Antihypertensive and Angiotensin-I-Converting Enzyme (ACE)-Inhibitory Peptides from Fish as Potential Cardioprotective Compounds
Abstract
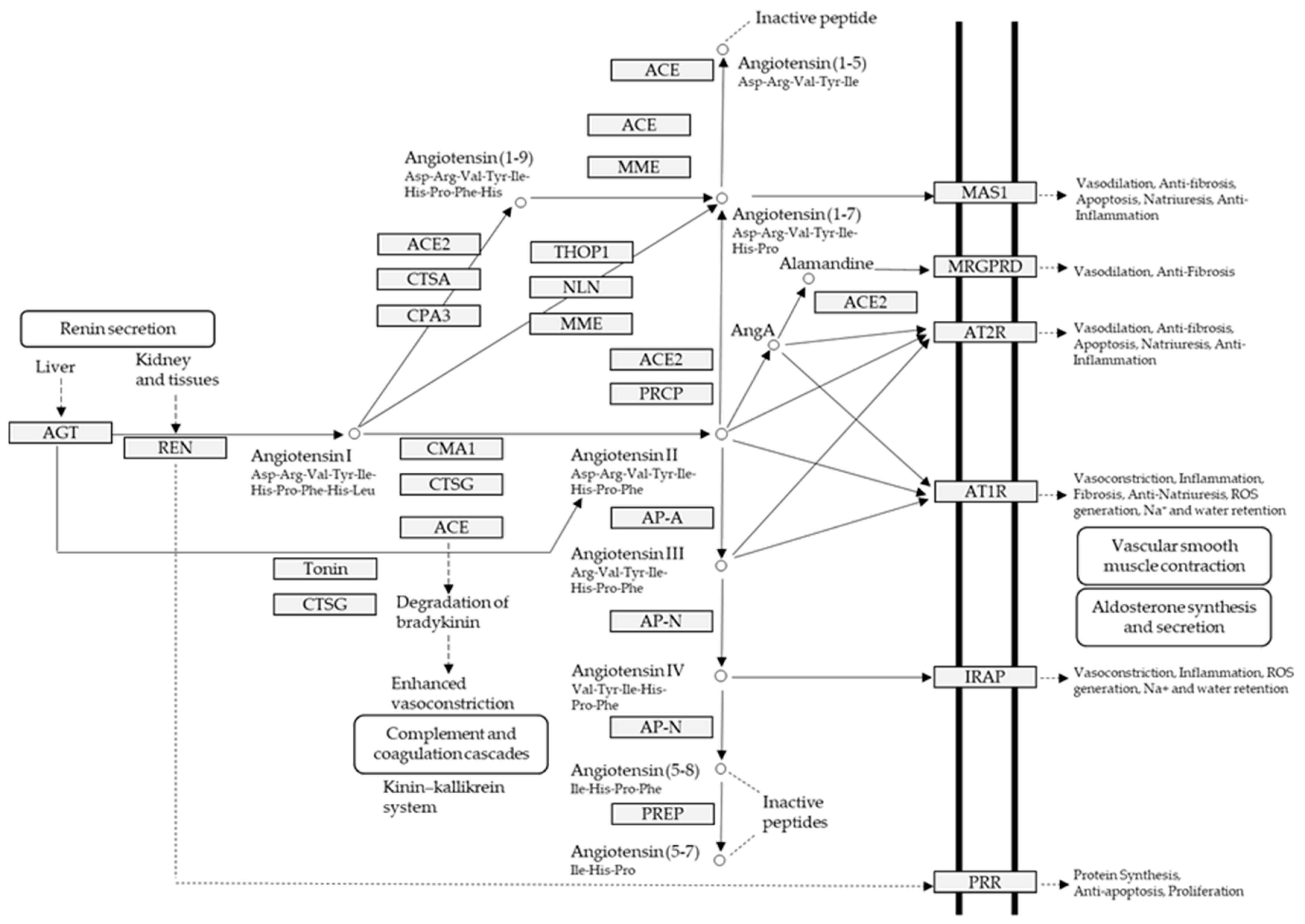
1. Introduction
2. Biological Activity of Fish, Fish By-Product Protein and Peptides on Hypertension
3. Impact of Enzymatic Digestion and Purification Processes on Antihypertension and ACE Inhibition Activity
3.1. Enzymatic Digestion
3.1.1. Effect of Enzymes on Different Peptide Bonds
3.1.2. Effect of Enzymes on the Separation of Blood Pressure Lowering Peptides
3.2. Isolation and Purification
Effect of Different Fractionation Processes on the Separation of Blood Pressure Lowering Peptides
4. Structure-Activity Relationship
5. Stability of ACE-Inhibiting Fish-Derived Peptides to Gastrointestinal Digestion
6. Conclusions and Possible Future Trends
Funding
Conflicts of Interest
References
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef]
- Isomaa, B.; Almgren, P.; Tuomi, T.; Forsén, B.; Lahti, K.; Nissén, M.; Taskinen, M.-R.; Groop, L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 2001, 24, 683–689. [Google Scholar] [CrossRef]
- Gomes, M.D.B. Impact of diabetes on cardiovascular disease: An update. Int. J. Hypertens. 2013, 2013, 653789. [Google Scholar]
- Kelli, H.M.; Kassas, I.; Lattouf, O.M. Cardio metabolic syndrome: A global epidemic. J. Diabetes Metab. 2015, 6, 2. [Google Scholar] [CrossRef]
- Deitel, M. Overweight and obesity worldwide now estimated to involve 1.7 billion people. Obes. Surg. 2003, 13, 329–330. [Google Scholar] [CrossRef] [PubMed]
- Shulman, G.I. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N. Engl. J. Med. 2014, 371, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Scholze, J.; Alegria, E.; Ferri, C.; Langham, S.; Stevens, W.; Jeffries, D.; Uhl-Hochgraeber, K. Epidemiological and economic burden of metabolic syndrome and its consequences in patients with hypertension in Germany, Spain and Italy; a prevalence-based model. BMC Public Health 2010, 10, 529. [Google Scholar] [CrossRef]
- Lind, L.; Berne, C.; Lithell, H. Prevalence of insulin resistance in essential hypertension. J. Hypertens. 1995, 13, 1457–1462. [Google Scholar] [CrossRef]
- Castro, J.P.; El-Atat, F.A.; McFarlane, S.I.; Aneja, A.; Sowers, J.R. Cardiometabolic syndrome: Pathophysiology and treatment. Curr. Hypertens. Rep. 2003, 5, 393–401. [Google Scholar] [CrossRef]
- Murray, C.J.; Lopez, A.D. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet 1997, 349, 1436–1442. [Google Scholar] [CrossRef]
- Giles, T.D.; Berk, B.C.; Black, H.R.; Cohn, J.N.; Kostis, J.B.; Izzo, J.L.; Weber, M.A. Expanding the definition and classification of hypertension. J. Clin. Hypertens. 2005, 7, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Angeli, F.; Reboldi, G.; Verdecchia, P. Hypertension, inflammation and atrial fibrillation. J. Hypertens. 2014, 32, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Bautista, L. Inflammation, endothelial dysfunction, and the risk of high blood pressure: Epidemiologic and biological evidence. J. Hum. Hypertens. 2003, 17, 223. [Google Scholar] [CrossRef] [PubMed]
- Chockalingam, A.; Campbell, N.R.; Fodor, J.G. Worldwide epidemic of hypertension. Can. J. Cardiol. 2006, 22, 553–555. [Google Scholar] [CrossRef]
- Harrison, D.G.; Guzik, T.J.; Lob, H.E.; Madhur, M.S.; Marvar, P.J.; Thabet, S.R.; Vinh, A.; Weyand, C.M. Inflammation, immunity, and hypertension. Hypertension 2011, 57, 132–140. [Google Scholar] [CrossRef]
- Berry, C.; Clark, A. Catabolism in chronic heart failure. Eur. Heart J. 2000, 21, 521–532. [Google Scholar] [CrossRef]
- Maksimowicz-McKinnon, K.; Bhatt, D.L.; Calabrese, L.H. Recent advances in vascular inflammation: C-reactive protein and other inflammatory biomarkers. Curr. Opin. Rheumatol. 2004, 16, 18–24. [Google Scholar] [CrossRef]
- Cardin, S.; Li, D.; Thorin-Trescases, N.; Leung, T.-K.; Thorin, E.; Nattel, S. Evolution of the atrial fibrillation substrate in experimental congestive heart failure: Angiotensin-dependent and-independent pathways. Cardiovasc. Res. 2003, 60, 315–325. [Google Scholar] [CrossRef]
- Nguyen, Q.; Dominguez, J.; Nguyen, L.; Gullapalli, N. Hypertension management: An update. Am. Health Drug Benefits 2010, 3, 47. [Google Scholar]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. JAMA J. Am. Med Assoc. 2003, 289, 2560–2571. [Google Scholar] [CrossRef]
- De Miguel, C.; Rudemiller, N.P.; Abais, J.M.; Mattson, D.L. Inflammation and hypertension: New understandings and potential therapeutic targets. Curr. Hypertens. Rep. 2015, 17, 507. [Google Scholar] [CrossRef] [PubMed]
- Drapala, A.; Sikora, M.; Ufnal, M. Statins, the renin–angiotensin–aldosterone system and hypertension–a tale of another beneficial effect of statins. J. Renin Angiotensin Aldosterone Syst. 2014, 15, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Hur, S.J. Antihypertensive peptides from animal products, marine organisms, and plants. Food Chem. 2017, 228, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Daskaya-Dikmen, C.; Yucetepe, A.; Karbancioglu-Guler, F.; Daskaya, H.; Ozcelik, B. Angiotensin-I-converting enzyme (ACE)-inhibitory peptides from plants. Nutrients 2017, 9, 316. [Google Scholar] [CrossRef]
- Norris, R.; FitzGerald, R.J. Antihypertensive peptides from food proteins. In Bioactive Food Peptides in Health and Disease; InTech Publishers: London, UK, 2013; pp. 45–72. [Google Scholar]
- Aluko, R.E. Antihypertensive peptides from food proteins. Annu. Rev. Food Sci. Technol. 2015, 6, 235–262. [Google Scholar] [CrossRef]
- Martínez-Maqueda, D.; Miralles, B.; Recio, I.; Hernández-Ledesma, B. Antihypertensive peptides from food proteins: A review. Food Funct. 2012, 3, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, J.; Malini, M.; Joshy, V.A. A critical review on food protein derived antihypertensive peptides. Drug Invent. Today 2019, 12, 474–479. [Google Scholar]
- Hernández-Ledesma, B.; del Mar Contreras, M.; Recio, I. Antihypertensive peptides: Production, bioavailability and incorporation into foods. Adv. Colloid Interface Sci. 2011, 165, 23–35. [Google Scholar] [CrossRef]
- Ono, S.; Hosokawa, M.; Miyashita, K.; Takahashi, K. Inhibition properties of dipeptides from salmon muscle hydrolysate on angiotensin I-converting enzyme. Int. J. Food Sci. Technol. 2006, 41, 383–386. [Google Scholar] [CrossRef]
- Ono, S.; Hosokawa, M.; Miyashita, K.; Takahashi, K. Isolation of Peptides with Angiotensin I-converting Enzyme Inhibitory Effect Derived from Hydrolysate of Upstream Chum Salmon Muscle. J. Food Sci. 2003, 68, 1611–1614. [Google Scholar] [CrossRef]
- Fang, L.; Geng, M.; Liu, C.; Wang, J.; Min, W.; Liu, J. Structural and molecular basis of angiotensin-converting enzyme by computational modeling: Insights into the mechanisms of different inhibitors. PLoS ONE 2019, 14, e0215609. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, M.; Jia, A.; Zhang, Y.; Zhu, H.; Zhang, C.; Sun, Z.; Liu, C. Purification and characterization of angiotensin I converting enzyme inhibitory peptides from jellyfish Rhopilema esculentum. Food Res. Int. 2013, 50, 339–343. [Google Scholar] [CrossRef]
