Structure Analysis and Anti-Tumor and Anti-Angiogenic Activities of Sulfated Galactofucan Extracted from Sargassum thunbergii
Abstract
1. Introduction
2. Results
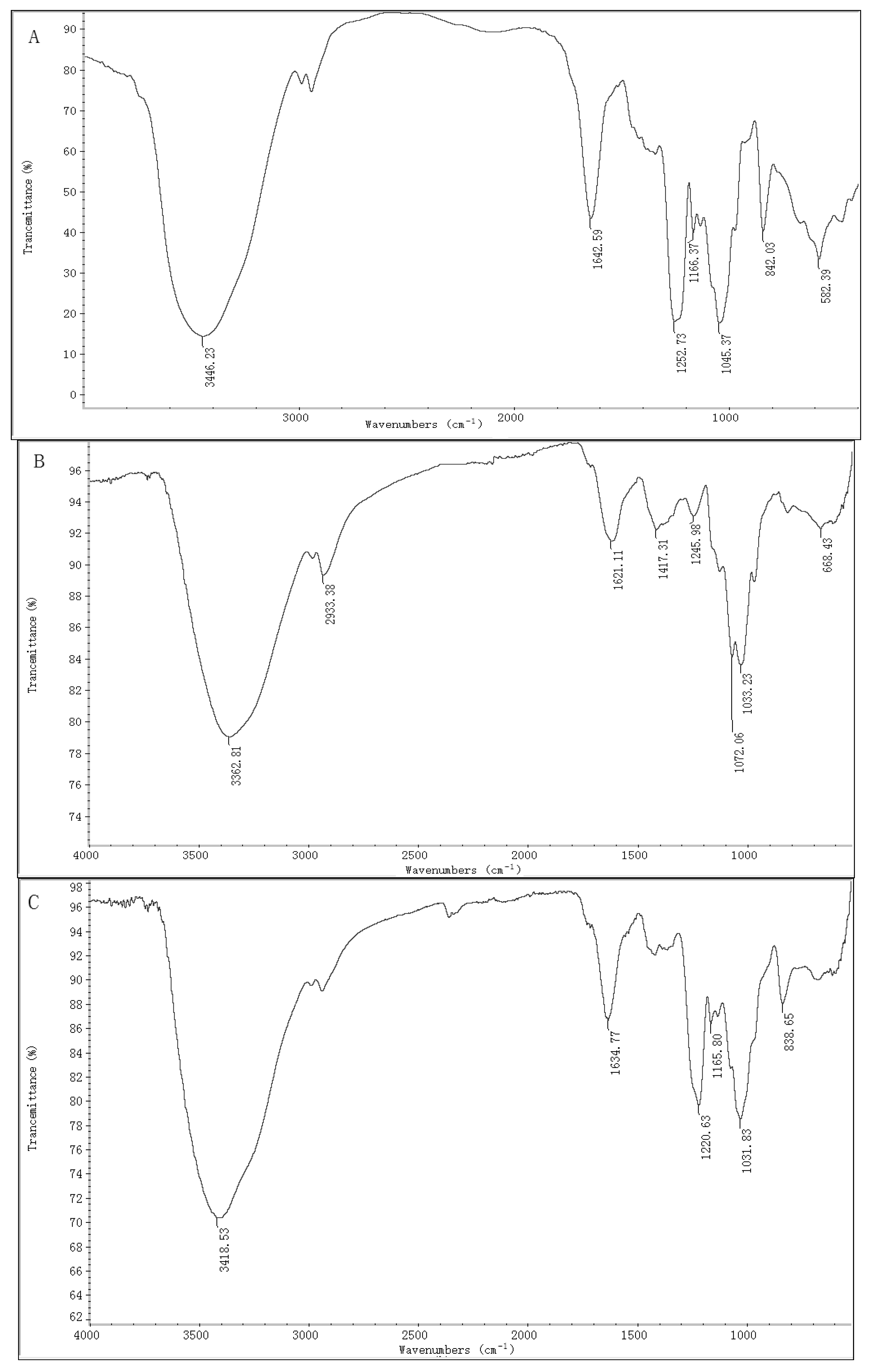
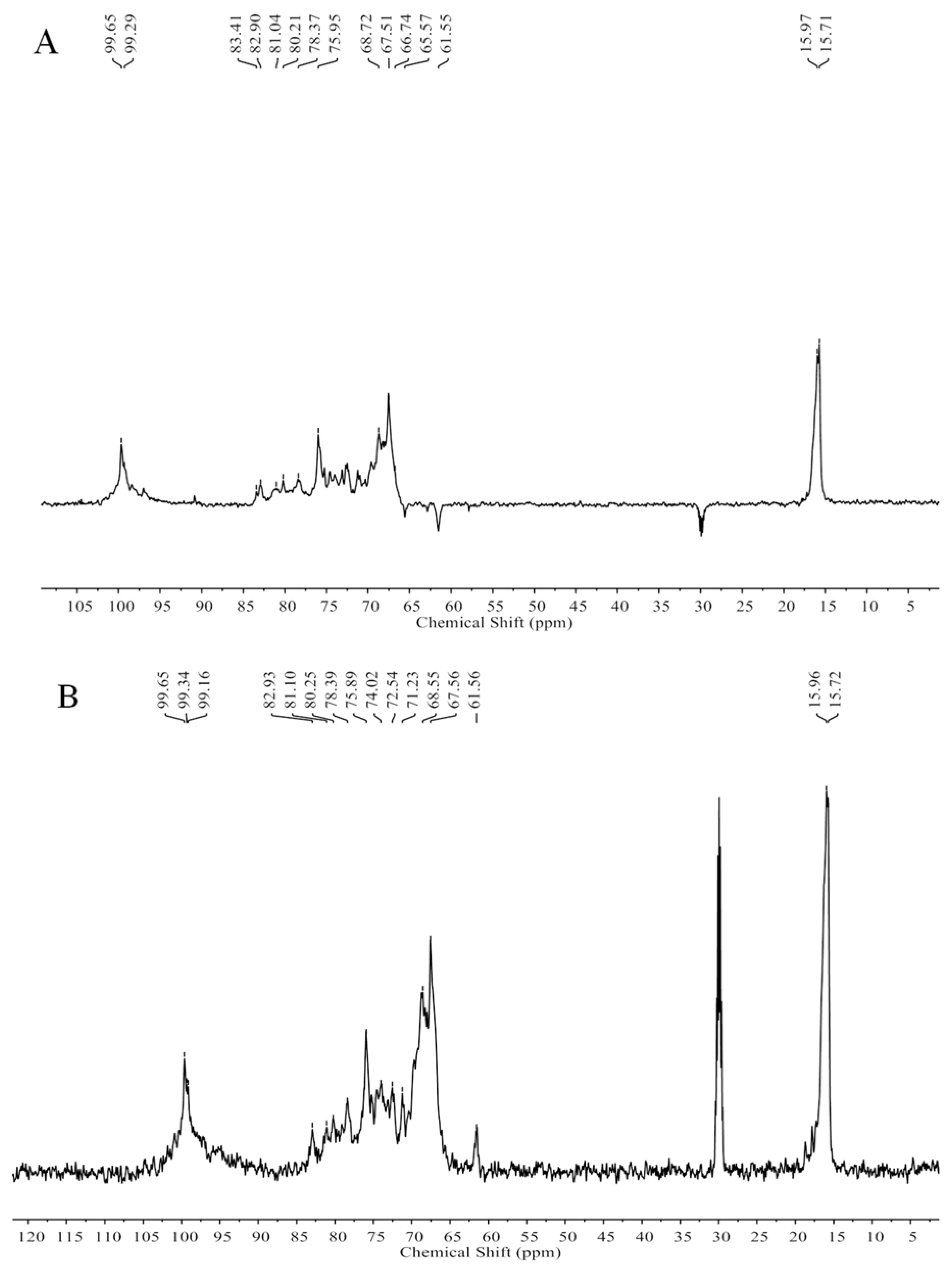
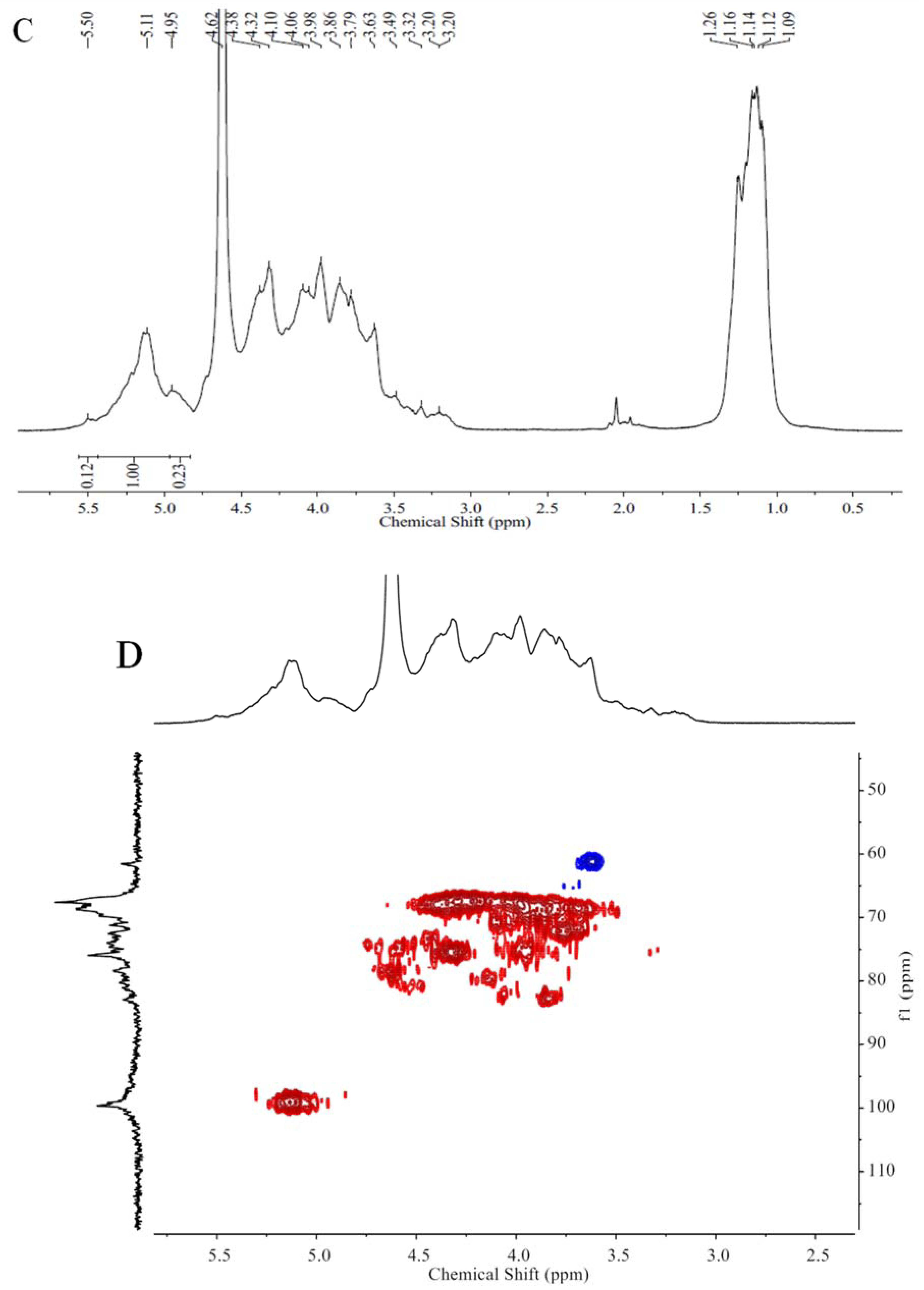
2.1. Chemical Compositions and Structrual Analyses of Sulfated Galactofucan and Its Derivates
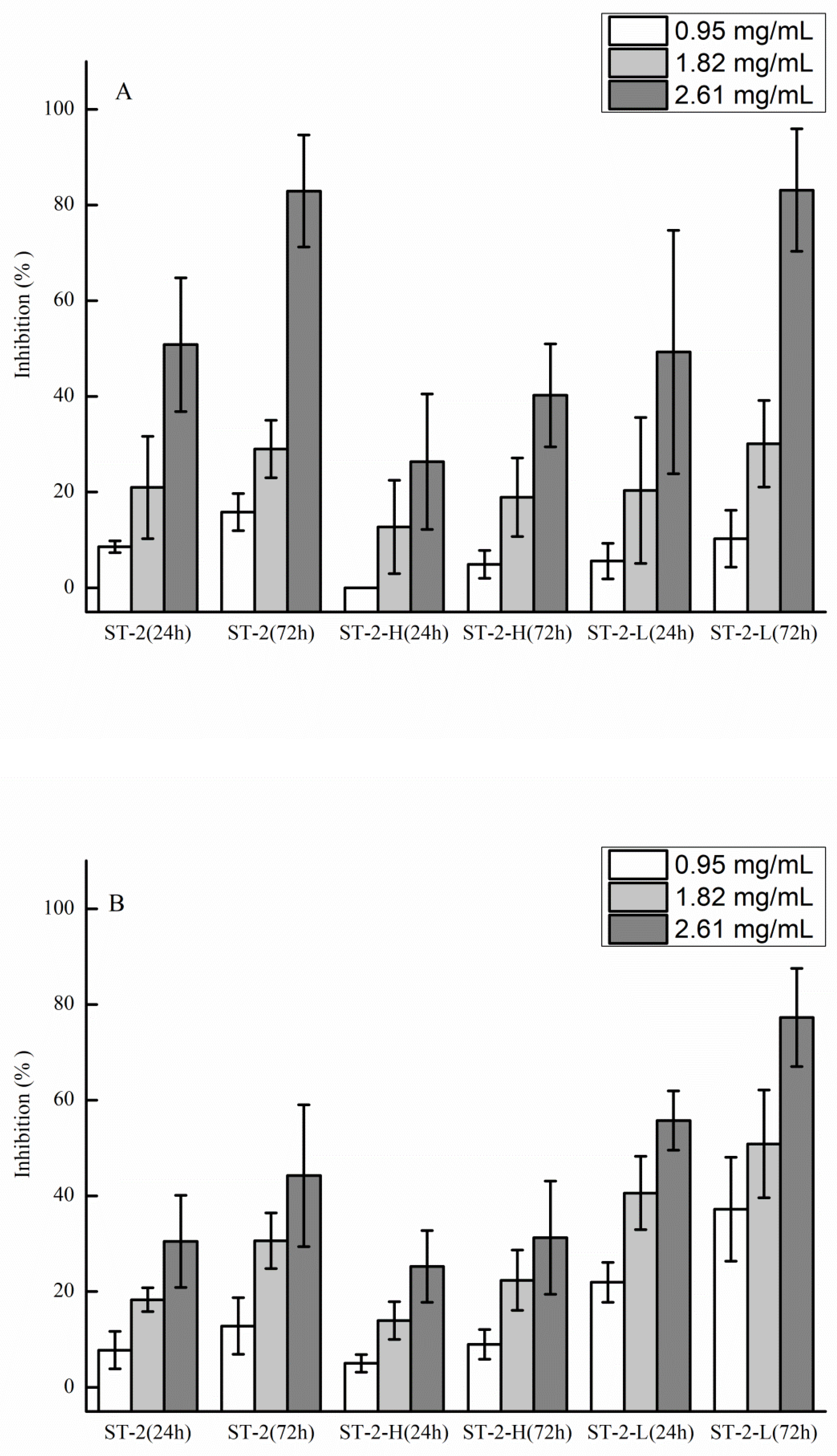
2.2. Anti-Tumor and Anti-Angiogenic Activities of Sulfated Galactofucan and Its Derivates
3. Materials and Methods
3.1. Materials
3.2. Preparation and Purification of Polysaccharides
3.3. Preparation of Desulfated Polysaccharides
3.4. Depolymerization of ST-2 by Autohydrolysis
3.5. Compositional Analysis
3.6. IR and MS Analysis of Oligosaccharides
3.7. NMR Spectroscopy
3.8. Anti-Tumor and Anti-Angiogenic Activities
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bilan, M.I.; Grachev, A.A.; Shashkov, A.S.; Kelly, M.; Sanderson, C.J.; Nifantiev, N.E.; Usov, A.I. Further studies on the composition and structure of a fucoidan preparation from the brown alga Saccharina latissima. Carbohydr. Res. 2010, 345, 2038–2047. [Google Scholar] [CrossRef]
- Bilan, M.I.; Zakharova, A.N.; Grachev, A.A.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. Polysaccharides of algae: 60. Fucoidan from the pacific brown alga Analipus japonicus (Harv.) winne (Ectocarpales, Scytosiphonaceae). Russ. J. Bioorg. Chem. 2007, 33, 38–46. [Google Scholar] [CrossRef]
- Chandía, N.P.; Matsuhiro, B. Characterization of a fucoidan from Lessonia vadosa (Phaeophyta) and its anticoagulant and elicitor properties. Int. J. Biol. Macromol. 2008, 42, 235–240. [Google Scholar] [CrossRef]
