Synthesis, Characterization, and Antifungal Property of Hydroxypropyltrimethyl Ammonium Chitosan Halogenated Acetates
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Synthesis and Characterization
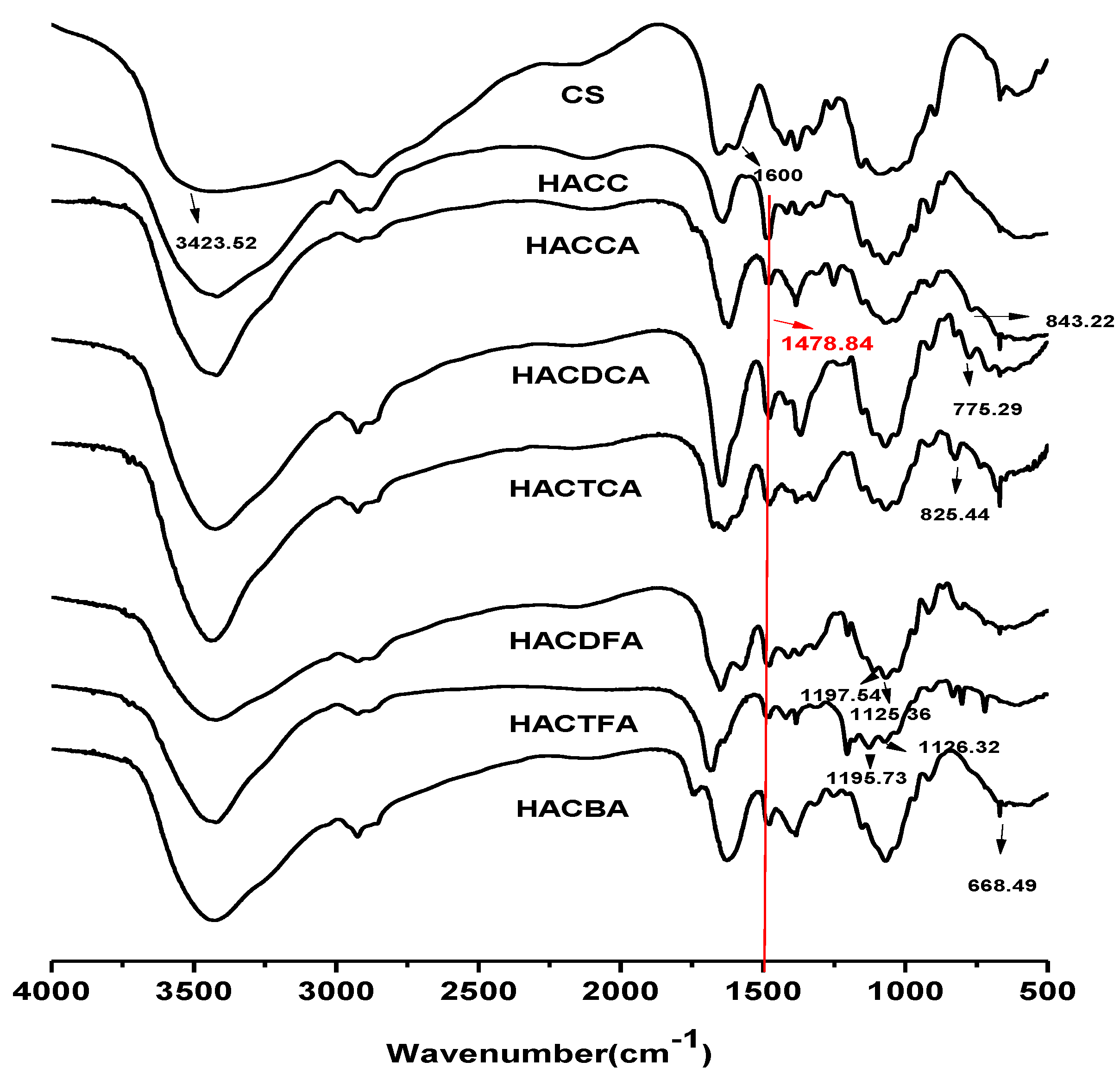
2.1.1. Fourier Transform Infrared Spectroscopy (FTIR) Spectra
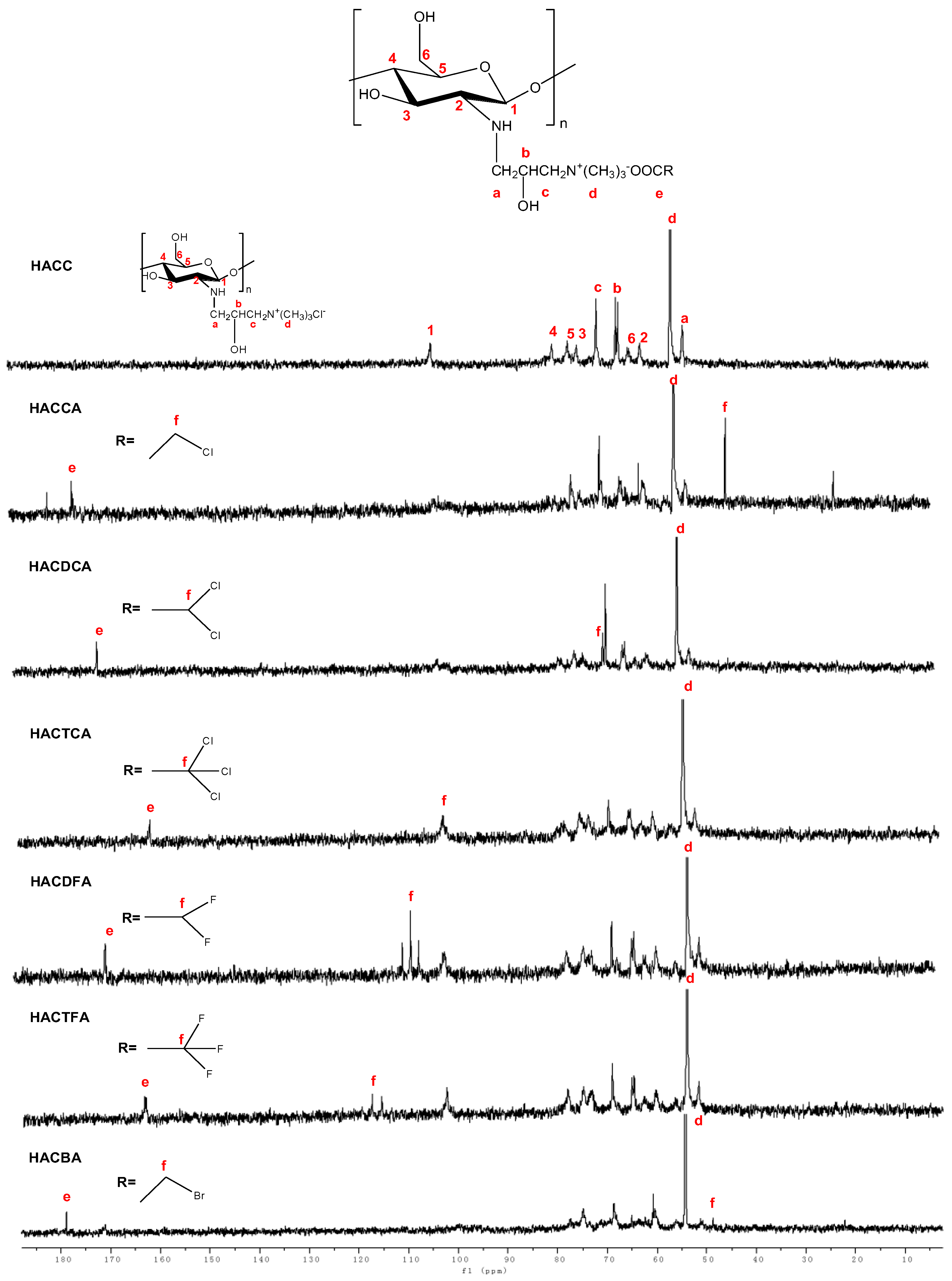
2.1.2. Nuclear Magnetic Resonance Spectrometer (NMR) Spectra
2.1.3. Elemental Analysis
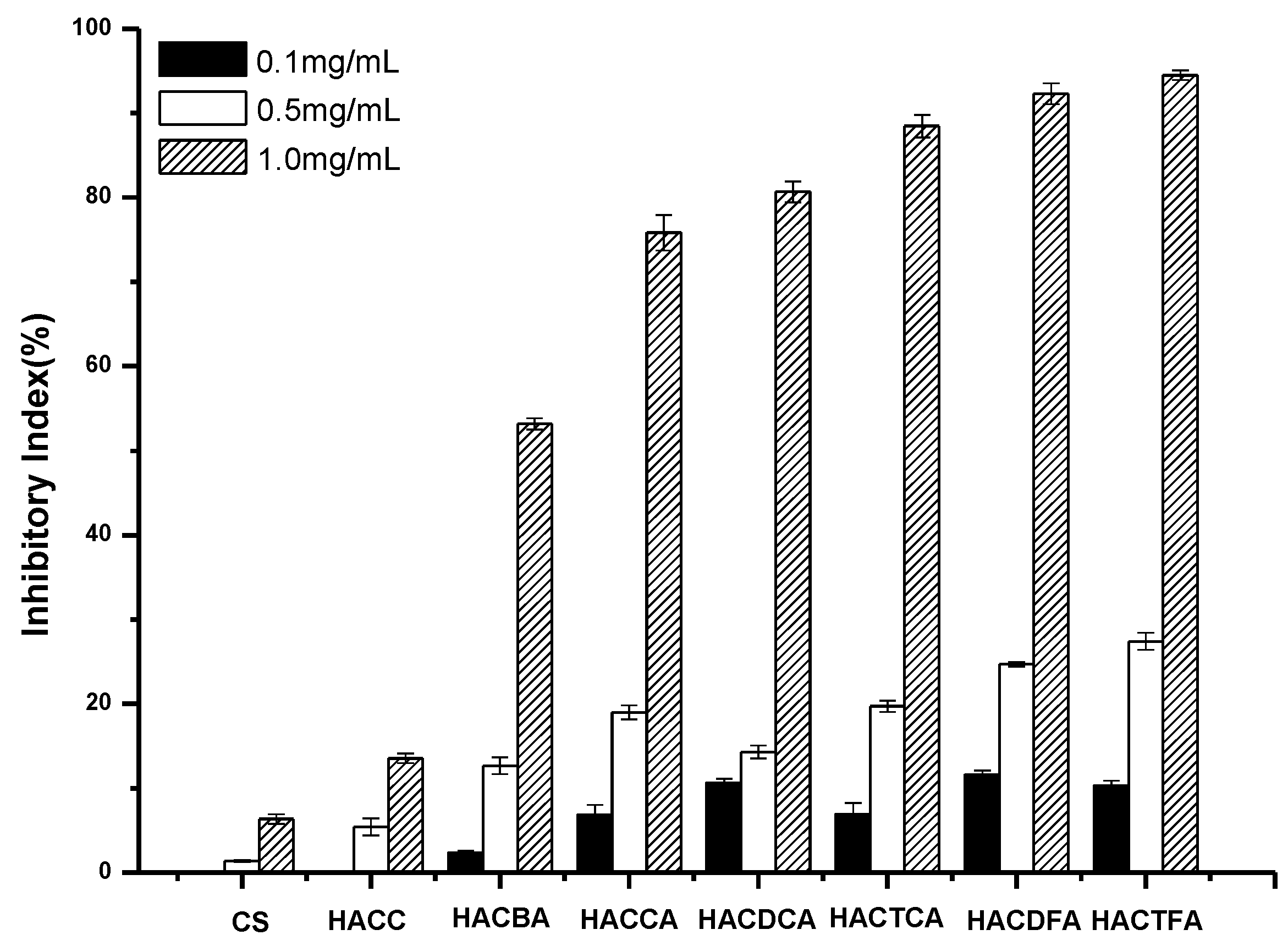
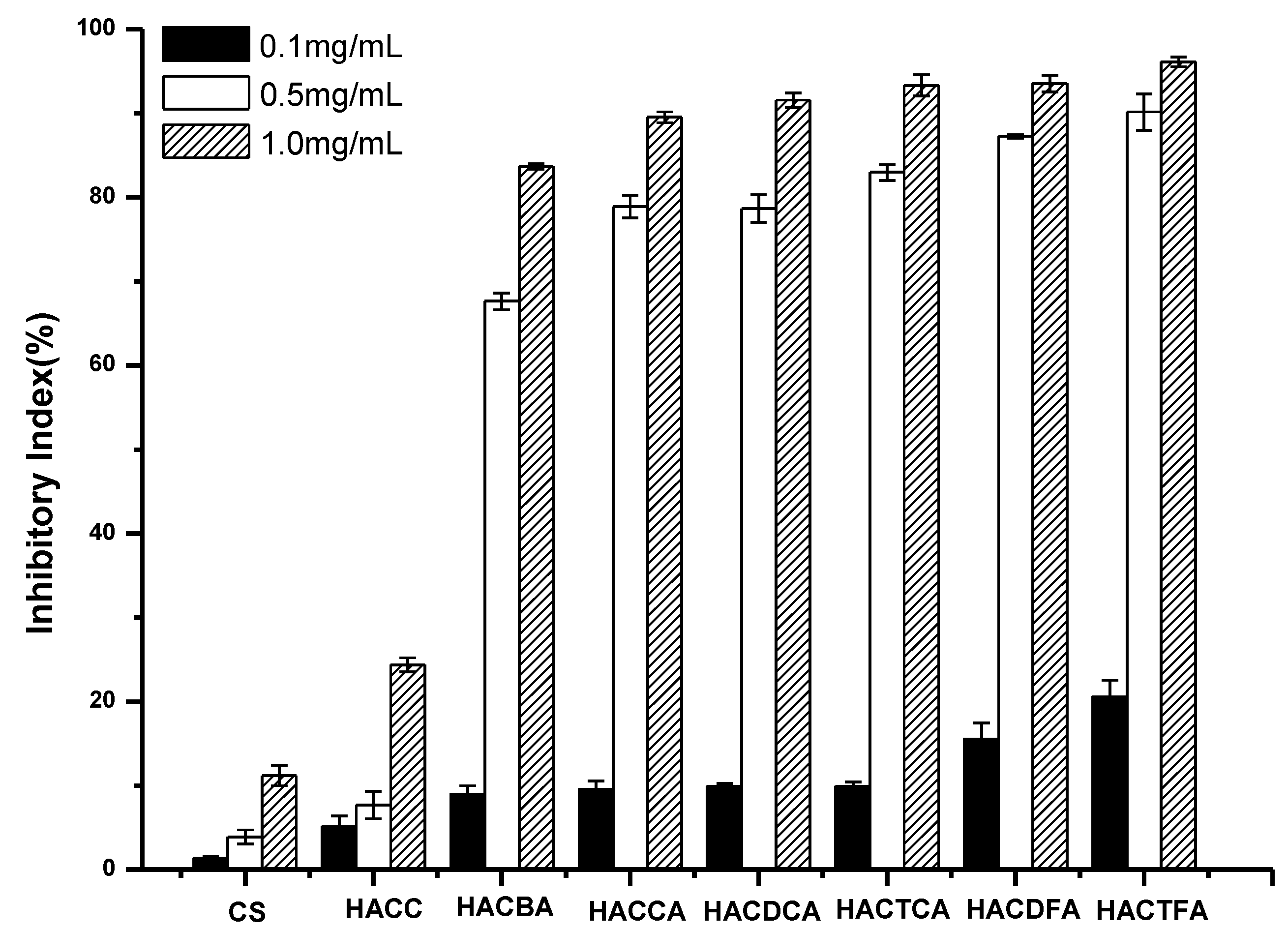
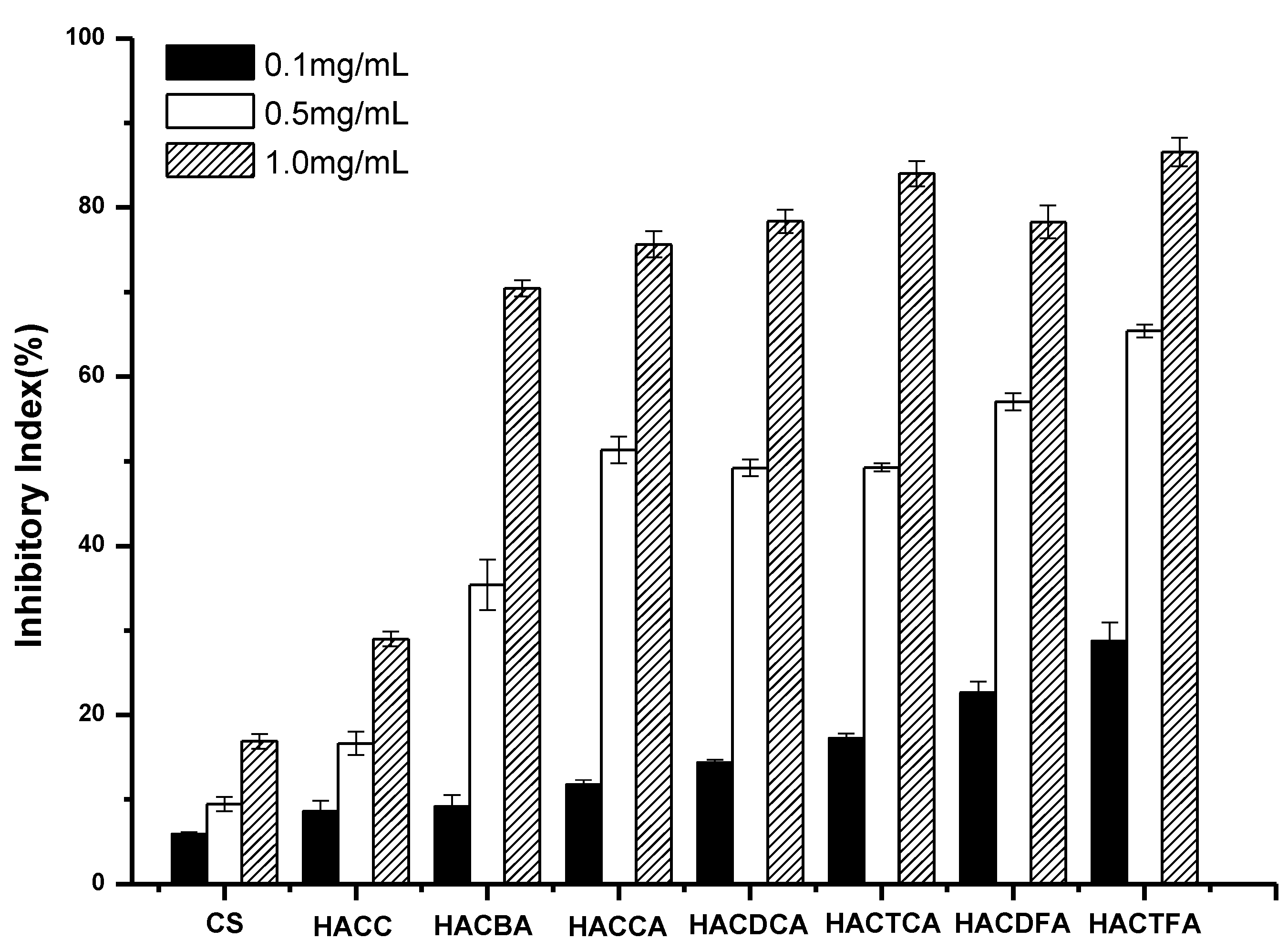
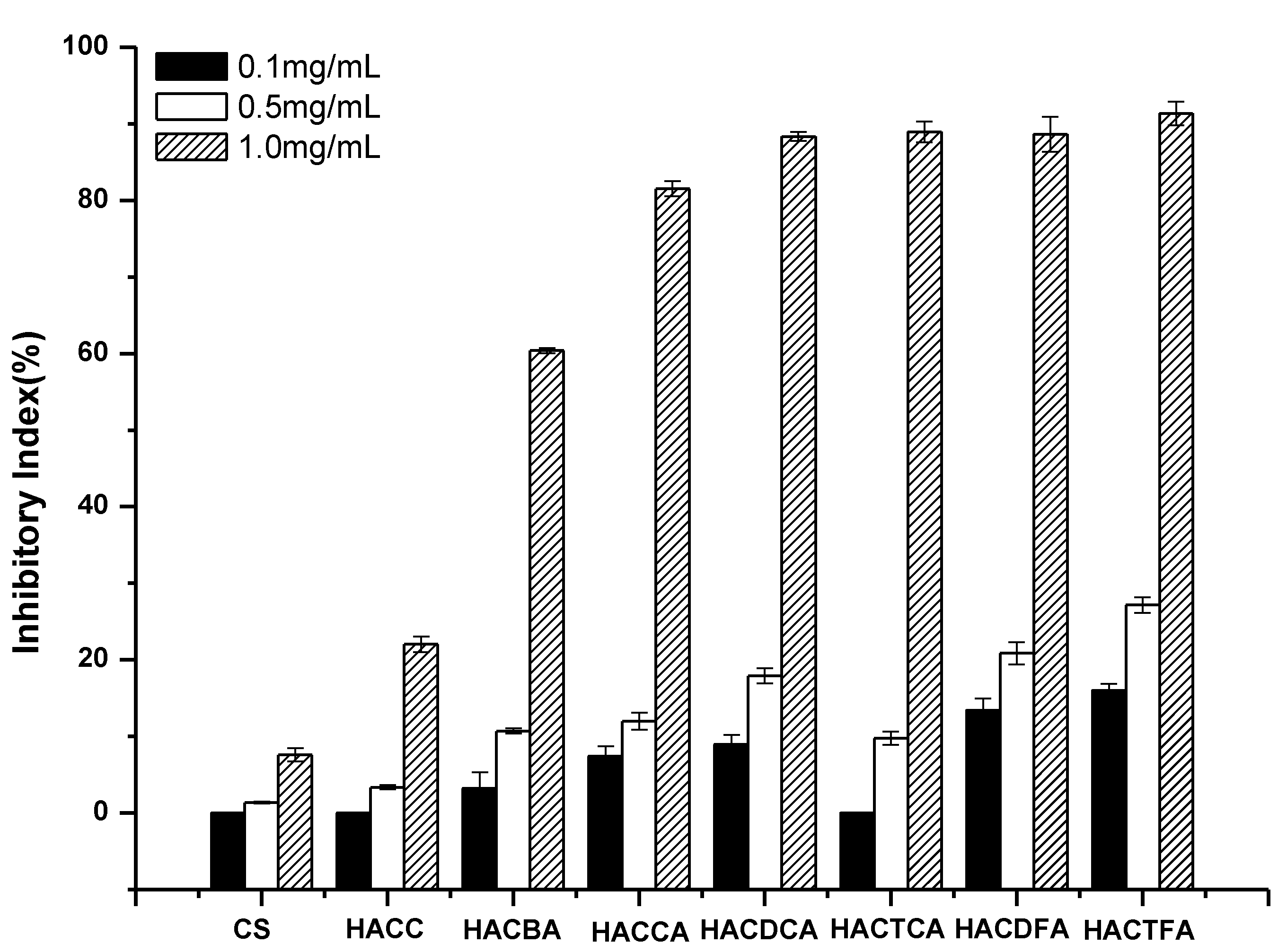
2.2. Antifungal Activity
3. Materials and Methods
3.1. Materials
3.2. Analytical Methods
3.2.1. Fourier Transform Infrared (FTIR) Spectroscopy
3.2.2. Nuclear Magnetic Resonance (NMR) Spectroscopy
3.2.3. Elemental Analysis
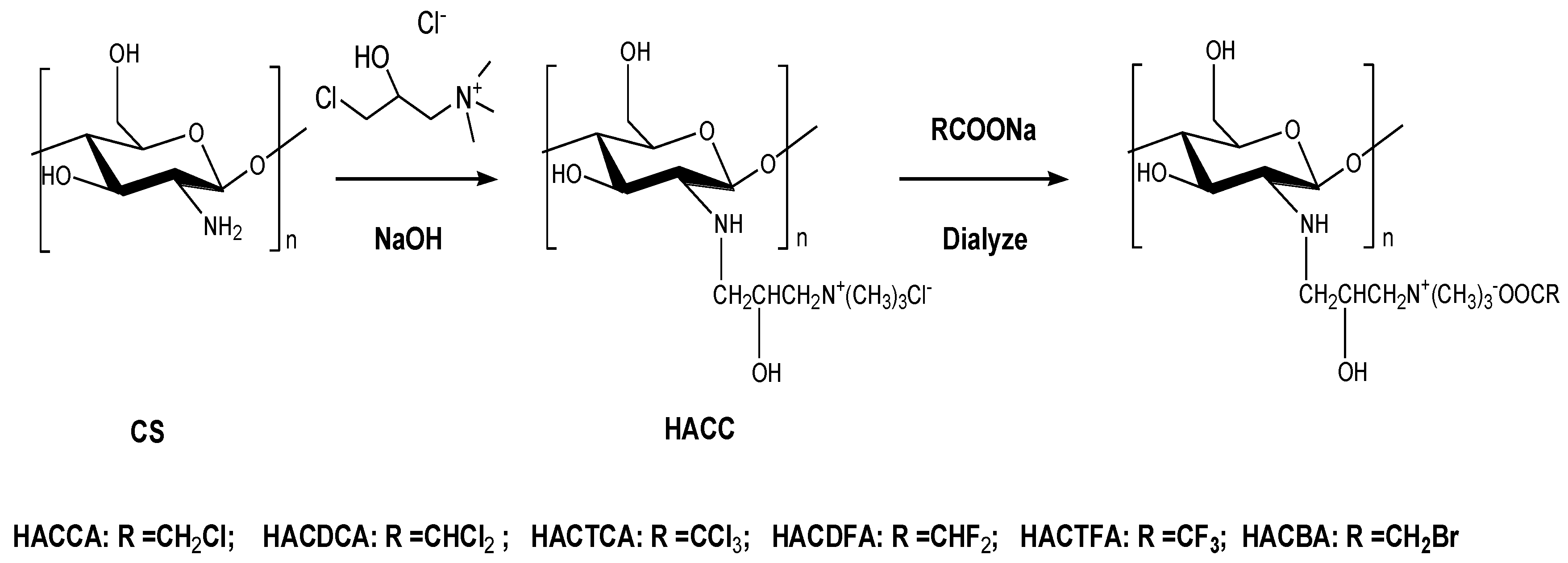
3.3. Synthesis of Chitosan Derivatives
3.3.1. Synthesis of Hydroxypropyltrimethyl Ammonium Chloride Chitosan (HACC)
3.3.2. Synthesis of Hydroxypropyltrimethyl Ammonium Chitosan Halogenated Acetates
3.4. Antifungal Assays
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jones, D.A.; Bertazzoni, S.; Turo, C.J.; Syme, R.A.; Hane, J.K. Bioinformatic prediction of plant-pathogenicity effector proteins of fungi. Curr. Opin. Microbiol. 2018, 46, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Qin, Y.; Liu, S.; Xing, R.; Yu, H.; Chen, X.; Li, K.; Li, P. Synthesis, characterization, and antifungal evaluation of diethoxyphosphoryl polyaminoethyl chitosan derivatives. Carbohydr. Polym. 