New 1,2,4-Oxadiazole Nortopsentin Derivatives with Cytotoxic Activity
Abstract
1. Introduction
2. Results and Discussion
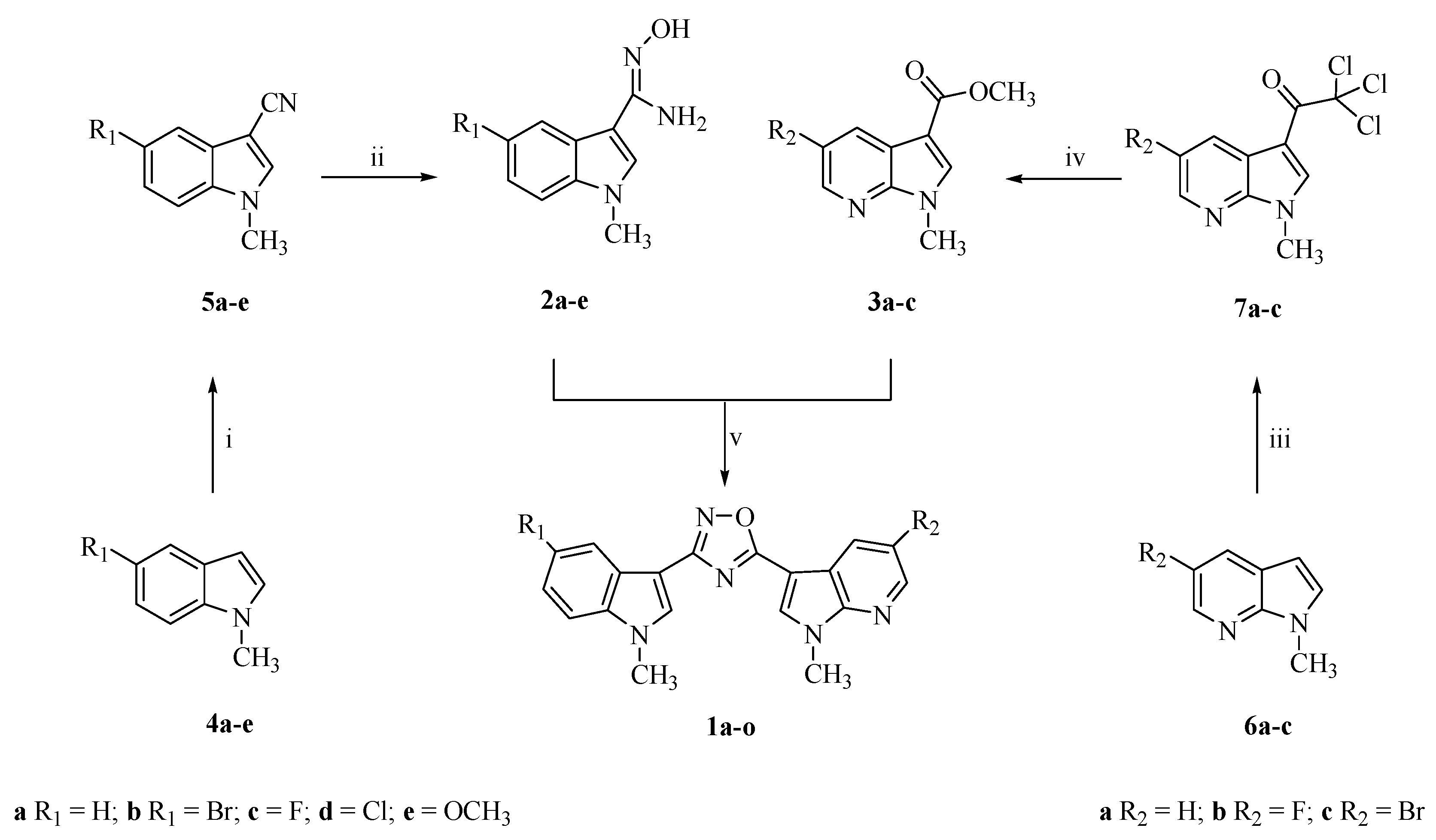
2.1. Chemistry
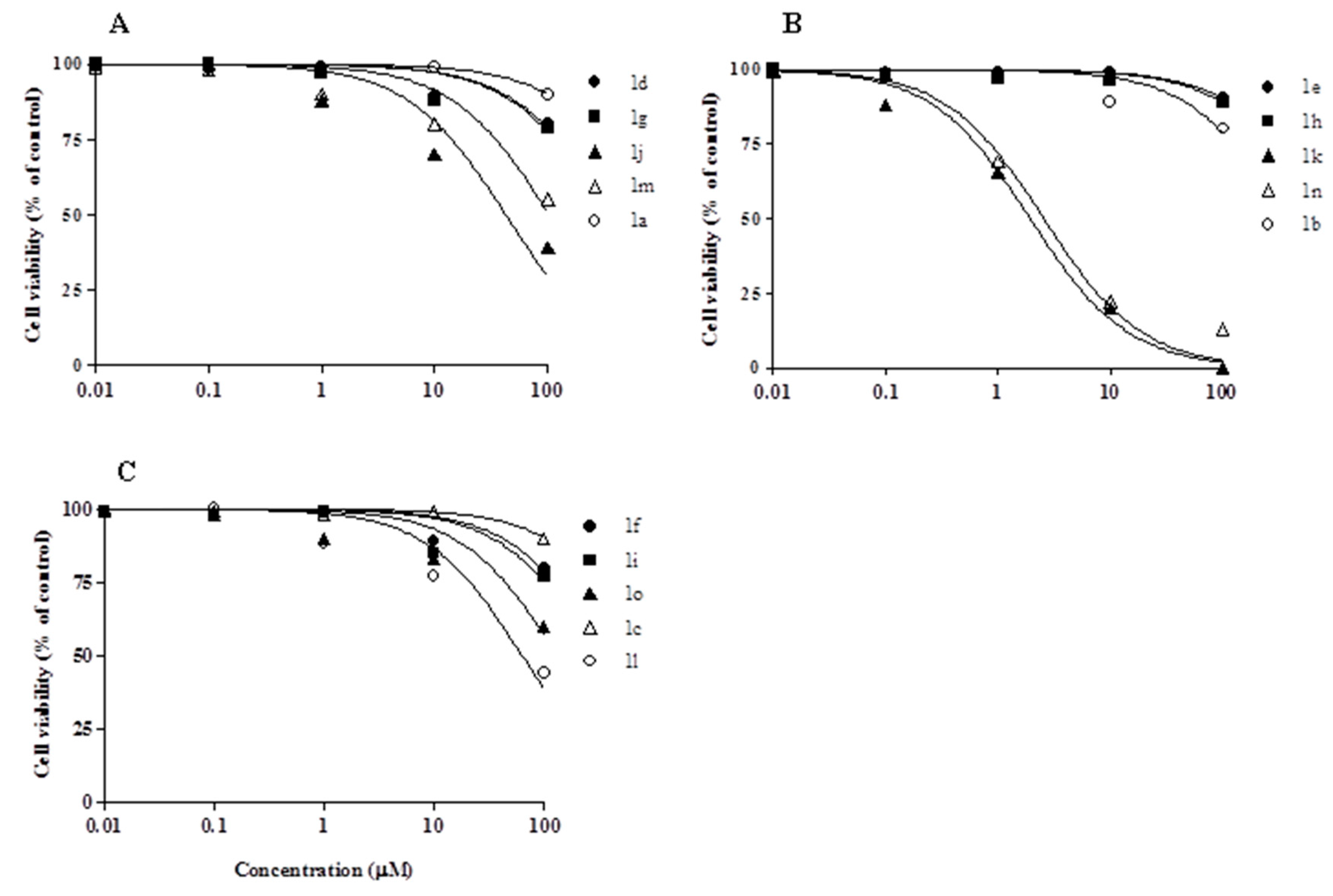
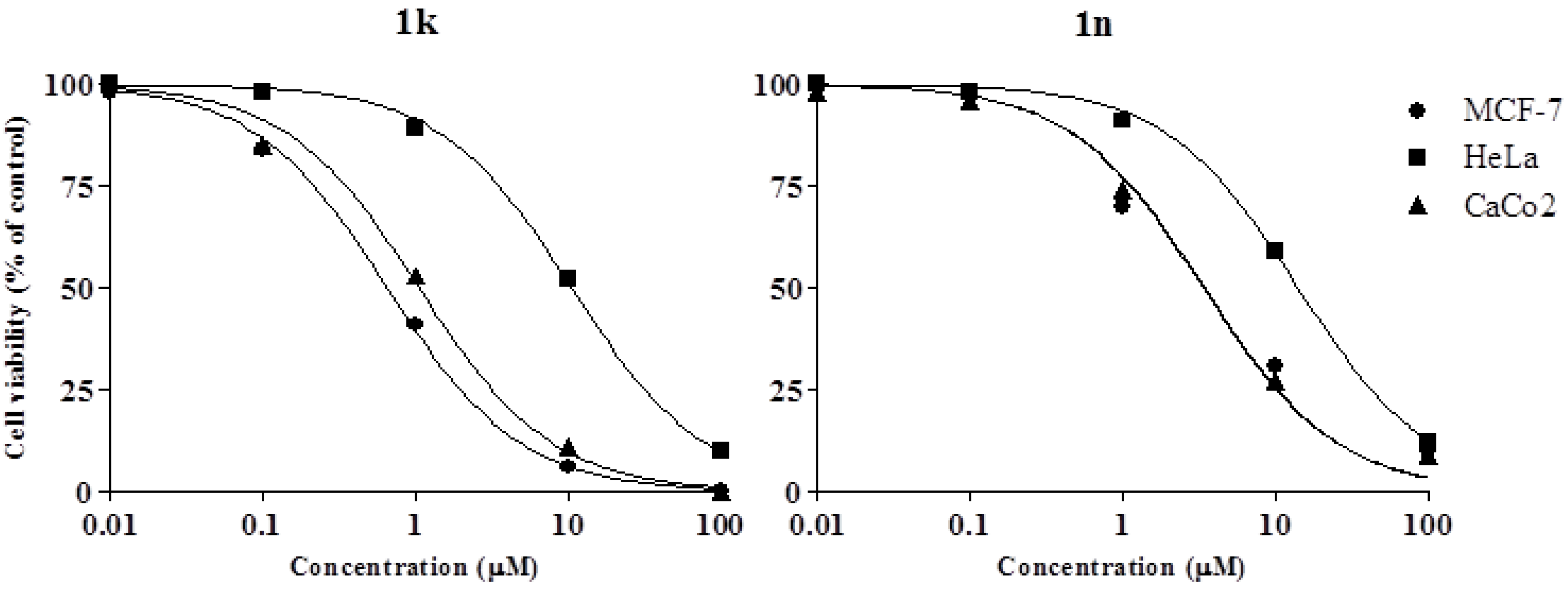
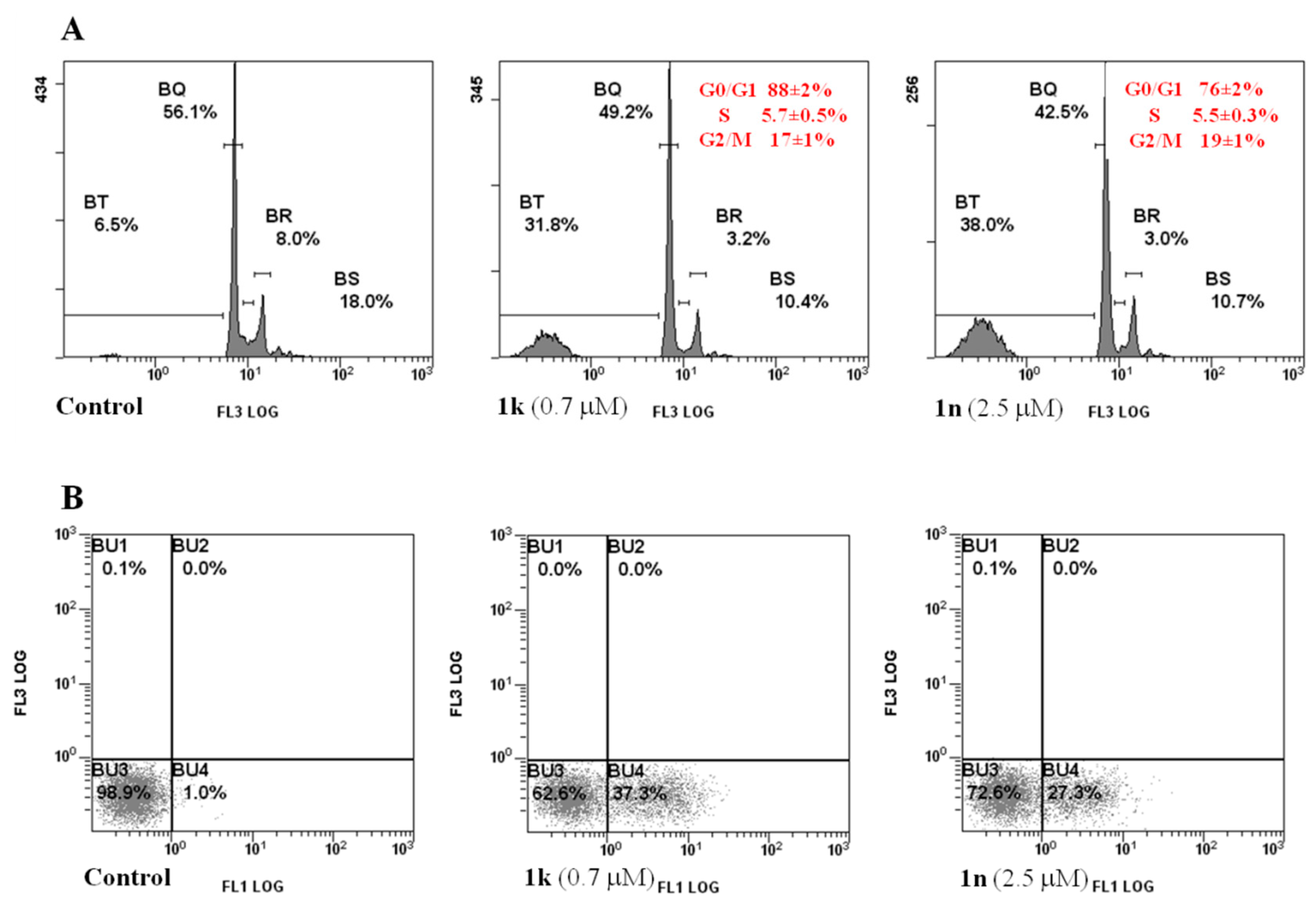
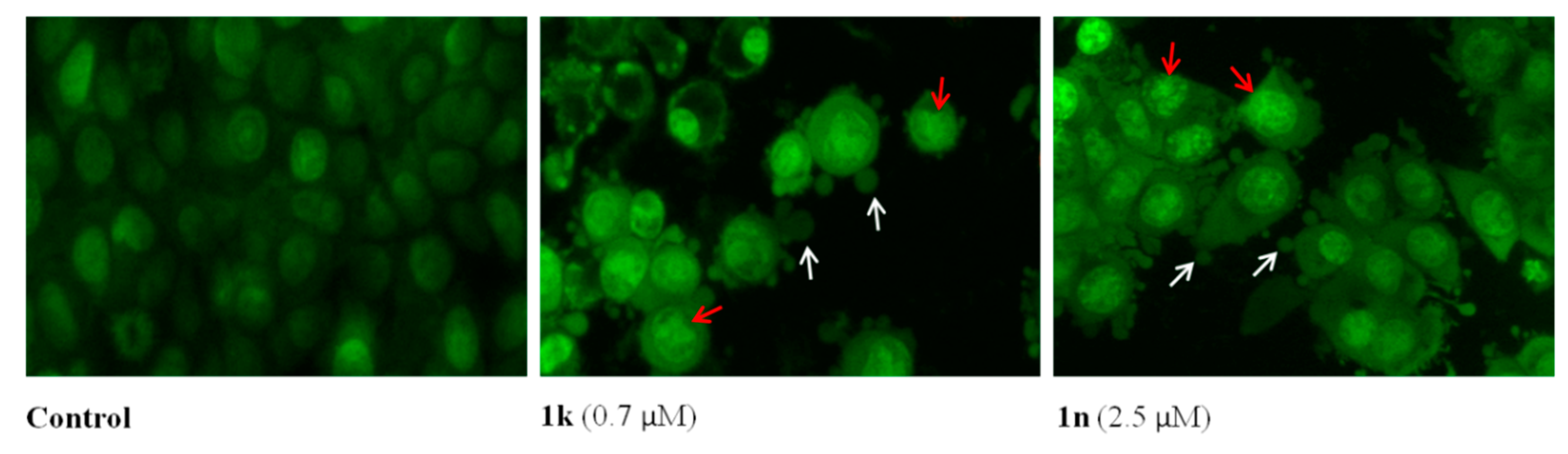
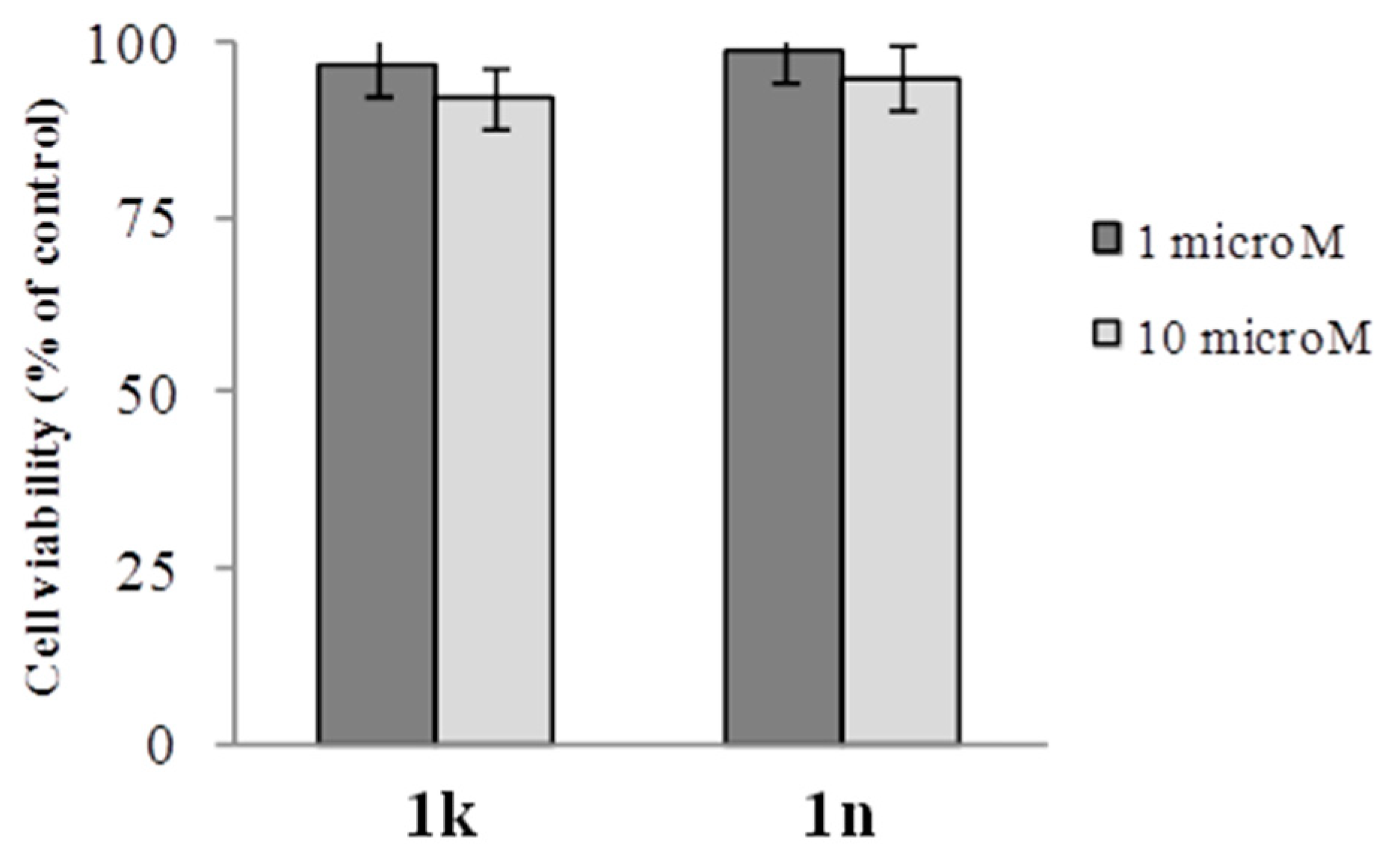
2.2. Biology
3. Materials and Methods
3.1. Chemistry
3.1.1. General
General Procedure for the Synthesis of 1-methyl-1H-indole-3-carbonitriles (5a–e)
General Procedure for the Synthesis of N′-hydroxy-1-methyl-1H-indole-3-carboximidamides (2a–e)
General Procedure for the Synthesis of 2,2,2-trichloro-1-(1-methyl-1H-pyrrolo[2,3-b]pyridin-3-yl)ethanones (7a–c)
General Procedure for the Synthesis of methyl-1-methyl-1H-pyrrolo[2,3-b]pyridine-3-carboxylate (3a–c)
General Procedure for the Synthesis of 1-methyl-3-[3-(1-methyl-1H-indol-3-yl)-1,2,4-oxadiazol-5-yl]-1H-pyrrolo[2,3-b]pyridines (1a–o)
3.2. Biology
3.2.1. Viability Assay in Vitro
3.2.2. Cell Cycle Analysis
3.2.3. Cell Death Detection
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.; Vieira, H.; Gaspar, H.; Santos, S. Marketed Marine Natural Products in the Pharmaceutical and Cosmeceutical Industries: Tips for Success. Mar. Drugs 2014, 12, 1066–1101. [Google Scholar] [CrossRef] [PubMed]
- Sakemi, S.; Sun, H.H. Nortopsentins A, B and C. Cytotoxic and antifungal imidazolediylbis[indoles] from the sponge Spongosorites ruetzleri. J. Org. Chem. 1991, 56, 4304–4307. [Google Scholar] [CrossRef]
- Diana, P.; Carbone, A.; Barraja, P.; Montalbano, A.; Martorana, A.; Dattolo, G.; Gia, O.; Dalla Via, L.; Cirrincione, G. Synthesis and antitumor properties of 2,5-bis(3′-indolyl)thiophenes: Analogues of marine alkaloid nortopsentin. Bioorg. Med. Chem. Lett. 2007, 17, 2342–2346. [Google Scholar] [CrossRef] [PubMed]