- Cong, Q.F.; Chen, H.J.; Liao, W.F.; Xiao, F.; Wang, P.P.; Qin, Y.; Dong, Q.; Ding, K. Structural characterization and effect on anti-angiogenic activity of a fucoidan from Sargassum fusiforme. Carbohydr. Polym. 2016, 136, 899–907. [Google Scholar] [CrossRef]
- Shevchenko, N.M.; Anastyuk, S.D.; Menshova, R.V.; Vishchuk, O.S.; Isakov, V.I.; Zadorozhny, P.A.; Sikorskaya, T.V.; Zvyagintseva, T.N. Further studies on structure of fucoidan from brown alga Saccharina gurjanovae. Carbohydr. Polym. 2015, 121, 207–216. [Google Scholar] [CrossRef]
- Bilan, M.I.; Grachev, A.A.; Ustuzhanina, N.E.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. Structure of a fucoidan from the brown seaweed Fucus evanescens C.Ag. Carbohydr. Res. 2002, 337, 719–730. [Google Scholar] [CrossRef]
- Bilan, M.I.; Grachev, A.A.; Ustuzhanina, N.E.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. A highly regular fraction of a fucoidan from the brown seaweed Fucus distichus L. Carbohydr. Res. 2004, 339, 511–517. [Google Scholar] [CrossRef]
- Bilan, M.I.; Grachev, A.A.; Shashkov, A.S.; Thuy, T.T.; Van, T.T.; Ly, B.M.; Nifantiev, N.E.; Usov, A.I. Preliminary investigation of a highly sulfated galactofucan fraction isolated from the brown alga Sargassum polycystum. Carbohydr. Res. 2013, 377, 48–57. [Google Scholar] [CrossRef]
- Duarte, M.E.R.; Cardoso, M.A.; Noseda, M.D.; Cerezo, A.S. Structural studies on fucoidans from the brown seaweed Sargassum stenophyllum. Carbohydr. Res. 2001, 333, 281–293. [Google Scholar] [CrossRef]
