Development and Characterization of Astaxanthin-Containing Whey Protein-Based Nanoparticles
Abstract
1. Introduction
2. Results and Discussion
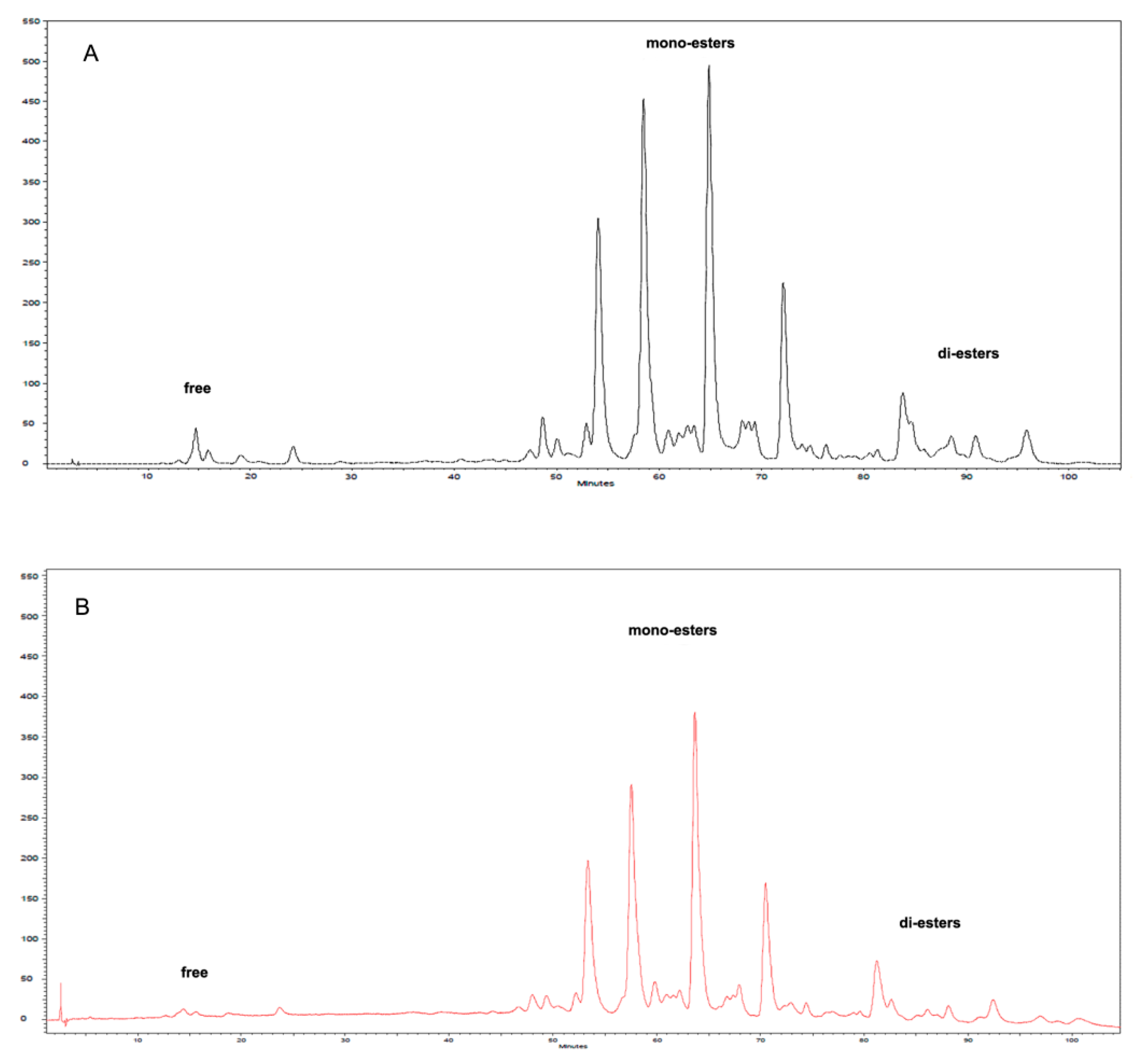
2.1. Encapsulation of Oleoresin
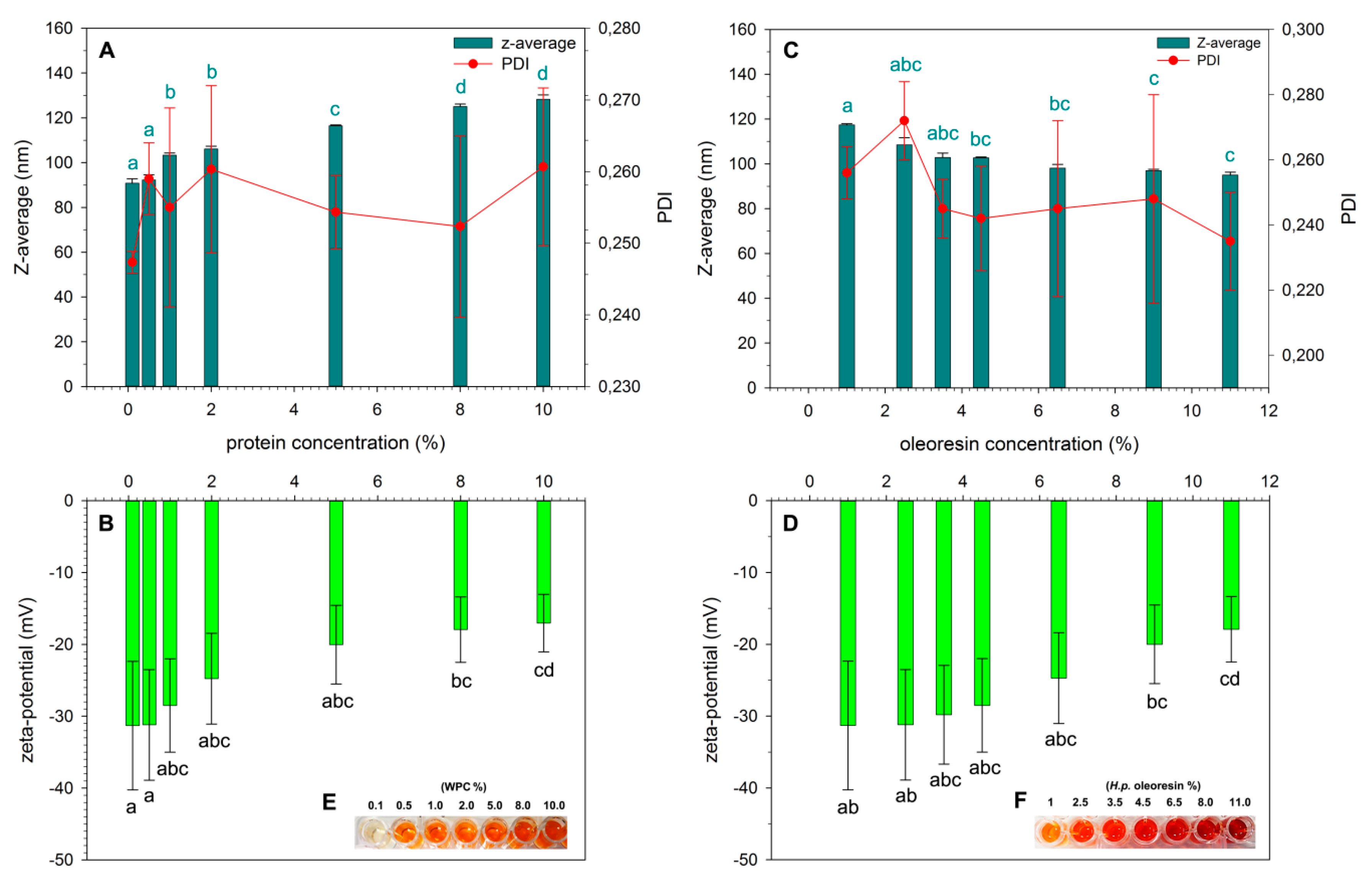
2.2. Characterization of NPs
2.3. Stability of the NPs
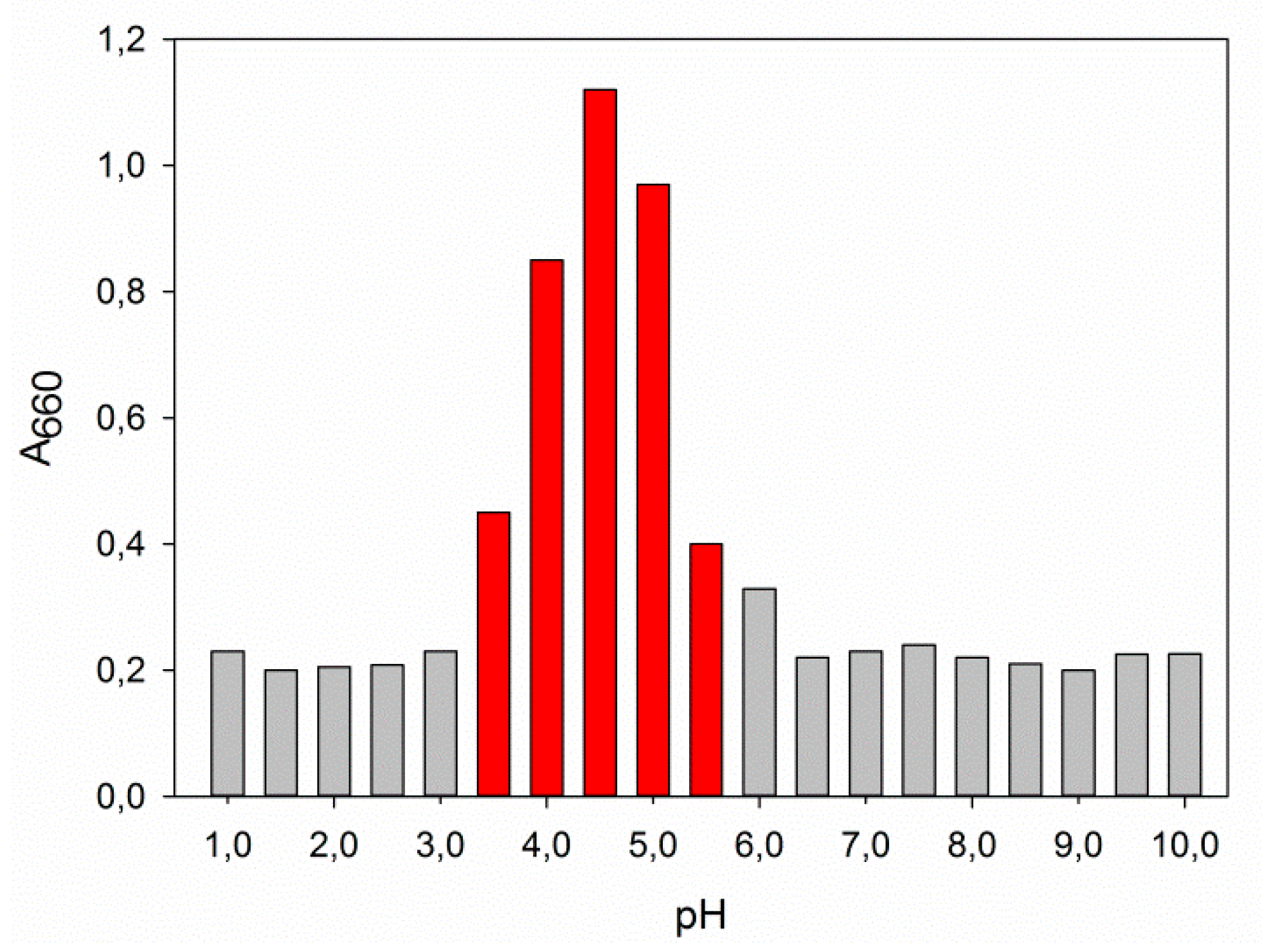
2.3.1. Effect of pH
2.3.2. UV Irradiation
2.3.3. Fe(III)-Induced Oxidation
2.3.4. Thermal Treatment
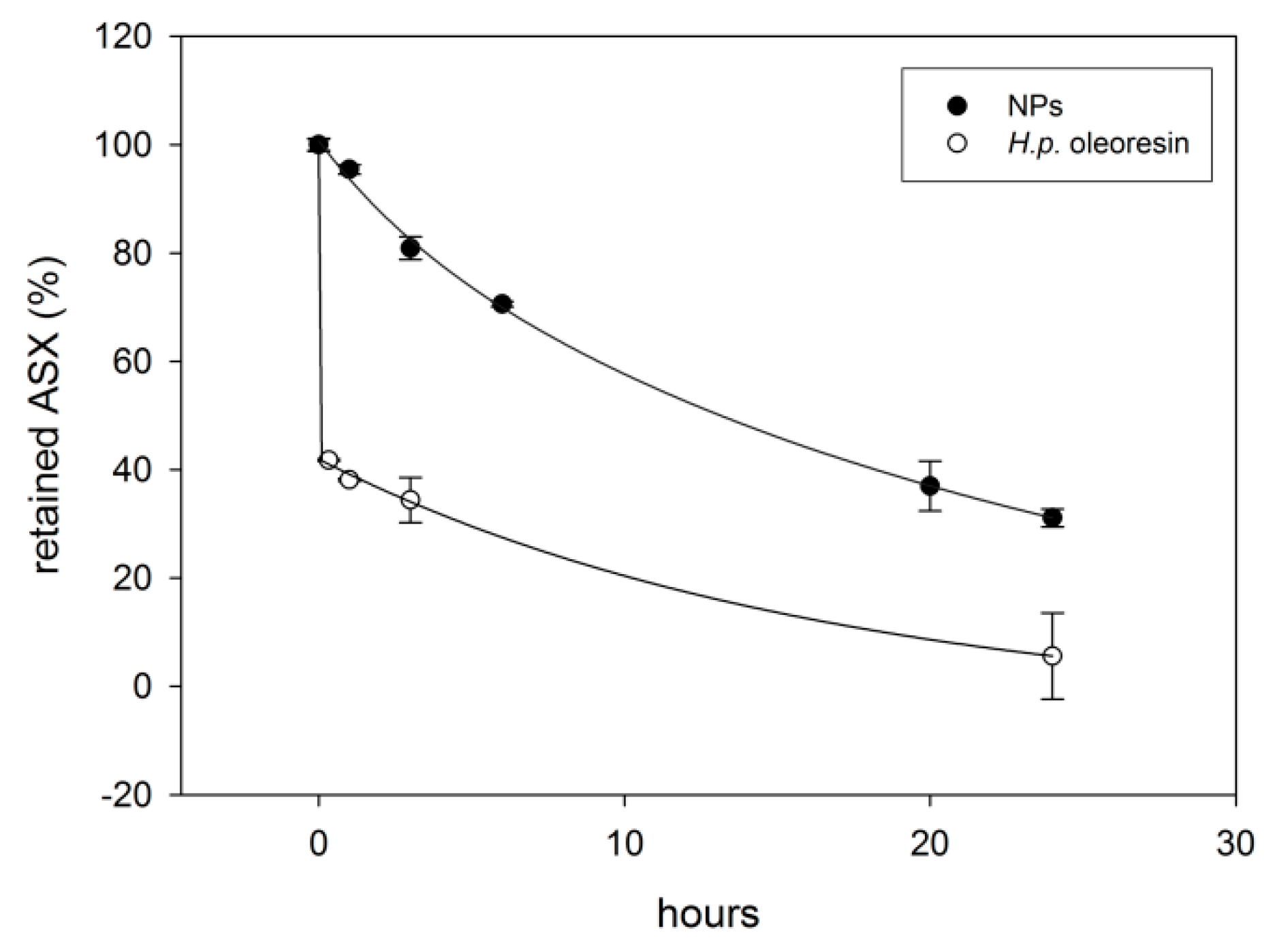
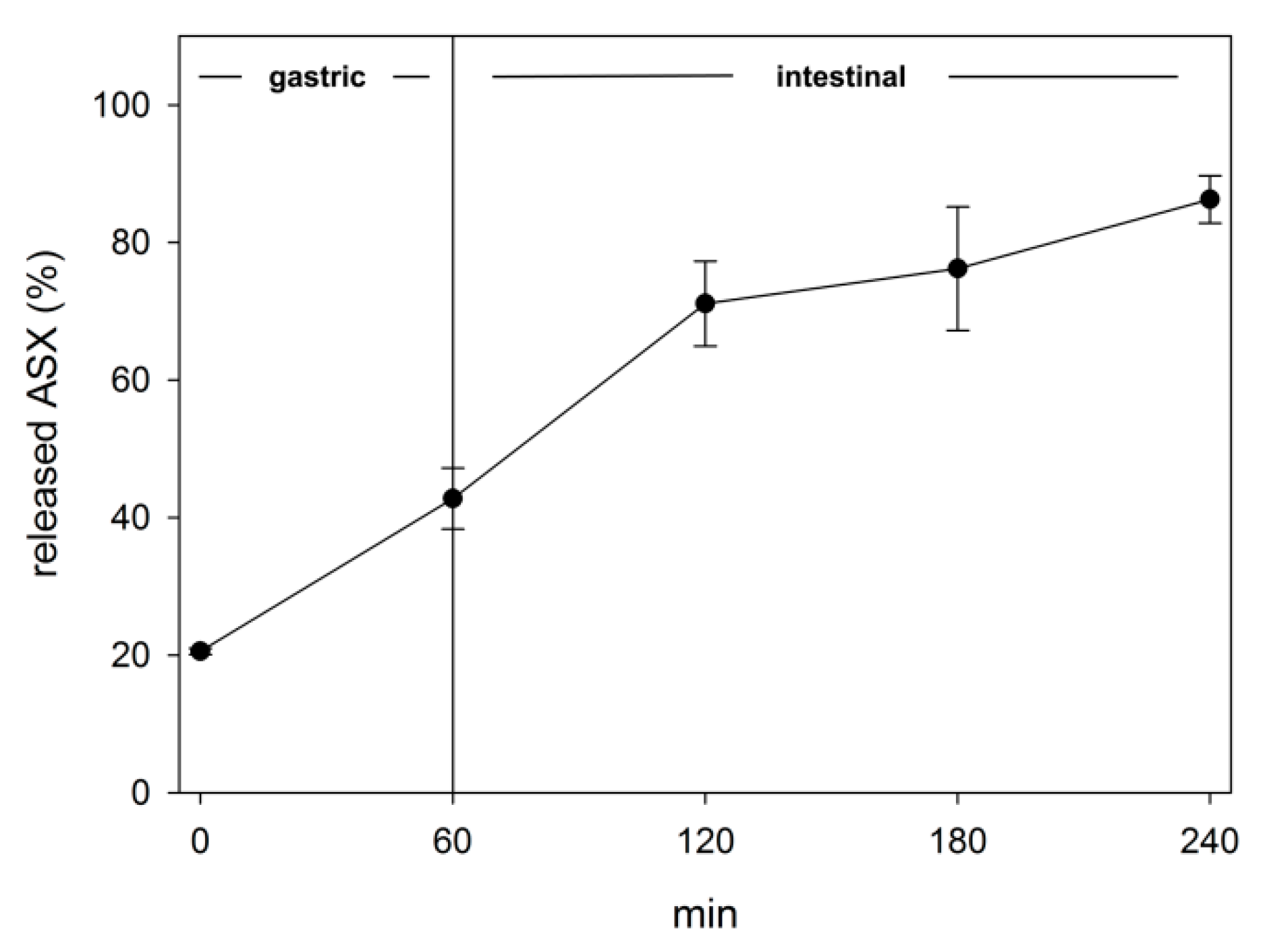
2.4. Evaluation of Bioaccessibility by In Vitro Simulated Digestion
3. Materials and Methods
3.1. Chemical and Reagents
3.2. Oleoresin Extraction
3.3. Spectrophotometric Analyses
3.3.1. ASX Quantification
3.3.2. Turbidity Analysis
3.4. HPLC Analysis
3.5. H.p. Oleoresin NP Preparation
3.6. Characterization of Astaxanthin Nanoparticles
3.6.1. Dynamic Light Scattering (DLS) Analysis
3.6.2. Encapsulation Efficiency
3.6.3. ABTS Radical Scavenging Activity (RSA)
3.7. Stability of the NPs
3.7.1. Effect of pH
3.7.2. UV Irradiation
3.7.3. Fe(III)-Induced Oxidation
3.7.4. Thermal Treatment
3.8. Simulated In Vitro Digestion
Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. 2006, 46, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Naguib, Y.M.A. Antioxidant activities of astaxanthin and related carotenoids. J. Agr. Food Chem. 2000, 48, 1150–1154. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Sakamoto, Y. Singlet oxygen quenching ability of astaxanthin esters from the green alga Haematococcus pluvialis. Biotechnol. Lett. 1999, 21, 265–269. [Google Scholar] [CrossRef]
- Guerra, B.A.; Otton, R. Impact of the carotenoid astaxanthin on phagocytic capacity and ROS/RNS production of human neutrophils treated with free fatty acids and high glucose. Int. Immunopharmacol. 2011, 11, 2220–2226. [Google Scholar] [CrossRef] [PubMed]
