Bioactive Peptides Produced by Cyanobacteria of the Genus Nostoc: A Review
Abstract
1. Introduction
2. Non-Ribosomal Peptides (NRPs) and Polyketides (PKs)
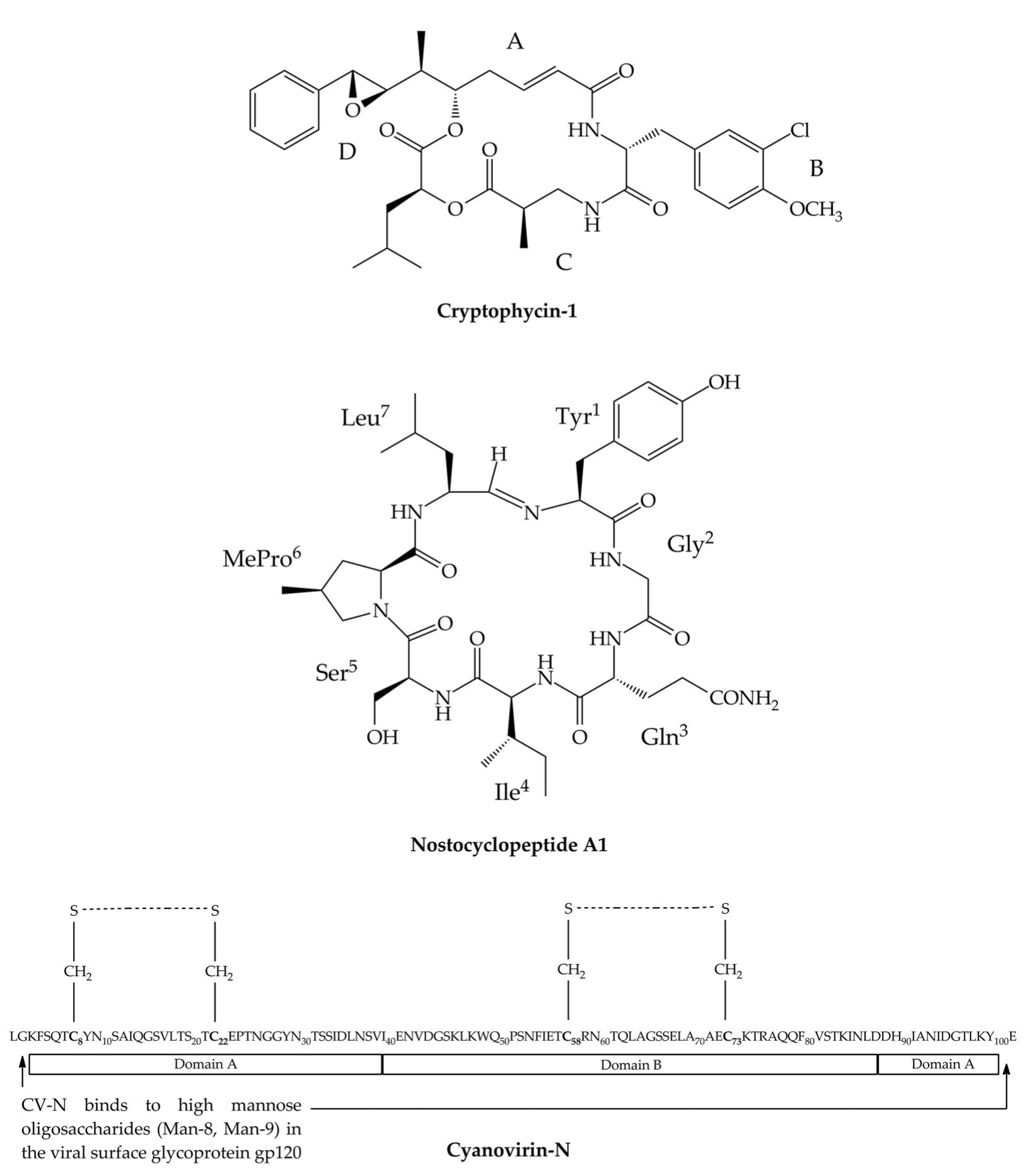
3. Cryptophycins
4. Nostocyclopeptides
5. Cyanovirin-N
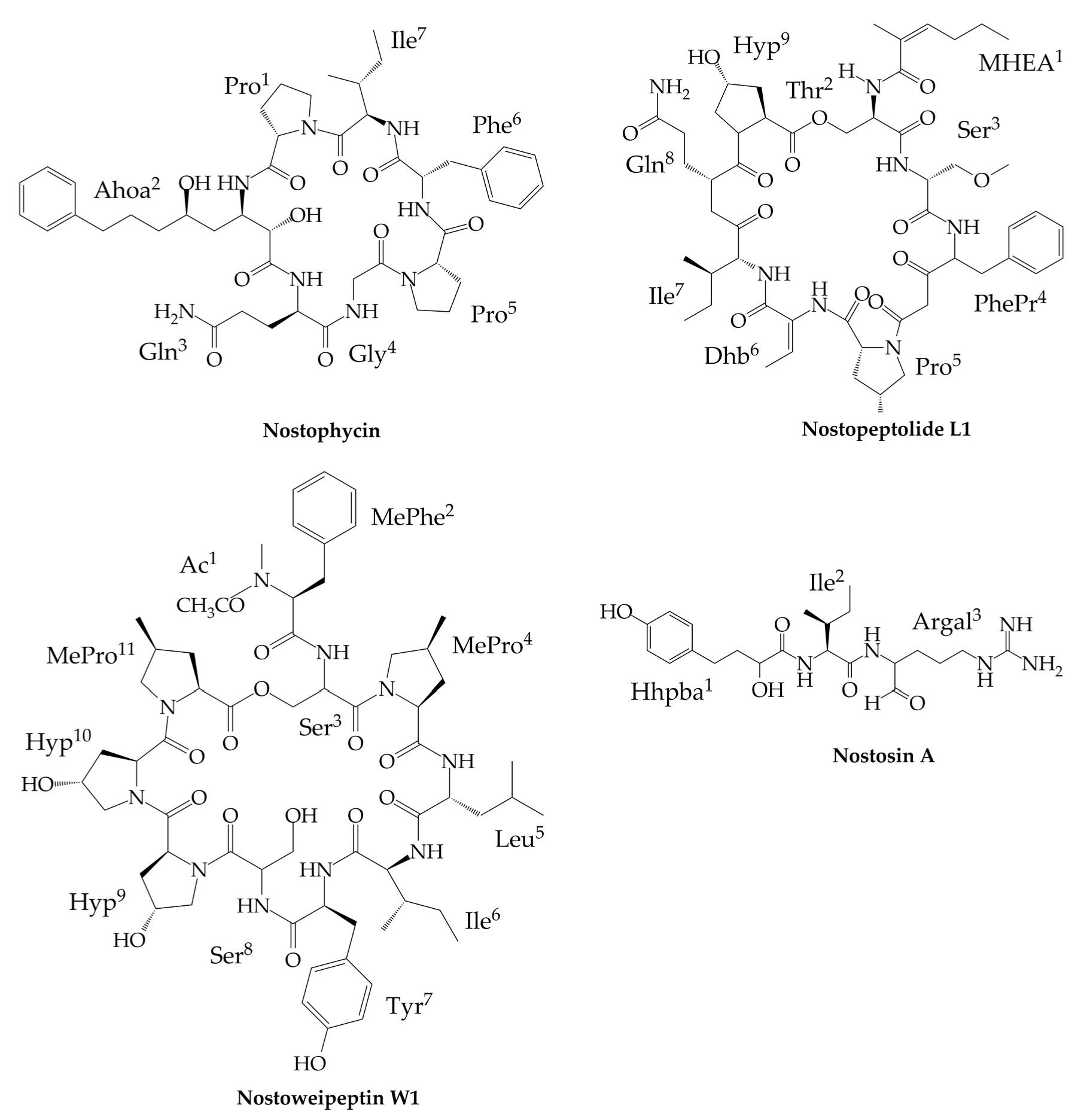
6. Other Peptides Exclusively Produced by Nostoc
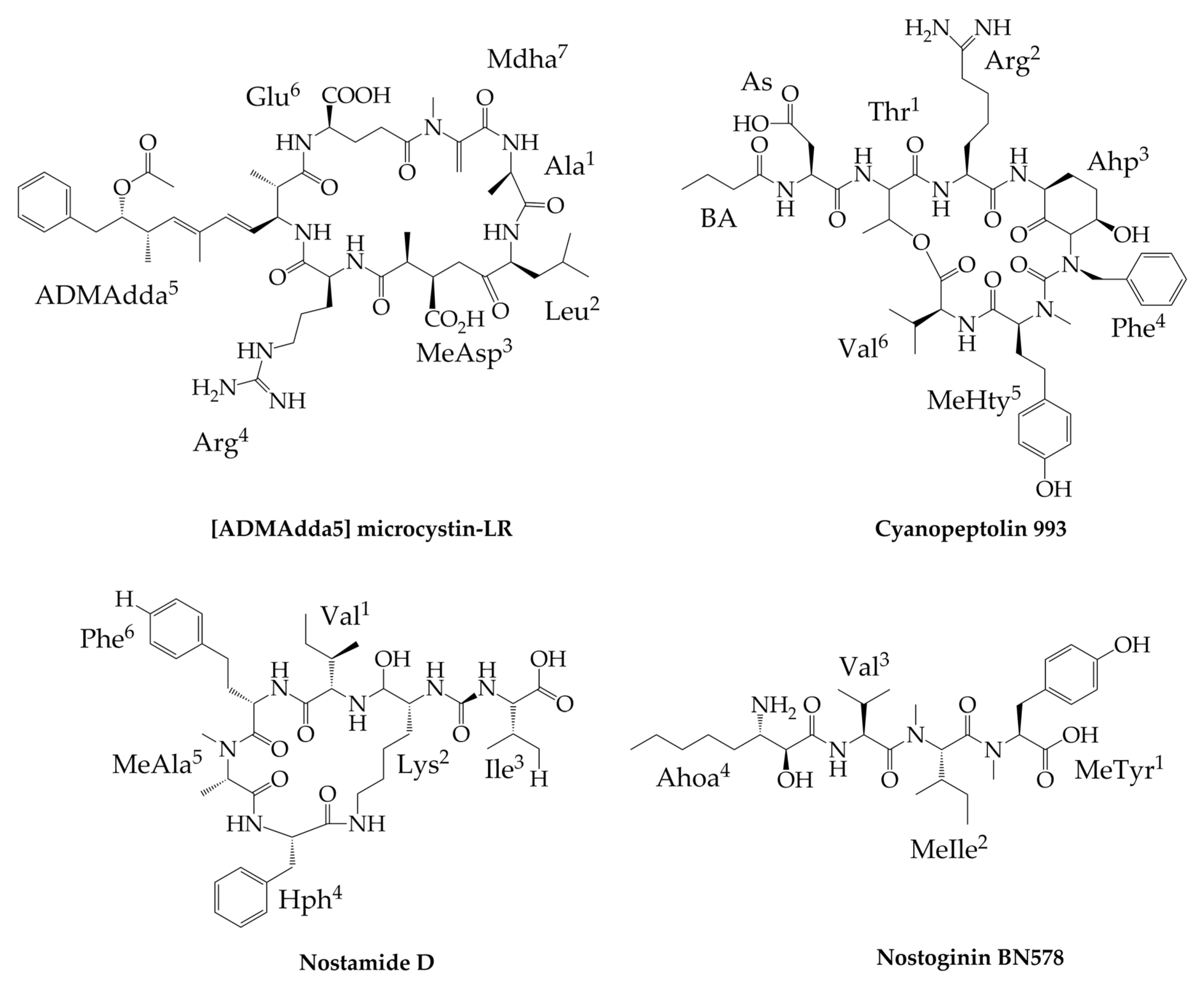
7. Peptides Produced by Nostoc and Other Cyanobacteria
8. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Gademann, K.; Portmann, C. Secondary Metabolites from Cyanobacteria: Complex Structures and Powerful Bioactivities. Curr. Org. Chem. 2008, 12, 326–341. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kajiyama, S.; Inawaka, K.; Kanzaki, H.; Kawazu, K. Nostodione A, a novel mitotic spindle poison from a blue-green alga Nostoc commune. Z. Naturforschung 1994, 49, 464–470. [Google Scholar] [CrossRef]
- Yamada, S.; Ohkubo, S.; Miyashita, H.; Setoguchi, H. Genetic diversity of symbiotic cyanobacteria in Cycas revoluta (Cycadaceae). FEMS Microbiol. Ecol. 2012, 81, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Kaasalainen, U.; Fewer, D.; Jokela, J.; Wahlsten, M.; Sivonen, K.; Rikkinen, J. Cyanobacteria produce a high variety of hepatotoxic peptides in lichen symbiosis. Proc. Natl. Acad. Sci. USA 2012, 109, 5886–5891. [Google Scholar] [CrossRef]
- Sand-Jensen, K. Ecophysiology of gelatinous Nostoc colonies: Unprecedented slow growth and survival in resource-poor and harsh environments. Ann. Bot. 2014, 114, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Hitzfeld, B.; Lampert, C.; Spaeth, N.; Mountfort, D.; Kaspar, H.; Dietrich, D. Toxin production in cyanobacterial mats from ponds on the McMurdo Ice Shelf, Antarctica. Toxicon 2000, 38, 1731–1748. [Google Scholar] [CrossRef]
- Jones, K. Interactions between desiccation and dark nitrogen fixation in tropical Nostoc commune. New Phytol. 1989, 113, 1–6. [Google Scholar] [CrossRef]
- Trnková, K.; Barták, M. Desiccation-induced changes in photochemical processes of photosynthesis and spectral reflectance in Nostoc commune (Cyanobacteria, Nostocales) colonies from polar regions. Phycol. Res. 2016, 65, 1. [Google Scholar]
- Thangaraj, B.; Rajasekar, D.; Vijayaraghavan, R.; Garlapati, D.; Devanesan, A.; Lakshmanan, U.; Dharmar, P. Cytomorphological and nitrogen metabolic enzyme analysis of psychrophilic and mesophilic Nostoc sp.: A comparative outlook. Biotech 2017, 7, 107. [Google Scholar] [CrossRef] [PubMed]
- Scherer, S.; Potts, M. Novel water stress protein from a desiccation-tolerant cyanobacterium: Purification and partial characterization. J. Biol. Chem. 1989, 264, 12546–12553. [Google Scholar] [PubMed]
- Potts, M. Mechanisms of desiccation tolerance in cyanobacteria. Eur. J. Phycol. 1999, 34, 319–328. [Google Scholar] [CrossRef]
- Sand-Jensen, K.; Raun, A.; Borum, J. Metabolism and resources of spherical colonies of Nostoc zetterstedtii. Limnol. Oceanogr. 2009, 54, 1282–1291. [Google Scholar] [CrossRef]
- Scherer, S.; Chen, T.; Böger, P. A new UV-A/B protecting pigment in the terrestrial cyanobacterium Nostoc commune. Plant Physiol. 1988, 88, 1055–1057. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Klmura, S.; Sato, S.; Katoh, H.; Abe, T.; Aral, M.; Tomlta-Yokotani, K. Evaluation of a cyanobacterium Nostoc sp. HK-01, as food material for space agriculture on mars. Biol. Sci. Space 2015, 29, 24–31. [Google Scholar] [CrossRef]
- Gao, K. Chinese studies on the edible blue-green alga, Nostoc flagelliforme: A review. J. Appl. Phycol. 1998, 10, 37–49. [Google Scholar] [CrossRef]