2018, 190, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.E.S.; da Cunha, F.S.; Honorato, A.; Capucho, A.S.; Dias, R.; Borel, J.C.; Ishikawa, F.H. Resistance to Fusarium Wilt in watermelon accessions inoculated by chlamydospores. Sci. Hortic. 2018, 228, 181–186. [Google Scholar] [CrossRef]
- Garmendia, G.; Umpierrez-Failache, M.; Ward, T.J.; Vero, S. Development of a PCR-RFLP method based on the transcription elongation factor 1-alpha gene to differentiate Fusarium graminearum from other species within the Fusarium graminearum species complex. Int. J. Food. Microbiol. 2018, 70, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Sevastos, A.; Kalampokis, I.F.; Panagiotopoulou, A.; Pelecanou, M.; Aliferis, K.A. Implication of Fusarium graminearum primary metabolism in its resistance to benzimidazole fungicides as revealed by 1H NMR metabolomics. Pestic. Biochem. Phys. 2018, 148, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Kheiri, A.; Moosawi Jorf, S.A.; Malihipour, A.; Saremi, H.; Nikkhah, M. Application of chitosan and chitosan nanoparticles for the control of Fusarium head blight of wheat (Fusarium graminearum) in vitro and greenhouse. Int. J. Biol. Macromol. 2016, 93 Pt A, 1261–1272. [Google Scholar] [CrossRef]
- Heneberg, P.; Svoboda, J.; Pech, P. Benzimidazole fungicides are detrimental to common farmland ants. Biol. Conserv. 2018, 221, 114–117. [Google Scholar] [CrossRef]