- Pashkow, F.J.; Watumull, D.G.; Campbell, C.L. Astaxanthin: A novel potential treatment for oxidative stress and inflammation in cardiovascular disease. Am. J. Cardiol. 2008, 101, 58d–68d. [Google Scholar] [CrossRef]
- Nakao, R.; Nelson, O.L.; Park, J.S.; Mathison, B.D.; Thompson, P.A.; Chew, B.P. Effect of Dietary Astaxanthin at Different Stages of Mammary Tumor Initiation in BALB/c Mice. Anticancer Res. 2010, 30, 2171–2175. [Google Scholar]
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications-A Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Poonkum, W.; Powtongsook, S.; Pavasant, P. Astaxanthin Induction in Microalga H. pluvialis with Flat Panel Airlift Photobioreactors Under Indoor and Outdoor Conditions. Prep. Biochem. Biotechnol. 2015, 45, 1–17. [Google Scholar] [CrossRef]
- Martinez-Delgado, A.A.; Khandual, S.; Villanueva-Rodriguez, S.J. Chemical stability of astaxanthin integrated into a food matrix: Effects of food processing and methods for preservation. Food Chem. 2017, 225, 23–30. [Google Scholar] [CrossRef]
- Kittikaiwan, P.; Powthongsook, S.; Pavasant, P.; Shotipruk, A. Encapsulation of Haematococcus pluvialis using chitosan for astaxanthin stability enhancement. Carbohyd. Polym. 2007, 70, 378–385. [Google Scholar] [CrossRef]
- Anarjan, N.; Tan, C.P.; Nehdi, I.A.; Ling, T.C. Colloidal astaxanthin: Preparation, characterisation and bioavailability evaluation. Food Chem. 2012, 135, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.E.; Choi, H.I.; Kwak, H.S.; Hwang, S.W.; Sung, Y.J.; Chang, W.S.; Sim, S.J. Rapid selection of astaxanthin-hyperproducing Haematococcus mutant via azide-based colorimetric assay combined with oil-based astaxanthin extraction. Bioresour. Technol. 2018, 267, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Liu, H.; Sun, W.W.; Xue, C.H.; Mao, X.Z. Effective Astaxanthin Extraction from Wet Haematococcus pluvialis Using Switchable Hydrophilicity Solvents. ACS Sustain. Chem. Eng. 2018, 6, 1560–1563. [Google Scholar] [CrossRef]
