Unique Aggregation of Sterigmatocystin in Water Yields Strong and Specific Circular Dichroism Response Allowing Highly Sensitive and Selective Monitoring of Bio-Relevant Interactions
Abstract
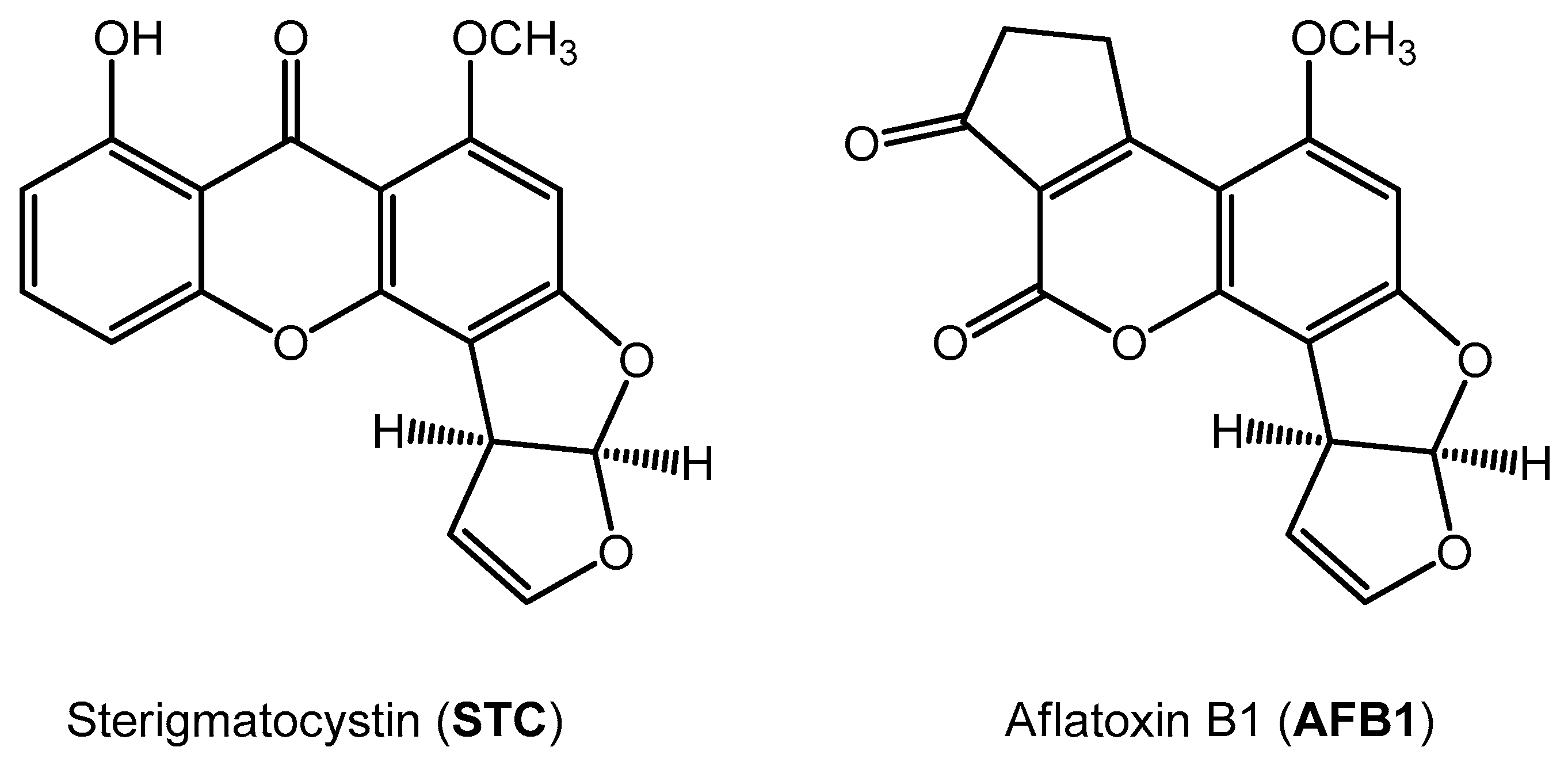
1. Introduction
2. Results and Discussion
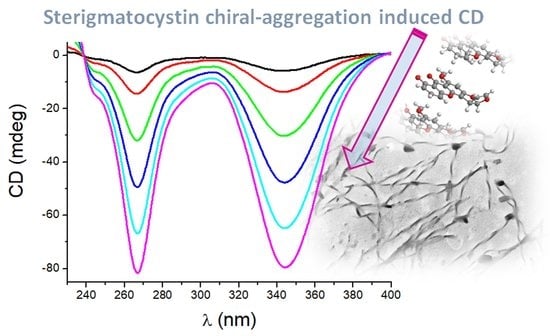
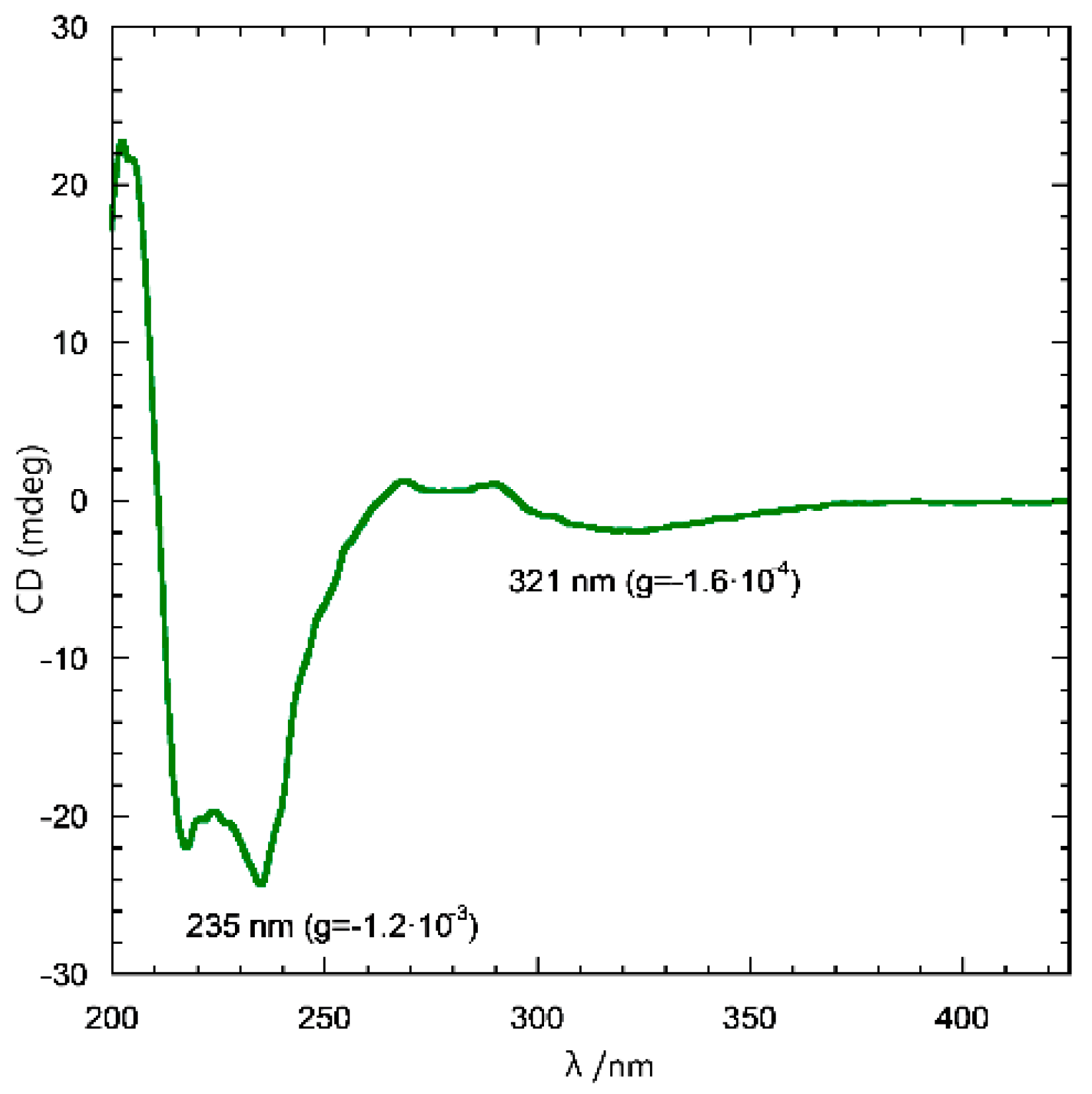
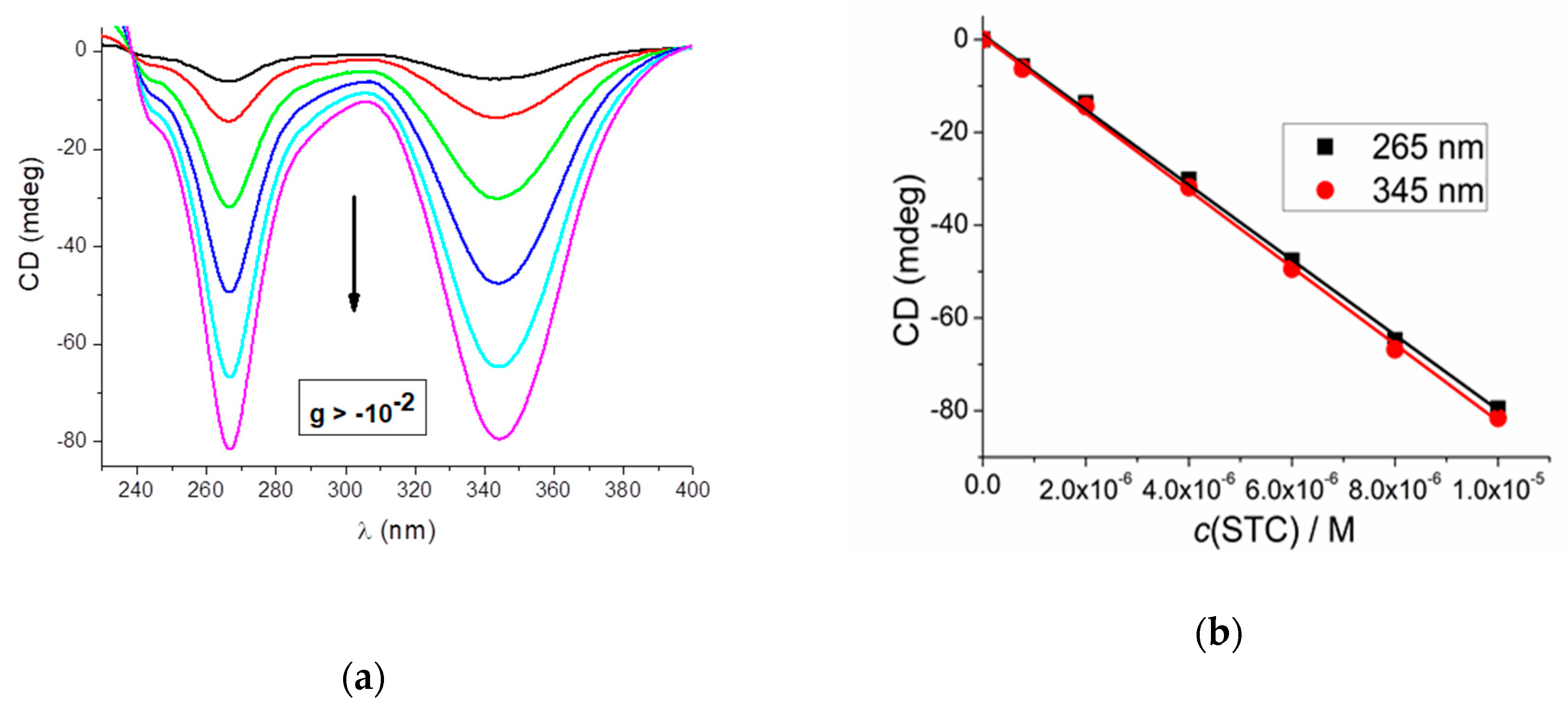
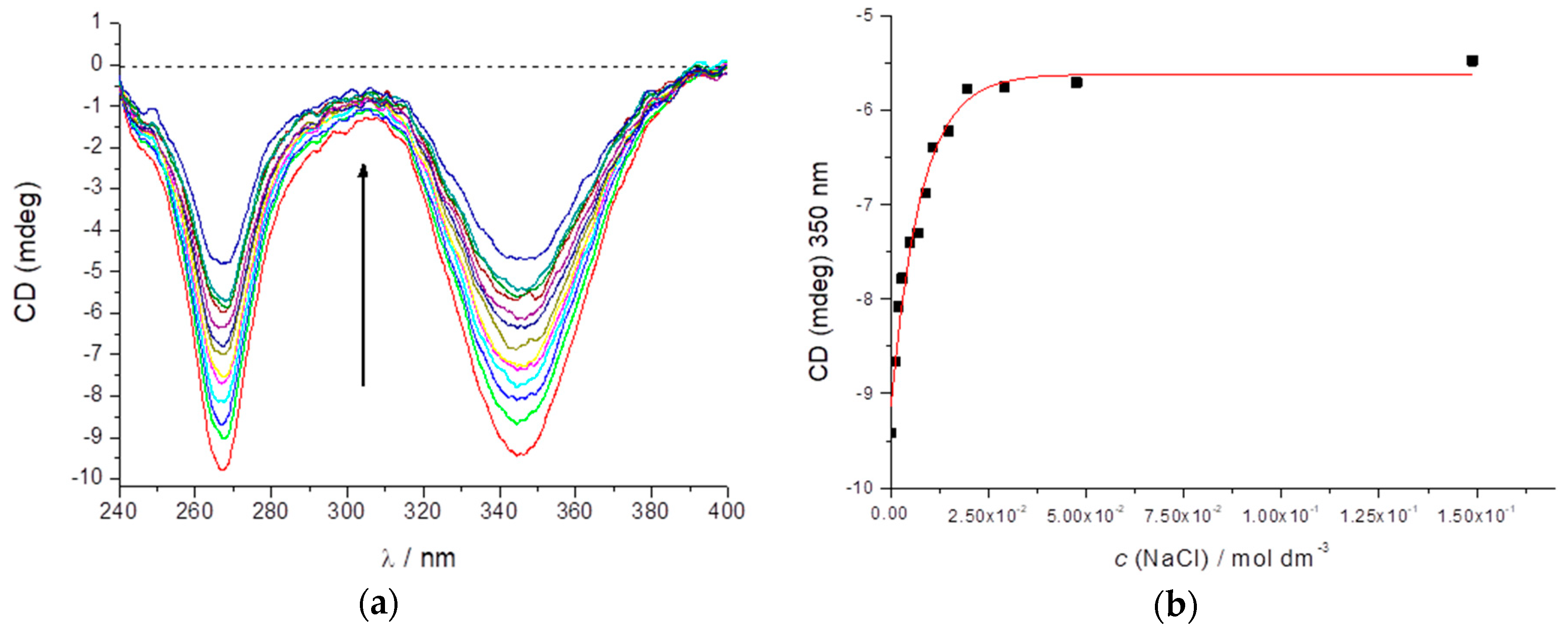
2.1. Preparation and CD Spectra of Aqueous Solutions of STC
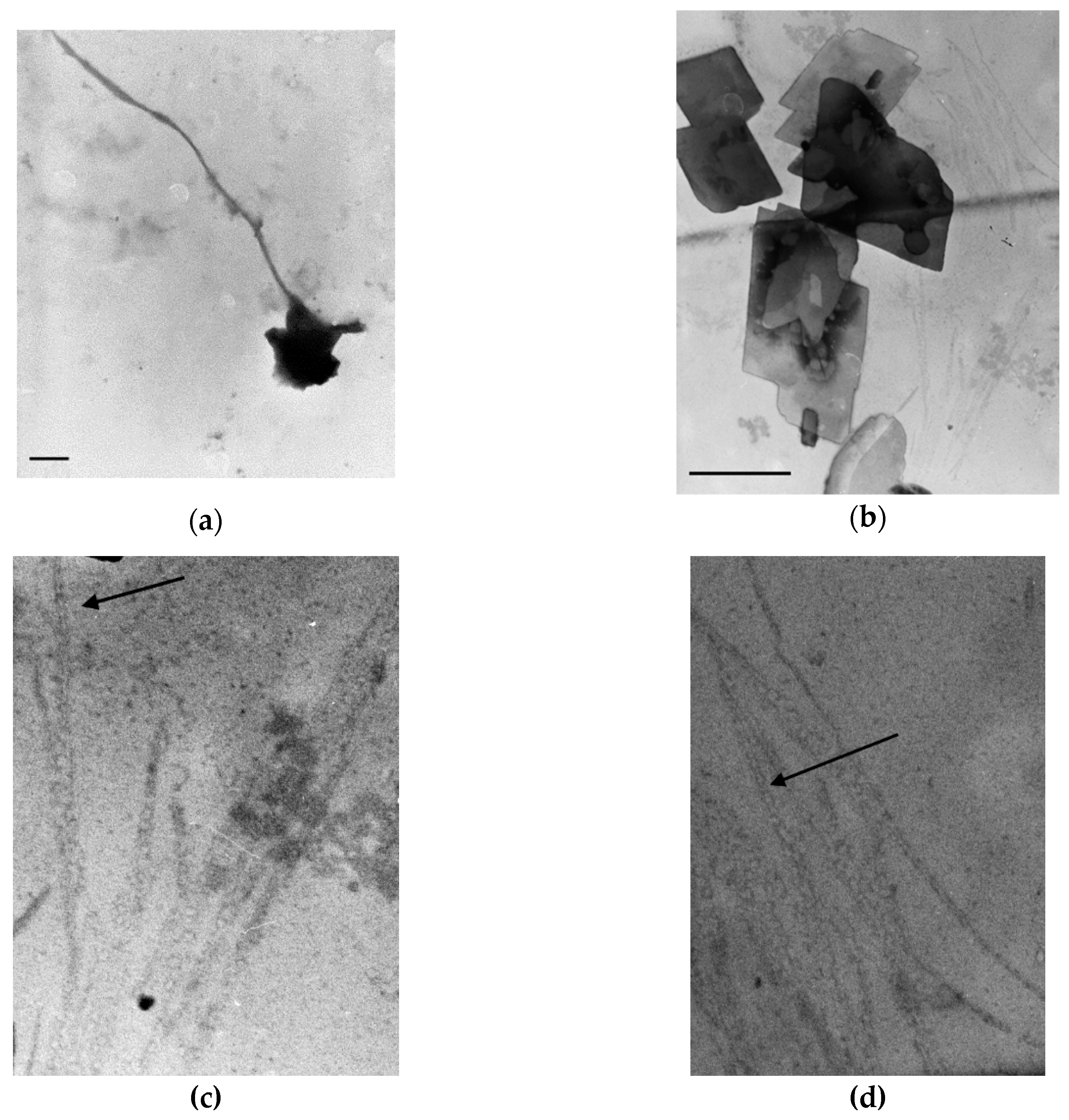
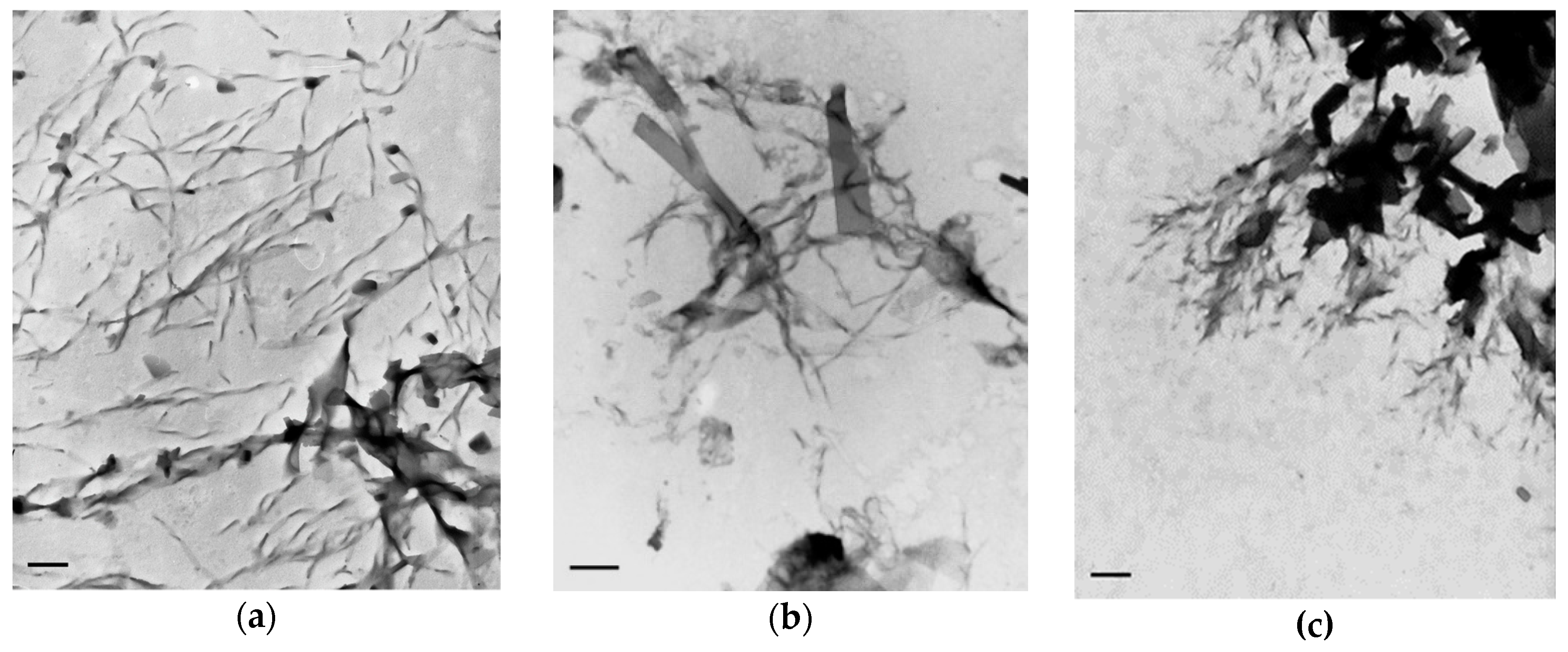
2.2. TEM Investigation
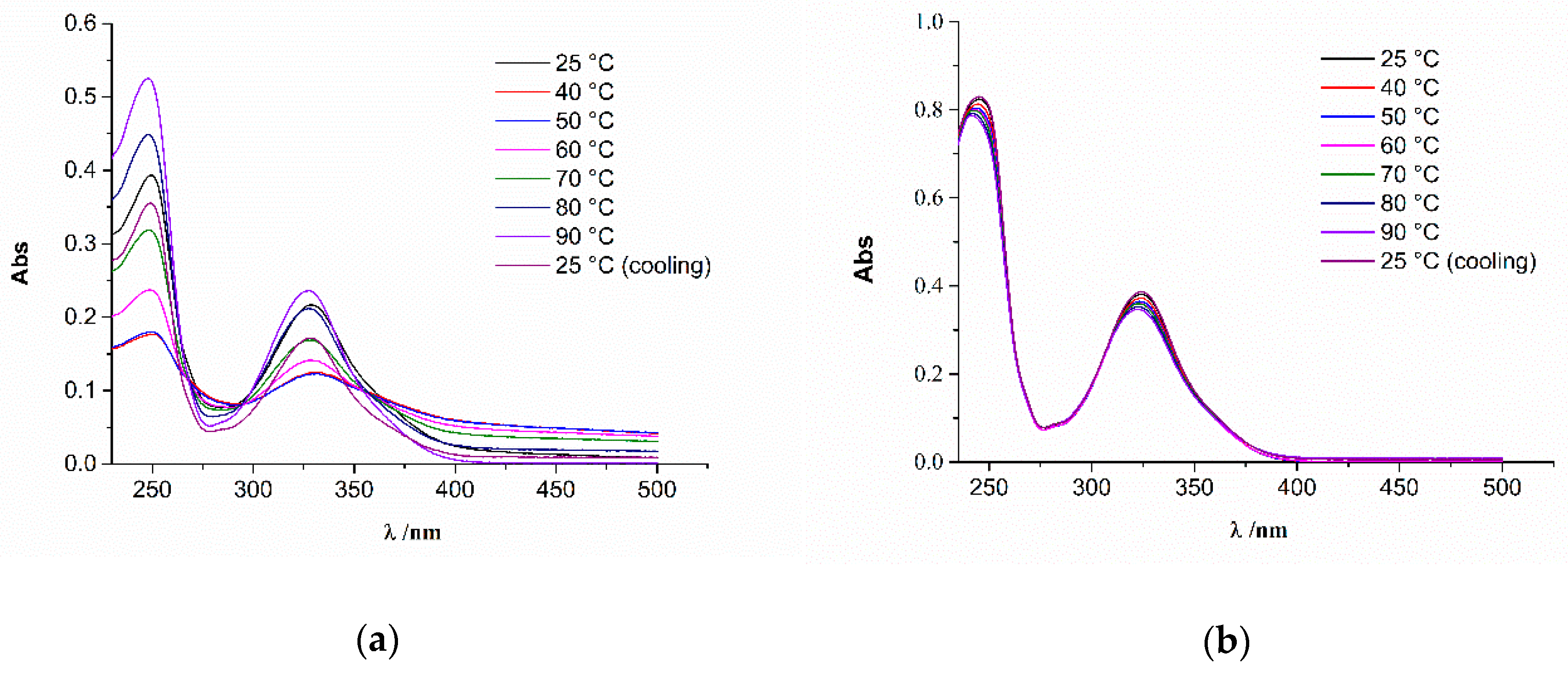
2.3. Thermodynamic Profiling of STC Aggregates by Differential Scanning Calorimetry (DSC)
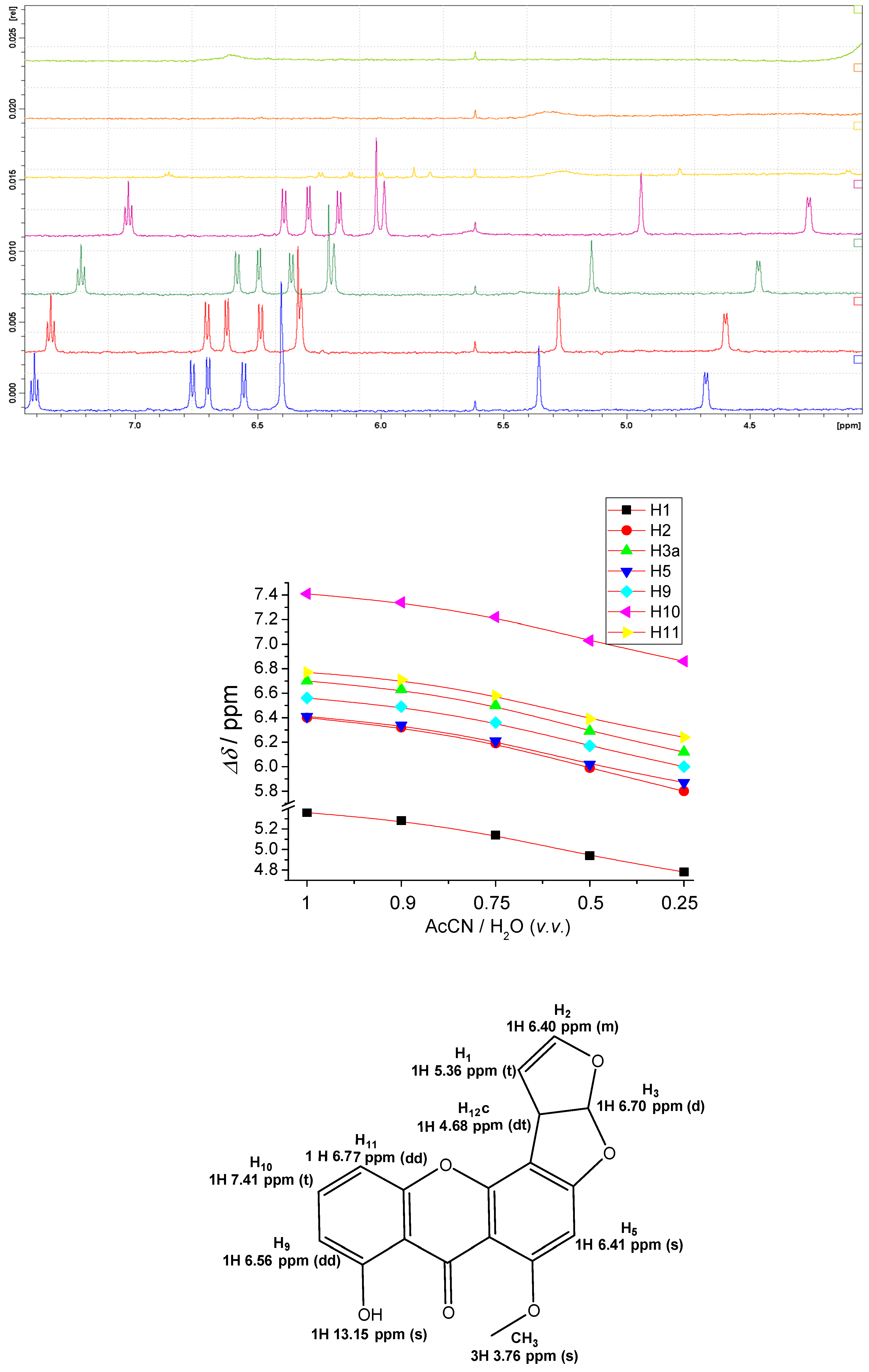
2.4. NMR Study of STC Aggregation
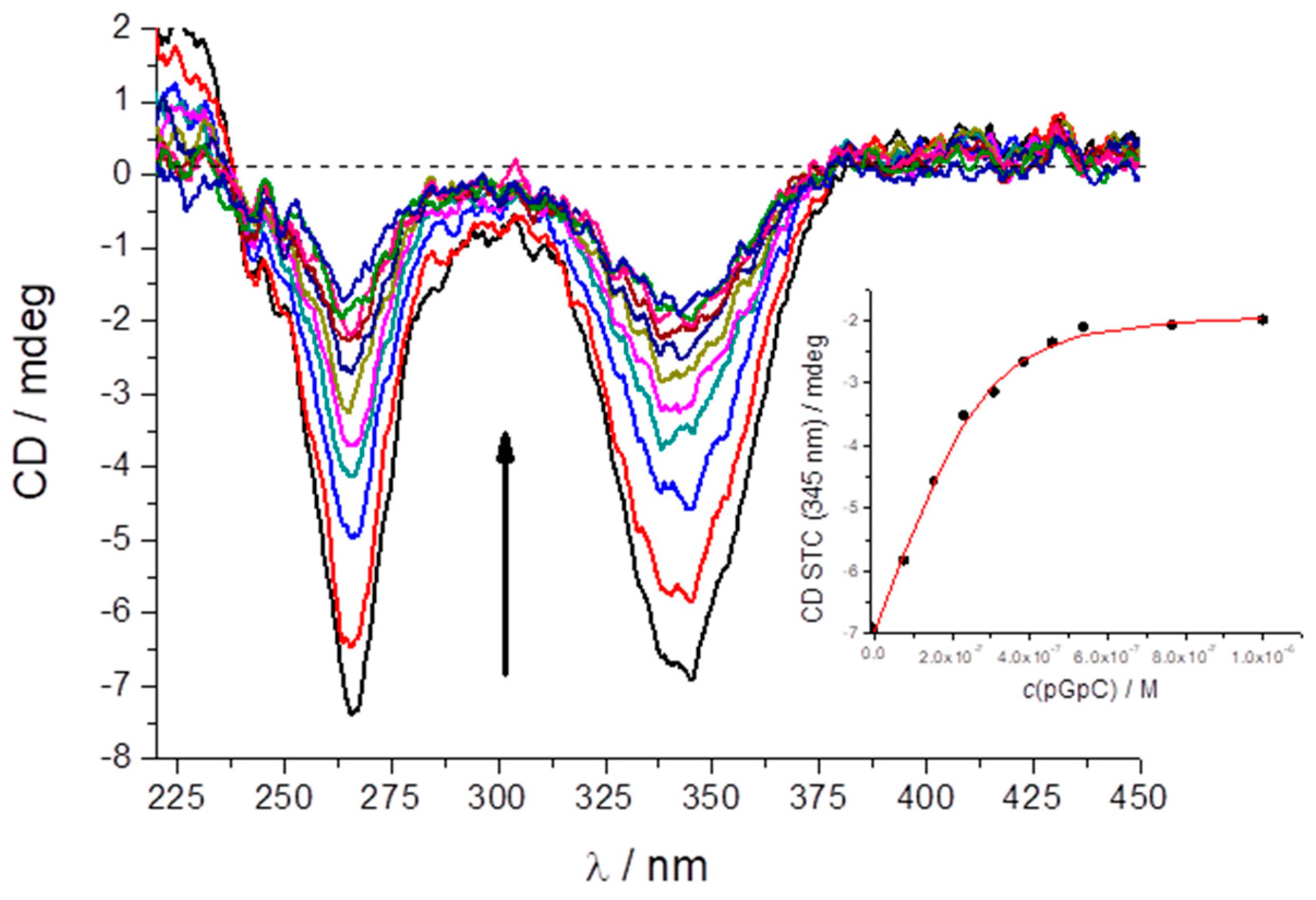
2.5. Study of STC Aggregation in Biorelevant Conditions
3. Materials and Methods
3.1. Chemicals
3.2. UV-Vis Spectroscopy
3.3. CD Spectroscopy
3.4. Differential Scanning Calorimetry (DSC)
3.5. DLS Method
3.6. Transmission Electron Microscope (TEM)
3.7. NMR Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IARC (International Agency for Research on Cancer) working group. Aflatoxins. In Biological Agents IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2012; Volume 100B, pp. 225–248. [Google Scholar]
- Jakšić Despot, D.; Kocsubé, S.; Bencsik, O.; Kecskeméti, A.; Szekeres, A.; Vágvölgyi, C.; Varga, J.; Šegvić Klarić, M. Species diversity and cytotoxic potency of airborne sterigmatocystin-producing Aspergilli from the section Versicolores. Sci. Total Environ. 2016, 562, 296–304. [Google Scholar] [CrossRef]
- Jakšić Despot, D.; Kocsubé, S.; Bencsik, O.; Kecskeméti, A.; Szekeres, A.; Vágvölgyi, C.; Varga, J.; Šegvić Klarić, M. New sterigmatocystin-producing species of Aspergillus section Versicolores from indoor air in Croatia. Mycol. Prog. 2016, 1–10. [Google Scholar] [CrossRef]
- Jurjević, Z.; Peterson, S.W.; Solfrizzo, M.; Peraica, M. Sterigmatocystin production by nine newly described Aspergillus species in section Versicolores grown on two different media. Mycotoxin Res. 2013, 29, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Rank, C.; Nielsen, K.F.; Larsen, T.O.; Varga, J.; Samson, R.A.; Frisvad, J.C. Distribution of sterigmatocystin in filamentous fungi. Fungal Biol. 2011, 115, 406–420. [Google Scholar] [CrossRef]
