Prenylated Phenol and Benzofuran Derivatives from Aspergillus terreus EN-539, an Endophytic Fungus Derived from Marine Red Alga Laurencia okamurai
Abstract
1. Introduction
2. Results and Discussion
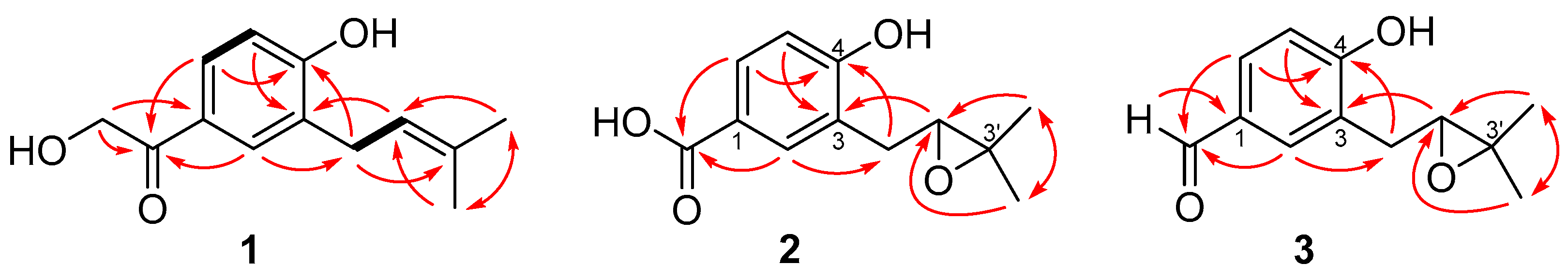
2.1. Structure Elucidation of the New Compounds
2.2. Biological Activities of the Isolated Compounds
3. Experimental Section
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation
3.4. Extraction and Isolation
3.5. Antioxidant and Antimicrobial Assays
3.6. Computational Section
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2019, 36, 122–173. [Google Scholar] [CrossRef]
- Pettit, R.K. Mixed fermentation for natural product drug discovery. Appl. Microbiol. Biot. 2009, 83, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Strobel, G.A. Endophytes as sources of bioactive products. Microbes Infect. 2003, 5, 535–544. [Google Scholar] [CrossRef]
- Samson, R.A.; Peterson, S.W.; Frisvad, J.C.; Varga, J. New species in Aspergillus section Terrei. Stud. Mycol. 2011, 69, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Xu, R.; Li, X.M.; Yang, S.Q.; Meng, L.H.; Wang, B.G. Simpterpenoid A, a meroterpenoid with a highly functionalized cyclohexadiene moiety featuring gem-propane-1,2-dione and methylformate groups, from the mangrove-derived Penicillium simplicissimum MA-332. Org. Lett. 2018, 20, 1465–1468. [Google Scholar] [CrossRef]
- Li, H.L.; Li, X.M.; Mándi, A.; Antus, S.; Li, X.; Zhang, P.; Liu, Y.; Kurtán, T.; Wang, B.G. Characterization of cladosporols from the marine algal-derived endophytic fungus Cladosporium cladosporioides EN-399 and configurational revision of the previously reported cladosporol derivatives. J. Org. Chem. 2017, 82, 9946–9954. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Li, X.M.; Li, X.; Yang, S.Q.; Wang, B.G. Structure, absolute configuration and biological evaluation of polyoxygenated meroterpenoids from the marine algal-derived Aspergillus terreus EN-539. Phytochem. Lett. 2019, 32, 138–142. [Google Scholar] [CrossRef]
- Cao, J.; Li, X.M.; Meng, L.H.; Konuklugil, B.; Li, X.; Li, H.L.; Wang, B.G. Isolation and characterization of three pairs of indolediketopiperazine enantiomers containing infrequent N-methoxy substitution from the marine algal-derived endophytic fungus Acrostalagmus luteoalbus TK-43. Bioorg. Chem. 2019, 90, 103030. [Google Scholar] [CrossRef]