- Berteau, O.; Mulloy, B. Sulfated fucans, fresh perspectives: Structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 2003, 13, 29R–40R. [Google Scholar] [CrossRef]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H. Chemical Structures and Bioactivities of Sulfated Polysaccharides from Marine Algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef]
- Kusaykin, M.; Bakunina, I.; Sova, V.; Ermakova, S.; Kuznetsova, T.; Besednova, N.; Zaporozhets, T.; Zvyagintseva, T. Structure, biological activity, and enzymatic transformation of fucoidans from the brown seaweeds. Biotechnol. J. 2008, 3, 904–915. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and Bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef]
- Mestechkina, N.M.; Shcherbukhin, V.D. Sulfated Polysaccharides and Their Anticoagulant Activity:A Review. Appl. Biochem. Microbiol. 2010, 46, 291–298. [Google Scholar] [CrossRef]
- Pomin, V.H. Structural and functional insights into sulfated galactans: A systematic review. Glycoconjuate. J. 2010, 27, 1–12. [Google Scholar] [CrossRef]
- Pomin, V.H.; Mourao, P.A.S. Structure, biology, evolution, and medical importance of sulfated fucans and galactans. Glycobiology 2008, 18, 1016–1027. [Google Scholar] [CrossRef]
- Usov, A.I.; Bilan, M.I. Fucoidans-sulfatedd polysaccharides of brown algae. Russ. Chem. Rev. 2009, 78, 785–799. [Google Scholar] [CrossRef]
- Wijesekara, I.; Pangestuti, R.; Kim, S.-K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2011, 84, 14–21. [Google Scholar] [CrossRef]