- Bertuzzi, T.; Romani, M.; Rastelli, S.; Mulazzi, A.; Pietri, A. Sterigmatocystin Occurrence in Paddy and Processed Rice Produced in Italy in the Years 2014-2015 and Distribution in Milled Rice Fractions. Toxins 2017, 9, 86. [Google Scholar] [CrossRef]
- Biancardi, A.; Dall’Asta, C. Determination of sterigmatocystin in feed by LC-MS/MS. Food Addit. Contam. Part A 2015, 32, 2093–2100. [Google Scholar] [CrossRef]
- Marley, E.; Brown, P.; Mackie, J.; Donnelly, C.; Wilcox, J.; Pietri, A.; Macdonald, S. Analysis of sterigmatocystin in cereals, animal feed, seeds, beer and cheese by immunoaffinity column clean-up and HPLC and LC-MS/MS quantification. Food Addit. Contam. Part A 2015, 32, 2131–2137. [Google Scholar] [CrossRef]
- Mo, H.G.J.; Pietri, A.; MacDonald, S.J.; Anagnostopoulos, C.; Spanjere, M. Survey on sterigmatocystin in food. EFSA Supporting Publ. 2015, 12, 774E. [Google Scholar] [CrossRef]
- Samson, R.A.; Houbraken, J.; Thrane, U.; Frisvad, J.C.; Andersen, B. Food and Indoor Fungi; CBS Laboratory Manual Series; Westerdijk Fungal Biodiversity Institute: Utrecht, The Netherlands, 2010; pp. 375–377. [Google Scholar]
- Tsang, C.-C.; Hui, T.W.S.; Lee, K.-C.; Chen, J.H.K.; Ngan, A.H.Y.; Tam, E.W.T.; Chan, J.F.W.; Wu, A.L.; Cheung, M.; Tse, B.P.H.; et al. Genetic diversity of Aspergillus species isolated from onychomycosis and Aspergillus hongkongensis sp. nov., with implications to antifungal susceptibility testing. Diagn. Microbiol. Infect. Dis. 2016, 84, 125–134. [Google Scholar] [CrossRef]
- Versilovskis, A.; De Saeger, S. Sterigmatocystin: Occurrence in foodstuffs and analytical methods—An overview. Mol. Nutr. Food Res. 2010, 54, 136–147. [Google Scholar] [CrossRef]
- Bloom, E.; Grimsley, L.F.; Pehrson, C.; Lewis, J.; Larsson, L. Molds and mycotoxins in dust from water-damaged homes in New Orleans after hurricane Katrina. Indoor Air 2009, 19, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Straumfors, A.; Uhlig, S.; Eriksen, G.S.; Heldal, K.K.; Eduard, W.; Krska, R.; Sulyok, M. Mycotoxins and other fungal metabolites in grain dust from Norwegian grain elevators and compound feed mills. World Mycotoxin J. 2015, 8, 361–373. [Google Scholar] [CrossRef]