- Yang, G.; Jin, Q.; Xu, C.; Fan, S.; Wang, C.; Xie, P. Synthesis, characterization and antifungal activity of coumarin-functionalized chitosan derivatives. Int. J. Biol. Macromol. 2018, 106, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Divya, K.; Smitha, V.; Jisha, M.S. Antifungal, antioxidant and cytotoxic activities of chitosan nanoparticles and its use as an edible coating on vegetables. Int. J. Biol. Macromol. 2018, 114, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Li, Q.; Dong, F.; Wei, L.; Guo, Z. Synthesis, characterization, and antifungal property of chitosan ammonium salts with halogens. Int. J. Biol. Macromol. 2016, 92, 293–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huczynski, A.; Antoszczak, M.; Kleczewska, N.; Lewandowska, M.; Maj, E.; Stefanska, J.; Wietrzyk, J.; Janczak, J.; Celewicz, L. Synthesis and biological activity of salinomycin conjugates with floxuridine. Eur. J. Med. Chem. 2015, 93, 33–41. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Ke, W.; Chen, X.; Kong, Y.; Zheng, H.; Yin, Y.; Cai, W. Preparation and properties of quaternary ammonium chitosan-g-poly(acrylic acid-co-acrylamide) superabsorbent hydrogels. React. Funct. Polym. 2017, 111, 14–21. [Google Scholar] [CrossRef]