- Diana, P.; Carbone, A.; Barraja, P.; Martorana, A.; Gia, O.; Dalla Via, L.; Cirrincione, G. 3,5-Bis(3′-indolyl)pyrazoles, analogues of marine alkaloid nortopsentin: Synthesis and antitumor properties. Bioorg. Med. Chem. Lett. 2007, 17, 6134–6137. [Google Scholar] [CrossRef] [PubMed]
- Diana, P.; Carbone, A.; Barraja, P.; Kelter, G.; Fiebig, H.H.; Cirrincione, G. Synthesis and antitumor activity of 2,5-bis(3′-indolyl)-furans and 3,5-bis(3′-indolyl)-isoxazoles, nortopsentin analogues. Bioorg. Med. Chem. 2010, 18, 4524–4529. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Parrino, B.; Barraja, P.; Spanò, V.; Cirrincione, G.; Diana, P.; Maier, A.; Kelter, G.; Fiebig, H.H. Synthesis and antiproliferative activity of 2,5-bis(3′-indolyl)pyrroles, analogues of the marine alkaloid nortopsentin. Mar. Drugs 2013, 11, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Kumar, N.M.; Chang, K.H.; Gupta, R.; Shah, K. Synthesis and in vitro anticancer activity of 3,5-bis(indolyl)-1,2,4-thiadiazoles. Bioorg. Med. Chem. Lett. 2011, 21, 5897–5900. [Google Scholar] [CrossRef]
- Carbone, A.; Parrino, B.; Cusimano, M.G.; Spanò, V.; Montalbano, A.; Barraja, P.; Schillaci, D.; Cirrincione, G.; Diana, P.; Cascioferro, S. New thiazole nortopsentin analogues inhibit bacterial biofilm formation. Mar. Drugs 2018, 16, 274. [Google Scholar] [CrossRef]
- Carbone, A.; Parrino, B.; Di Vita, G.; Attanzio, A.; Spanò, V.; Montalbano, A.; Barraja, P.; Tesoriere, L.; Livrea, M.A.; Diana, P.; et al. Synthesis and antiproliferative activity of thiazolyl-bis-pyrrolo[2,3-b]pyridines and indolyl-thiazolyl-pyrrolo[2,3-c]pyridines, nortopsentin analogues. Mar. Drugs 2015, 13, 460–492. [Google Scholar] [CrossRef]