- Nagelkerken, I.; Buchan, K.; Smith, G.W.; Bonair, K.; Bush, P.; Garzon-Ferreira, J.; Botero, L.; Gayle, P.; Harvell, C.D.; Heberer, C.; et al. Widespread disease in Caribbean sea fans: II. Patterns of infection and tissue loss. Mar. Ecol. Prog. Ser. 1997, 160, 255–263. [Google Scholar] [CrossRef]
- Soler-Hurtado, M.M.; Sandoval-Sierra, J.V.; Machordom, A.; Dieguez-Uribeondo, J. Aspergillus sydowii and Other Potential Fungal Pathogens in Gorgonian Octocorals of the Ecuadorian Pacific. PLoS ONE 2016, 11, e0165992. [Google Scholar] [CrossRef] [PubMed]
- Hallegraeff, G.; Coman, F.; Davies, C.; Hayashi, A.; McLeod, D.; Slotwinski, A.; Whittock, L.; Richardson, A.J. Australian Dust Storm Associated with Extensive Aspergillus sydowii Fungal “Bloom” in Coastal Waters. Appl. Environ. Microb. 2014, 80, 3315–3320. [Google Scholar] [CrossRef]
- Greco, G.; Capello, M.; Cecchi, G.; Cutroneo, L.; Di Piazza, S.; Zotti, M. Another possible risk for the Mediterranean Sea? Aspergillus sydowii discovered in the Port of Genoa (Ligurian Sea, Italy). Mar. Pollut. Bull. 2017, 122, 470–474. [Google Scholar] [CrossRef]
- Lee, S.; Park, M.S.; Lim, Y.W. Diversity of Marine-Derived Aspergillus from Tidal Mudflats and Sea Sand in Korea. Mycobiology 2016, 44, 237–247. [Google Scholar] [CrossRef]
- Hu, J.S.; Li, Z.; Gao, J.Y.; He, H.T.; Dai, H.Q.; Xia, X.K.; Liu, C.H.; Zhang, L.X.; Song, F.H. New Diketopiperazines from a Marine-Derived Fungus Strain Aspergillus versicolor MF180151. Mar. Drugs 2019, 17, 262. [Google Scholar] [CrossRef]
- Lee, Y.M.; Li, H.; Hong, J.; Cho, H.Y.; Bae, K.S.; Kim, M.A.; Kim, D.K.; Jung, J.H. Bioactive Metabolites from the Sponge-Derived Fungus Aspergillus versicolor. Arch. Pharm. Res. 2010, 33, 231–235. [Google Scholar] [CrossRef]
- Song, F.H.; Ren, B.; Chen, C.X.; Yu, K.; Liu, X.R.; Zhang, Y.H.; Yang, N.; He, H.T.; Liu, X.T.; Dai, H.Q.; et al. Three new sterigmatocystin analogues from marine-derived fungus Aspergillus versicolor MF359. Appl. Microbiol. Biotechnol. 2014, 98, 3753–3758. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Kawabata, T.; Kato, H.; Greshock, T.J.; Hirota, H.; Ohta, T.; Williams, R.M. Isolation of Antipodal (-)-Versicolamide B and Notoamides L-N from a Marine-Derived Aspergillus sp. Org. Lett. 2009, 11, 1297–1300. [Google Scholar] [CrossRef] [PubMed]