- Wang, B.G.; Gloer, J.B.; Ji, N.Y.; Zhao, J.C. Halogenated organic molecules of Rhodomelaceae origin: Chemistry and biology. Chem. Rev. 2013, 113, 3632–3685. [Google Scholar] [CrossRef]
- Gil, D.M.; Lizárraga, E.; Echeverría, G.A.; Piro, O.E.; Catalán, C.A.N.; Altabef, A.B. A combined experimental and theoretical study of the supramolecular self-assembly of the natural benzopyran 2,2-dimethyl-3-hydroxy-6-acetyl-chromane and its isomeric benzofuran 10,11-dihydro-10-hydroxytremetone. J. Mol. Struct. 2017, 1146, 164–178. [Google Scholar] [CrossRef]
- Lizárraga, E.; Romano, E.; Rudyk, R.A.; Catalán, C.A.N.; Brandán, S.A. Structural study, coordinated normal analysis and vibrational spectra of 4-hydroxy-3-(3-methyl-2-butenyl) acetophenone. Spectrochim. Acta A 2012, 97, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Venkatasubbaiah, P.; Chilton, W.S. Toxins produced by the dogwood anthracnose fungus Discula SP. J. Nat. Prod. 1991, 54, 1293–1297. [Google Scholar] [CrossRef]
- Sharma, R.; Bulger, P.G.; McNevin, M.; Dormer, P.G.; Ball, R.G.; Streckfuss, E.; Cuff, J.F.; Yin, J.J.; Chen, C.Y. A cascade approach to cyclic aminonitrones: Reaction discovery, mechanism, and scope. Org. Lett. 2009, 11, 3194–3197. [Google Scholar] [CrossRef] [PubMed]
- Mohammat, M.F.; Osman, N.S.; Shaameri, Z.; Hamzah, A.S. First consecutive linear synthesis of hostmaniene, 5-formyl-2-(isopropyl-1′-ol) benzofuran and anadendroic acid using prenylated phenol. Org. Commun. 2017, 10, 130–134. [Google Scholar] [CrossRef]
- Shima, K.; Hisada, S.; Inagaki, I. Studies on the constituents of Anodendron affine Durce. V. Isolation and structure of two new constituents. Yakugaku Zasshi 1972, 92, 1410–1414. [Google Scholar] [CrossRef]
- Zhao, M.B.; Zhou, S.X.; Zhang, Q.Y.; Wei, W.F.; Li, M.H.; Xing, J.Y.; Jiang, Y.; Tu, P.F. Prenylated benzoic acid derivatives from the stem of Euodia lepta. Nat. Prod. Res. 2017, 31, 1589–1593. [Google Scholar] [CrossRef]
- Wu, Z.D.; Li, D.Y.; Zeng, F.R.; Tong, Q.Y.; Zheng, Y.Y.; Liu, J.J.; Zhou, Q.; Li, X.N.; Chen, C.M.; Lai, Y.J.; et al. Brasilane sesquiterpenoids and dihydrobenzofuran derivatives from Aspergillus terreus [CFCC 81836]. Phytochemistry 2018, 156, 159–166. [Google Scholar] [CrossRef]
