Bioactive Prenyl- and Terpenyl-Quinones/Hydroquinones of Marine Origin †
Abstract
1. Introduction
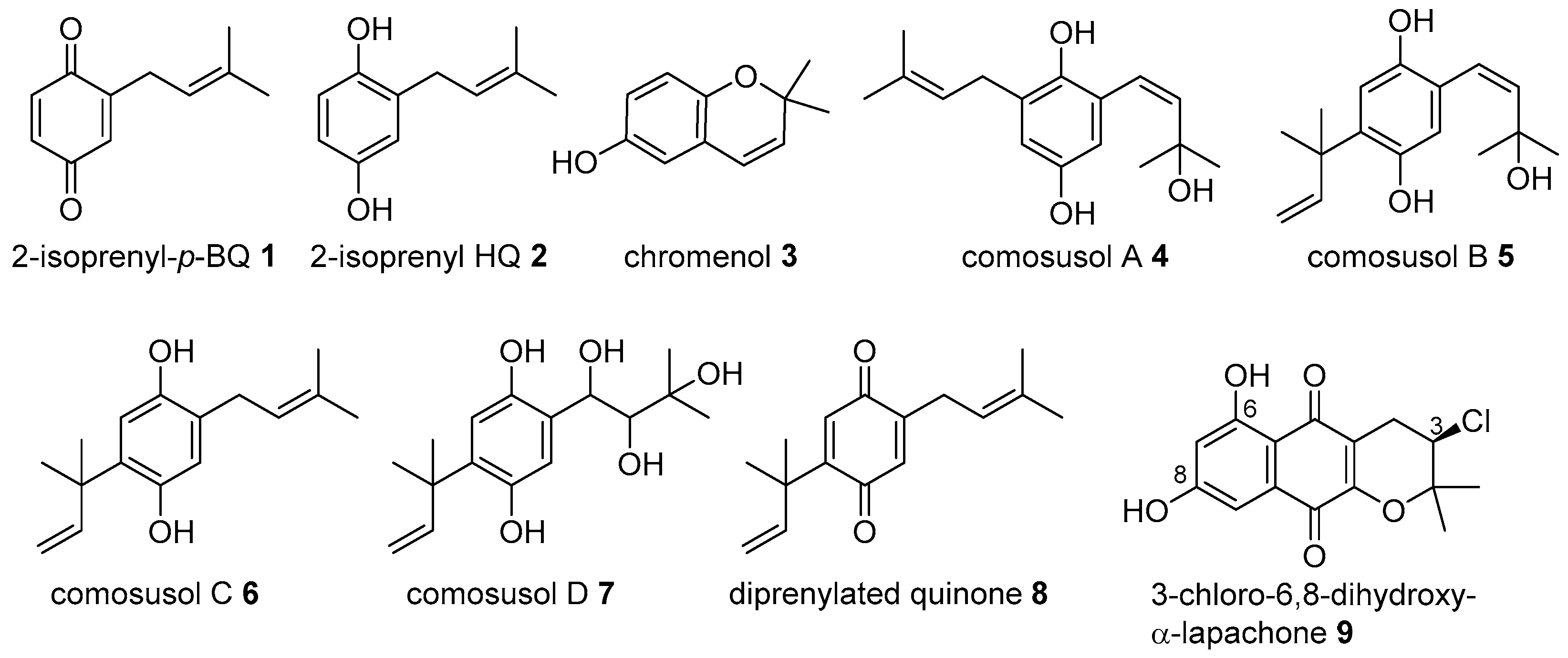
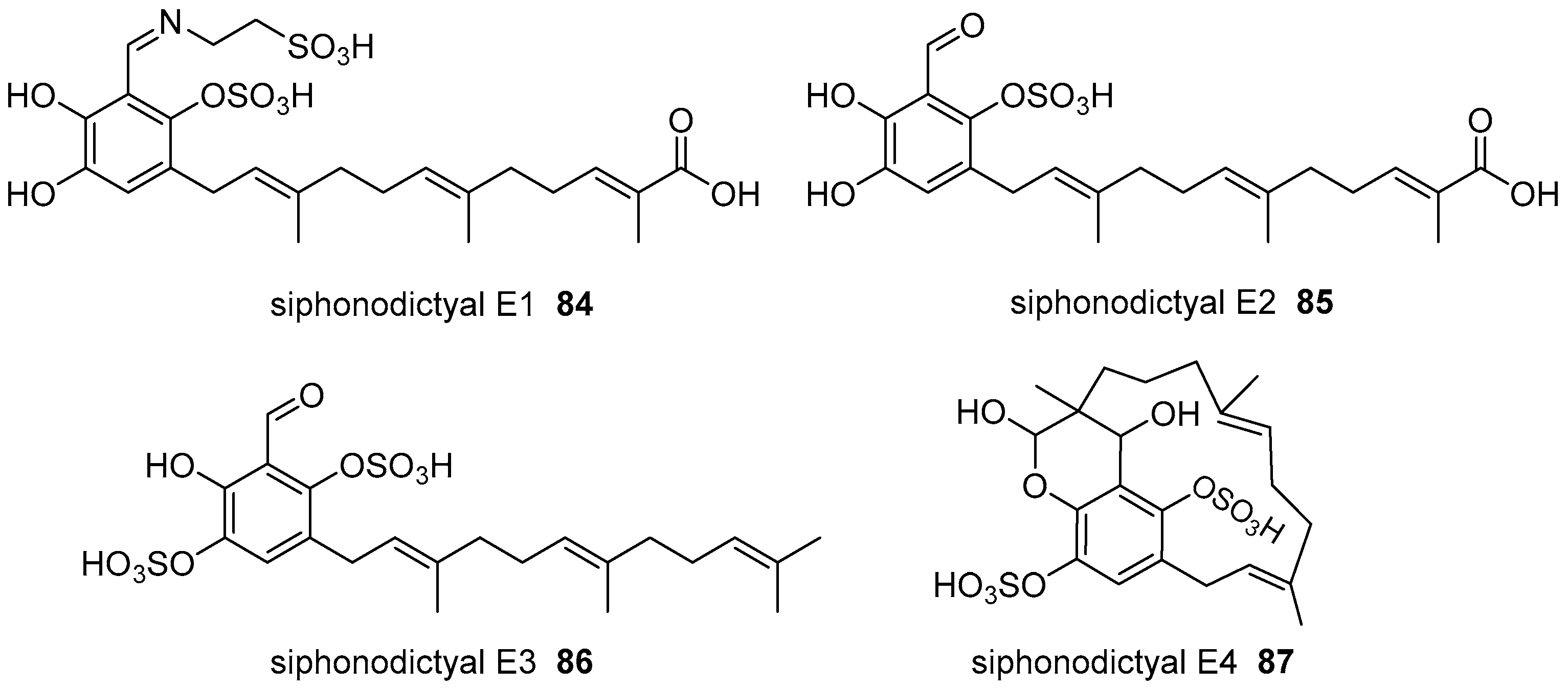
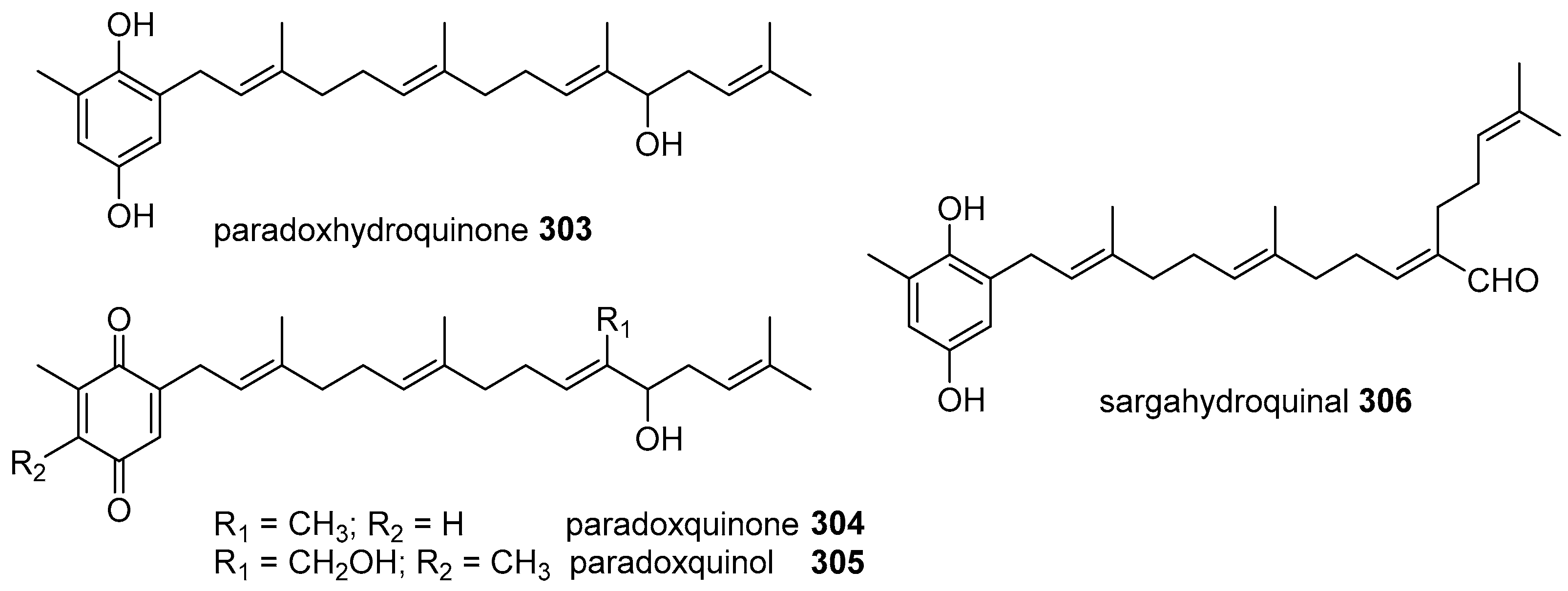