- Parrino, B.; Carbone, A.; Di Vita, G.; Ciancimino, C.; Attanzio, A.; Spanò, V.; Montalbano, A.; Barraja, P.; Tesoriere, L.; Diana, P.; et al. 3-[4-(1H-Indol-3-yl)-1,3-thiazol-2-yl]-1H-pyrrolo[2,3-b]pyridines, nortopsentin analogues with antiproliferative activity. Mar. Drugs 2015, 13, 1901–1924. [Google Scholar] [CrossRef] [PubMed]
- Spanò, V.; Attanzio, A.; Cascioferro, S.; Carbone, A.; Montalbano, A.; Barraja, P.; Tesoriere, L.; Cirrincione, G.; Diana, P.; Parrino, B. Synthesis and antitumor activity of new thiazole nortopsentin analogs. Mar. Drugs 2016, 14, 226. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Pennati, M.; Parrino, B.; Lopergolo, A.; Barraja, P.; Montalbano, A.; Spanò, V.; Sbarra, S.; Doldi, V.; de Cesare, M.; et al. Novel 1H-pyrrolo[2,3-b]pyridine derivatives nortopsentin analogues: Synthesis and antitumor activity in peritoneal mesothelioma experimental models. J. Med. Chem. 2013, 56, 7060–7072. [Google Scholar] [CrossRef] [PubMed]
- Diana, P.; Carbone, A.; Barraja, P.; Montalbano, A.; Parrino, B.; Lopergolo, A.; Pennati, M.; Zaffaroni, N.; Cirrincione, G. Synthesis and antitumor activity of 3-(2-phenyl-1,3-thiazol-4-yl)-1H-indoles and 3-(2-phenyl-1,3-thiazol-4-yl)-1H-7-azaindoles. ChemMedChem 2011, 6, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Pennati, M.; Barraja, P.; Montalbano, A.; Parrino, B.; Spanò, V.; Lopergolo, A.; Sbarra, S.; Doldi, V.; Zaffaroni, N.; et al. Synthesis and antiproliferative activity of substituted 3[2-(1H-indol-3-yl)-1,3-thiazol-4-yl]-1H-pyrrolo[3,2-b]pyridines, marine alkaloid nortopsentin analogues. Curr. Med. Chem. 2014, 21, 1654–1666. [Google Scholar] [CrossRef]
- Parrino, B.; Attanzio, A.; Spanò, V.; Cascioferro, S.; Montalbano, A.; Barraja, P.; Tesoriere, L.; Diana, P.; Cirrincione, G.; Carbone, A. Synthesis, antitumor activity and CDK1 inhibiton of new thiazole nortopsentin analogues. Eur. J. Med. Chem. 2017, 138, 371–383. [Google Scholar] [CrossRef]