- Kawase, Y.; Yamaguchi, S.; Inoue, O.; Sannomiya, M.; Kawabe, K. The syntheses and absolute configurations of fomannoxin, (−)-5-acetyl-2-(1-hydroxymethylvinyl)-2,3-dihydrobenzofuran, and anodendroic acid. Chem. Lett. 1980, 12, 1581–1584. [Google Scholar] [CrossRef]
- Prompanya, C.; Dethoup, T.; Gales, L.; Lee, M.; Pereira, J.A.; Silva, A.M.S.; Pinto, M.M.M.; Kijjoa, A. New polyketides and new benzoic Aacid derivatives from the marine sponge-associated fungus Neosartorya quadricincta KUFA 0081. Mar. Drug. 2016, 14, 134. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Donnelly, D.M.X.; Fukuda, N.; Kouno, I.; Martin, M.; O’Reilly, J. Dihydrobenzofurans from Heterobasidion annosum. Phytochemistry 1988, 27, 2709–2713. [Google Scholar] [CrossRef]
- Moriarty, R.M.; Grubjesic, S.; Surve, B.C.; Chandersekera, S.N.; Prakash, O.; Naithani, R. Synthesis of Abyssinone II and related compounds as potential chemopreventive agents. Eur. J. Med. Chem. 2006, 41, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Meng, L.H.; Mándi, A.; Li, X.M.; Kurtán, T.; Wang, B.G. Structure, absolute configuration, and conformational study of resorcylic acid derivatives and related congeners from the fungus Penicillium brocae. RSC Adv. 2015, 5, 39870–39877. [Google Scholar] [CrossRef]
- Pierce, C.G.; Uppuluri, P.; Tristan, A.R.; Wormley, F.L., Jr.; Mowat, E.; Ramage, G.; Lopez-Ribot, J.L. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Protoc. 2008, 3, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Li, X.M.; Yang, S.Q.; Cao, J.; Li, Y.H.; Wang, B.G. Induced terreins production from marine red algal-derived endophytic fungus Aspergillus terreus EN-539 co-cultured with symbiotic fungus Paecilomyces lilacinus EN-531. J. Antibiot. 2019. [Google Scholar] [CrossRef]
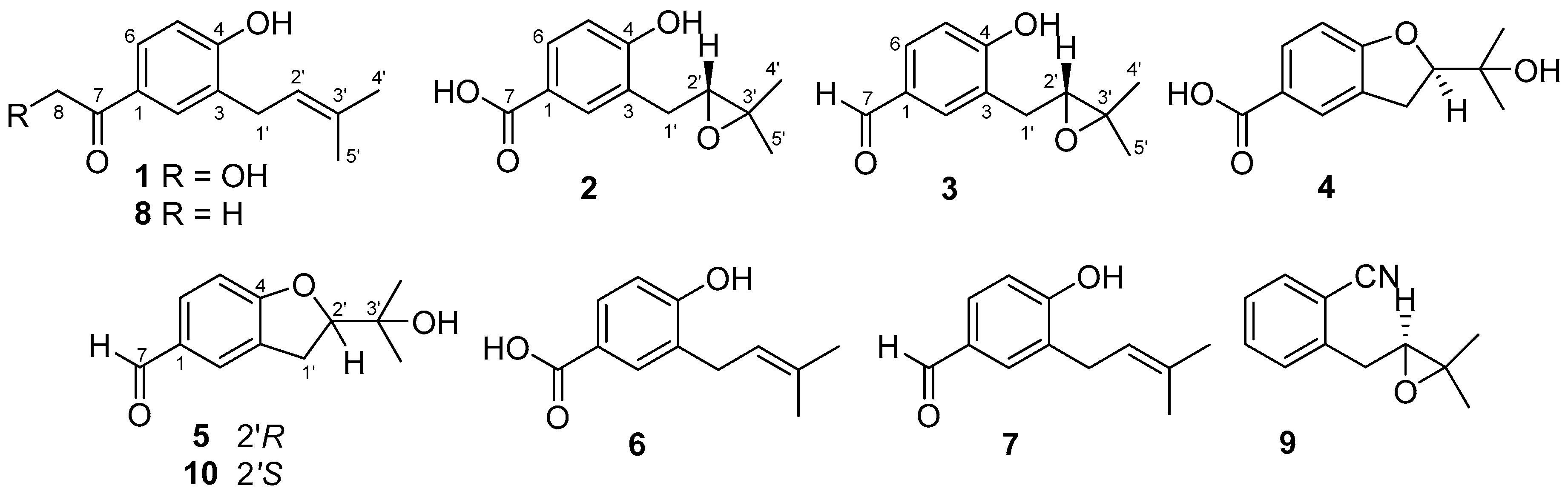

| No. | 1 a | 2 b | 3 a | |||
|---|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 128.1, C | 129.1, C | 129.8, C | |||
| 2 | 128.9, CH | 7.59, s | 130.6, CH | 7.71, s | 133.2, CH | 8.50, s |
| 3 | 125.8, C | 118.2, C | 121.8, C | |||
| 4 | 160.8, C | 154.5, C | 159.2, C | |||
| 5 | 115.0, CH | 6.77, d (9.1) | 115.1, CH | 6.69, d (8.3) | 117.9, CH | 7.72, d (8.4) |
| 6 | 127.7, CH | 7.58, d (9.1) | 128.0, CH | 7.70, d (8.3) | 129.8, CH | 8.46, d (8.4) |
| 7 | 196.4, C | 173.4, C | 191.9, CH | 10.64, s | ||
| 8 | 64.3, CH2 | 4.63, s | ||||