- Johnson, H.; King, S.; Banack, S.; Webster, C.; Callanaupa, W.; Cox, P. Cyanobacteria (Nostoc commune) used as a dietary item in the Peruvian highlands produce the neurotoxic amino acid BMAA. J Ethnopharmacol. 2008, 118, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Roney, B.; Renhui, L.; Banack, S.; Murch, S.; Honegger, R.; Cox, P. Consumption of fa cai Nostoc soup: A potential for BMAA exposure from Nostoc cyanobacteria in China? Amyotroph. Lateral Scler. 2009, 10, 44–49. [Google Scholar] [CrossRef]
- Li, Z.; Guo, M. Healthy efficacy of Nostoc commune Vaucher. Oncotarget 2018, 9, 14669–14679. [Google Scholar] [CrossRef] [PubMed]
- Win, T.; Barone, G.; Secundo, F.; Fu, P. Algal biofertilizers and plant growth stimulants for sustainable agriculture. Ind. Biotechnol. 2018, 14, 203–211. [Google Scholar] [CrossRef]
- Ghazal, F.; Mahdy, E.; El-Fattah, M.; El-Sadany, A.; Doha, N. The use of cyanobacteria as biofertilizer in wheat cultivation under different nitrogen rates. Nat. Sci. 2018, 16, 30–35. [Google Scholar]
- Moore, R. Cyclic peptides and depsipeptides from cyanobacteria: A review. J. Ind. Microbiol. 1996, 16, 134–143. [Google Scholar] [CrossRef] [PubMed]
- El-Sheekh, M.; Osman, M.; Dyan, M.; Amer, M. Production and characterization of antimicrobial active substance from the cyanobacterium Nostoc muscorum. Environ. Toxicol. Pharmacol. 2006, 21, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Ploutno, A.; Carmeli, S. Nostocyclyne A, a novel antimicrobial cyclophane from the cyanobacterium Nostoc sp. J. Nat. Prod. 2000, 63, 1524–1526. [Google Scholar] [CrossRef] [PubMed]
- Boyd, M.; Gustafson, K.; McMahon, J.; Shoemaker, R.; O’Keefe, B.; Mori, T.; Gulakowski, R.; Wu, L.; Rivera, M.; Laurencot, C.; et al. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: Potential applications to microbicide development. Antimicrob. Agents Chemother. 1997, 41, 1521–1530. [Google Scholar] [CrossRef]
- Botos, I.; Wlodawer, A. Cyanovirin-N: A sugar-binding antiviral protein with a new twist. Cell. Mol. Life Sci. 2003, 60, 277–287. [Google Scholar] [CrossRef]
- Dey, B.; Lerner, D.; Lusso, P.; Boyd, M.; Elder, J.; Berger, E. Multiple antiviral activities of cyanovirin-N: Blocking of human immunodeficiency virus type 1 gp120 interaction with CD4 and coreceptor and inhibition of diverse enveloped viruses. J. Virol. 2000, 74, 4562–4569. [Google Scholar] [CrossRef]
- Mazur-Marzec, H.; Fidor, A.; Cegłowska, M.; Wieczerzak, E.; Kropidłowska, M.; Goua, M.; Macaskill, J.; Edwards, C. Cyanopeptolins with trypsin and chymotrypsin inhibitory activity from the cyanobacterium Nostoc edaphicum CCNP 1411. Mar. Drugs 2018, 16, 220. [Google Scholar] [CrossRef]
- Dittmann, E.; Neilan, B.; Börner, T. Molecular biology of peptide and polyketide biosynthesis in cyanobacteria. Appl. Microbiol. Biotechnol. 2001, 57, 467–473. [Google Scholar]
- Dembitsky, D.; Řezanka, T. Metabolites produced by nitrogen-fixing Nostoc species. Folia Microbiol. 2005, 50, 363–391. [Google Scholar] [CrossRef]
- Nowruzi, B.; Khavari-Nejad, R.; Sivonen, K.; Kazemi, B.; Najafi, F.; Nejadsattari, T. Indentification and toxigenic potential of Nostoc sp. Algae 2012, 27, 303–313. [Google Scholar] [CrossRef]
- Řezanka, T.; Dor, I.; Dembitsky, V. Fatty acid composition of six freshwater wild cyanobacterial species. Folia Microbiol. 2003, 48, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Welker, M.; von Döhren, H. Cyanobacterial peptides—Nature’s own combinatorial biosynthesis. FEMS Microbiol. Rev. 2006, 30, 530–563. [Google Scholar]
- Shah, S.; Akhter, N.; Auckloo, B.; Khan, I.; Lu, Y.; Wang, K.; Wu, B.; Guo, Y.-W. Structural diversity, biological properties and applications on natural products from cyanobacteria. A review. Mar. Drugs 2017, 15, 354. [Google Scholar] [CrossRef]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.; Dunn, M. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorg. Med. Chem. 2018, 26, 2700–2707. [Google Scholar] [CrossRef]