- Wijesekara, I.; Kim, S.-K. Angiotensin-I-converting enzyme (ACE) inhibitors from marine resources: Prospects in the pharmaceutical industry. Mar. Drugs 2010, 8, 1080–1093. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kumar, A.; Sharma, R.; Baruwa, A. Pharmacological review on natural ACE inhibitors. Der. Pharm. Lett. 2010, 2, 273–293. [Google Scholar]
- Iwaniak, A.; Minkiewicz, P.; Darewicz, M. Food-originating ACE inhibitors, including antihypertensive peptides, as preventive food components in blood pressure reduction. Compr. Rev. Food Sci. Food Saf. 2014, 13, 114–134. [Google Scholar] [CrossRef]
- Attique, S.A.; Hassan, M.; Usman, M.; Atif, R.M.; Mahboob, S.; Al-Ghanim, K.A.; Bilal, M.; Nawaz, M.Z. A Molecular Docking Approach to Evaluate the Pharmacological Properties of Natural and Synthetic Treatment Candidates for Use against Hypertension. Int. J. Environ. Res. Public Health 2019, 16, 923. [Google Scholar] [CrossRef]
- Bhuyan, B.J.; Mugesh, G. Synthesis, characterization and antioxidant activity of angiotensin converting enzyme inhibitors. Org. Biomol. Chem. 2011, 9, 1356–1365. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Kang, K.-H.; Ryu, B.; Vo, T.-S.; Jung, W.-K.; Byun, H.-G.; Kim, S.-K. Angiotensin-I converting enzyme inhibitory peptides from antihypertensive skate (Okamejei kenojei) skin gelatin hydrolysate in spontaneously hypertensive rats. Food Chem. 2015, 174, 37–43. [Google Scholar] [CrossRef]
- Izzo, J.; Joseph, L.; Weir, M.R. Angiotensin-converting enzyme inhibitors. J. Clin. Hypertens. 2011, 13, 667–675. [Google Scholar] [CrossRef]
- Elliott, W.J.; Meyer, P.M. Incident diabetes in clinical trials of antihypertensive drugs: A network meta-analysis. Lancet 2007, 369, 201–207. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Y.; Xia, W.; Xiong, Y.L.; Ye, R.; Wang, H. Grass carp peptides hydrolysed by the combination of Alcalase and Neutrase: Angiotensin-I converting enzyme (ACE) inhibitory activity, antioxidant activities and physicochemical profiles. Int. J. Food Sci. Technol. 2016, 51, 499–508. [Google Scholar] [CrossRef]
- Wang, S.; Lin, L.-M.; Wu, Y.-N.; Fang, M.; Yu, Y.-Q.; Zhou, J.; Gong, Z.-Y. Angiotensin I Converting Enzyme (ACE) inhibitory activity and antihypertensive effects of grass carp peptides. Food Sci. Biotechnol. 2014, 23, 1661–1666. [Google Scholar] [CrossRef]
- Ahn, C.-B.; Jeon, Y.-J.; Kim, Y.-T.; Je, J.-Y. Angiotensin I converting enzyme (ACE) inhibitory peptides from salmon byproduct protein hydrolysate by Alcalase hydrolysis. Process. Biochem. 2012, 47, 2240–2245. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Zhong, Q.; Wu, Y.; Xia, W. Purification and characterization of a novel angiotensin-I converting enzyme (ACE) inhibitory peptide derived from enzymatic hydrolysate of grass carp protein. Peptides 2012, 33, 52–58. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Z.; Xu, S. Macroporous resin purification of grass carp fish (Ctenopharyngodon idella) scale peptides with in vitro angiotensin-I converting enzyme (ACE) inhibitory ability. Food Chem. 2009, 117, 387–392. [Google Scholar] [CrossRef]
- Borawska, J.; Darewicz, M.; Vegarud, G.; Iwaniak, A.; Minkiewicz, P. Ex vivo digestion of carp muscle tissue–ACE inhibitory and antioxidant activities of the obtained hydrolysates. Food Funct. 2015, 6, 210–217. [Google Scholar] [CrossRef]
- Nakajima, K.; Yoshie-Stark, Y.; Ogushi, M. Comparison of ACE inhibitory and DPPH radical scavenging activities of fish muscle hydrolysates. Food Chem. 2009, 114, 844–851. [Google Scholar] [CrossRef]
- Itou, K.; Nagahashi, R.; Saitou, M.; Akahane, Y. Antihypertensive effect of narezushi, a fermented mackerel product, on spontaneously hypertensive rats. Fish. Sci. 2007, 73, 1344–1352. [Google Scholar]
- Neves, A.C.; Harnedy, P.A.; O’Keeffe, M.B.; FitzGerald, R.J. Bioactive peptides from Atlantic salmon (Salmo salar) with angiotensin converting enzyme and dipeptidyl peptidase IV inhibitory, and antioxidant activities. Food Chem. 2017, 218, 396–405. [Google Scholar] [CrossRef]
- Enari, H.; Takahashi, Y.; Kawarasaki, M.; Tada, M.; Tatsuta, K. Identification of angiotensin I-converting enzyme inhibitory peptides derived from salmon muscle and their antihypertensive effect. Fish. Sci. 2008, 74, 911–920. [Google Scholar] [CrossRef]
- Kawasaki, T.; Seki, E.; Osajima, K.; Yoshida, M.; Asada, K.; Matsui, T.; Osajima, Y. Antihypertensive effect of valyl-tyrosine, a short chain peptide derived from sardine muscle hydrolyzate, on mild hypertensive subjects. J. Human Hypertens. 2000, 14, 519. [Google Scholar] [CrossRef]
- Toopcham, T.; Roytrakul, S.; Yongsawatdigul, J. Characterization and identification of angiotensin I-converting enzyme (ACE) inhibitory peptides derived from tilapia using Virgibacillus halodenitrificans SK1-3-7 proteinases. J. Funct. Foods 2015, 14, 435–444. [Google Scholar] [CrossRef]
- Ghassem, M.; Babji, A.S.; Said, M.; Mahmoodani, F.; Arihara, K. Angiotensin I–converting enzyme inhibitory peptides from snakehead fish sarcoplasmic protein hydrolysate. J. Food Biochem. 2014, 38, 140–149. [Google Scholar] [CrossRef]
- Wijesekara, I.; Qian, Z.-J.; Ryu, B.; Ngo, D.-H.; Kim, S.-K. Purification and identification of antihypertensive peptides from seaweed pipefish (Syngnathus schlegeli) muscle protein hydrolysate. Food Res. Int. 2011, 44, 703–707. [Google Scholar] [CrossRef]
- Fahmi, A.; Morimura, S.; Guo, H.-C.; Shigematsu, T.; Kida, K.; Uemura, Y. Production of angiotensin I converting enzyme inhibitory peptides from sea bream scales. Process. Biochem. 2004, 39, 1195–1200. [Google Scholar] [CrossRef]
- Lee, S.-H.; Qian, Z.-J.; Kim, S.-K. A novel angiotensin I converting enzyme inhibitory peptide from tuna frame protein hydrolysate and its antihypertensive effect in spontaneously hypertensive rats. Food Chem. 2010, 118, 96–102. [Google Scholar] [CrossRef]
- Matsui, T.; Matsufuji, H.; Seki, E.; Osajima, K.; Nakashima, M.; Osajima, Y. Inhibition of angiotensin I-converting enzyme by Bacillus licheniformis alkaline protease hydrolyzates derived from sardine muscle. Biosci. Biotechnol. Biochem. 1993, 57, 922–925. [Google Scholar] [CrossRef]
- Otani, L.; Ninomiya, T.; Murakami, M.; Osajima, K.; Kato, H.; Murakami, T. Sardine peptide with angiotensin I-converting enzyme inhibitory activity improves glucose tolerance in stroke-prone spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 2009, 73, 2203–2209. [Google Scholar] [CrossRef]
- Yokoyama, K.; Chiba, H.; Yoshikawa, M. Peptide inhibitors for angiotensin i-converting enzyme from thermolysin digest of dried bonitot. Biosci. Biotechnol. Biochem. 1992, 56, 1541–1545. [Google Scholar] [CrossRef]
- Ktari, N.; Nasri, R.; Mnafgui, K.; Hamden, K.; Belguith, O.; Boudaouara, T.; El Feki, A.; Nasri, M. Antioxidative and ACE inhibitory activities of protein hydrolysates from zebra blenny (Salaria basilisca) in alloxan-induced diabetic rats. Process. Biochem. 2014, 49, 890–897. [Google Scholar] [CrossRef]
