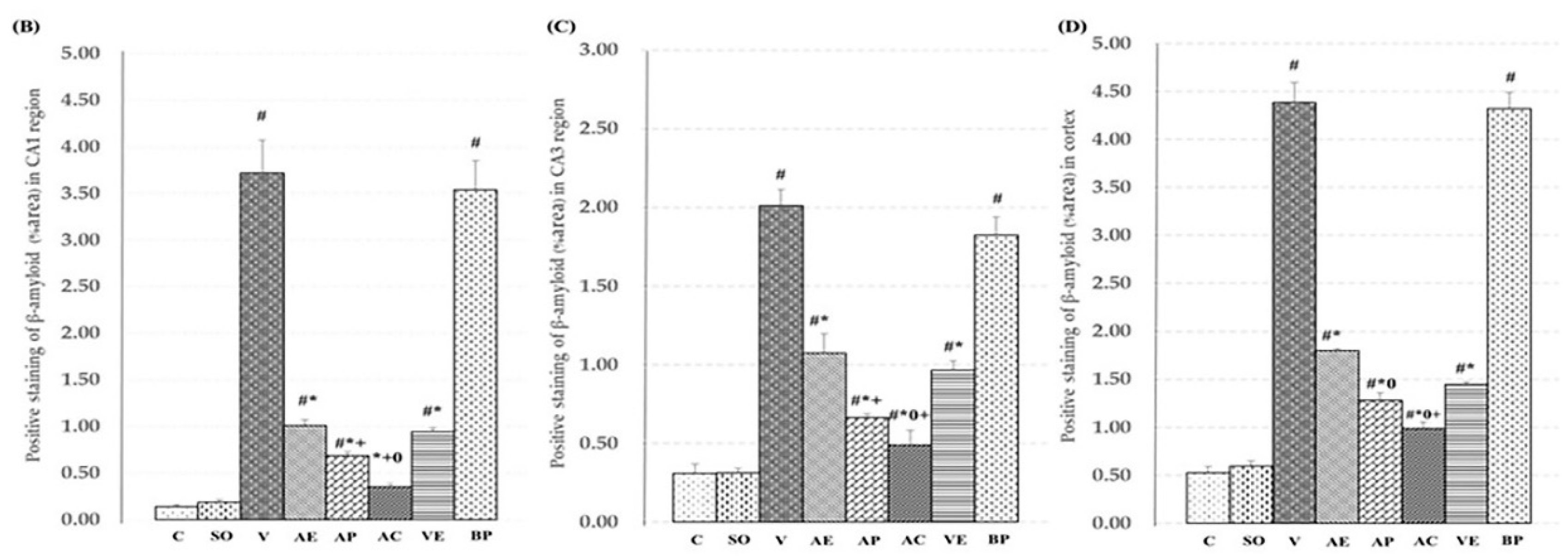
2.3. Effects of ASX on Neuronal Survival and Amyloidosis in Hippocampal and Cortex Regions
3. Discussion
4. Materials and Methods
4.1. Drugs and Chemicals
4.2. Animals
4.3. Extraction of ASX from Shrimp Shells and Preparation of Encapsulated ASX (ASX-powder)
4.4. Experimental Procedures
4.5. Behavioral Studies
4.5.1. Morris Water Maze Test
4.5.2. Object Location Test
4.5.3. Object Recognition Test
4.6. Preparation of Brain (Cortex and Hippocampus) Tissues Homogenate and Protein Extraction
4.6.1. Measurement of Glutathione Peroxidase (GPx) Activity
4.6.2. Superoxide (O2−) Anion Assay
4.6.3. Measurement of Protein
4.6.4. Measurement of Malondialdehyde (MDA)
4.7. Histopathological Analysis of Brain
4.7.1. Cresyl Violet Staining for Nissl Substance
4.7.2. Immunohistochemistry Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bui, T.T.; Nguyen, T.H. Natural product for the treatment of Alzheimer’s disease. J. Basic Clin. Physiol. Pharmacol. 2017, 28, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Leal, S.L.; Landau, S.M.; Bell, R.K.; Jagust, W.J. Hippocampal activation is associated with longitudinal amyloid accumulation and cognitive decline. Elife 2017, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Reilly, J.F.; Games, D.; Rydel, R.E.; Freedman, S.; Schenk, D.; Young, W.G.; Morrison, J.H.; Bloom, F.E. Amyloid deposition in the hippocampus and entorhinal cortex: Quantitative analysis of a transgenic mouse model. Proc. Natl. Acad. Sci. USA 2003, 100, 4837–4842. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Swomley, A.M.; Sultana, R. Amyloid β-peptide (1-42)-induced oxidative stress in Alzheimer disease: Importance in disease pathogenesis and progression. Antioxid. Redox Signal. 2013, 19, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Ambati, R.R.; Phand, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications-a review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Sila, A.; Kamoun, Z.; Ghlissi, A.; Makni, M.; Nasri, M.; Sahnoun, Z.; Arroume, N.N.; Bougatef, A. Ability of natural astaxanthin from shrimp by products to attenuate liver oxidative stress in diabetic rats. Pharmacol. Rep. 2015, 67, 310–316. [Google Scholar] [CrossRef]
- Rahman, S.O.; Panda, B.P.; Parvez, S.; Kaundal, M.; Hussain, S.; Akhtar, M.; Najmi, A.K. Neuroprotective role of astaxanthin in hippocampal insulin resistance induced by Aβ peptides in animal model of Alzheimer’s disease. Biomed. Pharmacother. 2019, 110, 47–58. [Google Scholar] [CrossRef]
- Wu, W.; Wang, X.; Xiang, Q.; Meng, X.; Peng, Y.; Da, N.; Liu, Z.; Sun, Q.; Wang, C.; Liu, X. Astaxanthin alleviates brain aging in rats by attenuating oxidative stress and increasing BDNF levels. Food Funct. 2014, 5, 158. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, F.; Hu, X.; Chen, J.; Wen, X.; Sun, Y.; Liu, Y.; Tang, R.; Zheng, K.; Song, Y. Inhibition of inflammation by astaxanthin alleviates cognition deficits in diabetic mic. Physiol. Behav. 2015, 151, 412–420. [Google Scholar] [CrossRef]