- Mendes-Pinto, M.M.; Raposo, M.F.J.; Bowen, J.; Young, A.J.; Morais, R. Evaluation of different cell disruption processes on encysted cells of Haematococcus pluvialis: Effects on astaxanthin recovery and implications for bio-availability. J. Appl. Phycol. 2001, 13, 19–24. [Google Scholar] [CrossRef]
- Krichnavaruk, S.; Shotipruk, A.; Goto, M.; Pavasant, P. Supercritical carbon dioxide extraction of astaxanthin from Haematococcus pluvialis with vegetable oils as co-solvent. Bioresour. Technol. 2008, 99, 5556–5560. [Google Scholar] [CrossRef]
- Vakarelova, M.; Zanoni, F.; Lardo, P.; Rossin, G.; Mainente, F.; Chignola, R.; Menin, A.; Rizzi, C.; Zoccatelli, G. Production of stable food-grade microencapsulated astaxanthin by vibrating nozzle technology. Food Chem. 2017, 221, 289–295. [Google Scholar] [CrossRef]
- Bustos-Garza, C.; Yanez-Fernandez, J.; Barragan-Huerta, B.E. Thermal and pH stability of spray-dried encapsulated astaxanthin oleoresin from Haematococcus pluvialis using several encapsulation wall materials. Food Res. Int. 2013, 54, 641–649. [Google Scholar] [CrossRef]
- Bustamante, A.; Masson, L.; Velasco, J.; del Valle, J.M.; Robert, P. Microencapsulation of H. pluvialis oleoresins with different fatty acid composition: Kinetic stability of astaxanthin and alpha-tocopherol. Food Chem. 2016, 190, 1013–1021. [Google Scholar] [CrossRef]
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goycoolea, F.M.; Arguelles-Monal, W. Microencapsulation of astaxanthin in a chitosan matrix. Carbohyd. Polym. 2004, 56, 41–45. [Google Scholar] [CrossRef]
- Anarjan, N.; Tan, C.P. Chemical stability of astaxanthin nanodispersions in orange juice and skimmed milk as model food systems. Food Chem. 2013, 139, 527–531. [Google Scholar] [CrossRef]