- Lassoued, I.; Mora, L.; Nasri, R.; Aydi, M.; Toldrá, F.; Aristoy, M.-C.; Barkia, A.; Nasri, M. Characterization, antioxidative and ACE inhibitory properties of hydrolysates obtained from thornback ray (Raja clavata) muscle. J. Proteom. 2015, 128, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.-J.; Je, J.-Y.; Kim, S.-K. Antihypertensive effect of angiotensin I converting enzyme-inhibitory peptide from hydrolysates of bigeye tuna dark muscle, Thunnus obesus. J. Agric. Food Chem. 2007, 55, 8398–8403. [Google Scholar] [CrossRef]
- Jung, W.-K.; Mendis, E.; Je, J.-Y.; Park, P.-J.; Son, B.W.; Kim, H.C.; Choi, Y.K.; Kim, S.-K. Angiotensin I-converting enzyme inhibitory peptide from yellowfin sole (Limanda aspera) frame protein and its antihypertensive effect in spontaneously hypertensive rats. Food Chem. 2006, 94, 26–32. [Google Scholar] [CrossRef]
- Yang, P.; Jiang, Y.; Hong, P.; Cao, W. Angiotensin I converting enzyme inhibitory activity and antihypertensive effect in spontaneously hypertensive rats of cobia (Rachycentron canadum) head papain hydrolysate. Food Sci. Technol. Int. 2013, 19, 209–215. [Google Scholar] [CrossRef]
- Balti, R.; Bougatef, A.; Sila, A.; Guillochon, D.; Dhulster, P.; Nedjar-Arroume, N. Nine novel angiotensin I-converting enzyme (ACE) inhibitory peptides from cuttlefish (Sepia officinalis) muscle protein hydrolysates and antihypertensive effect of the potent active peptide in spontaneously hypertensive rats. Food Chem. 2015, 170, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.-H.; Ryu, B.; Kim, S.-K. Active peptides from skate (Okamejei kenojei) skin gelatin diminish angiotensin-I converting enzyme activity and intracellular free radical-mediated oxidation. Food Chem. 2014, 143, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Je, J.-Y.; Park, P.-J.; Kwon, J.Y.; Kim, S.-K. A novel angiotensin I converting enzyme inhibitory peptide from Alaska pollack (Theragra chalcogramma) frame protein hydrolysate. J. Agric. Food Chem. 2004, 52, 7842–7845. [Google Scholar] [CrossRef]
- Himaya, S.; Ngo, D.-H.; Ryu, B.; Kim, S.-K. An active peptide purified from gastrointestinal enzyme hydrolysate of Pacific cod skin gelatin attenuates angiotensin-1 converting enzyme (ACE) activity and cellular oxidative stress. Food Chem. 2012, 132, 1872–1882. [Google Scholar] [CrossRef]
- Khantaphant, S.; Benjakul, S.; Kishimura, H. Antioxidative and ACE inhibitory activities of protein hydrolysates from the muscle of brownstripe red snapper prepared using pyloric caeca and commercial proteases. Process. Biochem. 2011, 46, 318–327. [Google Scholar] [CrossRef]
- Raghavan, S.; Kristinsson, H.G. ACE-inhibitory activity of tilapia protein hydrolysates. Food Chem. 2009, 117, 582–588. [Google Scholar] [CrossRef]
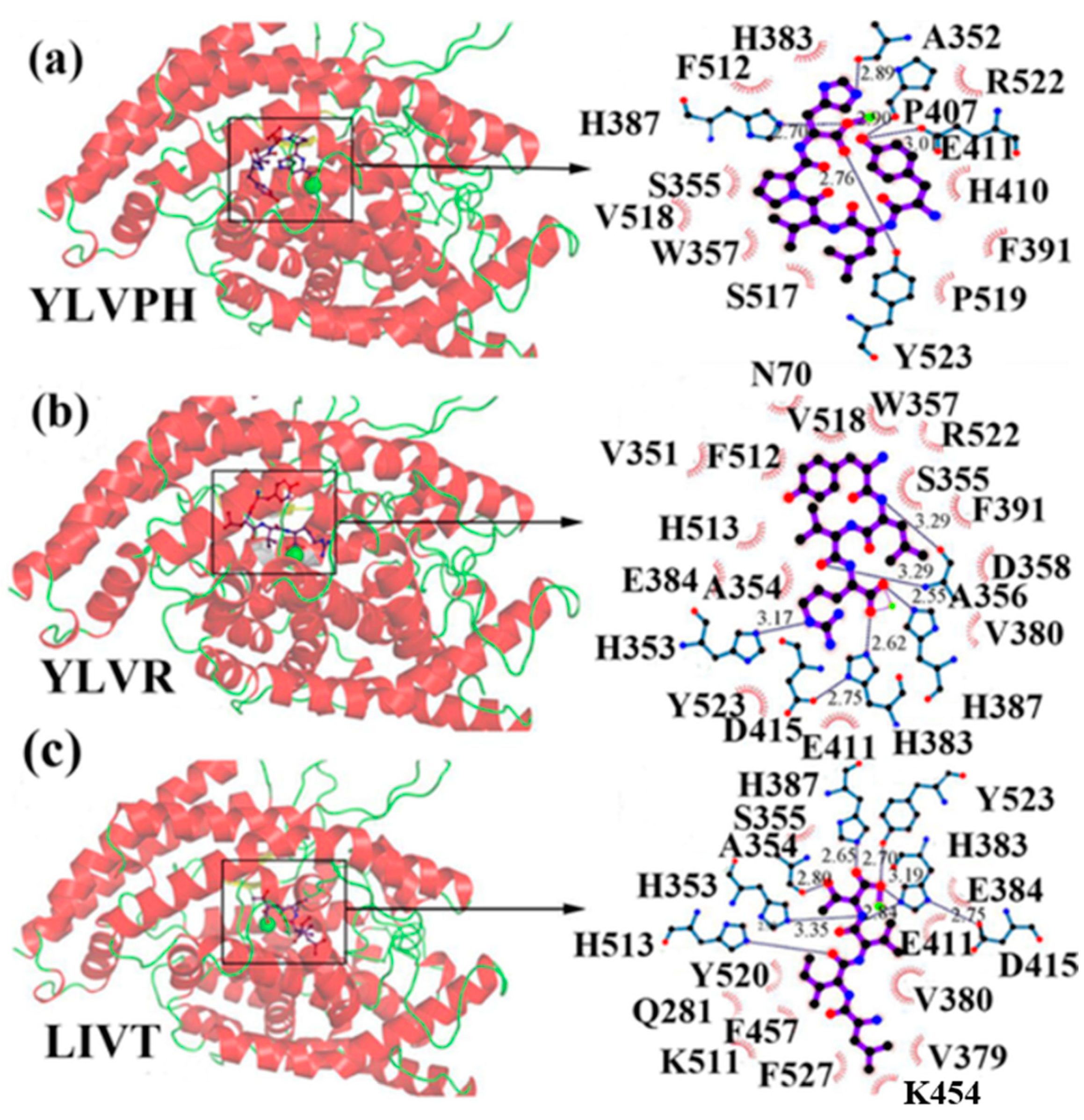
- Auwal, S.M.; Abidin, N.Z.; Zarei, M.; Tan, C.P.; Saari, N. Identification, structure-activity relationship and in silico molecular docking analyses of five novel angiotensin I-converting enzyme (ACE)-inhibitory peptides from stone fish (Actinopyga lecanora) hydrolysates. PLoS ONE 2019, 14, e0197644. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.-H.; Vo, T.-S.; Ryu, B.; Kim, S.-K. Angiotensin-I-converting enzyme (ACE) inhibitory peptides from Pacific cod skin gelatin using ultrafiltration membranes. Process. Biochem. 2016, 51, 1622–1628. [Google Scholar] [CrossRef]
- Erkkilä, A.T.; Schwab, U.S.; de Mello, V.D.; Lappalainen, T.; Mussalo, H.; Lehto, S.; Kemi, V.; Lamberg-Allardt, C.; Uusitupa, M.I. Effects of fatty and lean fish intake on blood pressure in subjects with coronary heart disease using multiple medications. Eur. J. Nutr. 2008, 47, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Yahia, D.A.; Madani, S.; Prost, J.; Bouchenak, M.; Belleville, J. Fish protein improves blood pressure but alters HDL 2 and HDL 3 composition and tissue lipoprotein lipase activities in spontaneously hypertensive rats. Eur. J. Nutr. 2005, 44, 10–17. [Google Scholar] [CrossRef]
- Ait-Yahia, D.; Madani, S.; Savelli, J.-L.; Prost, J.; Bouchenak, M.; Belleville, J. Dietary fish protein lowers blood pressure and alters tissue polyunsaturated fatty acid composition in spontaneously hypertensive rats. Nutrition 2003, 19, 342–346. [Google Scholar] [CrossRef]
- Fujita, H.; Yoshikawa, M. LKPNM: A prodrug-type ACE-inhibitory peptide derived from fish protein. Immunopharmacology 1999, 44, 123–127. [Google Scholar] [CrossRef]
- Xu, W.; Kong, B.-H.; Zhao, X.-H. Optimization of some conditions of Neutrase-catalyzed plastein reaction to mediate ACE-inhibitory activity in vitro of casein hydrolysate prepared by Neutrase. J. Food Sci. Technol. 2014, 51, 276–284. [Google Scholar] [CrossRef]
- Li, G.H.; Wan, J.Z.; Le, G.W.; Shi, Y.H. Novel angiotensin I-converting enzyme inhibitory peptides isolated from Alcalase hydrolysate of mung bean protein. J. Peptide Sci. Off. Publ. Eur. Pept. Soc. 2006, 12, 509–514. [Google Scholar] [CrossRef]
- Mahmoodani, F.; Ghassem, M.; Babji, A.S.; Yusop, S.M.; Khosrokhavar, R. ACE inhibitory activity of pangasius catfish (Pangasius sutchi) skin and bone gelatin hydrolysate. J. Food Science Technol. 2014, 51, 1847–1856. [Google Scholar] [CrossRef]
- Schwab, L.W.; Kloosterman, W.M.; Konieczny, J.; Loos, K. Papain catalyzed (co) oligomerization of α-amino acids. Polymers 2012, 4, 710–740. [Google Scholar] [CrossRef]
- Nchienzia, H.; Morawicki, R.; Gadang, V. Enzymatic hydrolysis of poultry meal with endo-and exopeptidases. Poult. Sci. 2010, 89, 2273–2280. [Google Scholar] [CrossRef] [PubMed]
- Karamać, M.; Kosińska-Cagnazzo, A.; Kulczyk, A. Use of different proteases to obtain flaxseed protein hydrolysates with antioxidant activity. Int. J. Mol. Sci. 2016, 17, 1027. [Google Scholar] [CrossRef] [PubMed]
- Kay, J. Specificity of Proteolysis; Wiley Online Library: Hoboken, NJ, USA, 1993. [Google Scholar]
- Li-Chan, E.C.; Hunag, S.-L.; Jao, C.-L.; Ho, K.-P.; Hsu, K.-C. Peptides derived from Atlantic salmon skin gelatin as dipeptidyl-peptidase IV inhibitors. J. Agric. Food Chem. 2012, 60, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Mullally, M.M.; O’Callaghan, D.M.; FitzGerald, R.J.; Donnelly, W.; Dalton, J.P. Proteolytic and peptidolytic activities in commercial pancreatic protease preparations and their relationship to some whey protein hydrolyzate characteristics. J. Agric. Food Chem. 1994, 42, 2973–2981. [Google Scholar] [CrossRef]
- Cushman, D.; Cheung, H. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem. Pharmacol. 1971, 20, 1637–1648. [Google Scholar] [CrossRef]
- Cheung, H.-S.; Wang, F.-L.; Ondetti, M.A.; Sabo, E.F.; Cushman, D.W. Binding of peptide substrates and inhibitors of angiotensin-converting enzyme. Importance of the COOH-terminal dipeptide sequence. J. Biol. Chem. 1980, 255, 401–407. [Google Scholar]
- Vitali, A. Proline-rich peptides: Multifunctional bioactive molecules as new potential therapeutic drugs. Curr. Protein Pept. Sci. 2015, 16, 147–162. [Google Scholar] [CrossRef]
- Morgan, A.A.; Rubenstein, E. Proline: The distribution, frequency, positioning, and common functional roles of proline and polyproline sequences in the human proteome. PLoS ONE 2013, 8, e53785. [Google Scholar] [CrossRef]
- De Luca, L.; Chiminazzo, A.; Sperni, L.; Strukul, G.; Scarso, A. Pyrrolidine-Containing Bisphosphonates as Potential Anti-Resorption Bone Drugs. Chem. A Eur. J. 2017, 23, 3474–3478. [Google Scholar] [CrossRef]