2. Meroterpenoids with Simple Isoprene Units
3. Meroterpenoids with Open-Chain and Cyclic Monoterpenoids
4. Meroterpenoids with Open-Chain and Cyclic Sesquiterpenoids
4.1. Open-Chain Sesquiterpenoids
4.2. Monocyclic Sesquiterpenoids
4.3. Bicyclic Sesquiterpenoids
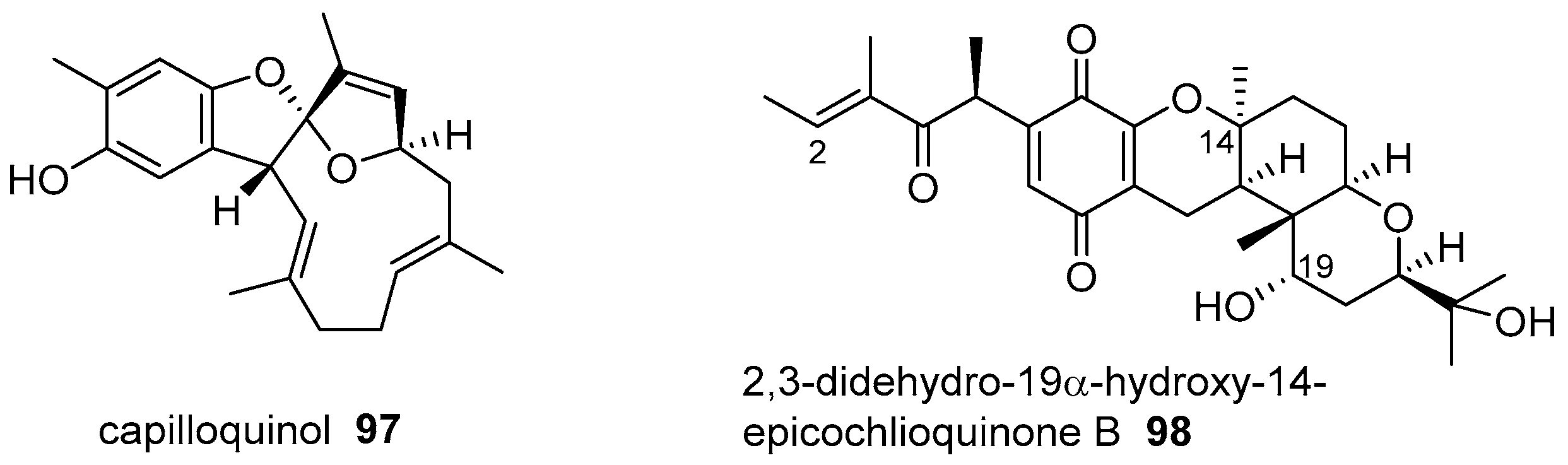
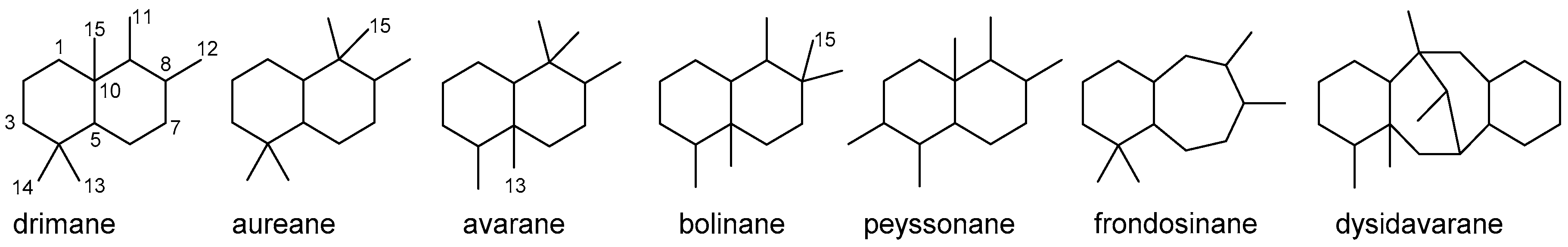