- Liu, X.J.; McClements, D.J.; Cao, Y.; Xiao, H. Chemical and Physical Stability of Astaxanthin-Enriched Emulsion-Based Delivery Systems. Food Biophys. 2016, 11, 302–310. [Google Scholar] [CrossRef]
- Tamjidi, F.; Shahedi, M.; Varshosaz, J.; Nasirpour, A. Stability of astaxanthin-loaded nanostructured lipid carriers as affected by pH, ionic strength, heat treatment, simulated gastric juice and freeze-thawing. J. Food Sci. Technol. 2017, 54, 3132–3141. [Google Scholar] [CrossRef] [PubMed]
- Li, M.M.; Zahi, M.R.; Yuan, Q.P.; Tian, F.B.; Liang, H. Preparation and stability of astaxanthin solid lipid nanoparticles based on stearic acid. Eur. J. Lipid Sci. Technol. 2016, 118, 592–602. [Google Scholar] [CrossRef]
- Tachaprutinun, A.; Udomsup, T.; Luadthong, C.; Wanichwecharungruang, S. Preventing the thermal degradation of astaxanthin through nanoencapsulation. Int. J. Pharm. 2009, 374, 119–124. [Google Scholar] [CrossRef]
- Liu, C.Z.; Liu, Z.Z.; Sun, X.; Zhang, S.Z.; Wang, S.H.; Feng, F.X.; Wang, D.F.; Xu, Y. Fabrication and Characterization of beta-Lactoglobulin-Based Nanocomplexes Composed of Chitosan Oligosaccharides as Vehicles for Delivery of Astaxanthin. J. Agr. Food Chem. 2018, 66, 6717–6726. [Google Scholar] [CrossRef]
- Britton, G. Structure and properties of carotenoids in relation to function. FASEB J. 1995, 9, 1551–1558. [Google Scholar] [CrossRef]
- Yi, J.; Lam, T.I.; Yokoyama, W.; Cheng, L.W.; Zhong, F. Beta-carotene encapsulated in food protein nanoparticles reduces peroxyl radical oxidation in Caco-2 cells. Food Hydrocoll. 2015, 43, 31–40. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Graham, D.E.; Phillips, M.C. Proteins at liquid interfaces. II. Adsorption isotherms. J. Colloid Interf. Sci. 1979, 70, 415–426. [Google Scholar] [CrossRef]