- IARC. Some Naturally Occurring and Synthetic Food Cornponents: Furocournarins and Ultraviolet Radiation. IARC Monogr. Eval. Carcinog. Risk Hum. 1987, 100B, 225–248. [Google Scholar]
- Kusunoki, M.; Misumi, J.; Shimada, T.; Aoki, K.; Matsuo, N.; Sumiyoshi, H.; Yamaguchi, T.; Yoshioka, H. Long-term administration of the fungus toxin, sterigmatocystin, induces intestinal metaplasia and increases the proliferative activity of PCNA, p53, and MDM2 in the gastric mucosa of aged Mongolian gerbils. Environ. Health Prev. Med. 2011, 16, 224–231. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ma, F.; Misumi, J.; Zhao, W.; Aoki, K.; Kudo, M. Long-term Treatment with Sterigmatocystin, a Fungus Toxin, Enhances the Development of Intestinal Metaplasia of Gastric Mucosa in Helicobacter pylori -infected Mongolian Gerbils. Scand. J. Gastroenterol. 2003, 38, 361–369. [Google Scholar] [CrossRef]
- You, W.C.; Blot, W.J.; Chang, Y.S.; Ershow, A.G.; Yang, Z.T.; An, Q.; Henderson, B.; Xu, G.W.; Fraumeni, J.F.; Wang, T.G. Diet and high risk of stomach cancer in Shandong, China. Cancer Res. 1988, 48, 3518–3523. [Google Scholar] [CrossRef]
- Oppliger, A.; Duquenne, P. Chapter 8—Highly Contaminated Workplaces. In Environmental Mycology in Public Health; Viegas, C., Pinheiro, A.C., Sabino, R., Viegas, S., Brandão, J., Veríssimo, C., Eds.; Academic Press: Amsterdam, The Netherlands, 2016; pp. 79–105. [Google Scholar]
- EFSA. Scientific Opinion on the risk for public and animal health related to the presence of sterigmatocystin in food and feed. EFSA J. 2013, 11, 3254. [Google Scholar] [CrossRef]
- Abdel-Wahhab, M.A.; Hasan, A.M.; Aly, S.E.; Mahrous, K.F. Adsorption of sterigmatocystin by montmorillonite and inhibition of its genotoxicity in the Nile tilapia fish (Oreachromis nilaticus). Mutat. Res.-Genet. Toxicol. Environ. Mutagenes. 2005, 582, 20–27. [Google Scholar] [CrossRef]