- Wijesinghe, W.A.J.P.; Jeon, Y.-J. Biological activities and potential industrial applications of fucose rich sulfated polysaccharides and fucoidans isolated from brown seaweeds: A review. Carbohydr. Polym. 2012, 88, 13–20. [Google Scholar] [CrossRef]
- Ito, H.; Sugiura, M. Antitumor polysaccharide fraction frm Sargassum thunbergii. Chem. Pharm. Bull. 1976, 24, 1114–1115. [Google Scholar] [CrossRef]
- Jin, W.; Liu, G.; Zhong, W.; Sun, C.; Zhang, Q. Polysaccharides from Sargassum thunbergii: Monthly variations and anti-complement and anti-tumour activities. Int. J. Biol. Macromol. 2017, 105, 1526–1531. [Google Scholar] [CrossRef]
- Jin, W.; Zhang, W.; Liu, G.; Yao, J.; Shan, T.; Sun, C.; Zhang, Q. The structure-activity relationship between polysaccharides from Sargassum thunbergii and anti-tumor activity. Int. J. Biol. Macromol. 2017, 105, 686–692. [Google Scholar] [CrossRef]
- Zhuang, C.; Itoh, H.; Mizuno, T.; Ito, H. Antitumor Active Fucoidan from the Brown Seaweed, Umitoranoo (Sargassum thunbergii). Biosci. Biotechnol. Biochem. 1995, 59, 563–567. [Google Scholar] [CrossRef]
- Han, Y.S.; Lee, J.H.; Lee, S.H. Fucoidan inhibits the migration and proliferation of HT-29 human colon cancer cells via the phosphoinositide-3 kinase/Akt/mechanistic target of rapamycin pathways. Mol. Med. Rep. 2015, 12, 3446–3452. [Google Scholar] [CrossRef]
- Kim, H.; Jeon, T.J. Fucoidan Induces Cell Aggregation and Apoptosis in Osteosarcoma MG-63 Cells. Anim. Cells Syst. 2016, 20, 186–192. [Google Scholar] [CrossRef]
- Liu, G.; Kuang, S.; Wu, S.; Jin, W.; Sun, C. A novel polysaccharide from Sargassum integerrimum induces apoptosis in A549 cells and prevents angiogensis in vitro and in vivo. Sci. Rep. 2016, 6, 26722. [Google Scholar] [CrossRef]