- Demetgul, C.; Beyazit, N. Synthesis, characterization and antioxidant activity of chitosan-chromone derivatives. Carbohydr. Polym. 2018, 181, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tan, W.; Zhang, Z.; Song, Y.; Li, Q.; Dong, F.; Guo, Z. Synthesis, characterization, and the antifungal activity of chitosan derivatives containing urea groups. Int. J. Biol. Macromol. 2018, 109, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Huber, D.; Grzelak, A.; Baumann, M.; Borth, N.; Schleining, G.; Nyanhongo, G.S.; Guebitz, G.M. Anti-inflammatory and anti-oxidant properties of laccase-synthesized phenolic-O-carboxymethyl chitosan hydrogels. New. Biotechnol. 2018, 40, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Kecen, X.; Xiaosai, Q. Grafting Modification of Chitosan. In Biopolymer Grafting, 1st ed.; Thakur, V.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 295–364. ISBN 9780128104613. [Google Scholar]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Comparative study on antimicrobial activity and biocompatibility of N-selective chitosan derivatives. React. Funct. Polym. 2018, 124, 149–155. [Google Scholar] [CrossRef]
- Peng, Z.-X.; Wang, L.; Du, L.; Guo, S.-R.; Wang, X.-Q.; Tang, T.-T. Adjustment of the antibacterial activity and biocompatibility of hydroxypropyltrimethyl ammonium chloride chitosan by varying the degree of substitution of quaternary ammonium. Carbohydr. Polym. 2010, 81, 275–283. [Google Scholar] [CrossRef]