- Trapero, A.; Llebaria, A. A prospect for pyrrolidine iminosugars as antidiabetic α-glucosidase inhibitors. J. Med. Chem. 2012, 55, 10345–10346. [Google Scholar] [CrossRef]
- Reyes, A.A.; Porras, B.H.; Chasalow, F.I.; Klahr, S. L-arginine decreases the infiltration of the kidney by macrophages in obstructive nephropathy and puromycin-induced nephrosis. Kidney Int. 1994, 45, 1346–1354. [Google Scholar] [CrossRef] [PubMed]
- Zoja, C.; Benigni, A.; Camozzi, D.; Corna, D.; Longaretti, L.; Todeschini, M.; Remuzzi, G. Combining lisinopril and l-arginine slows disease progression and reduces endothelin-1 in passive Heymann nephritis. Kidney Int. 2003, 64, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Guang, C.; Phillips, R.D. Plant food-derived angiotensin I converting enzyme inhibitory peptides. J. Agric. Food Chem. 2009, 57, 5113–5120. [Google Scholar] [CrossRef]
- Liu, M.; Du, M.; Zhang, Y.; Xu, W.; Wang, C.; Wang, K.; Zhang, L. Purification and identification of an ACE inhibitory peptide from walnut protein. J. Agric. Food Chem. 2013, 61, 4097–4100. [Google Scholar] [CrossRef] [PubMed]
- Quirós, A.; del Mar Contreras, M.; Ramos, M.; Amigo, L.; Recio, I. Stability to gastrointestinal enzymes and structure–activity relationship of β-casein-peptides with antihypertensive properties. Peptides 2009, 30, 1848–1853. [Google Scholar] [CrossRef] [PubMed]
- Gevaert, B.; Veryser, L.; Verbeke, F.; Wynendaele, E.; De Spiegeleer, B. Fish hydrolysates: A regulatory perspective of bioactive peptides. Protein Pept. Lett. 2016, 23, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.; Tiwari, B. Bioactive carbohydrates and peptides in foods: An overview of sources, downstream processing steps and associated bioactivities. Int. J. Mol. Sci. 2015, 16, 22485–22508. [Google Scholar] [CrossRef]

| Organization | Criteria for Cardiometabolic Syndrome |
|---|---|
| NCEP-ATP III | Dyslipidemia Central obesity Systemic arterial hypertension and hyperglycemia |
| IDF, EGIR, AACE | Central obesity Insulin resistance |
| WHO | Diabetes mellitus Insulin resistance any of two risk factors (obesity, hypertension, high triglycerides, reduced HDL-C level, or micro-albuminuria) |
| Country | Approximate Mean Annual Cost (Euros, 2008) Per Individual | Total Annual Cost-of-Illness (Million Euros, 2008) | Forecasted Total Annual Cost-of-Illness (Million Euros, 2020) |
|---|---|---|---|
| Germany | 1750 | 24,427 | 38,955 |
| Spain | 1125 | 1900 | 5329 |
| Italy | 2000 | 4877 | 12,523 |
| Peptide Properties | ||||
|---|---|---|---|---|
| Sequence | Mass (Da) | Hydrophobicity (Kcal mol−1) | Sources Other than Fish | Lowest IC50 (μM) Reported |
| Dipeptides | ||||
| DP | 230.0900 | +11.68 | Marine | 2.15 |
| FL | 278.1626 | +4.94 | Plant | 1.33 |
| FY | 328.1419 | +5.48 | Animal, plant, marine, insect | 1.67 |
| GW | 261.1111 | +6.96 | Animal, plant | 30.00 |
| IF | 278.1626 | +5.07 | Animal, plant, insect | 1.67 |
| LF | 278.1626 | +4.94 | Animal, plant | 4.00 |
| LW | 317.1735 | +4.56 | Animal, plant, marine | 2.50 |
| PP | 212.1158 | +8.1 | Animal, plant | - |
| VF | 264.1470 | +5.73 | Animal, plant, marine, insect | 2.70 |
| VL | 230.1626 | +6.19 | Plant | 13.00 |
| VP | 214.1314 | +7.58 | Animal, plant | 420.00 |
| VW | 303.1579 | +5.35 | Animal, plant, marine | 1.10 |
| YP | 278.1263 | +7.33 | Animal, plant | 720.00 |
| Tripeptides | ||||
| AFL | 349.1996 | +5.44 | Marine | 63.80 |
| IVF | 377.2308 | +4.61 | Animal, plant | 33.11 |
| LVL | 343.2464 | +4.94 | Animal, plant | 12.30 |
| Properties | Values |
|---|---|
| Chemical Formula | C6787H10263N1825O1930S44 |
| Number of amino acids | 1306 |
| Total number of atoms | 20849 |
| Molecular weight | 149714.86 Da |
| Charge | Negative |
| Grand average of hydropathicity (GRAVY) | −0.398 |
| Total no. of negatively charged residues | (Asp + Glu): 147 |
| Total no. of positively charged residues | (Arg + Lys): 121 |
| Fish Name (Common, Scientific) and Part Used | Hydrolysis, Fractionation, Purification | Condition and Resin/Material | Characterization | References |
|---|---|---|---|---|
| Grass carp fresh whole | 1—enzymatic hydrolysis 2—ultrafiltration (UF) 3—column chromatography, two-steps Reversed-phase high-performance liquid chromatography (RP-HPLC) | 1—alcalase (degree of hydrolysis (DH): 17.25%) 2—molecular weight cut-off (MWCO) 10, 3 kDa 3—DA 201-C macroporous adsorption resin column, Jupiter and Luna C18 column | Automated protein/peptide sequencer, Dawn Eos multi-angle laser light scattering combined with gel permeation chromatography (GPC/MALLS), RP-HPLC (cation exchange resin column) | [43,45] |
| Grass carp (Ctenopharyngodon idellus) fresh scales | 1—enzymatic hydrolysis 2—column chromatography | 1—neutral protease AS1398 (DH: 16%) 2—DA 201-C macroporous adsorption resin | Amino acid analyzer, advanced protein purification system (TSK gel column) | [46] |
| Grass carp (Ctenopharyngodon idellus) fresh whole | 1—enzymatic hydrolysis 2—UF 3—desalting | 1—alcalase, neutrase 2—MWCO 10 kDa 3—mixed ion exchange resins (cation exchange resin (001 × 16, Na type) and anion exchange resin (D301-G, Cl type)) | HPLC with gel permeation chromatography (TSK gel column), HPLC (cation exchange column) | [42] |
| Carp (Cyprinus carpio) fresh muscle tissue | 1—ex-vivo digestion | 1—human gastric and duodenal enzymes | Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) | [47] |
| Atlantic salmon (Salmo salar), Coho salmon (Onchorhynchus kisutch), Alaska pollack (Theragra chalcogramma), southern blue whiting (Micromesistius australis) fresh muscle tissues | 1—enzymatic hydrolysis 2—UF 3—size exclusion chromatography | 1—pepsin, pancreatin, thermolysin (Bacillus thermoproteolyticus), 2—MWCO 5 kDa 3—Bio-Gel P-2 column | [48] | |
| Chub mackerel (Scomber japonicus) fresh whole fish followed by fermentation | 1—aqueous extraction 2—ion-exchange chromatography | 1—hot water 2—cation-exchange resin (TSK-Gel) | HPLC (COSMOSIL 5C18-AR column) | [49] |
| Salmon pectoral fin by-product | 1—enzymatic hydrolysis 2—ion-exchange chromatography, size exclusion chromatography, RP-HPLC | 1—alcalase, flavourzyme, neutrase, protamex, pepsin, trypsin (DH: 10%) 2—HiPrep 16/10 diethylaminoethyl (DEAE) fast flow (FF) ion-exchange column, Sephadex G-25 gel filtration column, octadecylsilyl (ODS) C18 column | Hybrid quadrupole- time-of-flight (TOF) LC/MS/MS mass spectrometer coupled with electrospray ionization (ESI) source | [44] |
| Chum salmon (Oncorhynchus keta) fresh muscle tissue | 1—enzymatic hydrolysis 2—column chromatography, size exclusion chromatography, RP-HPLC | 1—thermolysin 2—octadecyl silica gel column, Sephadex G-25 column, preparative ODS column | Protein sequencer after automated Edman degradation, amino acid analyzer, ESI-MS | [30,31] |
| Salmon (Salmo salar) trimmings | 1—enzymatic hydrolysis 2—semi-preparative RP-HPLC | 1—different preparations and 1, 2 and 4 h durations (alcalase 2.4 L (DH: 13.98–18.35%), alcalase 2.4 L + flavourzyme 500 L (DH: 15.15–16.02%), corlolase PP (DH: 21.83–24.34%), PROMOD 144MG (DH: 20.34–22.11%) 2—C18 semi-preparative column | SDS-PAGE, RP-HPLC, gel permeation HPLC, ultra-performance liquid chromatography (UPLC)-MS/MS (Peptide XB-C18 column) | [50] |