- Kumar, D.; Patel, G.; Chavers, A.K.; Chang, K.-H.; Shah, K. Design and synthesis of 3,5-disubstituted boron-containing 1,2,4-oxadiazoles as potential combretastatin A-4 (CA-4) analogs. Eur. J. Med. Chem. 2011, 46, 3085–3092. [Google Scholar] [CrossRef]
- Zhang, H.-Z.; Kasibhatla, S.; Kuemmerle, J.; Kemnitzer, W.; Ollis-Mason, K.; Qiu, L.; Crogan-Grundy, C.; Tseng, B.; Drewe, J.; Cai, S.X. Discovery and structure-activity relationship of 3-aryl-5-aryl-1,2,4-oxadiazoles as a new series of apoptosis inducers and potential anticancer agents. J. Med. Chem. 2005, 48, 5215–5223. [Google Scholar] [CrossRef]
- Krasavin, M.; Sosnov, A.V.; Karapetian, R.; Konstantinov, I.; Soldatkina, O.; Godovykh, E.; Zubkov, F.; Bai, R.; Hamel, E.; Gakh, A.A. Antiproliferative 4-(1,2,4-oxadiazol-5-yl)piperidine-1-carboxamides, a new tubulin inhibitor chemotype. Bioorg. Med. Chem. Lett. 2014, 24, 4477–4481. [Google Scholar] [CrossRef]
- Lin, H.Y.; Snider, B.B. Synthesis of phidianidines A and B. J. Org. Chem. 2012, 77, 4832–4836. [Google Scholar] [CrossRef]
- Brogan, J.T.; Stoops, S.L.; Lindsley, C.W.T. Total synthesis and biological evaluation of phidianidines A and B uncovers unique pharmacological profiles at CNS targets. ACS Chem. Neurosci. 2012, 3, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.S.; Fu, Y.; Zhang, L.; Gong, J.X.; Wang, Z.Z.; Xiao, W.; Zhang, H.Y.; Guo, Y.W. Synthesis and biological evaluation of novel marine-derived indole-based 1,2,4-oxadiazoles derivatives as multifunctional neuroprotective agents. Bioorg. Med. Chem. Lett. 2015, 25, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Montalbano, A.; Parrino, B.; Diana, P.; Barraja, P.; Carbone, A.; Spanò, V.; Cirrincione, G. Synthesis of the new oligopeptide pyrrole derivative isonetropsin and its one pyrrole unit analogue. Tetrahedron 2013, 69, 2550–2554. [Google Scholar] [CrossRef]