- Zhang, W.; Jin, W.; Sun, D.; Zhao, L.; Wang, J.; Duan, D.; Zhang, Q. Structural Analysis and Anti-Complement Activity of Polysaccharides from Kjellmaniella crsaaifolia. Mar. Drugs 2015, 13, 1360–1374. [Google Scholar] [CrossRef]
- Jin, W.; Zhang, W.; Wang, J.; Ren, S.; Song, N.; Duan, D.; Zhang, Q. Characterization of laminaran and a highly sulfated polysaccharide from Sargassum fusiforme. Carbohydr. Res. 2014, 385, 58–64. [Google Scholar] [CrossRef]
- Foley, S.A.; Mulloy, B.; Tuohy, M.G. An Unfractionated Fucoidan from Ascophyllum nodosum: Extraction, Characterization, and Apoptotic Effects in Vitro. J. Nat. Prod. 2011, 74, 1851–1861. [Google Scholar] [CrossRef]
- Synytsya, A.; Kim, W.-J.; Kim, S.-M.; Pohl, R.; Synytsya, A.; Kvasnička, F.; Čopíková, J.; Il Park, Y. Structure and antitumour activity of fucoidan isolated from sporophyll of Korean brown seaweed Undaria pinnatifida. Carbohydr. Polym. 2010, 81, 41–48. [Google Scholar] [CrossRef]
- Anastyuk, S.D.; Shevchenko, N.M.; Ermakova, S.P.; Vishchuk, O.S.; Nazarenko, E.L.; Dmitrenok, P.S.; Zvyagintseva, T.N. Anticancer activity in vitro of a fucoidan from the brown alga Fucus evanescens and its low-molecular fragments, structurally characterized by tandem mass-spectrometry. Carbohydr. Polym. 2012, 87, 186–194. [Google Scholar] [CrossRef]
- Anastyuk, S.D.; Shevchenko, N.M.; Nazarenko, E.L.; Imbs, T.I.; Gorbach, V.I.; Dmitrenok, P.S.; Zvyagintseva, T.N. Structural analysis of a highly sulfated fucan from the brown alga Laminaria cichorioides by tandem MALDI and ESI mass spectrometry. Carbohydr. Res. 2010, 345, 2206–2212. [Google Scholar] [CrossRef]