- Golakoti, T.; Ogino, J.; Heltzel, C.; Le Husebo, T.; Jensen, C.; Larsen, L.; Patterson, G.; Moore, R.; Mooberry, S.; Corbett, T.; et al. Structure determination, conformational analysis, chemical stability studies, and antitumor evaluation of the cryptophycins. Isolation of new 18 analogs from Nostoc sp. strain GSV 224. J. Am. Chem. Soc. 1995, 117, 12030–12049. [Google Scholar] [CrossRef]
- Ploutno, A.; Carmeli, S. Modified peptides from a water bloom of the cyanobacterium Nostoc sp. Tetrahedron 2002, 58, 9949–9957. [Google Scholar] [CrossRef]
- Kapuścik, A.; Hrouzek, P.; Kuzma, M.; Bártová, S.; Novák, P.; Jokela, J.; Pflüger, M.; Eger, A.; Hundsberger, H.; Kopecký, J. Novel aeruginosin-865 from Nostoc sp. as a potent anti-inflammatory agent. ChemBioChem 2013, 14, 2329–2337. [Google Scholar] [CrossRef]
- Nowruzi, B.; Khavari-Nejad, R.; Sivonen, K.; Kazemi, B.; Najafi, F.; Nejadsattari, T. A gene expression study on strains of Nostoc (Cyanobacteria) revealing antimicrobial activity under mixotrophic conditions. Afr. J. Biotech. 2012, 11, 11296–11308. [Google Scholar] [CrossRef]
- Karjiyama, S.-I.; Kanzaki, H.; Kawazu, K.; Kobayashi, A. Nostofungicidine, an antifungal lipopeptide from the field-grown terrestrial blue-green alga Nostoc commune. Tetrahedron Lett. 1998, 39, 3737–3740. [Google Scholar] [CrossRef]
- Wang, H.; Fewer, D.; Holm, L.; Rouhiainen, L.; Sivonen, K. Atlas of nonribosomal peptide and polyketide biosynthetic pathways reveals common occurrence of nonmodular enzymes. Proc. Natl. Acad. Sci. USA 2014, 111, 9259–9264. [Google Scholar] [CrossRef]
- Finking, R.; Marahiel, M. Biosynthesis of nonribosomal peptides. Annu. Rev. Microbiol. 2004, 58, 453–488. [Google Scholar] [CrossRef]
- Dittmann, E.; Gugger, M.; Sivonen, K.; Fewer, D. Natural product biosynthetic diversity and comparative genomics of the cyanobacteria. Trends Microbiol. 2015, 23, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.; Kehr, J.; Mainz, A.; Dehm, D.; Petras, D.; Süssmuth, R.; Dittmann, E. Biochemical dissection of the natural diversification of microcystin provides lessons for synthetic biology of NRPS. Cell. Chem. Biol. 2016, 23, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Patel, A.; Hur, G.; Tufar, P.; Wuo, M.; McCammon, J.; Burkart, M. Mechanistic probes for the epimerization domain of nonribosomal peptide synthetases. ChemBioChem Commun. 2019, 20, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Burja, A.; Banaigs, B.; Abou-Mansour, E.; Burgess, J.; Wright, P. Marine cyanobacteria—A prolific source of natural products. Tetrahedron 2001, 57, 9347–9377. [Google Scholar] [CrossRef]
- Schwartz, R.; Hirsch, S.; Sesin, D.; Flor, J.; Chartrain, M.; Fromtling, R.; Harris, G.; Salvatore, M.; Liesch, J.; Yudin, K. Pharmaceuticals from cultured algae. J. Ind. Microbiol. 1990, 5, 113–124. [Google Scholar] [CrossRef]
- Eggen, M.; Georg, G. The cryptophycins: Their synthesis and anticancer activity. Med. Res. Rev. 2002, 22, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Weiss, C.; Figueras, E.; Borbely, A.; Sewald, N. Cryptophycins: Cytotoxic cyclodepsipeptides with potential for tumor targeting. J. Pept. Sci. 2017, 23, 514–531. [Google Scholar] [CrossRef] [PubMed]
- Magarvey, N.; Beck, Z.; Golakoti, T.; Ding, Y.; Huber, U.; Hemscheidt, T.; Abelson, D.; Moore, R.; Sherman, D. Biosynthetic characterization and chemoenzymatic assembly of the cryptophycins. Potent anticancer agents from Nostoc cyanobionts. ACS Chem. Biol. 2006, 1, 766–779. [Google Scholar] [CrossRef]
- Golakoti, T.; Ohtani, I.; Patterson, G.; Moore, R.; Corbett, T.; Valeriote, F.; Demchik, L. Total structures of cryptophycins, potent antitumor depsipeptides from the blue-green alga Nostoc sp. strain GSV 224. J. Am. Chem. Soc. 1994, 116, 4729–4737. [Google Scholar]