- Barraja, P.; Caracausi, L.; Diana, P.; Spanò, V.; Montalbano, A.; Carbone, A.; Parrino, B.; Cirrincione, G. Synthesis and Antiproliferative Activity of the Ring System [1,2]Oxazolo[4,5-g]indole. ChemMedChem 2012, 7, 1901–1904. [Google Scholar] [CrossRef]
- Parrino, B.; Ullo, S.; Attanzio, A.; Spanò, V.; Cascioferro, S.; Montalbano, A.; Barraja, P.; Tesoriere, L.; Diana, P.; Cirrincione, G. New tripentone analogs with antiproliferative activity. Molecules 2017, 22, 2005. [Google Scholar] [CrossRef] [PubMed]
- Diana, P.; Stagno, A.; Barraja, P.; Montalbano, A.; Carbone, A.; Parrino, B.; Cirrincione, G. Synthesis of the new ring system pyrrolizino[2,3-b]indol-4(5H)-one. Tetrahedron 2011, 67, 3374–3379. [Google Scholar] [CrossRef]
- Barraja, P.; Spanò, V.; Giallombardo, D.; Diana, P.; Montalbano, A.; Carbone, A.; Parrino, B.; Cirrincione, G. Synthesis of [1,2]oxazolo[5,4-e]indazoles as antitumour agents. Tetrahedron 2013, 69, 6474–6477. [Google Scholar] [CrossRef]
- Spanò, V.; Montalbano, A.; Carbone, A.; Parrino, B.; Diana, P.; Cirrincione, G.; Castagliuolo, I.; Brun, P.; Issinger, O.-G.; Tisi, S.; et al. Synthesis of a new class of pyrrolo[3,4-h]quinazolines with antimitotic activity. Eur. J. Med. Chem. 2014, 74, 340–357. [Google Scholar] [CrossRef]
- Parrino, B.; Carbone, A.; Muscarella, M.; Spanò, V.; Montalbano, A.; Barraja, P.; Salvador, A.; Vedaldi, D.; Cirrincione, G.; Diana, P. 11H-Pyrido[3′,2′:4,5]pyrrolo[3,2-c]cinnoline and pyrido[3′,2′:4,5]pyrrolo[1,2-c][1,2,3]benzotriazine: Two new ring systems with antitumor activity. J. Med. Chem. 2014, 57, 9495–9511. [Google Scholar] [CrossRef]
- Parrino, B.; Carbone, A.; Ciancimino, C.; Spanò, V.; Montalbano, A.; Barraja, P.; Cirrincione, G.; Diana, P.; Sissi, C.; Palumbo, M.; et al. Water-soluble isoindolo[2,1-a]quinoxalin-6-imines: In vitro antiproliferative activity and molecular mechanism(s) of action. Eur. J. Med. Chem. 2015, 94, 149–162. [Google Scholar] [CrossRef]