- Ciancia, M.; Satob, Y.; Nonamic, H.; Cerezod, A.S.; Erra-Balsellsd, R.; Matulewicz, M.C. Autohydrolysis of a partially cyclized mu/nu-carrageenan and structural elucidation of the oligosaccharides by chemical analysis, NMR spectroscopy and UV-MALDI mass spectrometry. Arkivoc 2005, 2005, 319–331. [Google Scholar]
- Menshova, R.V.; Anastyuk, S.D.; Ermakova, S.P.; Shevchenko, N.M.; Isakov, V.I.; Zvyagintseva, T.N. Structure and anticancer activity in vitro of sulfated galactofucan from brown alga Alaria angusta. Carbohydr. Polym. 2015, 132, 118–125. [Google Scholar] [CrossRef]
- Jin, W.; Liu, B.; Li, S.; Chen, J.; Tang, H.; Jiang, D.; Zhang, Q.; Zhong, W. The structural features of the sulfated heteropolysaccharide (ST-1) from Sargassum thunbergii and its neuroprotective activities. Int. J. Biol. Macromol. 2018, 108, 307–313. [Google Scholar] [CrossRef]
- Jin, W.; Zhang, W.; Wang, J.; Ren, S.; Song, N.; Zhang, Q. Structural analysis of heteropolysaccharide from Saccharina japonica and its derived oligosaccharides. Int. J. Biol. Macromol. 2013, 62C, 697–704. [Google Scholar] [CrossRef]
- Anastyuk, S.D.; Imbs, T.I.; Shevchenko, N.M.; Dmitrenok, P.S.; Zvyagintseva, T.N. ESIMS analysis of fucoidan preparations from Costaria costata, extracted from alga at different life-stages. Carbohydr. Polym. 2012, 90, 993–1002. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Q.; Wang, J.; Shi, X.; Zhang, Z. Analysis of the Monosaccharide Composition of Fucoidan by Precolumn Derivation HPLC. Chin. J. Oceanol. Limnol. 2009, 27, 1–5. [Google Scholar] [CrossRef]