- Wagner, M.; Paul, D.; Shih, C.; Jordan, M.; Wilson, L.; Williams, D. In vitro pharmacology of cryptophycin 52(LY355703) in human tumor cell lines. Cancer Chemother. Pharmacol. 1999, 43, 115–125. [Google Scholar] [CrossRef]
- Corbett, T.; Valeriote, F.; Demchik, L.; Lowichik, N.; Polin, L.; Panchapor, C.; Pugh, S.; White, K.; Kushner, J.; Rake, J.; et al. Discovery of cryptophycin-1 and BCN-183577: Examples of strategies and problems in the detection of antitumor activity in mice. IND 1997, 15, 207–218. [Google Scholar]
- Smith, C.; Zhang, X.; Mooberry, S.; Patterson, G.; Moore, R. Cryptophycin: A new antimicrotubule agent active against drug-resistant cells. Cancer Res. 1994, 54, 3779–3784. [Google Scholar]
- Boinpally, R.; Polin, L.; Zhou, S.-L.; Jasti, B.; Wiegand, R.; White, K.; Kushner, J.; Horwitz, J.; Corbett, T.; Parchment, R. Pharmacokinetics and tissue distribution of cryptophycin 52 (C-52) epoxide and cryptophycin 55 (C-55) chlorohydrin in mice with subcutaneous tumors. Cancer Chemother. Pharmacol. 2003, 52, 25–33. [Google Scholar] [CrossRef]
- Liang, J.; Moore, R.; Moher, E.; Munroe, J.; Al-awar, R.; Hay, D.; Varie, D.; Zhang, T.; Aikins, J.; Martinelli, M.; et al. Cryptophycins-309, 249 and other cryptophycin analogs: Preclinical efficacy studies with mouse and human tumors. Investig. New Drug 2005, 23, 213–224. [Google Scholar] [CrossRef]
- Weiss, C.; Sammet, B.; Sewald, N. Recent approaches for the synthesis of modified cryptophycins. Nat. Prod. Rep. 2013, 30, 924–940. [Google Scholar] [CrossRef]
- Edelman, M.; Gandara, D.; Hausner, P.; Israel, V.; Thornton, D.; DeSanto, J.; Doyle, L. Phase 2 study of cryptophycin 52 (LY355703) in patients previously treated with platinum based chemotherapy for advanced non-small cell lung cancer. Lung Cancer 2003, 39, 197–199. [Google Scholar] [CrossRef]
- D’Agostino, G.; del Campo, J.; Mellado, B.; Izquierdos, M.; Minarik, T.; Cirri, L.; Marini, L.; Perez-Gracia, J.; Scambia, G. A multicancer phase II study of the cryptophycin analog LY355703 in patients with platinum-resistant ovarian cancer. Int. J. Gynecol. Cancer 2006, 16, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Pillow, T.; DePalatis, L.; Li, G.; Phillips, G.; Polson, A.; Raab, H.; Spencer, S.; Zheng, B. The Cryptophycins as potent payloads for antibody drug conjugates. Bioorg. Med. Chem. Lett. 2015, 25, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Borbély, A.; Figueras, E.; Martins, A.; Esposito, S.; Auciello, G.; Monteagudo, E.; Di Marco, A.; Summa, V.; Cordella, P.; Perego, R.; et al. Synthesis and biological evaluation of RGD-cryptophycin conjugates for targeted drug delivery. Pharmaceutics 2019, 11, 151. [Google Scholar] [CrossRef]
- Golakoti, T.; Yoshida, W.; Chaganty, S.; Moore, R. Isolation and structure determination of nostocyclopeptides A1 and A2 from the terrestrial cyanobacterium Nostoc sp. ATCC53789. J. Nat. Prod. 2001, 64, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Jokela, J.; Herfindal, L.; Wahlsten, M.; Permi, P.; Selheim, F.; Vasconçelos, V.; Døskeland, S.; Sivonen, K. A novel cyanobacterial nostocyklopeptide is a potent antitoxin against Microcystis. ChemBioChem 2010, 11, 1594–1599. [Google Scholar] [CrossRef]
- Enck, S.; Kopp, F.; Marahiel, M.; Geyer, A. The entropy balance of nostocyklopeptide macrocyclization analysed by NMR spectroscopy. ChemBioChem 2008, 9, 2597–2601. [Google Scholar] [CrossRef]
- Becker, J.E.; Moore, R.E.; Moore, B.S. Cloning, sequencing, and biochemical characterization of the nostocycyclopeptide biosynthetic gene cluster: Molecular basis for imine macrocyclization. Gene 2004, 325, 35–42. [Google Scholar] [CrossRef]
- Herfindal, L.; Myhren, L.; Kleppe, R.; Krakstad, C.; Selheim, F.; Jokela, J.; Sivonen, K.; Døskeland, S. Nostocyclopeptide-M1: A potent, nontoxic inhibitor of the hepatocyte drug trasporters OATP1B3 and OATP1B1. Mol. Pharm. 2011, 8, 360–367. [Google Scholar] [CrossRef]