- Essigmann, J.M.; Barker, L.J.; Fowler, K.W.; Francisco, M.A.; Reinhold, V.N.; Wogan, G.N. Sterigmatocystin-DNA interactions: Identification of a major adduct formed after metabolic activation in vitro. Proc. Natl. Acad. Sci. USA 1979, 76, 179–183. [Google Scholar] [CrossRef]
- Essigmann, J.M.; Croy, R.G.; Nadzan, A.M.; Busby, W.F.; Reinhold, V.N.; Büchi, G.; Wogan, G.N. Structural identification of the major DNA adduct formed by aflatoxin B1 in vitro. Proc. Natl. Acad. Sci. USA 1977, 74, 1870–1874. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Harris, T.M.; Stone, M.P. Intercalation of aflatoxin B1 in two oligodeoxynucleotide adducts: Comparative 1H NMR analysis of d(ATCAFBGAT).d(ATCGAT) and d(ATAFBGCAT)2. Biochemistry 1990, 29, 10438–10448. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Liu, X.C.; Patel, D.J. Solution Structure of the Covalent Sterigmatocystin-DNA Adduct. Biochemistry 1992, 31, 10790–10801. [Google Scholar] [CrossRef] [PubMed]
- Jones, W.R.; Stone, M.P. Site-specific targeting of aflatoxin adduction directed by triple helix formation in the major groove of oligodeoxyribonucleotides. Nucleic Acids Res. 1998, 26, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Stark, A.A. Mutagenicity and carcinogenicity of mycotoxins: DNA binding as a possible mode of action. Ann. Rev. Microbiol. 1980, 34, 235–262. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraj Reddy, M.; Rick Irvin, T.; Randerath, K. Formation and persistence of sterigmatocystin-DNA adducts in rat liver determined via 32P-postlabeling analysis. Mutat. Res. 1985, 152, 85–96. [Google Scholar] [CrossRef]
- Martin, C.N.; Garner, R.C. Aflatoxin B-oxide generated by chemical or enzymic oxidation of aflatoxin B1 causes guanine substitution in nucleic acids. Nature 1977, 267, 863–865. [Google Scholar] [CrossRef]
- Raney, V.M.; Harris, T.M.; Stone, M.P. DNA conformation mediates aflatoxin B1-DNA binding and the formation of guanine N7 adducts by aflatoxin B1 8,9-exo-epoxide. Chem. Res. Toxicol. 1993, 6, 64–68. [Google Scholar] [CrossRef]
- Gao, W.; Jiang, L.; Ge, L.; Chen, M.; Geng, C.; Yang, G.; Li, Q.; Ji, F.; Yan, Q.; Zou, Y.; et al. Sterigmatocystin-induced oxidative DNA damage in human liver-derived cell line through lysosomal damage. Toxicol. In Vitro 2015, 29, 1–7. [Google Scholar] [CrossRef]