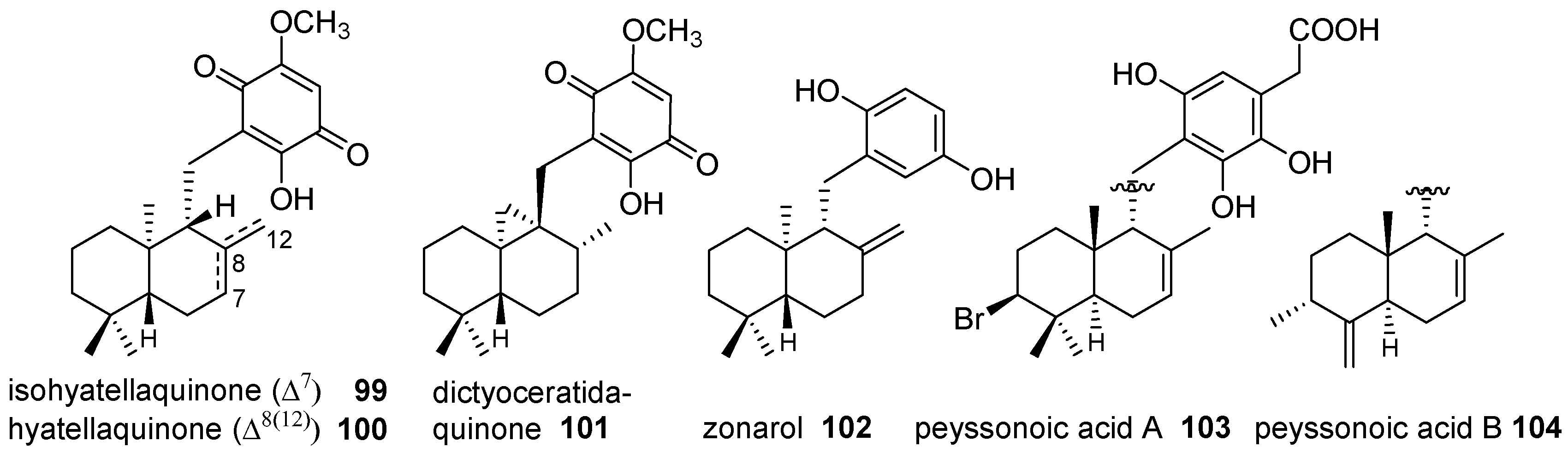
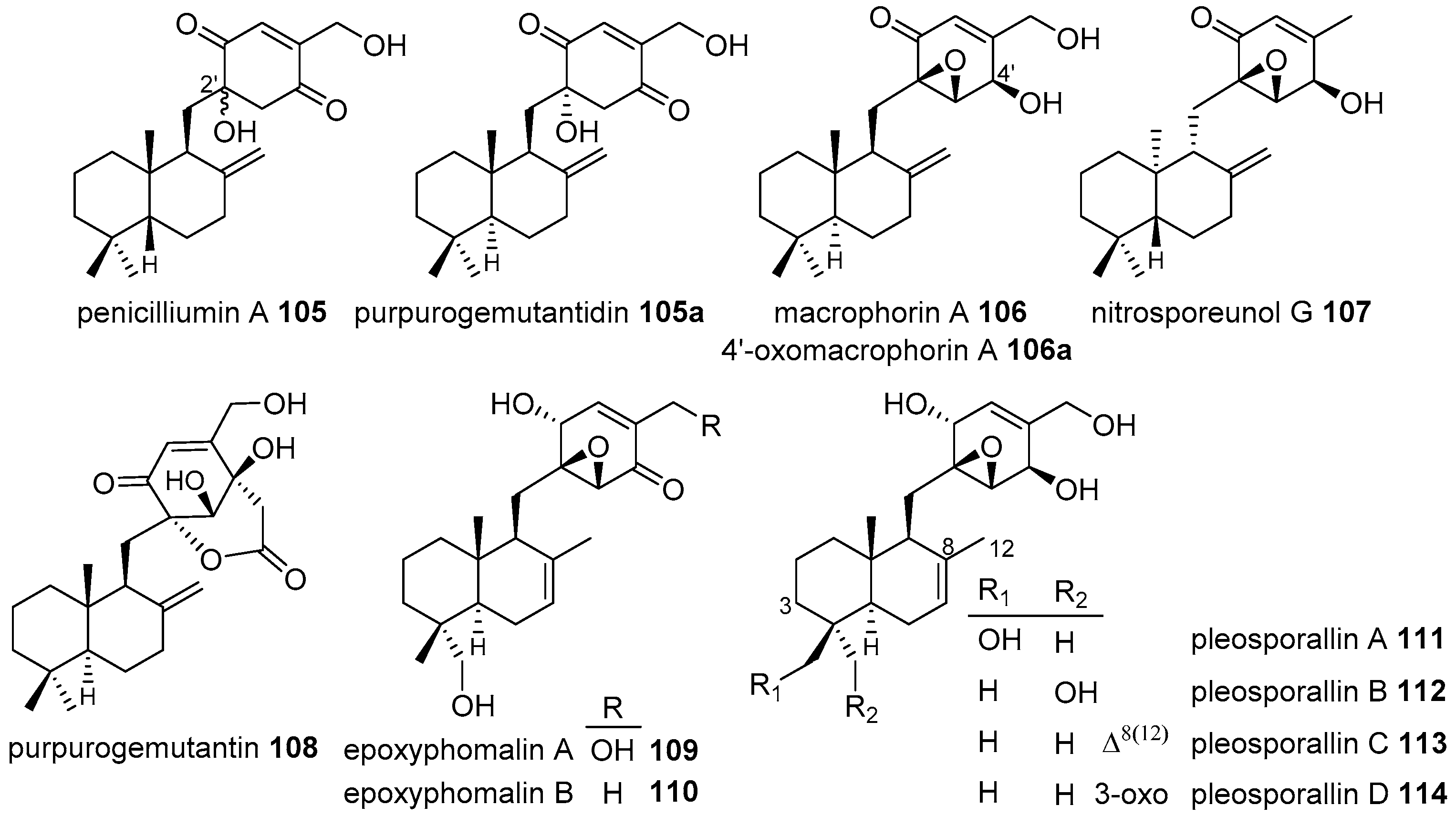
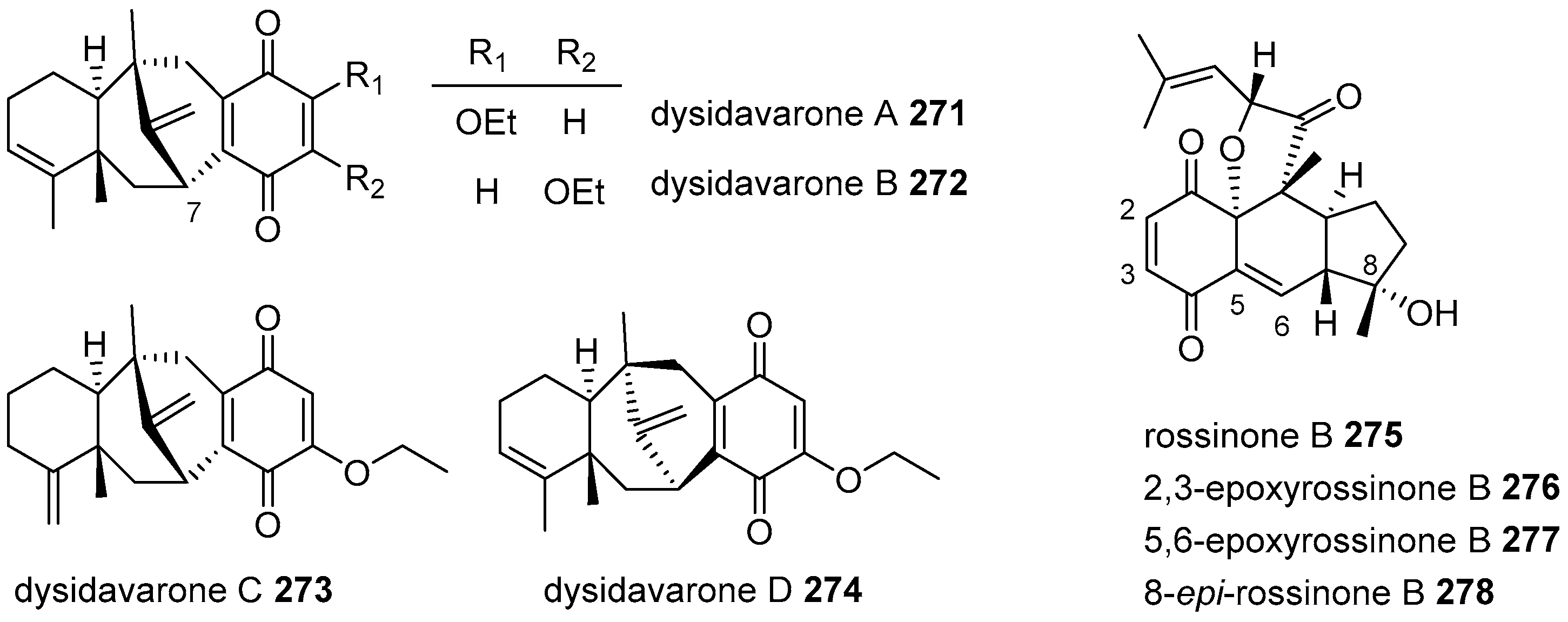