- Gustafson, K.; Sowder, R.; Henderson, L.; Cardellina, J.; McMahon, J.; Rajamani, U.; Pannell, L.; Boyd, M. Isolation, primary sequence determination, and disulfide bond structure of cyanovirin-N, an anti-HIV (Human Immunodeficiency Virus) protein from the cyanobacterium Nostoc ellipsosporum. Biochem. Biophys. Res. Commun. 1997, 238, 223–228. [Google Scholar] [CrossRef]
- Yang, F.; Bewley, C.; Louis, J.; Gustafson, K.; Boyd, M.; Gronenborn, A.; Clore, G.; Wlodawer, A. Crystal structure of cyanovirin-N, a potent HIV-inactivating protein, shows unexpected domain swapping. J. Mol. Biol. 1999, 288, 403–412. [Google Scholar] [CrossRef]
- Bewley, C.; Otero-Quintero, S. The potent anti-HIV cyanovirin-N contains two novel carbohydrate binding sites that selectively bind to Man8 D1D3 and Man9 with nanomolar affinity: Implications for binding to the HIV envelope protein gp120. J. Am. Soc. 2001, 123, 3892–3902. [Google Scholar] [CrossRef]
- Barrientos, L.; Louis, J.; Botos, I.; Mori, T.; Han, Z.; O’Keefe, B.; Boyd, M.; Wlodawer, A.; Gronenborn, A. The domain-swapped dimer of cyanovirin-N is in a metastable folded state: Reconciliation of X-Ray and NMR structures. Structure 2002, 10, 673–686. [Google Scholar] [CrossRef]
- Barrientos, L.; O’Keefe, B.; Bray, M.; Sanchez, A.; Gronenborn, A.; Boyd, M. Cyanovirin-N binds to the viral surface glycoprotein, GP1,2 and inhibits infectivity of Ebola virus. Antivir. Res. 2003, 58, 47–56. [Google Scholar] [CrossRef]
- Barrientos, L.; Gronenborn, A. The highly specific carbohydrate-binding protein cyanovirin-N: Structure anti-HIV/Ebola activity and possibilities for therapy. Mini Rev. Med. Chem. 2005, 5, 21–31. [Google Scholar] [CrossRef]
- Esser, M.; Mori, T.; Mondor, I.; Sattentau, Q.; Dey, B.; Berger, E.; Boyd, M.; Lifson, J. Cyanovirin-N binds to gp120 to interfere with CD4-dependent human immunodeficiency virus type 1 virion binding, fusion, and infectivity but does not affect the CD4 binding site on gp120 or soluble CD4-induced conformational changes in gp120. J. Virol. 1999, 73, 4360–4371. [Google Scholar]
- Mori, T.; Boyd, M. Cyanovirin-N, a potent human immunodeficiency virus-inactivating protein, blocks both CD4-dependent and CD4-independent binding of soluble gp120 (sgp120) to target cells, inhibits sCD4-induced binding of sgp120 to cell-associated CXCR4, and dissociates bound sgp120 from target cells. Antimicrob. Agents Chemother. 2001, 45, 664–672. [Google Scholar] [PubMed]
- Tsai, C.-C.; Emau, P.; Jiang, Y.; Tian, B.; Morton, W.; Gustafson, K.; Boyd, M. Cyanovirin-N gel as a topical microbicide prevents rectal transmission of SHIV89.6P in macaques. AIDS Res. Hum. Retrovir. 2003, 19, 535–541. [Google Scholar] [CrossRef]
- Lagenaur, L.; Sanders-Beer, B.; Brichacek, B.; Pal, R.; Liu, X.; Liu, Y.; Yu, R.; Venzon, D.; Lee, P.P.; Hamer, D.H. Prevention of vaginal SHIV transmission in macaques by a live recombinant Lactobacillus. Mucosal. Immunol. 2011, 4, 648–657. [Google Scholar] [CrossRef]
- Lagenaur, L.; Swedek, I.; Lee, P.; Parks, T.P. Robust vaginal colonization of Macaques with a novel vaginally disintegrating tablet containing a live biotherapeutic product to prevent HIV infection in women. PLoS ONE 2015, 10, 1–17. [Google Scholar] [CrossRef]
- Yu, R.; Cheng, A.; Lagenaur, L.; Huang, W.; Weiss, D.; Treece, J.; Sanders-Beer, B.; Hamer, D.; Lee, P.; Xu, Q.; et al. A Chinese rhesus macaque (Macaca mulatta) model for vaginal Lactobacillus colonization and live microbicide development. J. Med. Primatol. 2009, 38, 125–136. [Google Scholar] [CrossRef]
- Lofti, H.; Sheervalilou, R.; Zarghami, N. An update of the recombinant protein expression systems of cyanovirin-N and challenges of preclinical development. BioImpacts 2018, 8, 139–151. [Google Scholar]