- Gursoy-Yuzugullu, O.; Yuzugullu, H.; Yilmaz, M.; Ozturk, M. Aflatoxin genotoxicity is associated with a defective DNA damage response bypassing p53 activation. Liver Int. 2011, 31, 561–571. [Google Scholar] [CrossRef]
- Mulder, J.E.; Bondy, G.S.; Mehta, R.; Massey, T.E. The impact of chronic Aflatoxin B-1 exposure and p53 genotype on base excision repair in mouse lung and liver. Mutat. Res. 2015, 773, 63–68. [Google Scholar] [CrossRef]
- Pfeiffer, E.; Fleck, S.C.; Metzler, M. Catechol formation: A novel pathway in the metabolism of sterigmatocystin and 11-methoxysterigmatocystin. Chem. Res. Toxicol. 2014, 27, 2093–2099. [Google Scholar] [CrossRef]
- Sivakumar, V.; Thanislass, J.; Niranjali, S.; Devaraj, H. Lipid peroxidation as a possible secondary mechanism of sterigmatocystin toxicity. Hum. Exp. Toxicol. 2001, 20, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Cui, Y.; Shen, H.; Xing, L.; Cui, J.; Wang, J.; Zhang, X. Sterigmatocystin-Induced DNA Damage Triggers G2 Arrest via an ATM/p53-Related Pathway in Human Gastric Epithelium GES-1 Cells in vitro. PLoS ONE 2013, 8, e65044. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.H.; Fang, G.Z.; Pan, M.F.; Wang, X.F.; Wang, S. One-pot synthesis of carbon dots-embedded molecularly imprinted polymer for specific recognition of sterigmatocystin in grains. Biosens. Bioelectron. 2016, 77, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Septien, I.; Cutuli, M.T.; Garcia, M.E.; Suarez, G.; Blanco, J.L. Solubility and Stability of Sterigmatocystin in Different Organic-Solvents. Toxicon 1993, 31, 1337–1340. [Google Scholar] [CrossRef]
- Pescitelli, G.; Bari, L.; Berova, N. Application of electronic circular dichroism in the study of supramolecular systems. Chem. Soc. Rev. 2014, 43, 5211–5233. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, T.; Usuki, T. Circular Dichroism Spectroscopy. In Supramolecular Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Tinoco, I.; Bustamante, C.; Maestre, M.F. The Optical-Activity of Nucleic-Acids and Their Aggregates. Annu. Rev. Biophys. 1980, 9, 107–141. [Google Scholar] [CrossRef]
- Garab, G.; van Amerongen, H. Linear dichroism and circular dichroism in photosynthesis research. Photosynth. Res. 2009, 101, 135–146. [Google Scholar] [CrossRef]