- Graham, D.E.; Phillips, M.C. Proteins at liquid interfaces. III. Molecular structures of adsorbed films. J. Colloid Interf. Sci. 1979, 70, 427–439. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Z.; Sun, P.P.; Chen, T.P.; Chen, F. Microalgal carotenoids: Beneficial effects and potential in human health. Food Funct. 2014, 5, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Singh, H.; Sarkar, A. Behaviour of protein-stabilised emulsions under various physiological conditions. Adv. Colloid Interfac. 2011, 165, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Decker, E.A.; Xiao, H.; McClements, D.J. Nanoemulsion delivery systems: Influence of carrier oil on beta-carotene bioaccessibility. Food Chem. 2012, 135, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- de Bruijn, W.J.C.; Weesepoel, Y.; Vincken, J.P.; Gruppen, H. Fatty acids attached to all-trans-astaxanthin alter its cis-trans equilibrium, and consequently its stability, upon light-accelerated autoxidation. Food Chem. 2016, 194, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Anarjan, N.; Tan, C.P. Effects of Storage Temperature, Atmosphere and Light on Chemical Stability of Astaxanthin Nanodispersions. J. Am. Oil Chem. Soc. 2013, 90, 1223–1227. [Google Scholar] [CrossRef]
- Chen, H.Q.; Zhong, Q.X. Thermal and UV stability of beta-carotene dissolved in peppermint oil microemulsified by sunflower lecithin and Tween 20 blend. Food Chem. 2015, 174, 630–636. [Google Scholar] [CrossRef]
- Boon, C.S.; McClements, D.J.; Weiss, J.; Decker, E.A. Factors Influencing the Chemical Stability of Carotenoids in Foods. Crit. Rev. Food Sci. 2010, 50, 515–532. [Google Scholar] [CrossRef]
- Woodall, A.A.; Lee, S.W.M.; Weesie, R.J.; Jackson, M.J.; Britton, G. Oxidation of carotenoids by free radicals: Relationship between structure and reactivity. Biochim. Biophys. Acta Gen. Subj. 1997, 1336, 33–42. [Google Scholar] [CrossRef]