| Pink salmon (Oncorhynchus gorbuscha) muscle | 1—enzymatic hydrolysis 2—column chromatography, ion-exchange chromatography | 1—papain 2—silica gel, cation exchange resin (CG50-type 1) | Nuclear magnetic resonance (NMR), ESI-MS, LC/MS (XTerra MS C18 column), HPLC (C18 PA-A column) | [51] |
| Sardine muscle | 1—alkali solubilization 2—enzymatic hydrolysis 3—RP-HPLC | 1—20% NaOH 2—Bacillus licheniformis alkaline protease 3—YMC ODS-AQ column | [52] | |
| Tilapia (Oreochromis niloticus) fresh whole | 1—enzymatic hydrolysis 2—UF 3— fast protein liquid chromatography (FPLC), ion exchange chromatography, size exclusion chromatography | 1—Virgibacillus halodenitrificans SK1-3-7 proteinases (DH: 48%) 2—MWCO 30, 5 kDa 3—DEAE-Sephacel anion exchange column, carboxymethyl (CM)-Sepharose cation exchange column, Superdex peptide 10/300 GL size exclusion column | LC-MS/MS (LC ( (Acclaim PepMap 100 C18 nanocolumn) coupled to ESI-Ion Trap MS with electrospray) | [53] |
| Snakehead fish muscle | 1—enzymatic hydrolysis 2—gel chromatography, RP-HPLC | 1—flavorzyme, neutrase, proteinase k, papain, alcalase 2—polyacrylamide Bio-Gel P-2 column, C18 column | LC-ESI-MS (RP C18 PepMap 100 column in HPLC connected to micro-TOF-Q-MS) and Tandem MS analysis | [54] |
| Seaweed pipefish (Syngnathus schlegeli) fresh muscle | 1—enzymatic hydrolysis 2—FPLC, RP-HPLC | 1—papain, alcalase, neutrase, pronase, pepsin, trypsin 2—HiPrep 16/10 DEAE FF ion exchange column, Primesphere C18 column | Q-TOF-MS coupled with ESI | [55] |
| Sea bream fresh scales | 1—enzymatic hydrolysis 2—size exclusion chromatography, ion-exchange chromatography, RP-HPLC | 1—enzyme L 2—Sephadex LH-20 gel filtration column, UNO Q-1 column, Superdex Peptide HR 10/30 gel filtration column, Sephasil Peptide C18 column | Protein sequencer after automated Edman degradation | [56] |
| Tuna frame | 1—enzymatic hydrolysis 2—UF 3—ion-exchange chromatography, RP-HPLC | 1—alcalase, α-chymotrypsin, papain, pepsin, neutrase, trypsin 2—MWCO 10, 5, 1 kDa 3—Hiprep 16/10 DEAE FF anion exchange column, Primesphere 10 C18 column, Synchropak RPP-100 analytical column | Q-TOF-MS coupled with ESI | [57] |
| Sardine muscle | 1—enzymatic hydrolysis 2—RP-HPLC | 1—Bacillus licheniformis alkaline protease (different enzyme: Product ratio and 1, 17 and 24 h duration) 2—YMC ODS-AQ column | Asahipak GS-320 gel filtration chromatography | [58,59] |
| Bonito dehydrated whole | 1—enzymatic hydrolysis 2—HPLC | 1—pepsin, trypsin, chymotrypsin, thermolysin 2—YMC ODS-AQ column, phenyl silica column, cyanopropyl silica column, ODS C18 column | Protein sequencer | [60] |
| Zebra blenny (Salaria basilisca) fresh muscle | 1—enzymatic hydrolysis | 1—crude alkaline protease extracts extracted from homogenized viscera of zebra blenny, sardinella and smooth hound | [61] | |
| Thornback ray (Raja clavata) muscle | 1—enzymatic hydrolysis | 1—alcalase 2.4L (DH: 22%), neutrase 0.5L (DH: 11%), B. subtilis A26 (DH: 18%), R. clavata crude alkaline protease (DH: 15%) | RP-HPLC (symmetry C18 column), MALDI-TOF/TOF | [62] |
| Bigeye tuna dark muscle | 1—enzymatic hydrolysis 2—UF 3—ion-exchange chromatography, RP-HPLC | 1—alcalase (DH: 67.22%), α-chymotrypsin (DH: 77.71%), neutrase (DH: 80.93%), papain (DH: 74.45%), pepsin (DH: 78.72%), trypsin (DH: 46.28%) 2—MWCO 3 kDa 3—HiPrep 16/10 DEAE FF ion-exchange column, Primesphere ODS C18 column, Synchropak RPP-100 RP-HPLC analytical column | Q-TOF-MS coupled with ESI | [63] |
| Yellowfin sole (Limanda aspera) fresh frame | 1—enzymatic hydrolysis 2—UF 3—ion-exchange chromatography, gel permeation chromatography, RP-HPLC | 1—α-chymotrypsin 2—MWCO 30, 10, 5 kDa 3—SP-Sephadex C-25 ion-exchange column, OHpak SB-803 HQ gel permeation HPLC column, SP Nucleosil 100-7 C18 RP semi-prep column, Zorbax SB C18 RP analytical column | Gel permeation chromatography (OHpak SB-803 HQ gel permeation HPLC column) | [64] |
| Cobia (Rachycentron canadum) fresh head | 1—enzymatic hydrolysis 2—UF | 1—papain, 2—MWCO 8, 5, 3 kDa | High performance size exclusion chromatography (Protein-Pak 60 column) | [65] |
| Cuttlefish (Sepia officinalis) fresh muscle | 1—enzymatic hydrolysis 2—gel filtration column chromatography, RP-HPLC | 1—Bacillus mojavensis A21 proteases (DH: 16%), cuttlefish hepatopancreas proteases (DH: 8%) 2—Sephadex G-25 gel filtration column, Vydac C18 column | ESI–MS, MS/MS | [66] |
| Skate (Okamejei kenojei) fresh skin | 1—alkaline and aqueous gelatin extraction 2—enzymatic hydrolysis 3—UF 4—FPLC, gel filtration chromatography | 1—1% Ca(OH)2, water 2—alcalase, flavourzyme, protamex, neutrase, protease, α-chymotrypsin 3—MWCO 1 kDa 4—HiPrep 16/10 DEAE FF anion-exchange column, Superdex Peptide 10/300 GL gel filtration column | Q-TOF-MS coupled with ESI | [39,67] |
| Alaska Pollack (Theragra chalcogramma) frame | 1—enzymatic hydrolysis 2—UF 3—ion-exchange chromatography, size exclusion chromatography, RP-HPLC | 1—pepsin 2—MWCO 30, 10, 5, 3, 1 kDa 3—SP-Sephadex C-25 ion-exchange column, Sephadex G-25 gel filtration column, Capcell Pak C18 UG-120 column | Protein sequencer after automated Edman degradation, | [68] |
| Pacific cod (Gadus macrocephalus) fresh skin | 1—alkaline and aqueous gelatin extraction 2—enzymatic hydrolysis 3—FPLC, HPLC, RP-HPLC | 1—1% Ca(OH)2, water 2—gastrointestinal endopeptidases (pepsin, trypsin and α-chymotrypsin) 3—HiPrep 16/10 DEAE FF anion exchange column, Primesphere 10 C18 column, YMC-Pack Pro C18 column | Q-TOF-MS coupled with ESI | [69] |
| Brownstripe red snapper (Lutjanus vitta) fresh flesh | 1—enzymatic hydrolysis (one step and two steps) | 1—one step: Proteases from pyloric caeca of brownstripe red snapper (DH: 20–40%), flavourzyme (DH: 20–40%), alcalase (DH: 20–40%), two steps: Hydrolysates with 40% DH (durations 1, 2, 3, 5 h) | Amino acid analyzer | [70] |
| Tilapia (Oreochromis niloticus) fresh white muscle | 1—alkaline solubilization 2—enzymatic hydrolysis 3-UF | 1—2 N NaOH 2—flavourzyme (DH: 7.5–25%), cryotin-F (DH: 7.5–25%) 3—MWCO 30, 10 kDa | SDS–PAGE | [71] |
| Stone fish (Actinopyga lecanora) fresh whole (excluding internal organs) | 1—enzymatic hydrolysis 2—RP-HPLC, isoelectric focusing (IEF)- electrophoresis | 1—bromelain 2—semi preparative C18 column | Q-TOF LC/MS | [72] |
| Pacific cod (Gadus macrocephalus) fresh skin | 1—alkaline and aqueous gelatin extraction 2—enzymatic hydrolysis 3—UF 4—FPLC, gel filtration chromatography, RP-HPLC | 1—1% Ca(OH)2, water 2—pepsin, papain, α-chymotrypsin, trypsin, neutrase, alcalase 3—MWCO 10, 5, 1 kDa 4—HiPrep 16/10 DEAE FF anion exchange column, Superdex Peptide 10/300 GL gel filtration column | Q-TOF LC/MS/MS coupled with ESI | [73] |
| Fish Name | Anti-ACE and Antihypertensive Effects | Sequence and Molecular Mass | References |
|---|---|---|---|
| Grass carp | - ACE inhibition, hydrolysate; IC50 0.872 mg mL−1, < 3 kDa; IC50 0.308 mg mL−1, < 3 kDa fraction 6; IC50 0.00553 mg mL−1, F6-I; IC50 0.00534 mg mL−1, VAP; IC50 0.00538 mg mL−1 | F6-I rich in VAP 285 Da | [45] |