- Keller, D.; Bustamante, C. Theory of the Interaction of Light with Large Inhomogeneous Molecular Aggregates. 2. Psi-Type Circular-Dichroism. J. Chem. Phys. 1986, 84, 2972–2980. [Google Scholar] [CrossRef]
- Keller, D.; Bustamante, C. Theory of the Interaction of Light with Large Inhomogeneous Molecular Aggregates. 1. Absorption. J. Chem. Phys. 1986, 84, 2961–2971. [Google Scholar] [CrossRef]
- Fukuyama, K.; Hamada, K.; Tsukihara, T.; Katsube, Y.; Hamasaki, T.; Hatsuda, Y. Crystal-Structures of Sterigmatocystin and O-Methylsterigmatocystin, Metabolites of Genus Aspergillus. Bull. Chem. Soc. Jpn. 1976, 49, 1153–1154. [Google Scholar] [CrossRef]
- Padula, D.; Di Pietro, S.; Capozzi, M.A.M.; Cardellicchio, C.; Pescitelli, G. Strong Intermolecular Exciton Couplings in Solid-State Circular Dichroism of Aryl Benzyl Sulfoxides. Chirality 2014, 26, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Pescitelli, G. Solid-state Circular Dichroism and Hydrogen Bonding, Part 2: The Case of Hypothemycin Re-investigated. Chirality 2012, 24, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Aggeli, A.; Nyrkova, I.A.; Bell, M.; Harding, R.; Carrick, L.; McLeish, T.C.B.; Semenov, A.N.; Boden, N. Hierarchical self-assembly of chiral rod-like molecules as a model for peptide beta-sheet tapes, ribbons, fibrils, and fibers. Proc. Natl. Acad. Sci. USA 2001, 98, 11857–11862. [Google Scholar] [CrossRef]
- Oda, R.; Huc, I.; Schmutz, M.; Candau, S.J.; MacKintosh, F.C. Tuning bilayer twist using chiral counterions. Nature 1999, 399, 566–569. [Google Scholar] [CrossRef]
- McGhee, J.D.; Hippel, P.H.V. Theoretical Aspects of DNA-Protein Interactions—Cooperative and Non-Cooperative Binding of Large Ligands to a One-Dimensional Homogeneous Lattice. J. Mol. Biol. 1974, 86, 469–489. [Google Scholar] [CrossRef]
- Fekry, M.I.; Szekely, J.; Dutta, S.; Breydo, L.; Zang, H.; Gates, K.S. Noncovalent DNA Binding Drives DNA Alkylation by Leinamycin: Evidence That the Z,E-5-(Thiazol-4-yl)-penta-2,4-dienone Moiety of the Natural Product Serves as an Atypical DNA Intercalator. J. Am. Chem. Soc. 2011, 133, 17641–17651. [Google Scholar] [CrossRef]