- Slater, T.F. Overview of methods used for detecting lipid peroxidation. Method Enzymol. 1984, 105, 283–293. [Google Scholar]
- Lim, A.S.L.; Burdikova, Z.; Sheehan, J.J.; Roos, Y.H. Carotenoid stability in high total solid spray dried emulsions with gum Arabic layered interface and trehalose-WPI composites as wall materials. Innov. Food Sci. Emerg. 2016, 34, 310–319. [Google Scholar] [CrossRef]
- Villalobos-Castillejos, F.; Cerezal-Mezquita, P.; Hernandez-De Jesus, M.L.; Barragan-Huerta, B.E. Production and stability of water-dispersible astaxanthin oleoresin from Phaffia rhodozyma. Int. J. Food Sci. Technol. 2013, 48, 1243–1251. [Google Scholar] [CrossRef]
- Takeungwongtrakul, S.; Benjakul, S. Astaxanthin degradation and lipid oxidation of Pacific white shrimp oil: Kinetics study and stability as affected by storage conditions. Int. Aquat. Res. 2016, 8, 15–27. [Google Scholar] [CrossRef]
- Wagner, L.A.; Warthesen, J.J. Stability of Spray-Dried Encapsulated Carrot Carotenes. J. Food Sci. 1995, 60, 1048–1053. [Google Scholar] [CrossRef]
- Kanasawud, P.; Crouzet, J.C. Mechanism of Formation of Volatile Compounds by Thermal-Degradation of Carotenoids in Aqueous-Medium. 1. Beta-Carotene Degradation. J. Agr. Food Chem. 1990, 38, 237–243. [Google Scholar] [CrossRef]
- Breithaupt, D.E.; Weller, P.; Wolters, M.; Hahn, A. Comparison of plasma responses in human subjects after the ingestion of 3R,3R′-zeaxanthin dipalmitate from wolfberry (Lycium barbarum) and non-esterified 3R,3R′-zeaxanthin using chiral high-performance liquid chromatography. Brit. J. Nutr. 2004, 91, 707–713. [Google Scholar] [CrossRef]
- Zhou, Q.X.; Xu, J.; Yang, L.; Gu, C.X.; Xue, C.H. Thermal stability and oral absorbability of astaxanthin esters from Haematococcus pluvialis in Balb/c mice. J. Sci. Food Agr. 2019, 99, 3662–3671. [Google Scholar] [CrossRef]