- Woraphatphadung, T.; Sajomsang, W.; Gonil, P.; Saesoo, S.; Opanasopit, P. Synthesis and characterization of pH-responsive N-naphthyl-N,O-succinyl chitosan micelles for oral meloxicam delivery. Carbohydr. Polym. 2015, 121, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, Y.; Wang, F.; Lv, L.; Liu, J.; Li, M.; Guo, A.; Jiang, J.; Shen, Y.; Guo, S. Synthesis, in vitro and in vivo evaluation of new norcantharidin-conjugated hydroxypropyltrimethyl ammonium chloride chitosan derivatives as polymer therapeutics. Int. J. Pharm. 2013, 453, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.; Dragonetti, C.; Magni, M.; Roberto, D. Degradation of toxic halogenated organic compounds by iron-containing mono-, bi- and tri-metallic particles in water. Inorg. Chim. Acta 2015, 431, 48–60. [Google Scholar] [CrossRef]
- Luo, J.; Hu, J.; Wei, X.; Fu, L.; Li, L. Dehalogenation of persistent halogenated organic compounds: A review of computational studies and quantitative structure-property relationships. Chemosphere 2015, 131, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Zhong, Z.; Lin, L. Evaluation of chitosan quaternary ammonium salt-modified resin denture base material. Int. J. Biol. Macromol. 2016, 85, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Tan, W.; Zhang, C.; Gu, G.; Guo, Z. Synthesis of water soluble chitosan derivatives with halogeno-1,2,3-triazole and their antifungal activity. Int. J. Biol. Macromol. 