| - ACE inhibition, < 3 kDa; IC50 0.23 mg mL−1, - Antihypertension activity, single oral administration of 100 mg kg−1 BW ~30 mmHg maximal drop of systolic blood pressure (SBP) at 6 h in SHR dose/time dependent and non-significant effect in normotensive rats, long-term study, 100 mg kg−1 BW ~20 mmHg maximal drop of SBP at four weeks in SHR and non-significant effect in normotensive rats | Fraction < 3 kDa rich in Leu, Asp, Phe, Gly, Pro, hydrophobic amino acids (AAs) at C-terminal (44.15%), 1022–1228 Da (56.6%), 725–834 Da (43.3%) | [43] | |
| Grass carp (C. idellus) | - ACE inhibition, hydrolysate; IC50 1.66 mg mL−1, after ethanol gradient elution (v/v), 20% ETOH; IC50 0.48mg mL−1, 40% ETOH; IC50 0.39 mg mL−1, 60% ETOH; IC50 0.25mg mL−1, 80% ETOH; IC50 0.13 mg mL−1, % ACE inhibition at 2.5 mg mL−1 hydrolysate; ~70%, after ethanol gradient elution (v/v), 20% ETOH; ~75%, 40% ETOH; ~85%, 60% ETOH; ~90%, 80% ETOH; >95% dose-dependent effects | Hydrolysate and fractions rich in hydrophobic AAs (31–38%) and Pro, Gly | [46] |
| Grass carp (C. idellus) | - ACE inhibition, hydrolysate; IC50 0.692 mg mL−1, < 10 kDa; IC50 0.272 mg mL−1, desalted < 10 kDa; IC50 0.105 mg mL−1, - Antihypertensive activity, 100 mg kg−1 BW 43 mm Hg maximal SBP drop in SHR | Desalted < 10 kDa rich in < 1000 Da (190–500 Da (59.2%), 500–1000 Da (32.3%)) | [42] |
| Carp (C. carpio) | - ACE inhibition, two-hour gastric and one-hour duodenal digested fraction; IC50 1.90 mg mL−1, two-hour gastric digested fraction; IC50 9.26 mg mL−1 | [47] | |
| Atlantic salmon (S. salar), Coho salmon (O. kisutch), Alaska pollack (T. chalcogramma), southern blue whiting (M. australis) | - ACE inhibition, Atlantic salmon; IC50 7.91 mg mL−1, Coho salmon; IC50 4.66 mg mL−1, Alaska pollack; IC50 3.41 mg mL−1, southern blue whiting; IC50 3.62 mg mL−1 | Hydrolysates and fractions rich in anserine | [48] |
| Chub mackerel (S. japonicus) | - ACE inhibition, hot-water extract of raw mackerel; IC50 0.2 mg mL−1, hot-water extract of fermented mackerel; IC50 0.06 mg mL−1, - Antihypertension activity, administration of 10 mg kg−1 BW, 27 mm Hg maximal SBP drop in SHR at 4 h | [49] | |
| Salmon | - ACE inhibition, alcalase hydrolysate; IC50 0.356 mg mL−1, P1; IC50 0.00912 mg mL−1, non- competitive mode, P2; IC50 0.01372 mg mL−1, mixed mode, P4; IC50 0.00679 mg mL−1, mixed mode | P1 (VWDPPKFD ~ 1003 Da), P2 (FEDYVPLSCF ~ 1219 Da), P4 (FNVPLYE ~ 881 Da) | [44] |
| Chum salmon (O. keta) | - ACE inhibition, hydrolysate; IC50 0.0381 mg mL−1, 10% ethanol fraction O-2; IC50 of 0.0286 mg mL−1, positive fraction of gel filtration chromatography S-4; IC50 of 0.0215 mg mL−1, FL; IC50 of 13.6 μM, non-competitive inhibition mode MW; IC50 of 9.8 μM, non-competitive inhibition mode | Rich in FL 278 Da | [30,31] |
| Salmon (S. salar) | - ACE inhibition, protein extract; IC50 3.68 mg mL−1, alcalase hydrolysate; IC50 0.74 mg mL−1, alcalase + flavourzyme hydrolysate; IC50 0.94 mg mL−1, Corolase hydrolysate; IC50 0.97 mg mL−1, PROMOD hydrolysate; IC50 1.35 mg mL−1, FF; IC50 59.15 μM, F; IC50 0.15 μM, Y; IC50 132.84 μM, YP; IC50 5.21 μM | Two selected corolase fraction rich in GPAV, VP, VC, YP, FF, PP, DP, I/LD, I/LH, W, L/I, F, Y | [50] |
| Pink Salmon (O. gorbuscha) | - ACE inhibition, hydrolysate; IC50 79 µg mL−1, IW; IC50 0.38 µg mL−1, LW; IC50 2.2 µg mL−1, % ACE inhibition, 20 µg mL−1 hydrolysate; 26%, aqueous fraction; 12%, 1-butanol soluble fraction; 51%, % ACE inhibition of sub-fractions of butanol-soluble fraction; 20 µg mL−1 1st-silica gel column fractions (Fr.1-3); ≤ 71%, 6 µg mL−1 2nd-silica gel column fractions (Fr.2-2); ≤ 48%, - Antihypertensive activity, single oral administration of 30 mg kg−1 BW salmon peptide, 20 mm Hg maximal SBP drop at 2 h in SHR, long-term administration of 1.0 g kg−1 BW d−1 of the salmon peptide; 5 mm Hg maximal SBP drop at 4-weeks in SHR | Ion exchange column fraction (Fr.3-5) rich in di-peptides (VW, GW, MW, LW, WA, WM, FL, LF, VL, LI, IL, VF, IF, YF, FY) and tri-peptides (VIF, IVF, FVL, AFL, LVL, VIL, IVL, FIA, YLV, IVW) | [51] |
| Sardine | - Antihypertensive activity, administration of 3 mg VY 9.7- and 5.3-mm Hg systolic/diastolic blood pressure (SBP/DBP) drop at 1 week, 9.3- and 5.2-mm Hg SBP/DBP drop at four weeks | VY 280 Da | [52] |
| Tilapia (O. niloticus) | - % ACE inhibition, hydrolysate; 85%, > 30 kDa; 51%, 5–30 kDa; 65%, < 5 kDa; 88%, fractions of < 5 kDa; DEAE-Sephacel; 59%, CM-Sepharose; 38%, Superdex peptide 10/300 GL; 35%, crude hydrolysate; IC50 0.54 mg mL−1, Superdex peptide 10/300 GL-P-I; IC50 0.15 mg mL−1 non-competitive mode, MILLLFR; IC50 0.12 µM, LNLQDF; IC50 0.51 µM, LNLQDFR; IC50 0.85 µM | Superdex peptide 10/300 GL-P-I rich in MILLLFR 905.83 Da, LNLQDFR 905.66 Da, KHQDFF 821.41 Da, KPLLLMQLLLLFR 1526.64 Da, QNLLNYR 920.80 Da, QDLLFR 791.64 Da, NLGALLFR 903.49 Da, NELLLFR 904.60 Da, ELLGFV 677.49 Da, AHLLLL 679.49 Da | [53] |
| Snakehead fish | - ACE inhibition, crude sarcoplasmic protein; IC50 3.34 mg mL−1, hydrolysate of papain; IC50 0.182 mg mL−1, neutrase; IC50 0.152 mg mL−1, flavourzyme; IC50 0.149 mg mL−1, proteinase K; IC50 0.137 mg mL−1, alcalase; IC50 0.038 mg mL−1, post digestion LYPPP; IC50 1.3 μM, YSMYPP; IC50 2.8 μM | Alcalase hydrolysate rich in LYPPP 585 Da, YSMYPP 756 Da | [54] |
| Seaweed pipefish (S. schlegeli) | - % ACE inhibition, alcalase; 86%, - ACE inhibition, alcalase hydrolysate F3; IC50 0.068 mg mL−1, F3-II; IC50 0.62 mg mL−1, F3-III; IC50 1.44 mg mL−1 | Alcalase hydrolysate rich in F3-II; TFPHGP 744 Da, F3-III; HWTTQR 917 Da | [55] |
| Sea bream | - ACE inhibition, hydrolysate; IC50 0.57 mg mL−1, fractions III; IC50 0.096 mg mL−1, III-1; IC50 0.052 mg mL−1, III-2; IC50 0.080 mg mL−1, III-1C; IC50 0.027 mg mL−1, III-1C-A; IC50 0.063 mg mL−1, III-1C-B; IC50 0.005 mg mL−1, III-1C-C; IC50 0.16 mg mL−1, III-1C-D; IC50 0.003 mg mL−1, - Antihypertensive activity, administration of 300 mg kg−1 BW d−1 hydrolysate significantly lowered blood pressure in SHR | Fractions rich in III-1C-A; GY 238 Da, III-1C-B; VY 280 Da, III-1C-C; GF 222 Da, III-1C-D; VIY 393 Da | [56] |
| Tuna | - % ACE inhibition, 2 mg mL−1 pepsin hydrolysate (PH); 88.2%, 1 mg mL−1 PHI (MW <1 kDa); ≥80%, 1 mg mL−1 PHII (MW 1–5 kDa); 91.6%, 1 mg mL−1 PHIII (MW 5–10 kDa); 89.4%, 0.2 mg mL−1 PHII-F3; 76.4%, 0.1 mg mL−1 PHII-F3-3; 86.5%, - ACE inhibition, pure peptide of PHII-F3-3; IC50 11.28 μM, non-competitive inhibition mode - Antihypertensive activity, single oral administration of 10 mg kg−1 BW pure PHII-F3-3; 21 mmHg maximal SBP drop in SHR at 6 h | PHII-F3-3 rich in GDLGKTTTVSNWSPPKYKDTP 2482 Da | [57] |
| Sardine | - ACE inhibition, 1–2 wt%, 1 h; IC50 0.24 mg mL−1, 0.1–0.5 wt%, 24 h; IC50 0.25-0.26 mg mL−1, 0.3 wt%, 17 h; IC50 0.26 mg mL−1, A-I (0.3 wt%, 17 h and 1 wt%, 1 h); IC50 0.26 mg mL−1, A-I-Y1; IC50 1.222 mg mL−1, A-I-Y2; IC50 0.015 mg mL−1, A-I-Y3; IC50 0.078 mg mL−1, A-I-Y4; IC50 0.041 mg mL−1 | A-I (0.3 wt%, 17 h and 1 wt%, 1 h) in decreasing order rich in AAs Glu > Asp > Lys > Leu 250–1000 Da | [58] |
| - ACE inhibition, Hydrolysate; IC50 62.4 mg mL−1, - Antihypertensive activity, single oral administration of 1 g kg−1 BW d−1; 13 mmHg maximal SBP drop in stroke-prone SHR at four weeks | [59] | ||