- Parrino, B.; Carbone, A.; Spanò, V.; Montalbano, A.; Giallombardo, D.; Barraja, P.; Attanzio, A.; Tesoriere, L.; Palumbo, M.; Sissi, C.; et al. Aza-isoindolo and isoindolo-azaquinoxaline derivatives with antiproliferative activity. Eur. J. Med. Chem. 2015, 94, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Spanò, V.; Parrino, B.; Carbone, A.; Montalbano, A.; Salvador, A.; Brun, P.; Vedaldi, D.; Diana, P.; Cirrincione, G.; Barraja, P. Pyrazolo[3,4-h]quinolines promising photosensitizing agents in the treatment of cancer. Eur. J. Med. Chem. 2015, 102, 334–351. [Google Scholar] [CrossRef] [PubMed]
- Diana, P.; Stagno, A.; Barraja, P.; Carbone, A.; Parrino, B.; Dall’Acqua, F.; Vedaldi, D.; Salvador, A.; Brun, P.; Castagliuolo, I.; et al. Synthesis of triazeno-azaindoles a new class of triazenes with antitumor activity. ChemMedChem 2011, 6, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Spanò, V.; Pennati, M.; Parrino, B.; Carbone, A.; Montalbano, A.; Cilibrasi, V.; Zuco, V.; Lopergolo, A.; Cominetti, D.; Diana, P.; et al. Preclinical activity of new [1,2]oxazolo[5,4-e]isoindole derivatives in diffuse malignant peritoneal mesothelioma. J. Med. Chem. 2016, 59, 7223–7238. [Google Scholar] [CrossRef] [PubMed]
- Barraja, P.; Diana, P.; Spanò, V.; Montalbano, A.; Carbone, A.; Parrino, B.; Cirrincione, G. An efficient synthesis of pyrrolo[3′,2′:4,5]thiopyrano[3,2-b]pyridin-2-one: A new ring system of pharmaceutical interest. Tetrahedron 2012, 68, 5087–5094. [Google Scholar] [CrossRef]
- Spanò, V.; Frasson, I.; Giallombardo, D.; Doria, F.; Parrino, B.; Carbone, A.; Montalbano, A.; Nadai, M.; Diana, P.; Cirrincione, G.; et al. Synthesis and antiproliferative mechanism of action of pyrrole[3′,2′:6,7]cyclohepta[1,2-d]pyrimidin-2-amines as singlet oxygen photosensitizers. Eur. J. Med. Chem. 2016, 123, 447–461. [Google Scholar] [CrossRef]