- Colleluori, D.; Tien, D.; Kang, F.; Pagliei, T.; Kuss, R.; McCormick, T.; Watson, K.; McFadden, K.; Chaiken, I.; Buckheit, R.; et al. Expression, purification, and characterization of recombinant cyanovirin-N for vaginal anti-HIV microbicide development. Protein Expr. Purif. 2005, 39, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Chen, W.; Guo, C.; Qian, C.; Liu, G.; Ge, F.; Huang, Y.; Kitazato, K.; Wang, Y.; Xiong, S. Soluble cytoplasmic expression, rapid purification, and characterization of cyanovirin-N as a his-SUMO fusion. Appl. Microbiol. Biotechnol. 2010, 85, 1051–1060. [Google Scholar] [CrossRef]
- Sexton, A.; Drake, P.; Mahmood, N.; Harman, S.; Shattock, R.; Ma, J. Transgenic plant production of cyanovirin-N, an HIV microbicide. FASEB J. 2006, 20, 356–358. [Google Scholar] [CrossRef]
- Sexton, A.; Harman, S.; Shattock, R.; Ma, J. Design, expression, and characterization of a multivalent, combination HIV microbicide. FASEB J. 2009, 23, 3590–3600. [Google Scholar] [CrossRef]
- Mori, T.; Shoemaker, R.; Gulakowski, R.; Krepps, B.; McMahon, J.; Gustafson, K.; Pannell, L.; Boyd, M. Analysis of sequence requirements for biological activity of cyanovirin-N, a potent HIV (Human Immunodeficiency Virus)-inactivating protein. Biochem. Biophys. Res. Commun. 1997, 238, 218–222. [Google Scholar] [CrossRef]
- Zappe, H.; Snell, M.; Bossard, M. PEGylation of cyanovirin-N, an entry inhibitor of HIV. Adv. Drug Deliv. Rev. 2008, 60, 79–87. [Google Scholar] [CrossRef]
- Chen, J.; Huang, D.; Chen, W.; Guo, C.; Wei, B.; Wu, C.; Peng, Z.; Fan, J.; Hou, Z.; Fang, Y.; et al. Linker-extended native cyanovirin-N facilitates PEGylation and potently inhibits HIV-1 by targeting the glycan ligand. PLoS ONE 2014, 9, 1–15. [Google Scholar] [CrossRef]
- Fujii, K.; Sivonen, K.; Kashiwagi, T.; Hirayama, K.; Harada, K.-I. Nostophycin, a novel cyclic peptide from the toxic cyanobacterium Nostoc sp. 152. J. Org. Chem. 1999, 64, 5777–5782. [Google Scholar] [CrossRef]
- Namikoshi, M.; Rinehart, K.; Sakai, R.; Sivonen, K.; Carmichael, W. Structures of three new cyclic heptapeptide hepatotoxins produced by the cyanobacterium (blue-green alga) Nostoc sp. strain 152. J. Org. Chem. 1990, 55, 6135–6139. [Google Scholar] [CrossRef]
- Fenner, A.; Engene, N.; Spadafora, C.; Gerwick, W.; Balunas, M. Medusamide A, a Panamanian cyanobacterial depsipeptide with multiple ß-amino acids. Org. Lett. 2016, 18, 352–355. [Google Scholar] [CrossRef]
- Kanamori, Y.; Iwasaki, A.; Sumimoto, S.; Suenaga, K. Urumamide, a novel chymotrypsin inhibitor with a ß-amino acid from a marine cyanobacterium Okeania sp. Tetrahedron Lett. 2016, 57, 4213–4216. [Google Scholar] [CrossRef]
- Fewer, D.P.; Ӧsterholm, J.; Rouhiainen, L.; Jokela, J.; Wahlsten, M.; Sivonen, K. Nostophycin biosynthesis is directed by a hybrid polyketide synthase-nonribosomal peptide synthetase in the toxic cyanobacterium Nostoc sp. strain 152. Appl. Environ. Microbiol. 2011, 77, 8034–8040. [Google Scholar] [CrossRef]
- Kurmayer, R. The toxic cyanobacterium Nostoc sp. strain 152 produces highest amounts of microcystin and nostophycin under stress conditions. J. Phycol. 2011, 47, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Golakoti, T.; Yoshida, W.; Chaganty, S.; Moore, R. Isolation and structures of nostopeptolides A1, A2 and A3 from the cyanobacterium Nostoc sp. GSV 224. Tetrahedron 2000, 56, 9093–9102. [Google Scholar] [CrossRef]
- Liu, L.; Jokela, J.; Herfindal, L.; Wahlsten, M.; Sinkkonen, J.; Permi, P.; Fewer, D.; Døskeland, S.; Sivonen, K. 4-methylproline guided natural product discovery: Co-occurrence of 4-hydroxy and 4-methylprolines in nostoweipeptins and nostopeptolides. ACS. Chem. Biol. 2014, 9, 2646–2655. [Google Scholar] [CrossRef] [PubMed]