- Che, H.; Li, Q.; Zhang, T.; Wang, D.; Yang, L.; Xu, J.; Yanagita, T.; Xue, G.; Cheng, Y.; Wang, Y. The effects of astaxanthin and docosahexaenoic acid-acylated astaxanthin on Alzheimer’s disease in APP/PS1 double transgenic mice. J. Agric. Food Chem. 2018, 66, 4948–4957. [Google Scholar] [CrossRef] [PubMed]
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goucoolea, F.M.; Arguelles-Monal, W. Microencapsulation of astaxanthin in a chitosan matrix. Carbohyd. Polym. 2004, 56, 41–45. [Google Scholar] [CrossRef]
- Taksima, T.; Limpawattana, M.; Klaypradit, W. Astaxanthin encapsulated in beads using ultrasonic atomizer and application in yogurt as evaluated by consumer sensory profile. LWT Food Sci. Technol. 2015, 62, 431–437. [Google Scholar] [CrossRef]
- Sangsuriyawong, A.; Limpawattana, M.; Siriwan, D.; Klaypradit, W. Properties and bioavailability assessment of shrimp astaxanthin loaded liposome. Food Sci. Biotechnol. 2019, 28, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Tachaprutinun, A.; Udomsup, T.; Luadthong, C.; Wanichwecharungruang, S. Preventing the thermal degradation of astaxanthin through nanoencapsulation. Int. J. Pharm. 2009, 374, 119–124. [Google Scholar] [CrossRef]
- Pu, J.; Bankston, J.D.; Sathivel, S. Developing microencapsulation flaxseed oil containing shrimp (Litopenaeus setiferus) astaxanthin using a pilot scale spay dryer. Biosyst Eng. 2011, 108, 121–132. [Google Scholar] [CrossRef]
- Grimmig, B.; Daly, L.; Hudson, C.; Nash, K.R.; Bickford, P.C. Astaxanthin attenuates neurotoxicity in a mouse model of Parkinson’s disease. Funct. Food Health Dis. 2017, 7, 562–576. [Google Scholar] [CrossRef]
- Ji, X.; Peng, D.; Zhang, Y.; Zhang, J.; Wang, Y.; Gao, Y.; Lu, H.; Tang, P. Astaxanthin improves cognitive performance in mice following mild traumatic brain injury. Brain Res. 2017, 1659, 88–95. [Google Scholar] [CrossRef]
- Grimmig, B.; Hudson, C.; Moss, L.; Peters, M.; Subbarayan, M.; Weeber, E.J.; Bickford, P.C. Astaxanthin supplementation modulates cognitive function and synaptic plasticity in young and aged mice. GeroScience 2019, 41, 77–87. [Google Scholar] [CrossRef]
- Yook, J.S.; Okamoto, M.; Rakwal, R.; Shibato, J.; Lee, M.C.I.; Matsui, T.; Chang, H.; Cho, J.Y.; Soya, H. Astaxanthin supplementation enhances adult hippocampal neurogenesis and spatial memory in mice. Mol. Nutr. Food Res. 2016, 60, 589–599. [Google Scholar] [CrossRef]
- Liu, X.; Osawa, T. Astaxanthin protects neuronal cells against oxidative damage and is a potent candidate for brain food. Forum Nutr. 2009, 61, 129–135. [Google Scholar] [PubMed]