- Granado-Lorencio, F.; Blanco-Navarro, I.; Perez-Sacristan, B.; Hernandez-Alvarez, E. Biomarkers of carotenoid bioavailability. Food Res. Int. 2017, 99, 902–916. [Google Scholar] [CrossRef]
- Chitchumroonchokcai, C.; Failla, M.L. Hydrolysis of zeaxanthin esters by carboxyl ester lipase during digestion facilitates micellarization and uptake of the xanthophyll by Caco-2 human intestinal cells. J. Nutr. 2006, 136, 588–594. [Google Scholar] [CrossRef]
- Versantvoort, C.H.M.; Oomen, A.G.; Van de Kamp, E.; Rompelberg, C.J.M.; Sips, A.J.A.M. Applicability of an in vitro digestion model in assessing the bioaccessibility of mycotoxins from food. Food Chem. Toxicol. 2005, 43, 31–40. [Google Scholar] [CrossRef]
- Kopf-Bolanz, K.A.; Schwander, F.; Gijs, M.; Vergeres, G.; Portmann, R.; Egger, L. Validation of an In Vitro Digestive System for Studying Macronutrient Decomposition in Humans. J. Nutr. 2012, 142, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Rich, G.T.; Faulks, R.M.; Wickham, M.S.J.; Fillery-Travis, A. Solubilization of carotenoids from carrot juice and spinach in lipid phases: II. Modeling the duodenal environment. Lipids 2003, 38, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Nidhi, B.; Baskaran, V. Influence of Vegetable Oils on Micellization of Lutein in a Simulated Digestion Model. J. Am. Oil Chem. Soc. 2011, 88, 367–372. [Google Scholar] [CrossRef]
- Bowen, P.E.; Mobarhan, S.; Smith, J.C. Carotenoid Absorption in Humans. Methods Enzymol. 1993, 214, 3–17. [Google Scholar]
- Montero, P.; Calvo, M.M.; Gomez-Guillen, M.C.; Gomez-Estaca, J. Microcapsules containing astaxanthin from shrimp waste as potential food coloring and functional ingredient: Characterization, stability, and bioaccessibility. LWT Food Sci. Technol. 2016, 70, 229–236. [Google Scholar] [CrossRef]
- Sarada, R.; Vidhyavathi, R.; Usha, D.; Ravishankar, G.A. An efficient method for extraction of astaxanthin from green alga Haematococcus pluvialis. J. Agr. Food Chem. 2006, 54, 7585–7588. [Google Scholar] [CrossRef]