4.3.1. Drimane Skeleton
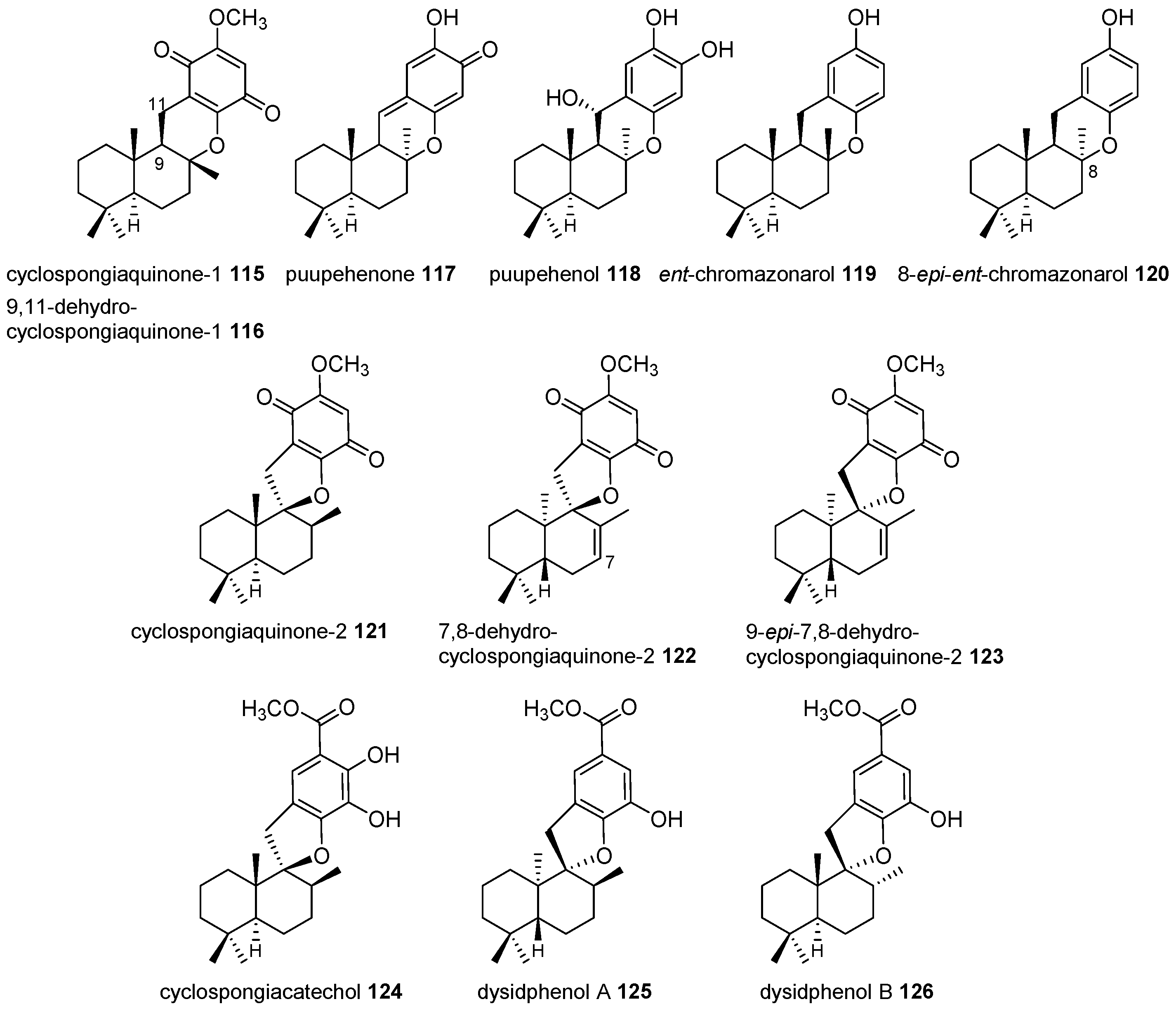
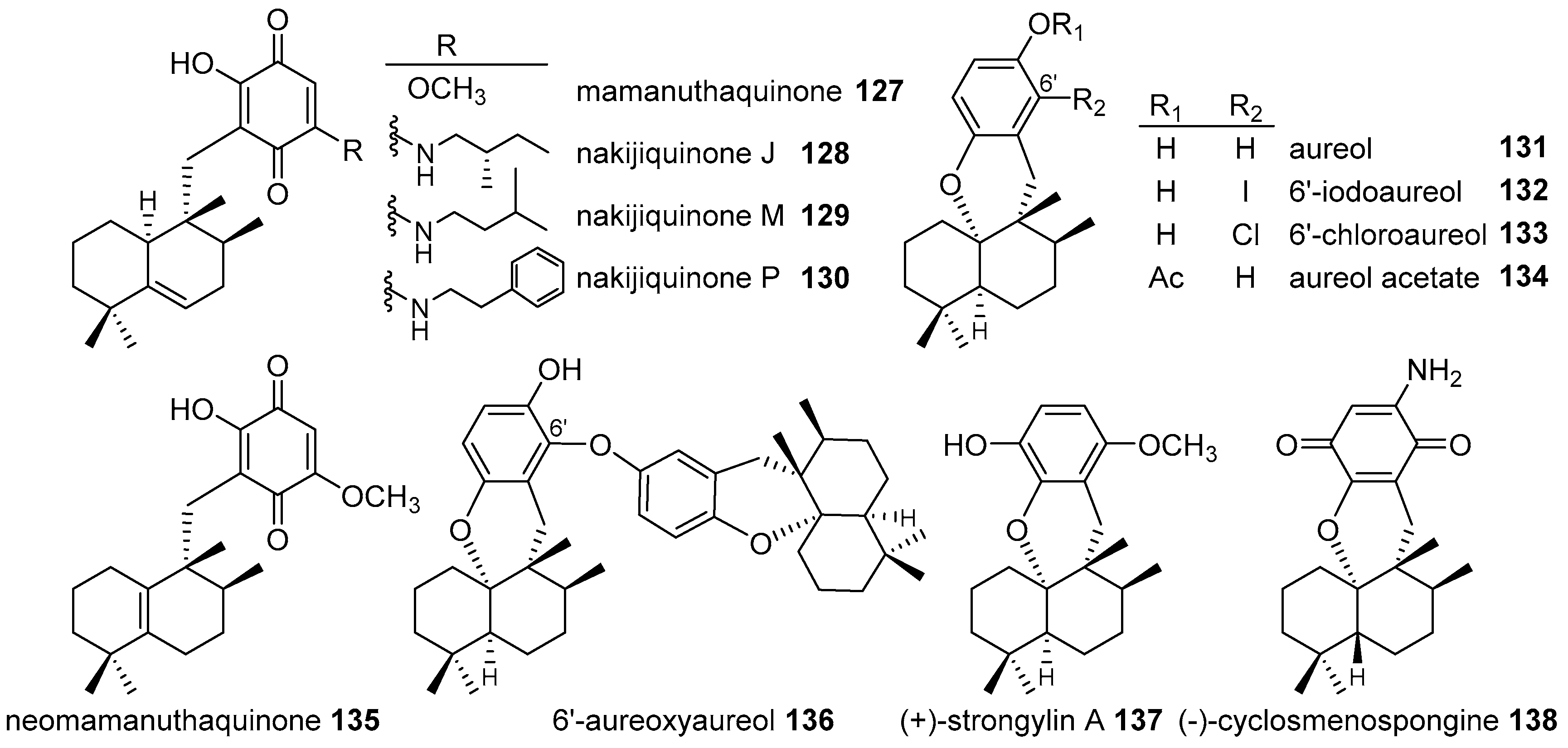
4.3.2. Aureane Skeleton