| 1’ | 28.1, CH2 | 30.3, CH2 | 2.75, dd (7.4, 16.6) 3.03, dd (5.3, 16.6) | 31.4, CH2 | 3.54, dd (7.1, 16.8) 3.85, dd (4.9, 16.8) | |
| 2’ | 122.7, CH | 3.22, d (7.3) | 68.6, CH | 3.77, dd (5.3, 7.4) | 68.0, CH | 4.54, dd (4.9, 7.1) |
| 3’ | 131.3, C | 5.29, t (7.3) | 76.6, C | 79.4, C | ||
| 4’ | 17.6, CH3 | 1.67, s | 19.2, CH3 | 1.26, s | 22.0, CH3 | 2.07, s |
| 5’ | 25.5, CH3 | 1.70, s | 24.1, CH3 | 1.33, s | 26.2, CH3 | 2.13, s |
| Compounds | 4 | 5 | ||
|---|---|---|---|---|
| 2’R | 2’S | 2’R | 2’S | |
| calculated OR | −60.9 | +61.0 | −91.3 | +95.5 |
| measured OR | −95.0 | −55.0 | ||
| Compounds | Ah | Et | Ec | Ml | Pa | Sa | Vh | Vp | Vv |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 32 | 32 | 16 | 2 | 8 | 4 | 8 | 32 |
| 2 | 64 | n.a. | 32 | 32 | 64 | n.a. | n.a. | n.a. | n.a. |
| 3 | n.a. | n.a. | 64 | 32 | n.a. | n.a. | n.a. | n.a. | n.a. |
| 4 | n.a. | 32 | n.a. | n.a. | n.a. | 64 | n.a. | n.a. | n.a. |
| 5 | n.a. | 64 | n.a. | 32 | n.a. | 32 | n.a. | n.a. | n.a. |
| 6 | 8 | 16 | 16 | n.a. | n.a. | 64 | 32 | 8 | n.a. |
| 7 | 4 | 16 | 32 | 8 | 16 | 16 | 8 | 8 | 64 |
| Chloramphenicol | 1 | 0.5 | 2 | 2 | 1 | 2 | 2 | 4 | 4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.-L.; Li, X.-M.; Yang, S.-Q.; Meng, L.-H.; Li, X.; Wang, B.-G. Prenylated Phenol and Benzofuran Derivatives from Aspergillus terreus EN-539, an Endophytic Fungus Derived from Marine Red Alga Laurencia okamurai. Mar. Drugs 2019, 17, 605. https://doi.org/10.3390/md17110605
Li H-L, Li X-M, Yang S-Q, Meng L-H, Li X, Wang B-G. Prenylated Phenol and Benzofuran Derivatives from Aspergillus terreus EN-539, an Endophytic Fungus Derived from Marine Red Alga Laurencia okamurai. Marine Drugs. 2019; 17(11):605. https://doi.org/10.3390/md17110605
Chicago/Turabian StyleLi, Hong-Lei, Xiao-Ming Li, Sui-Qun Yang, Ling-Hong Meng, Xin Li, and Bin-Gui Wang. 2019. "Prenylated Phenol and Benzofuran Derivatives from Aspergillus terreus EN-539, an Endophytic Fungus Derived from Marine Red Alga Laurencia okamurai" Marine Drugs 17, no. 11: 605. https://doi.org/10.3390/md17110605
APA StyleLi, H.-L., Li, X.-M., Yang, S.-Q., Meng, L.-H., Li, X., & Wang, B.-G. (2019). Prenylated Phenol and Benzofuran Derivatives from Aspergillus terreus EN-539, an Endophytic Fungus Derived from Marine Red Alga Laurencia okamurai. Marine Drugs, 17(11), 605. https://doi.org/10.3390/md17110605