| Bonito | - ACE inhibition, thermolysin; IC50 29 µg mL−1, trypsin; IC50 161 µg mL−1, pepsin; IC50 47 µg mL−1, chymotrypsin; IC50 117 µg mL−1, trypsin/chymotrypsin; IC50 175 µg mL−1, further digested peptic hydrolysate; trypsin; IC50 65 µg mL−1, chymotrypsin; IC50 41 µg mL−1, trypsin/chymotrypsin; IC50 38 µg mL−1, further digested thermolysin hydrolysate; pepsin; IC50 22 µg mL−1, pepsin → trypsin; IC50 26 µg mL−1, pepsin → chymotrypsin; IC50 29 µg mL−1, pepsin → trypsin/chymotrypsin; 26 IC50 of µg mL−1, IY; 3.7 μM, IWHHT; 5.1 μM, IVGRPRHQG; 6.2 μM, ALPHA; 10 μM, FQP; 12 μM, LKPNM; 17 μM, IKPLNY; 43 μM, DYGLYP; 62 mΜ - Antihypertensive activity, administration of 10 mg kg−1 BW of IKP/IVGRPRHQG significantly inhibited the elevation of blood pressure in normotensive rat | Thermolysin hydrolysate rich in; IKPLNY 746 Da, IVGRPRHQG 1019 Da, IWHHT 692 Da, ALPHA 507 Da, LKPNM 601 Da, IY 294 Da, FQP 390 Da, DYGLYP 726 Da | [60] |
| Zebra blenny (S. basilisca) | - ACE inhibition, hydrolysate of zebra blenny protease; IC50 93.6 µg mL−1, smooth hound protease; IC50 130 µg mL−1, sardinella protease; IC50 182 µg mL−1 dose-dependent inhibition, - in-vivo % ACE inhibition in serum of surviving diabetic rats; zebra blenny protease; 52.8%, smooth hound protease; 53%, sardinella protease; 56%, - Antihypertensive activity, adverse effect on diabetic (alloxan-induced) rats | [61] | |
| Thornback ray (R. clavata) | - % ACE inhibition at 5 mg mL−1, crude muscle protein; 15%, hydrolysate of alcalase; 84%, neutrase; 87%, B. subtilis A26; 87%, R. clavata crude alkaline protease; 51% | Crude mix and hydrolysates rich in Glu, Gly, Pro | [62] |
| Bigeye tuna | - ACE inhibition, purified PIII-2; IC50 21.6 µM, non-competitive inhibition mode, - % ACE inhibition at 2 mg mL−1, hydrolysate of alcalase; 48%, α-chymotrypsin; 57%, neutrase; 64%, papain; 20%, pepsin; 81%, trypsin; 36%, pepsin hydrolysate fraction PIII; 78%, PIII-2; ~80%, - Antihypertensive activity, oral administration of 10 mg kg−1 BW ~17 mm Hg maximal SBP drop in SHR at 3 h and 6 h | PIII-2 rich in WPEAAELMMEVDP 1581 Da | [63] |
| Yellowfin sole (L. aspera) | - % ACE inhibition; 30–10 kDa; 47.6%, 10–5 kDa; 34.5%, <5 kDa; 68.8%, - ACE inhibition, <5 kDa; IC50 0.883 mg mL−1 (22.3 μM), fractions of < 5 kDa; cation exchange chromatography; IC50 0.210 mg mL−1, gel permeation chromatography; IC50 0.093 mg mL−1, 1st RP-HPLC; IC50 0.056 mg mL−1, 2nd RP-HPLC; IC50 0.029 mg mL−1, undigested protein; significant ACE inhibition at 250 µM, 500 mM, non-competitively, - Antihypertensive activity, single oral administration of 10 mg kg−1 BW 22 mmHg maximal SBP drop in SHR at 3 h | Undigested protein rich in MIFPGAGGPEL 1.2 kDa and <5 kDa fraction rich in hydrophobic AAs | [64] |
| Cobia (R. canadum) | - ACE inhibition, hydrolysate; IC50 0.57 mg mL−1, >8 kDa; IC50 1.06 mg mL−1, 8–5 kDa; IC50 0.73 mg mL−1, 5–3 kDa; IC50 0.36 mg mL−1, <3 kDa; IC50 0.24 mg mL−1, - Antihypertensive activity, oral administration of 150, 600, 1200 mg kg−1 BW significantly lowered SBP in SHR dose-dependently at 2–8 h, 1200 mg kg−1 BW, 57 mmHg maximal SBP drop in SHR at 4 h | <3 kDa fraction; 67% (1749–173 Da), 11% (2831–1747 Da), 16% (7875–2831 Da) | [65] |
| Cuttlefish (S. officinalis) | - ACE inhibition; A21 proteases hydrolysate; IC50 1.12 mg mL−1, Cuttlefish proteases hydrolysate; IC50 1.19 mg mL−1, Peptides of A21 proteases hydrolysate: SFHPYFSY; IC50 82.71 μM AFVGYVLP; IC50 18.02 μM KNGDGY; IC50 51.63 μM STHGVW; IC50 19.30 μM RSIKGF; IC50 32.74 μM GS; IC50 1156.3 μM Peptides of cuttlefish proteases hydrolysate: GIHETTY; IC50 25.66 μM EKSYELP; IC50 14.41 μM VELYP; IC50 5.22 μM - Antihypertensive activity, oral administration of VELYP 10 mg kg−1 BW d−1 decreased SBP and DBP at 2–8 h post-administration non-cytotoxically (20 mmHg maximal SBP drop) in SHR | Hydrolysates rich in SFHPYFSY 1047.1 Da, AFVGYVLP 865.4 Da, KNGDGY 653.2 Da, STHGVW 663.1 Da, RSIKGF 665.0 Da, GS 163.0 Da, GIHETTY 820.3 Da, EKSYELP 865.1 Da, VELYP 620.1 Da | [66] |
| Skate (O. kenojei) | - % ACE inhibition, 2 mg mL−1 Alcalase gelatin hydrolysate; 72.8%, 1 mg mL−1 alcalase/protease gelatin hydrolysate < 1 kDa (SAP); 86%, 100 µg mL−1 SAP-I; 73%, 100 µg mL−1 SAP-I3; 85% MVGSAPGVL; IC50 3.09 μM, LGPLGHQ; IC50 4.22 μM | SAP-I3 rich in MVGSAPGVL 829 Da, LGPLGHQ 720 Da | [67] |
| - Antihypertensive activity, oral administration of 1 g SAP kg−1 BW d−1, 20 days, 127.2 mmHg maximal SBP drop, 77.6 mmHg maximal DBP drop, 94.2 mmHg maximal mean blood pressure drop at day 20, in SHR | SAP rich in Lys 31% > Gly 16% > Glu 7%, SAPI-3 rich in | [39] | |
| Alaska Pollack (T. chalcogramma) | - ACE inhibition <1 kDa; IC50 0.457 mg mL−1 SP-Sephadex C-25; IC50 0.11 mg mL−1 Sephadex G-25; IC50 0.066 mg mL−1 1st RP-HPLC; IC50 0.023 mg mL−1 2nd RP-HPLC; IC50 0.013 mg mL−1 FGASTRGA; IC50 14.7 μM | 2nd RP-HPLC fraction rich in FGASTRGA 765 Da | [68] |
| Pacific cod (G. macrocephalus) | - ACE inhibition 100 µg mL−1 Hydrolysate; 60.40%, FPLC; ≤71.81%, HPLC; ≤77.85%, LLMLDNDLPP; IC50 35.7 μM | HPLC fraction rich in LLMLDNDLPP 1301 Da | [69] |
| Brownstripe red snapper (Lutjanus vitta) | - % ACE inhibition ~ ≤33% | Hydrolysates rich in hydrophobic AAs ~ 44% > charged AAs ~ 43% > polar AAs 13% | [70] |
| Tilapia (Oreochromis niloticus) | - % ACE inhibition, 0.2% w/v protein cryotin hydrolysates 62–71%, flavourzyme hydrolysates 66–73% | MW range 3.5–110 kDa | [71] |
| Stone fish (Actinopyga lecanora) | - % ACE inhibition, RP-HPLC fractions; 43.50%, IEF electrophoresis sub-fractions; 69.21%, ALGPQFY; IC50 0.012 mM, KVPPKA; IC50 0.980 mM, LAPPTM; IC50 1.310 mM, EVLIQ; IC50 1.440 mM, EHPVL; IC50 1.680 mM | ALGPQFY 794.44 Da, KVPPKA 638.88 Da, LAPPTM 628.85 Da, EVLIQ 600.77 Da, EHPVL 593.74 Da | [72] |
| Pacific cod (Gadus macrocephalus) | - % ACE inhibition, 1 mg mL−1 pepsin hydrolysate; 91%, 500 µg mL−1 pepsin hydrolysate < 1 kDa; 70%, 250 µg mL−1 FPLC fraction of < 1 kDa (F1); 81%, 100 µg mL−1 gel column fraction of F1; 96%, GASSGMPG; IC50 6.9 μM, LAYA; IC50 14.5 μM | GASSGMPG 662 Da, LAYA 436 Da | [73] |
| Lean (white) fish | - Antihypertensive activity, 4 meals/week lean fish, 8-week controlled study, 5 mm Hg maximal SBP drop (3.5 ± 3.2% decreased), 4 mm Hg maximal DBP drop (4.6 ± 3.6% decreased) in coronary heart disease patients using multiple medications | [74] | |
| Purified fish protein (salmon, tuna, cod, mix of white fishes) (94% protein) | - Antihypertensive activity, 20.4 g kg−1 BW d−1 (20% protein diet), 2-months controlled study, 30.9 mm Hg maximal SBP drop (14% decrease compare to casein-diet) in SHR at 4, 6, 7 wk up to 2-months | Diet rich in Glu 13% > Gly 12.3% > Asp 8% > Lys 7% > Ala 6.9% > Arg 6.5% | [75,76] |
| Bonito, LKP from muscle | - ACE inhibition, LKPNM; IC50 2.4 μM, LKP; IC50 0.3 μM, - Antihypertensive activity, intravenous injection of 100 μg kg−1 of LKPNM; 30 mm Hg maximal SBP drop, 30 μg kg−1 of LKP; 50 mm Hg maximal SBP drop dose-dependently in SHR oral administration of 10.5 mg kg−1 LKPNM at 4 h and 4.2 mg kg−1 LKP at 2 h; 10 mm Hg maximal SBP drop in SHR | [77] |