- Bitter, T.; Muir, H.M. A modified uronic acid carbazole reaction. Anal. Biochem. 1962, 4, 330–334. [Google Scholar] [CrossRef]

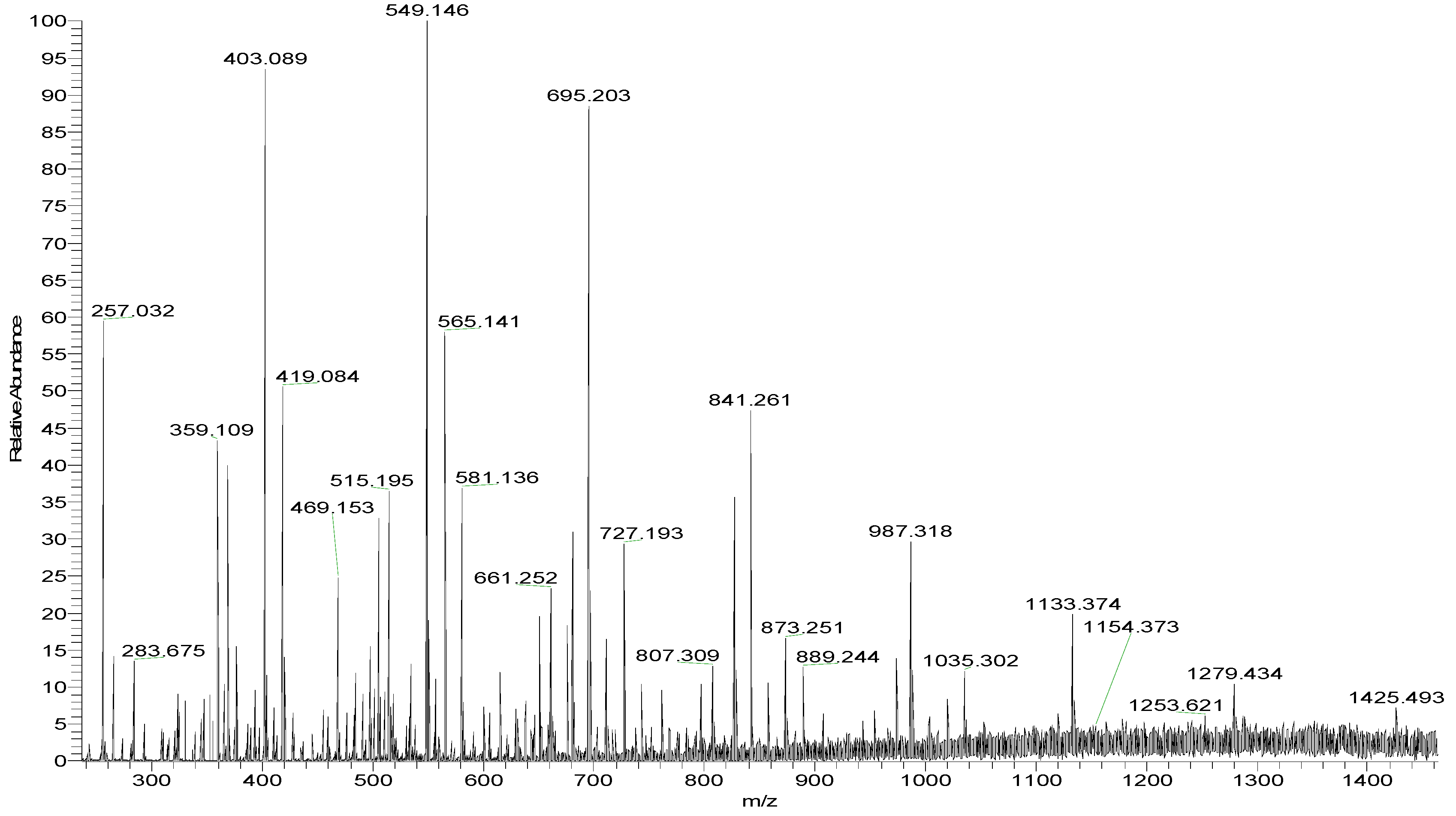
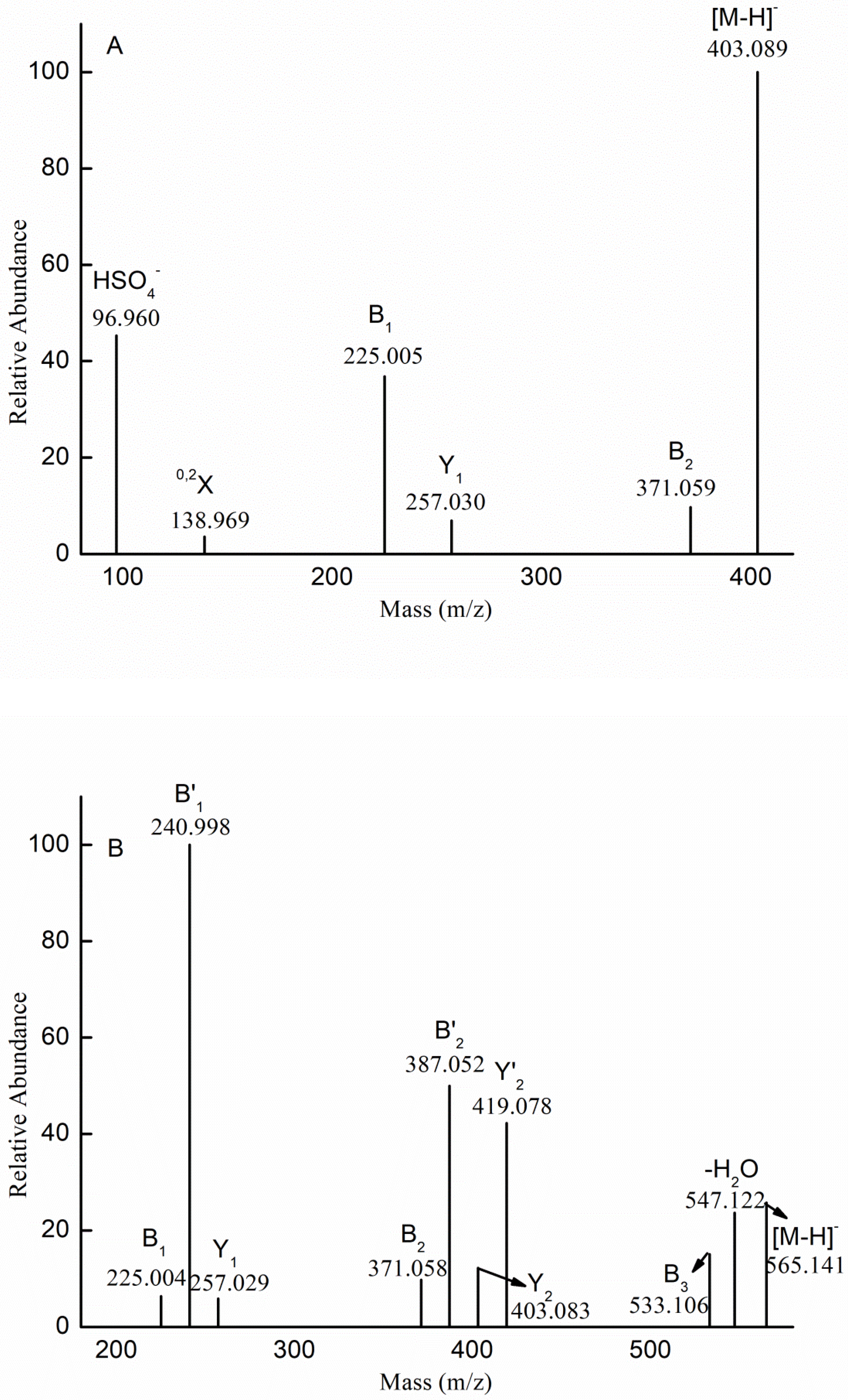
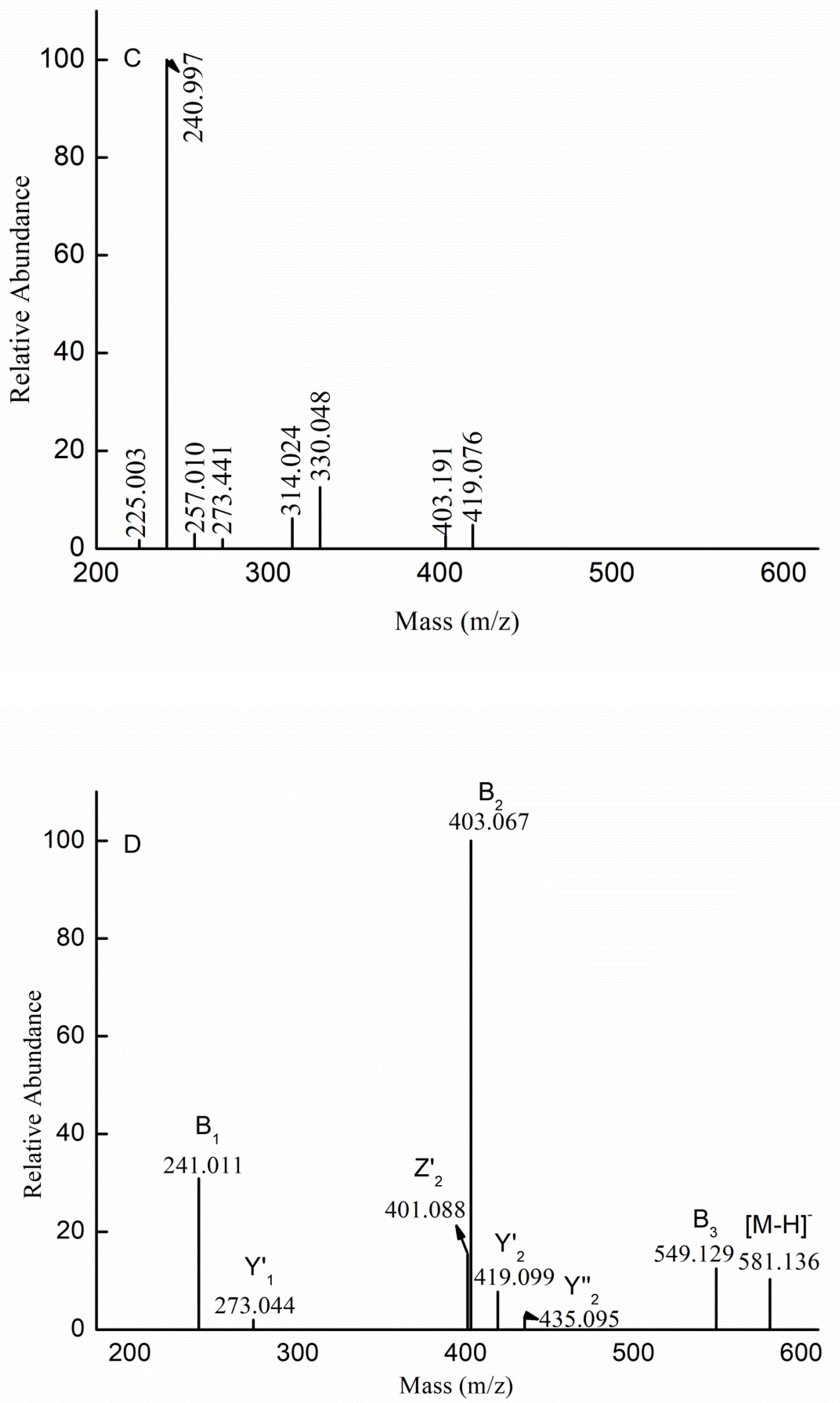
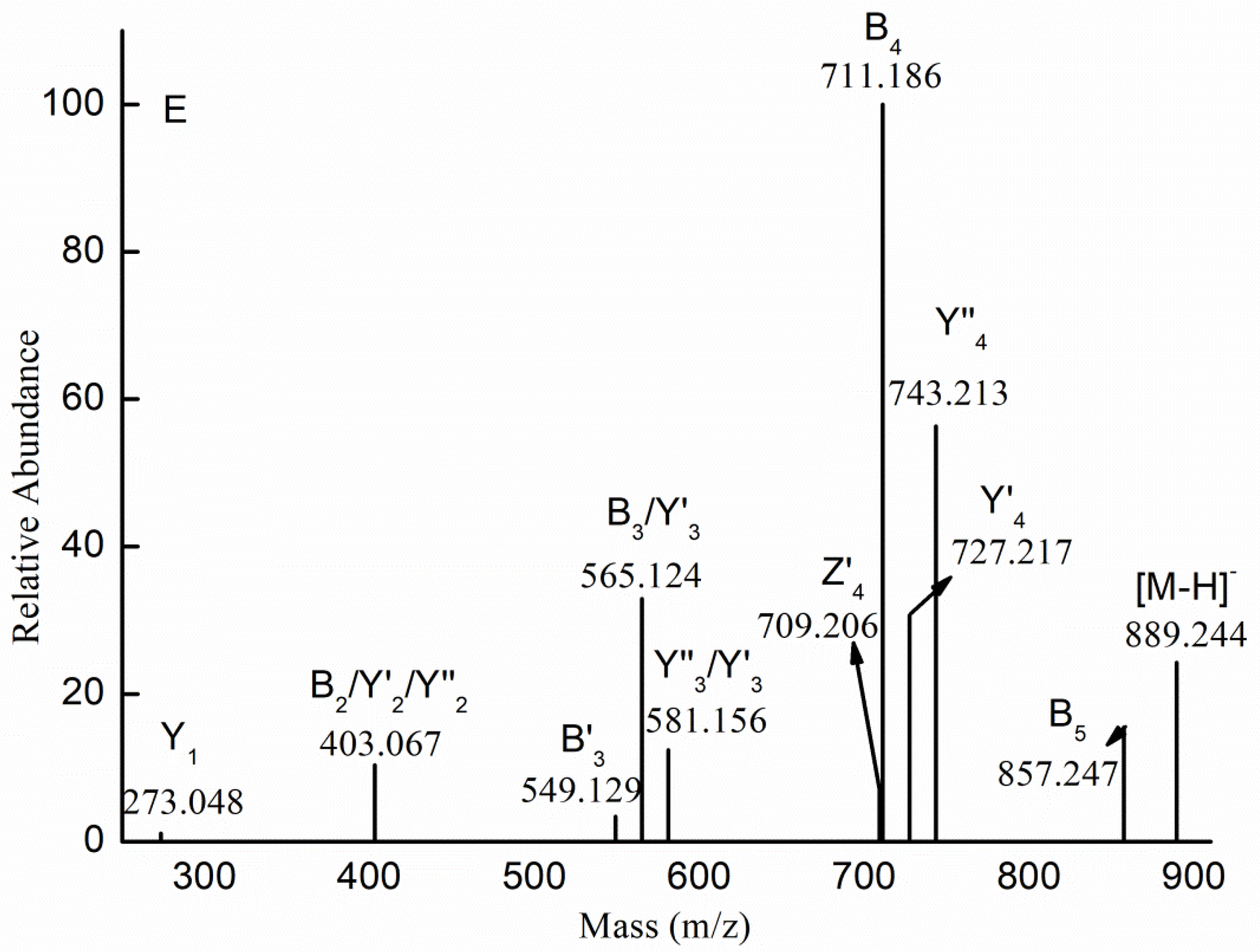
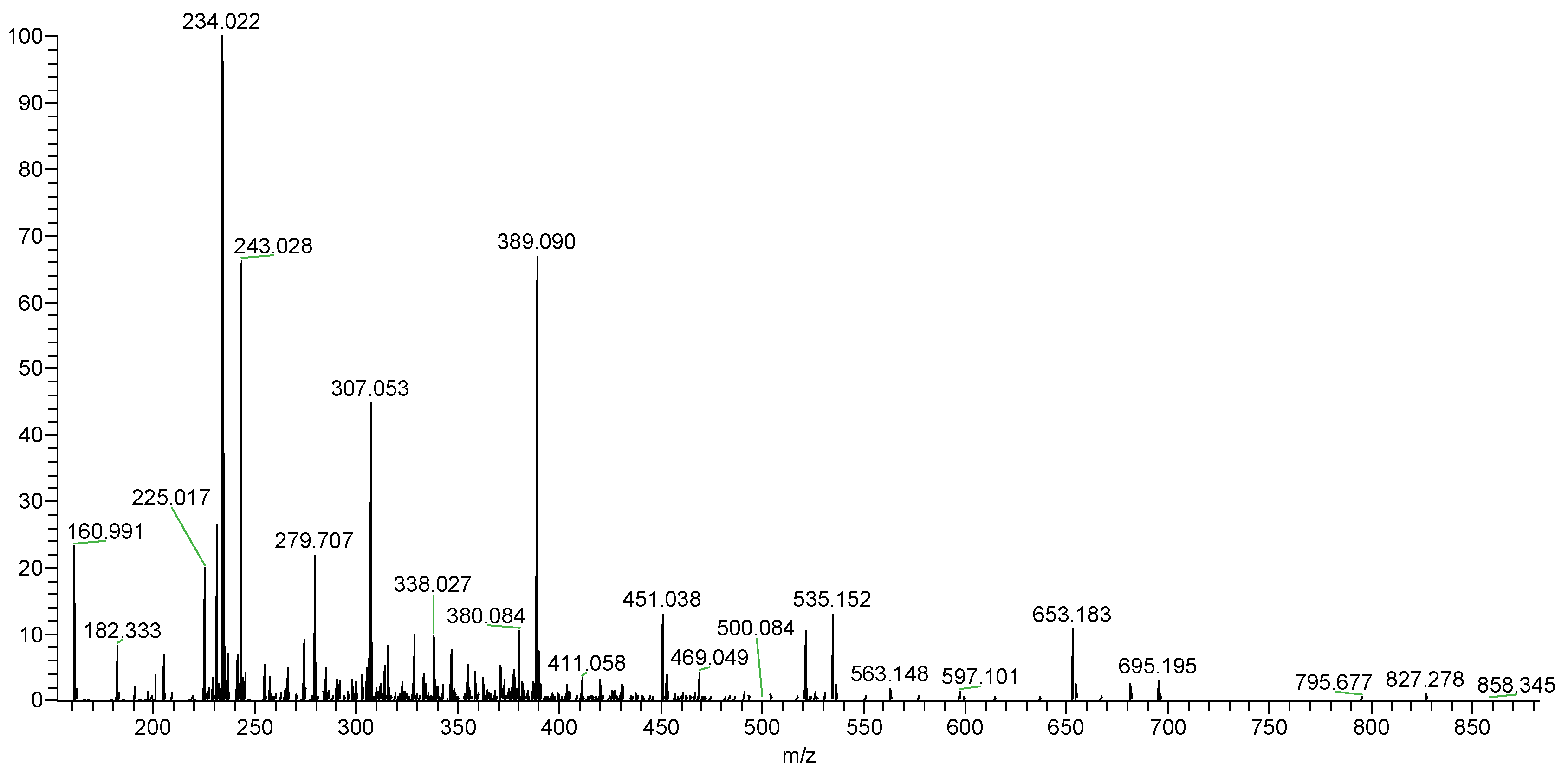

| m/z | Charge | Composition | m/z | Charge | Composition |
|---|---|---|---|---|---|
| 257.032 | 1 | [Me Fuc(SO3H)-H]− | 330.024 | 2 | [Me Gal2Fuc(SO3H)2-2H]2− |
| 403.089 | 1 | [Me Fuc2(SO3H)-H]− | 807.309 | 1 | [Me Gal2Fuc2(SO3H)2-H]− |
| 549.146 | 1 | [Me Fuc3(SO3H)-H]− | 476.059 | 2 | [Me Gal2Fuc3(SO3H)2-2H]2− |
| 695.203 | 1 | [Me Fuc4(SO3H)-H]− | 419.084 | 1 | [Me GalFuc(SO3H)-H]− |
| 841.261 | 1 | [Me Fuc5(SO3H)-H]− | 565.141 | 1 | [Me GalFuc2(SO3H)-H]− |
| 987.318 | 1 | [Me Fuc6(SO3H)-H]− | 711.198 | 1 | [Me GalFuc3(SO3H)-H]− |
| 1133.374 | 1 | [Me Fuc7(SO3H)-H]− | 857.257 | 1 | [Me GalFuc4(SO3H)-H]− |
| 1279.434 | 1 | [Me Fuc8(SO3H)-H]− | 1003.316 | 1 | [Me GalFuc5(SO3H)-H]− |
| 1425.493 | 1 | [Me Fuc9(SO3H)-H]− | 581.136 | 1 | [Me Gal2Fuc(SO3H)-H]− |
| 389.073 | 1 | [Fuc2(SO3H)-H]− | 727.193 | 1 | [Me Gal2Fuc2(SO3H)-H]− |
| 535.130 | 1 | [Fuc3(SO3H)-H]− | 873.251 | 1 | [Me Gal2Fuc3(SO3H)-H]− |
| 681.188 | 1 | [Fuc4(SO3H)-H]− | 1019.305 | 1 | [Me Gal2Fuc4(SO3H)-H]− |
| 827.247 | 1 | [Fuc5(SO3H)-H]− | 743.188 | 1 | [Me Gal3Fuc(SO3H)-H]− |
| 973.303 | 1 | [Fuc6(SO3H)-H]− | 889.244 | 1 | [Me Gal3Fuc2(SO3H)-H]− |
| 1119.361 | 1 | [Fuc7(SO3H)-H]− | 1035.302 | 1 | [Me Gal3Fuc3(SO3H)-H]− |