2016, 91, 623–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Liu, X.; Jin, X.; Li, H.; Sun, J.; Gu, X. The novel application of chitosan: Effects of cross-linked chitosan on the fire performance of thermoplastic polyurethane. Carbohydr. Polym. 2018, 189, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Qin, Y.; Liu, S.; Xing, R.; Yu, H.; Chen, X.; Li, K.; Li, P. Synthesis, characterization and antifungal efficacy of chitosan derivatives with triple quaternary ammonium groups. Int. J. Biol. Macromol. 2018, 114, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Li, Q.; Wang, G.; Dong, F.; Zhou, H.; Zhang, J. Synthesis, characterization, and antifungal activity of novel inulin derivatives with chlorinated benzene. Carbohydr. Polym. 2014, 99, 469–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Tan, W.; Luan, F.; Yin, X.; Dong, F.; Li, Q.; Guo, Z. Synthesis of quaternary ammonium salts of chitosan bearing halogenated acetate for antifungal and antibacterial activities. Polymers 2018, 10, 530. [Google Scholar] [CrossRef]
- Kaushal, J.; Seema; Singh, G.; Arya, S.K. Immobilization of catalase onto chitosan and chitosan-bentonite complex: A comparative study. Biotechnol. Rep. 2018, 18, 251–258. [Google Scholar]
- Rui, L.; Xie, M.; Hu, B.; Zhou, L.; Saeeduddin, M.; Zeng, X. Enhanced solubility and antioxidant activity of chlorogenic acid-chitosan conjugates due to the conjugation of chitosan with chlorogenic acid. Carbohydr. Polym. 