- Tang, C.H.; Wang, X.Y.; Yang, X.Q.; Li, L. Formation of soluble aggregates from insoluble commercial soy protein isolate by means of ultrasonic treatment and their gelling properties. J. Food Eng. 2009, 92, 432–437. [Google Scholar] [CrossRef]
- Reyes, F.A.; Mendiola, J.A.; Ibanez, E.; del Valle, J.M. Astaxanthin extraction from Haematococcus pluvialis using CO2-expanded ethanol. J. Supercrit. Fluids 2014, 92, 75–83. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Pan, X.Y.; Yao, P.; Jiang, M. Simultaneous nanoparticle formation and encapsulation driven by hydrophobic interaction of casein-graft-dextran and beta-carotene. J. Colloid Interf. Sci. 2007, 315, 456–463. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]




| Sample | ASX Concentration (mg/mL) | RSA (%) |
|---|---|---|
| H.p. oleoresin | 0.2 | 72.1 |
| NPs | 0.025 | 95.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanoni, F.; Vakarelova, M.; Zoccatelli, G. Development and Characterization of Astaxanthin-Containing Whey Protein-Based Nanoparticles. Mar. Drugs 2019, 17, 627. https://doi.org/10.3390/md17110627
Zanoni F, Vakarelova M, Zoccatelli G. Development and Characterization of Astaxanthin-Containing Whey Protein-Based Nanoparticles. Marine Drugs. 2019; 17(11):627. https://doi.org/10.3390/md17110627
Chicago/Turabian StyleZanoni, Francesca, Martina Vakarelova, and Gianni Zoccatelli. 2019. "Development and Characterization of Astaxanthin-Containing Whey Protein-Based Nanoparticles" Marine Drugs 17, no. 11: 627. https://doi.org/10.3390/md17110627
APA StyleZanoni, F., Vakarelova, M., & Zoccatelli, G. (2019). Development and Characterization of Astaxanthin-Containing Whey Protein-Based Nanoparticles. Marine Drugs, 17(11), 627. https://doi.org/10.3390/md17110627