- Pescitelli, G.; Kurtán, T.; Flörke, U.; Krohn, K. Absolute Structural Elucidation of Natural Products—A Focus on Quantum-Mechanical Calculations of Solid-State CD Spectra. Chirality 2009, 21, E181–E201. [Google Scholar] [CrossRef]

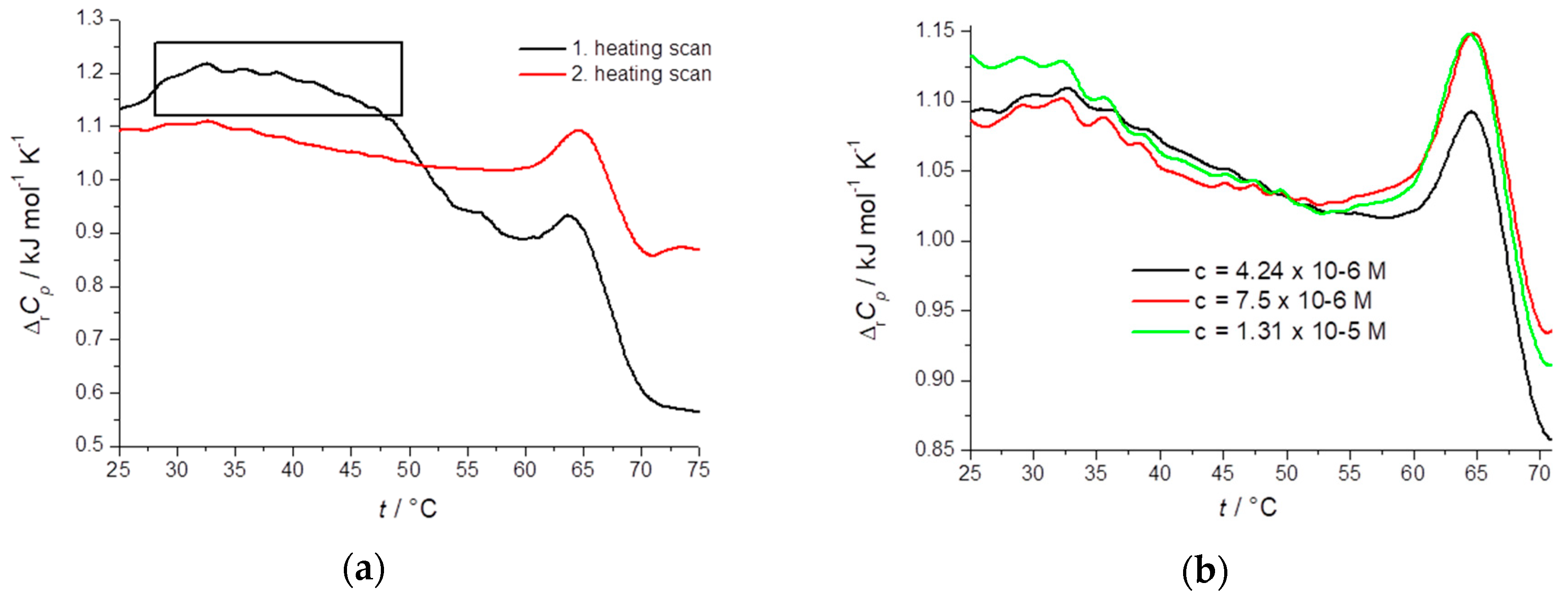
| c(STC)/M | Scan | Tmax/°C | ΔrHc/kJ mol−1 | ΔrSc/J K−1 mol−1 |
|---|---|---|---|---|
| 1 4.24 × 10−6 | #1 | 65.7 | 57.1 | 168.5 |
| #2 | 66.1 | 40.2 | 118.5 | |
| 7.5 × 10−6 | #1 | 66.3 | 27.2 | 80.0 |
| #2 | 65.9 | 21.8 | 64.3 | |
| #3 | 65.2 | 31.1 | 91.9 | |
| #4 | 64.8 | 32.7 | 96.7 | |
| 1.31 × 10−5 | #1 | 65.9 | 21.7 | 64.1 |
| #2 | 65.9 | 16.6 | 49.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakšić, D.; Šegvić Klarić, M.; Crnolatac, I.; Šijaković Vujičić, N.; Smrečki, V.; Górecki, M.; Pescitelli, G.; Piantanida, I. Unique Aggregation of Sterigmatocystin in Water Yields Strong and Specific Circular Dichroism Response Allowing Highly Sensitive and Selective Monitoring of Bio-Relevant Interactions. Mar. Drugs 2019, 17, 629. https://doi.org/10.3390/md17110629
Jakšić D, Šegvić Klarić M, Crnolatac I, Šijaković Vujičić N, Smrečki V, Górecki M, Pescitelli G, Piantanida I. Unique Aggregation of Sterigmatocystin in Water Yields Strong and Specific Circular Dichroism Response Allowing Highly Sensitive and Selective Monitoring of Bio-Relevant Interactions. Marine Drugs. 2019; 17(11):629. https://doi.org/10.3390/md17110629
Chicago/Turabian StyleJakšić, Daniela, Maja Šegvić Klarić, Ivo Crnolatac, Nataša Šijaković Vujičić, Vilko Smrečki, Marcin Górecki, Gennaro Pescitelli, and Ivo Piantanida. 2019. "Unique Aggregation of Sterigmatocystin in Water Yields Strong and Specific Circular Dichroism Response Allowing Highly Sensitive and Selective Monitoring of Bio-Relevant Interactions" Marine Drugs 17, no. 11: 629. https://doi.org/10.3390/md17110629
APA StyleJakšić, D., Šegvić Klarić, M., Crnolatac, I., Šijaković Vujičić, N., Smrečki, V., Górecki, M., Pescitelli, G., & Piantanida, I. (2019). Unique Aggregation of Sterigmatocystin in Water Yields Strong and Specific Circular Dichroism Response Allowing Highly Sensitive and Selective Monitoring of Bio-Relevant Interactions. Marine Drugs, 17(11), 629. https://doi.org/10.3390/md17110629