2017, 170, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Li, Q.; Li, W.; Dong, F.; Guo, Z. Synthesis and antioxidant property of novel 1,2,3-triazole-linked starch derivatives via ‘click chemistry’. Int. J. Biol. Macromol. 2016, 82, 404–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Du, Y.; Fan, L.; Chen, X.; Yang, J. Preparation, characterization and antimicrobial activity of quaternized carboxymethyl chitosan and application as pulp-cap. Polymer 2006, 47, 1796–1804. [Google Scholar] [CrossRef]
- Dananjaya, S.H.S.; Erandani, W.; Kim, C.H.; Nikapitiya, C.; Lee, J.; De Zoysa, M. Comparative study on antifungal activities of chitosan nanoparticles and chitosan silver nano composites against Fusarium oxysporum species complex. Int. J. Biol. Macromol. 2017, 105, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Sajomsang, W.; Gonil, P.; Tantayanon, S. Antibacterial activity of quaternary ammonium chitosan containing mono or disaccharide moieties: Preparation and characterization. Int. J. Biol. Macromol. 2009, 44, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Yang, J.; Wu, H.; Hu, Z.; Yi, J.; Tong, J.; Zhu, X. Preparation and characterization of quaternary ammonium chitosan hydrogel with significant antibacterial activity. Int. J. Biol. Macromol. 2015, 79, 830–836. [Google Scholar] [CrossRef] [PubMed]

| Componds | Yields (%) | Elemental Analyses (%) | Degrees of Substitution (%) | ||
|---|---|---|---|---|---|
| C | N | C/N | |||
| CS | - | 41.47 | 7.56 | 5.48 | - |
| HACC | 65.27 | 39.71 | 7.41 | 5.35 | 67.71 |