- Yang, S.; Zhou, Q.; Yang, L.; Xue, Y.; Xu, J.; Xue, C. Effect of thermal processing on astaxanthin and astaxanthin esters in pacific white shrimp Litopenaeus vannamei. J. Oleo Sci. 2015, 64, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, S.; Sherief, P.M. Extraction, characterization, antioxidant and anti-inflammatory properties of Carotenoids from the shell waste of Arabian red shrimp Aristeus alcocki, Ramadan 1938. Open Conf. Proc. J. 2011, 2, 95–103. [Google Scholar]
- Miao, F.; Lu, D.; Li, Y.; Zeng, M. Characterization of astaxanthin esters in Heamatococcus pluvialis by Liquid Chromatography–Atmospheric Pressure Chemical Ionization Mass Spectrometry. Anal. Bio-Chem. 2006, 352, 176–181. [Google Scholar]
- Hritcu, L.; Noumedem, J.A.; Cioanca, O.; Hancianu, M.; Kuete, V.; Mihasan, M. Methanolic extract of piper nigrum fruits improves memory impairment by decreasing brain oxidative stress in amyloid beta (1-42) rat model of Alzheimer’s disease. Cell. Mol. Neurobiol. 2014, 34, 437–449. [Google Scholar] [CrossRef]
- Cioanca, O.; Hritcu, L.; Mihasan, M.; Hancianu, M. Cognitive-enhancing and anti-oxidant activities of inhaled coriander volatile oil in amyloid β (1-42) rat model of Alzheimer’s disease. Physiol. Behav. 2013, 120, 193–202. [Google Scholar] [CrossRef]
- Shichiri, M. The role of lipid peroxidation in neurological disorders. J. Clin. Biochem. Nutr. 2014, 54, 151–160. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta. 2003, 329, 23–38. [Google Scholar] [CrossRef]
- Kuedo, Z.; Sangsuriyawong, A.; Klaypradit, W.; Tipmanee, V.; Chonpathompikunlert, P. Effects of Astaxanthin from Litopenaeus Vannamei on Carrageenan-Induced Edema and Pain Behavior in Mice. Molecules 2016, 21, 382. [Google Scholar] [CrossRef]
- Ito, N.; Saito, H.; Seki, S.; Ueda, F.; Asada, T. Effects of composite supplement containing astaxanthin and sesamin on cognitive functions in people with mile cognitive impairment: A randomized, double-blind, placebo-controlled trial. J. Alzheimer Dis. 2018, 62, 1767–1775. [Google Scholar] [CrossRef]
- Asadbegi, M.; Yaghmaei, P.; Salehi, I.; Komaki, A.; Ebrahim-Habibi, A. Investigation of thymol effect on learning and memory impairment induced by intrahippocampal injection of amyloid beta in high fat diet-fed rats. Metab. Brain Dis. 2017, 32, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Fan, Y.G.; Wang, M.; Wang, D.; Li, X.H. Atorvastatin attenuates the production of IL-1β, IL-6, and TNF-α in the hippocampus of an amyloid β 1-42 induced rat model of Alzheimer’s disease. Clin. Interv. Aging 2013, 8, 103. [Google Scholar] [PubMed]
- Chotiko, A.; Sathivel, S. Development of a combined low-methoxyl-pectin and rice-bran extract delivery system to improve the viability of Lactobacillus plantarum under acid and bile conditions. LWT Food Sci. Technol. 2016, 66, 420–427. [Google Scholar] [CrossRef]