- Dehm, D.; Krumbholz, J.; Baunach, M.; Wiebach, V.; Hinrichs, K.; Guljamow, A.; Tabuchi, T.; Jenke-Kodama, H.; Süssmuth, R.; Dittmann, E. Unlocking the spatial control of secondary metabolism uncovers hidden natural product diversity in Nostoc punctiforme. ACS Chem. Biol. 2019, 14, 1271–1279. [Google Scholar] [CrossRef]
- Hoffmann, D.; Hevel, J.; Moore, R.; Moore, B. Sequence analysis and biochemical characterization of the nostopeptolide A biosynthetic gene cluster from Nostoc sp. GSV 224. Gene 2003, 311, 171–180. [Google Scholar] [CrossRef]
- Liu, L.; Jokela, J.; Wahlsten, M.; Nowruzi, B.; Permi, P.; Zhang, Y.; Xhaard, H.; Fewer, D.; Sivonen, K. Nostosins, trypsin inhibitors isolated from the terrestrial cyanobacterium Nostoc sp. strain FSN. J. Nat. Prod. 2014, 77, 1784–1790. [Google Scholar] [CrossRef] [PubMed]
- Fewer, D.; Jokela, J.; Rouhiainen, L.; Wahlsten, M.; Koskenniemi, K.; Stal, L.; Sivonen, K. The non-ribosomal assembly and frequent occurrence of the protease inhibitors spumigins in the bloom-forming cyanobacterium Nodularia spumigena. Mol. Microbiol. 2009, 73, 924–937. [Google Scholar] [CrossRef]
- Ishida, K.; Okita, Y.; Matsuda, H.; Okino, T.; Murakami, M. Aeruginosins, protease inhibitors from the cyanobacterium Microcystis aeruginosa. Tetrahedron 1999, 55, 10971–10988. [Google Scholar] [CrossRef]
- Murakami, M.; Sun, Q.; Ishida, K.; Matsuda, H.; Okino, T.; Yamaguchi, K. Microvirdins, elastase inhibitors from the cyanobacterium Nostoc minutum (NIES-26). Phytochemistry 1997, 45, 1197–1202. [Google Scholar] [CrossRef]
- Sivonen, K.; Leikoski, N.; Fewer, D.; Jokela, J. Cyanobactins—Ribosomal cyclic peptides produced by cyanobacteria. Appl. Microbiol. Biotechnol. 2010, 86, 1213–1225. [Google Scholar] [CrossRef]
- Janssen, E. Cyanobacterial peptides beyond microcystins—A review on co-occurrence, toxicity, and challenges for risk assessment. Water Res. 2019, 151, 488–499. [Google Scholar] [CrossRef]
- Sivonen, K.; Carmichael, W.; Namikoshi, M.; Rinehart, K.; Dahlem, A.; Niemela, S. Isolation and characterization of hepatotoxic microcystin homologs from the filamentous freshwater cyanobacterium Nostoc sp. strain 152. Appl. Environ. Microbiol. 1990, 56, 2650–2657. [Google Scholar]
- Beattie, K.; Kaya, K.; Sano, T.; Codd, G. Three dehydrobotyrine-containing microcystins from Nostoc. Phytochemistry 1998, 47, 1289–1292. [Google Scholar] [CrossRef]
- Laub, J.; Henriksen, P.; Brittain, S.; Wang, J.; Carmichael, W.; Rinehart, K.; Moestrup, Ø. [ADMAdda5]-microcystins in Planktothrix agardhii strain PH-123 (cyanobacteria)—Importance for monitoring of microcystins in the environment. Environ. Toxicol. 2002, 17, 351–357. [Google Scholar] [CrossRef]
- Oksanen, I.; Jokela, J.; Fewer, D.; Wahlsten, M.; Rikkinen, J.; Sivonen, K. Discovery of rare and highly toxic microcystins from lichen-associated cyanobacterium Nostoc sp. strain IO-102-I. Appl. Environ. Microbiol. 2004, 70, 5756–5763. [Google Scholar] [CrossRef]
- Fewer, D.; Wahlsten, M.; Ӧsterholm, J.; Jokela, J.; Rouhiainen, L.; Kaasalainen, U.; Rikkinen, J.; Sivonen, K. The genetic basis for O-acetylation of the microcystin toxin in cyanobacteria. Chem. Biol. 2013, 20, 861–869. [Google Scholar] [CrossRef]
- Schmidt, J.; Wilhelm, S.; Boyer, G. The fate of microcystins in the environment and challenges for monitoring. Toxins 2014, 6, 3354–3387. [Google Scholar] [CrossRef]
- Gehringer, M.; Adler, L.; Roberts, A.; Moffitt, M.; Mihali, T.; Mills, T.; Fieker, C.; Neilan, B. Nodularin, a cyanobacterial toxin, is synthesized in planta by symbiotic Nostoc sp. ISME J. 2012, 6, 1834–1847. [Google Scholar] [CrossRef]
- Jokela, J.; Heinila, L.; Shishido, T.; Wahlsten, M.; Fewer, D.; Fiore, M.; Wang, H.; Haapaniemi, E.; Permii, P.; Sivonen, K. Production of high amounts of hepatotoxin nodularin and new protease inhibitors pseudospumigins by the Brazilian benthic Nostoc sp. CENA543. Front. Microbiol. 2017, 8, 1963. [Google Scholar] [CrossRef]