- Parrino, B.; Ciancimino, C.; Carbone, A.; Spanò, V.; Montalbano, A.; Barraja, P.; Cirrincione, G.; Diana, P. Synthesis of isoindolo[1,4]benzoxazinone and isoindolo[1,5]benzoxazepine: Two new ring systems of pharmaceutical interest. Tetrahedron 2015, 71, 7332–7338. [Google Scholar] [CrossRef]
- Spanò, V.; Pennati, M.; Parrino, B.; Carbone, A.; Montalbano, A.; Lopergolo, A.; Zuco, V.; Cominetti, D.; Diana, P.; Cirrincione, G.; et al. [1,2]oxazolo[5,4-e]isoindoles as promising tubulin polymerization inhibitors. Eur. J. Med. Chem. 2016, 124, 840–851. [Google Scholar] [CrossRef]
- Parrino, B.; Ullo, S.; Attanzio, A.; Cascioferro, S.; Spanò, V.; Carbone, A.; Montalbano, A.; Barraja, P.; Cirrincione, G.; Tesoriere, L. Synthesis of 5H-pyrido[3,2-b]pyrrolizin-5-one tripentone analogs with antitumor activity. Eur. J. Med. Chem. 2018, 158, 236–246. [Google Scholar] [CrossRef]
- Spanò, V.; Giallombardo, D.; Cilibrasi, V.; Parrino, B.; Carbone, A.; Montalbano, A.; Frasson, I.; Salvador, A.; Richter, S.N.; Doria, F.; et al. Pyrrolo[3′,2′:6,7]cyclohepta[1,2-b]pyridines with potent photo-antiproliferative activity. Eur. J. Med. Chem. 2017, 128, 300–318. [Google Scholar] [CrossRef]
- Ren, X.; Chen, J.; Chen, F.; Cheng, J. The palladium-catalyzed cyanation of indole C-H bonds with the combination of NH4HCO3 and DMSO as a safe cyanide source. Chem. Commun. 2011, 47, 6725–6727. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhang, W.; Shen, Z. Cu-Catalyzed Cyanation of Indoles with Acetonitrile as a Cyano Source. J. Org. Chem. 2015, 80, 8868–8873. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.X.; Lennernas, H.; Welage, L.S.; Barnett, J.L.; Landowski, C.P.; Foster, D.; Fleischer, D.; Lee, K.D.; Amidon, G.L. Comparison of human duodenum and Caco-2 gene expression profiles for 12,000 gene sequence tags and correlation with permeability of 26 drugs. Pharm. Res. 2002, 19, 1400–1416. [Google Scholar] [CrossRef] [PubMed]