- Morris, G. Spatial localization does not require the presence of local cues. Learn. Motiv. 1981, 12, 239–260. [Google Scholar] [CrossRef]
- Kolb, B.; Cioe, J.; Comeau, W. Contrasting effects of motor and visual spatial learning tasks on dendritic arborization and spine density in rats. Neurobiol. Learn. Mem. 2008, 90, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Barker, G.R.; Warburton, E.C. Object-in-place associative recognition memory depends on glutamate receptor neurotransmission within two defined hippocampal-cortical circuits: A critical role for AMPA and NMDA receptors in the hippocampus, perirhinal, and prefrontal cortices. Cereb. cortex 2013, 25, 472–481. [Google Scholar] [CrossRef]
- Kayanoki, Y.; Fujii, J.; Islam, K.N.; Suzuki, K.; Kawata, S.; Matsuzawa, Y.; Taniguchi, N. The protective role of glutathione peroxidase in apoptosis induced by reactive oxygen species. J. Biochem. 1996, 199, 817–822. [Google Scholar] [CrossRef]
- Ukeda, H.; Maeda, S.; Ishii, T.; Sawamura, M. Spectrophotometric assay for superoxide dismutase based on tetrazolium salt 3′-1-[(phenylamino)-carbonyl-3,4-tetrazolium]-bis (4-methoxy-6-nitro) benzenesulfonic acid hydrate reduction by xanthine-xanthine oxidase. Anal. Biochem. 1997, 251, 206–209. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L. Protein measurement with folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Paxions, G.; Chorles, W. Cresyl violet. In The Rat Brain in Stereotaxic Coordinates; Paxions, G., Chorles, W., Eds.; Academic Press: Cambridge, MA, USA, 1981; pp. 9–17. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taksima, T.; Chonpathompikunlert, P.; Sroyraya, M.; Hutamekalin, P.; Limpawattana, M.; Klaypradit, W. Effects of Astaxanthin from Shrimp Shell on Oxidative Stress and Behavior in Animal Model of Alzheimer’s Disease. Mar. Drugs 2019, 17, 628. https://doi.org/10.3390/md17110628
Taksima T, Chonpathompikunlert P, Sroyraya M, Hutamekalin P, Limpawattana M, Klaypradit W. Effects of Astaxanthin from Shrimp Shell on Oxidative Stress and Behavior in Animal Model of Alzheimer’s Disease. Marine Drugs. 2019; 17(11):628. https://doi.org/10.3390/md17110628
Chicago/Turabian StyleTaksima, Takunrat, Pennapa Chonpathompikunlert, Morakot Sroyraya, Pilaiwanwadee Hutamekalin, Maruj Limpawattana, and Wanwimol Klaypradit. 2019. "Effects of Astaxanthin from Shrimp Shell on Oxidative Stress and Behavior in Animal Model of Alzheimer’s Disease" Marine Drugs 17, no. 11: 628. https://doi.org/10.3390/md17110628
APA StyleTaksima, T., Chonpathompikunlert, P., Sroyraya, M., Hutamekalin, P., Limpawattana, M., & Klaypradit, W. (2019). Effects of Astaxanthin from Shrimp Shell on Oxidative Stress and Behavior in Animal Model of Alzheimer’s Disease. Marine Drugs, 17(11), 628. https://doi.org/10.3390/md17110628