| 160.991 | 2 | [Fuc(SO3H)2-2H]2− | 347.032 | 2 | [Fuc3(SO3H)3-2H]2− |
| 182.332 | 3 | [Fuc2(SO3H)3-3H]3− | 380.084 | 2 | [Fuc4(SO3H)2-2H]2− |
| 234.022 | 2 | [Fuc2(SO3H)2-2H]2− | 469.049 | 1 | [Fuc2(SO3H)2-H]− |
| 243.028 | 1 | [Fuc(SO3H)-H]− | 695.195 | 1 | [Fuc3(SO3H)3-H]− |
| 279.707 | 3 | [Fuc4(SO3H)3-3H]3− | 521.136 | 1 | [XylFuc2(SO3H)-H]− |
| 307.053 | 2 | [Fuc3(SO3H)2-2H]2− | 653.183 | 1 | [Xyl2Fuc2(SO3H)-H]− |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, W.; Wu, W.; Tang, H.; Wei, B.; Wang, H.; Sun, J.; Zhang, W.; Zhong, W. Structure Analysis and Anti-Tumor and Anti-Angiogenic Activities of Sulfated Galactofucan Extracted from Sargassum thunbergii. Mar. Drugs 2019, 17, 52. https://doi.org/10.3390/md17010052
Jin W, Wu W, Tang H, Wei B, Wang H, Sun J, Zhang W, Zhong W. Structure Analysis and Anti-Tumor and Anti-Angiogenic Activities of Sulfated Galactofucan Extracted from Sargassum thunbergii. Marine Drugs. 2019; 17(1):52. https://doi.org/10.3390/md17010052
Chicago/Turabian StyleJin, Weihua, Wanli Wu, Hong Tang, Bin Wei, Hong Wang, Jiadong Sun, Wenjing Zhang, and Weihong Zhong. 2019. "Structure Analysis and Anti-Tumor and Anti-Angiogenic Activities of Sulfated Galactofucan Extracted from Sargassum thunbergii" Marine Drugs 17, no. 1: 52. https://doi.org/10.3390/md17010052
APA StyleJin, W., Wu, W., Tang, H., Wei, B., Wang, H., Sun, J., Zhang, W., & Zhong, W. (2019). Structure Analysis and Anti-Tumor and Anti-Angiogenic Activities of Sulfated Galactofucan Extracted from Sargassum thunbergii. Marine Drugs, 17(1), 52. https://doi.org/10.3390/md17010052