| HACCA | 69.43 | 38.10 | 6.27 | 6.05 | 67.31 |
| HACDCA | 50.86 | 38.86 | 6.63 | 5.86 | 56.23 |
| HACTCA | 64.85 | 37.22 | 6.07 | 6.13 | 71.97 |
| HACDFA | 58.97 | 40.86 | 7.13 | 5.73 | 48.64 |
| HACTFA | 67.56 | 38.27 | 6.25 | 6.12 | 71.39 |
| HACBA | 65.84 | 40.64 | 6.70 | 6.06 | 67.89 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mi, Y.; Tan, W.; Zhang, J.; Wei, L.; Chen, Y.; Li, Q.; Dong, F.; Guo, Z. Synthesis, Characterization, and Antifungal Property of Hydroxypropyltrimethyl Ammonium Chitosan Halogenated Acetates. Mar. Drugs 2018, 16, 315. https://doi.org/10.3390/md16090315
Mi Y, Tan W, Zhang J, Wei L, Chen Y, Li Q, Dong F, Guo Z. Synthesis, Characterization, and Antifungal Property of Hydroxypropyltrimethyl Ammonium Chitosan Halogenated Acetates. Marine Drugs. 2018; 16(9):315. https://doi.org/10.3390/md16090315
Chicago/Turabian StyleMi, Yingqi, Wenqiang Tan, Jingjing Zhang, Lijie Wei, Yuan Chen, Qing Li, Fang Dong, and Zhanyong Guo. 2018. "Synthesis, Characterization, and Antifungal Property of Hydroxypropyltrimethyl Ammonium Chitosan Halogenated Acetates" Marine Drugs 16, no. 9: 315. https://doi.org/10.3390/md16090315
APA StyleMi, Y., Tan, W., Zhang, J., Wei, L., Chen, Y., Li, Q., Dong, F., & Guo, Z. (2018). Synthesis, Characterization, and Antifungal Property of Hydroxypropyltrimethyl Ammonium Chitosan Halogenated Acetates. Marine Drugs, 16(9), 315. https://doi.org/10.3390/md16090315