| Peptide’s Primary Structure | Peptide Sequence IC50 Mass (Da) Isoelectric point Net Charge and Hydrophobicity (Kcal mol−1) | Peptide’s Primary Structure | Peptide Sequence IC50 Mass (Da) Isoelectric Point Net Charge and Hydrophobicity (Kcal mol−1) |
|---|---|---|---|
| Penta-peptides | |||
 | ALPHA 10 μM [60] 507.2798 7.95 0 +10.12 | 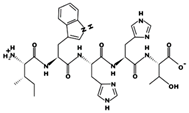 | IWHHT 5.1 μM [60] 692.3386 8.05 0 +9.60 |
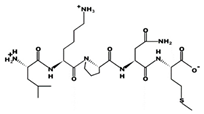 | LKPNM 17 μM [60] 601.3248 10.14 +1 +9.77 | 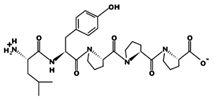 | LYPPP 1.3 μM [54] 585.3153 5.22 0 +6.36 |
 | VELYP 5.22 μM [66] 619.3207 3.01 −1 +9.25 |  | EHPVL 1.680 mM [72] 593.3164 5.06 −1 +12.29 |
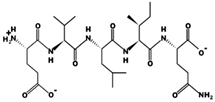 | EVLIQ 1.440 mM [72] 600.3472 3.12 −1 +9.47 | ||
| Hexa-peptides | |||
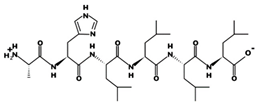 | AHLLLL - 678.4416 7.95 0 +5.73 | 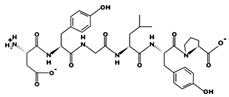 | DYGLYP 62 mΜ [60] 349.1633 5.48 0 +7.83 |
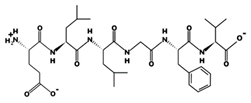 | ELLGFV - 676.3784 3.23 −1 +8.01 | 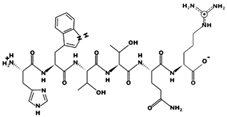 | HWTTQR 1.74 mM [55] 827.4028 10.91 +1 +11.22 |
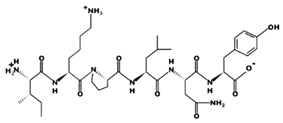 | IKPLNY 43 μM [60] 746.4314 9.79 +1 +8.61 |  | KHQDFF - 820.3857 7.55 0 +14.02 |
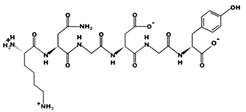 | KNGDGY 51.63 μM [66] 652.2808 6.42 0 +16.78 | 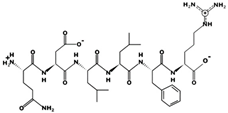 | QDLLFR - 790.4325 6.48 0 +9.91 |
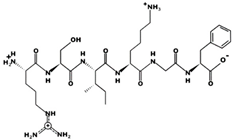 | RSIKGF 32.74 μM [66] 706.4115 11.52 +2 +11.29 | 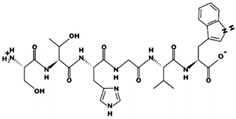 | STHGVW 19.30 μM [66] 685.3175 7.63 0 +9.54 |
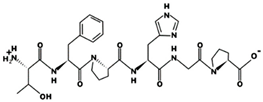 | TFPHGP 947.56 μM [55] 654.3117 7.57 0 +10.20 | 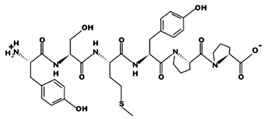 | YSMYPP 2.8 μM [54] 756.3142 5.17 0 +6.55 |
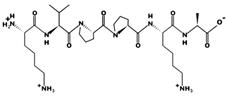 | KVPPKA 0.980 mM [72] 638.4104 10.57 +2 +13.82 | 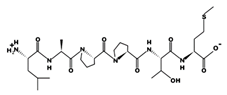 | LAPPTM 1.310 mM [72] 628.3244 5.40 0 +7.01 |
| Hepta-peptides | |||
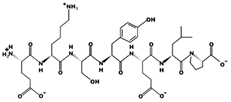 | EKSYELP 14.41 μM [66] 864.4215 4.08 −1 +16.60 | 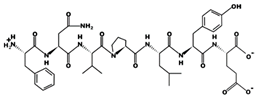 | FNVPLYE 7.71 μM [44] 880.4317 3.09 −1 +8.39 |
 | GIHETTY 25.66 μM [66] 819.3751 5.06 −1 +13.68 | 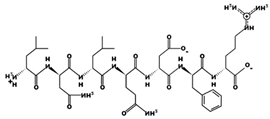 | LNLQDFR 0.85 µM [53] 904.4753 6.74 0 +10.76 |
 | MILLLFR 0.12 µM 904.5552 [53] 10.88 +1 +2.46 | 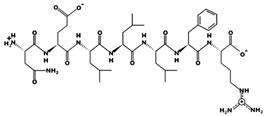 | NELLLFR - 903.5163 6.38 0 +8.73 |
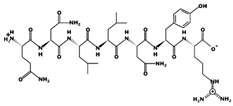 | QNLLNYR - 919.4862 9.60 +1 +8.97 |  | LGPLGHQ 4.22 μM [67] 720.3908 7.89 0 +10.94 |
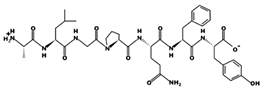 | ALGPQFY 0.012 mM [72] 794.3951 5.46 0 +6.79 | ||
| Octa-peptides | |||
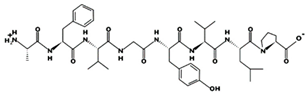 | AFVGYVLP 18.02 μM [66] 864.4731 5.23 0 +5.10 | 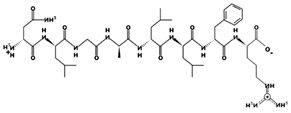 | NLGALLFR - 902.5323 10.60 +1 +6.75 |
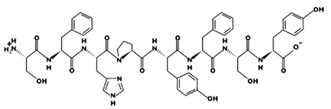 | SFHPYFSY 82.71 μM [66] 1046.4484 7.57 0 +6.45 | 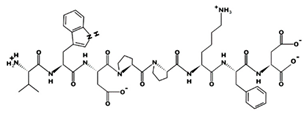 | VWDPPKFD 9.1 μM [44] 1002.4796 3.91 −1 +14.00 |
 | FGASTRGA 14.7 μM [68] 765.3759 10.90 +1 +12.01 | 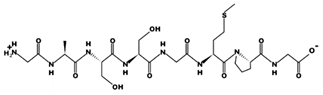 | GASSGMPG 6.9 μM [73] 662.2685 5.60 0 +12.24 |
| Nona-peptides | |||
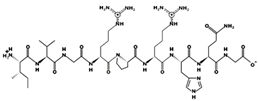 | IVGRPRHQG 6.2 μM [60] 1018.5770 12.49 +2 +15.48 | 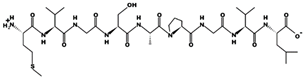 | MVGSAPGVL 3.09 μM [67] 829.4354 5.51 0 +8.46 |
| Deca-peptides | |||
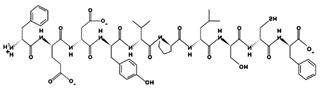 | FEDYVPLSCF 11.26 μM [44] 1218.5249 2.92 −2 +9.91 | 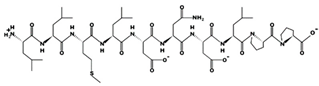 | LLMLDNDLPP 35.7 μM [69] 1139.5877 2.76 −2 +10.64 |
| Synthesized Peptide | Fragment of the Synthesized Peptide | |
|---|---|---|
| IC50 (µM) | Activity Improved By | |
| IVGRPRHQG | VGRPRHQG | |
| 6.2 | 5.4 | 13% |
| IWHHTF | IW | |
| 2.5 | 2.0 | 20% |
| IKPLNY | IKP | |
| 43 | 1.7 | 96% |
| LKPNM | LKP | |
| 17 | 1.6 | 90.5% |
| DYGLYP | LYP | |
| 62 | 6.6 | 89% |
| Sequence | Not pre-Incubated with ACE IC50 (µM) | Pre-Incubated with ACE IC50 (µM) | Decrease of SBP in SHR (60 mg kg−1 BW) (max, Δmm Hg) | Treatment Time (h) |
|---|---|---|---|---|
| Inhibitor-type | ||||
| IY | 2.31 | 1.9 | 19 | 2 |
| IKP | 6.9 | 3.4 | 20 | 6 |
| LKP | 0.32 | 0.32 | 18 | 4 |
| IWH | 3.5 | 3.5 | 30 | 4 |
| Prodrug-type | ||||
| LKPNM | 2.4 | 0.76 | 23 | 6 |
| IWHHT | 5.8 | 3.5 | 26 | 6 |
| Lipinski’s rule of five | ||||||
|---|---|---|---|---|---|---|
| Molecular Weight (g mol−1) | Lipophilicity (MLog P) | Hydrogen Bond Donors | Hydrogen Bond Acceptors | No. of Rule Violations | Drug-Likeness | |
| Sequence | Less than 500 Dalton | Less than 5 | Less than 5 | Less than 10 | Less than two violations | Follows Lipinski’s rule |
| Tri-peptides | ||||||
| AFL, FIA | ~349 | 0.85 | 4 | 5 | 0 | Yes |
| FQP | 390.43 | −0.55 | 5 | 6 | 0 | Yes |
| IVW | 416.51 | 0.68 | 5 | 5 | 0 | Yes |
| LVL, VIL, IVL | ~343 | 0.87 | 4 | 5 | 0 | Yes |
| YLV, VIY | 393.48 | 0.79 | 5 | 6 | 0 | Yes |
| VAP | 285.34 | −0.53 | 4 | 5 | 0 | Yes |
| VIF, IVF, FVL | ~377 | 1.31 | 4 | 5 | 0 | Yes |
| Tetra-peptides | ||||||
| GPAV | 342.39 | −1.26 | 4 | 6 | 0 | Yes |
| LAYA | 436.5 | −0.21 | 6 | 7 | 0 | Yes |
| Penta-peptides | ||||||
| ALPHA | 507.58 | −1.93 | 6 | 8 | 2 | No |
| IWHHT | 692.77 | −2.76 | 10 | 10 | 3 | No |
| LKPNM | 601.76 | −1.93 | 7 | 9 | 2 | No |
| LYPPP | 585.69 | 0.06 | 5 | 8 | 2 | No |
| VELYP | 619.71 | −0.7 | 8 | 10 | 3 | No |
| EHPVL | 593.67 | −1.85 | 7 | 10 | 3 | No |
| EVLIQ | 600.7 | −1.34 | 8 | 10 | 3 | No |
| Fish Name | Peptide Sequence | Product | Producer | Type of Product |
|---|---|---|---|---|
| Bonito (Sarda orientalis) | LKPNM | PeptACE™, Vasotensin®, Levenorm®, Peptide ACE 3000, Peptide Tea, Katsuobushi | Natural factors nutritional products Ltd., Canada, Metagenics, USA, Ocean nutrition Canada Ltd., Canada, Nippon supplement Inc., Japan | Tablet, capsule, powder, dietary supplement and functional food |
| Sardine (Sardinops sagax) | VY | Lapis Support, Valtyron®, Sato Marine Super P | Tokiwa Yakuhin Co., Japan, Senmi Ekisu Co., Japan, Sato pharmaceutical Co., Japan | Tablet, ingredient, beverage |
| Blue ling (Molva dypterygia) | Molval | Dielen, France | Nutritional supplement | |
| Mackerel | Tensideal | ABYSS’ ingredients, France | Dietary supplement |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abachi, S.; Bazinet, L.; Beaulieu, L. Antihypertensive and Angiotensin-I-Converting Enzyme (ACE)-Inhibitory Peptides from Fish as Potential Cardioprotective Compounds. Mar. Drugs 2019, 17, 613. https://doi.org/10.3390/md17110613
Abachi S, Bazinet L, Beaulieu L. Antihypertensive and Angiotensin-I-Converting Enzyme (ACE)-Inhibitory Peptides from Fish as Potential Cardioprotective Compounds. Marine Drugs. 2019; 17(11):613. https://doi.org/10.3390/md17110613
Chicago/Turabian StyleAbachi, Soheila, Laurent Bazinet, and Lucie Beaulieu. 2019. "Antihypertensive and Angiotensin-I-Converting Enzyme (ACE)-Inhibitory Peptides from Fish as Potential Cardioprotective Compounds" Marine Drugs 17, no. 11: 613. https://doi.org/10.3390/md17110613
APA StyleAbachi, S., Bazinet, L., & Beaulieu, L. (2019). Antihypertensive and Angiotensin-I-Converting Enzyme (ACE)-Inhibitory Peptides from Fish as Potential Cardioprotective Compounds. Marine Drugs, 17(11), 613. https://doi.org/10.3390/md17110613