| Compound | R1 | R2 | Yield (%) |
|---|---|---|---|
| 1a | H | H | 75 |
| 1b | H | Br | 72 |
| 1c | H | F | 62 |
| 1d | Br | H | 75 |
| 1e | Br | Br | 82 |
| 1f | Br | F | 75 |
| 1g | F | H | 72 |
| 1h | F | Br | 60 |
| 1i | F | F | 55 |
| 1j | Cl | H | 65 |
| 1k | Cl | Br | 75 |
| 1l | Cl | F | 85 |
| 1m | OCH3 | H | 70 |
| 1n | OCH3 | Br | 65 |
| 1o | OCH3 | F | 80 |
| Compound | IC50 (µM) a | |||
|---|---|---|---|---|
| MCF-7 | HCT-116 | HeLa | CaCo2 | |
| 1k | 0.65 ± 0.05 | 1.93 ± 0.06 | 10.56 ± 0.98 | 1.06 ± 0.09 |
| 1n | 2.41 ± 0.23 | 3.55 ± 0.1 | 13.96 ± 1.41 | 3.33 ± 0.25 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cascioferro, S.; Attanzio, A.; Di Sarno, V.; Musella, S.; Tesoriere, L.; Cirrincione, G.; Diana, P.; Parrino, B. New 1,2,4-Oxadiazole Nortopsentin Derivatives with Cytotoxic Activity. Mar. Drugs 2019, 17, 35. https://doi.org/10.3390/md17010035
Cascioferro S, Attanzio A, Di Sarno V, Musella S, Tesoriere L, Cirrincione G, Diana P, Parrino B. New 1,2,4-Oxadiazole Nortopsentin Derivatives with Cytotoxic Activity. Marine Drugs. 2019; 17(1):35. https://doi.org/10.3390/md17010035
Chicago/Turabian StyleCascioferro, Stella, Alessandro Attanzio, Veronica Di Sarno, Simona Musella, Luisa Tesoriere, Girolamo Cirrincione, Patrizia Diana, and Barbara Parrino. 2019. "New 1,2,4-Oxadiazole Nortopsentin Derivatives with Cytotoxic Activity" Marine Drugs 17, no. 1: 35. https://doi.org/10.3390/md17010035
APA StyleCascioferro, S., Attanzio, A., Di Sarno, V., Musella, S., Tesoriere, L., Cirrincione, G., Diana, P., & Parrino, B. (2019). New 1,2,4-Oxadiazole Nortopsentin Derivatives with Cytotoxic Activity. Marine Drugs, 17(1), 35. https://doi.org/10.3390/md17010035