- Yamaki, H.; Sitachitta, N.; Sano, T.; Kaya, K. Two new chymotrypsin inhibitors isolated from the cyanobacterium Microcystis aeruginosa NIES-88. J. Nat. Prod. 2005, 68, 14–18. [Google Scholar] [CrossRef]
- Okino, T.; Qi, S.; Matsuda, H.; Murakami, M.; Yamaguchi, K. Nostopeptins A and B, elastase inhibitors from the cyanobacterium Nostoc minutum. J. Nat. Prod. 1997, 60, 158–161. [Google Scholar] [CrossRef]
- Mehner, C.; Müller, D.; Kehraus, S.; Hautmann, S.; Gütschow, M.; König, G. New peptolides from the cyanobacterium Nostoc insulare as selective and potent inhibitors of human leukocyte elastase. ChemBioChem 2008, 9, 2692–2703. [Google Scholar] [CrossRef]
- Kaya, K.; Sano, T.; Beattie, K.; Codd, G. Nostocyclin, a novel 3-amino-6-hydroxy-2-piperidone-containing cyclic depsipeptide from the cyanobacterium Nostoc sp. Tetrahedron Lett. 1996, 37, 6725–6728. [Google Scholar] [CrossRef]
- Rouhiainen, L.; Jokela, J.; Fewer, D.; Urmann, M.; Sivonen, K. Two alternative starter modules for the Non-ribosomal biosynthesis of specific anabaenopeptin variants in Anabaena (cyanobacteria). Chem. Biol. 2010, 17, 265–273. [Google Scholar] [CrossRef]
- Guljamow, A.; Kreische, M.; Ishida, K.; Liaimer, A.; Altermark, B.; Bähr, L.; Hertweck, C.; Ehwald, R.; Dittmann, E. High-density cultivation of terrestrial Nostoc strains leads to reprogramming of secondary metabolome. Appl. Environ. Microbiol. 2017, 83, 1–15. [Google Scholar] [CrossRef]
- Shishido, T.; Jokela, J.; Fewer, D.; Wahlsten, M.; Fiore, M.; Sivonen, K. Simultaneous production of anabaenopeptins and namalides by the cyanobacterium Nostoc sp. CENA543. ACS Chem. Biol. 2017, 12, 2746–2755. [Google Scholar] [CrossRef]
- Cheruku, P.; Plaza, A.; Lauro, G.; Keffer, J.; Lloyd, J.; Bifulco, G.; Bewley, C. Discovery and synthesis of namalide reveals a new anabaenopeptin scaffold and peptidase inhibitor. J. Med. Chem. 2012, 55, 735–742. [Google Scholar] [CrossRef]
- Sanz, M.; Salinas, R.; Pinto, E. Namalides B and C and spumigins K-N from the cultured freshwater cyanobacterium Sphaerospermopsis torques-reginae. J. Nat. Prod. 2017, 80, 2492–2501. [Google Scholar] [CrossRef]
- Murakami, M.; Suzuki, S.; Itou, Y.; Kodani, S.; Ishida, K. New anabaenopeptins, potent carboxypeptidase-A inhibitors from cyanobacterium Aphanizomenon flos-aquae. J. Nat. Prod. 2000, 63, 1280–1282. [Google Scholar] [CrossRef]
- Harms, H.; Kurita, K.; Pan, L.; Wahome, P.; He, H.; Kinghorn, A.; Carter, G.; Linington, R. Discovery of anabaenopeptin 679 from freshwater algal bloom material: Insights into the structure-activity relationship of anabaenopeptin protease inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 4960–4965. [Google Scholar] [CrossRef]
- Sano, T.; Usui, T.; Ueda, K.; Osada, H.; Kaya, K. Isolation of new protein phosphatase inhibitors from two cyanobacteria species, Planktothrix spp. J. Nat. Prod. 2001, 64, 1052–1055. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fidor, A.; Konkel, R.; Mazur-Marzec, H. Bioactive Peptides Produced by Cyanobacteria of the Genus Nostoc: A Review. Mar. Drugs 2019, 17, 561. https://doi.org/10.3390/md17100561
Fidor A, Konkel R, Mazur-Marzec H. Bioactive Peptides Produced by Cyanobacteria of the Genus Nostoc: A Review. Marine Drugs. 2019; 17(10):561. https://doi.org/10.3390/md17100561
Chicago/Turabian StyleFidor, Anna, Robert Konkel, and Hanna Mazur-Marzec. 2019. "Bioactive Peptides Produced by Cyanobacteria of the Genus Nostoc: A Review" Marine Drugs 17, no. 10: 561. https://doi.org/10.3390/md17100561
APA StyleFidor, A., Konkel, R., & Mazur-Marzec, H. (2019). Bioactive Peptides Produced by Cyanobacteria of the Genus Nostoc: A Review. Marine Drugs, 17(10), 561. https://doi.org/10.3390/md17100561