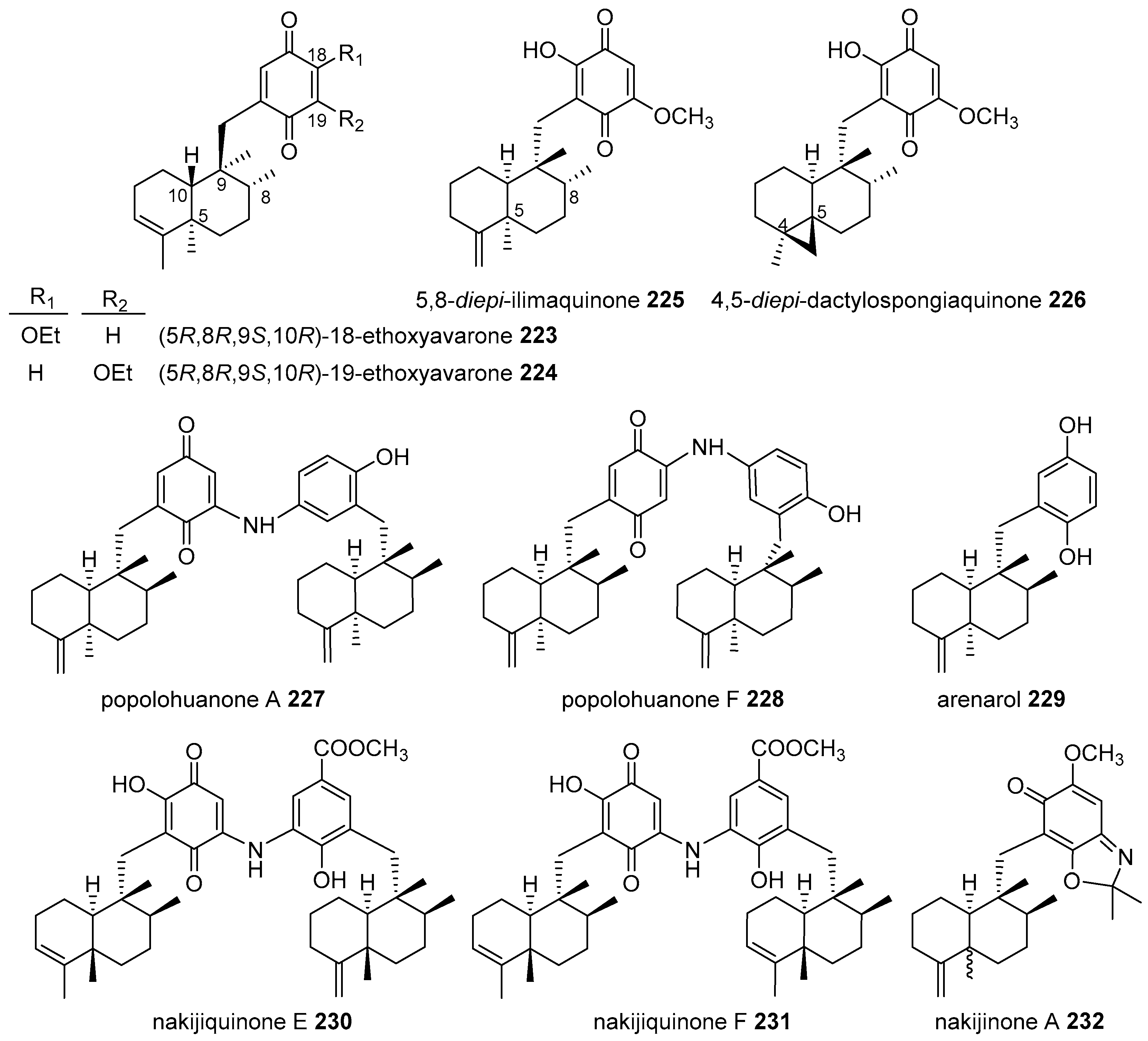
4.3.3. Avarane Skeleton
4.3.4. Other Bicyclic Sesquiterpenoids
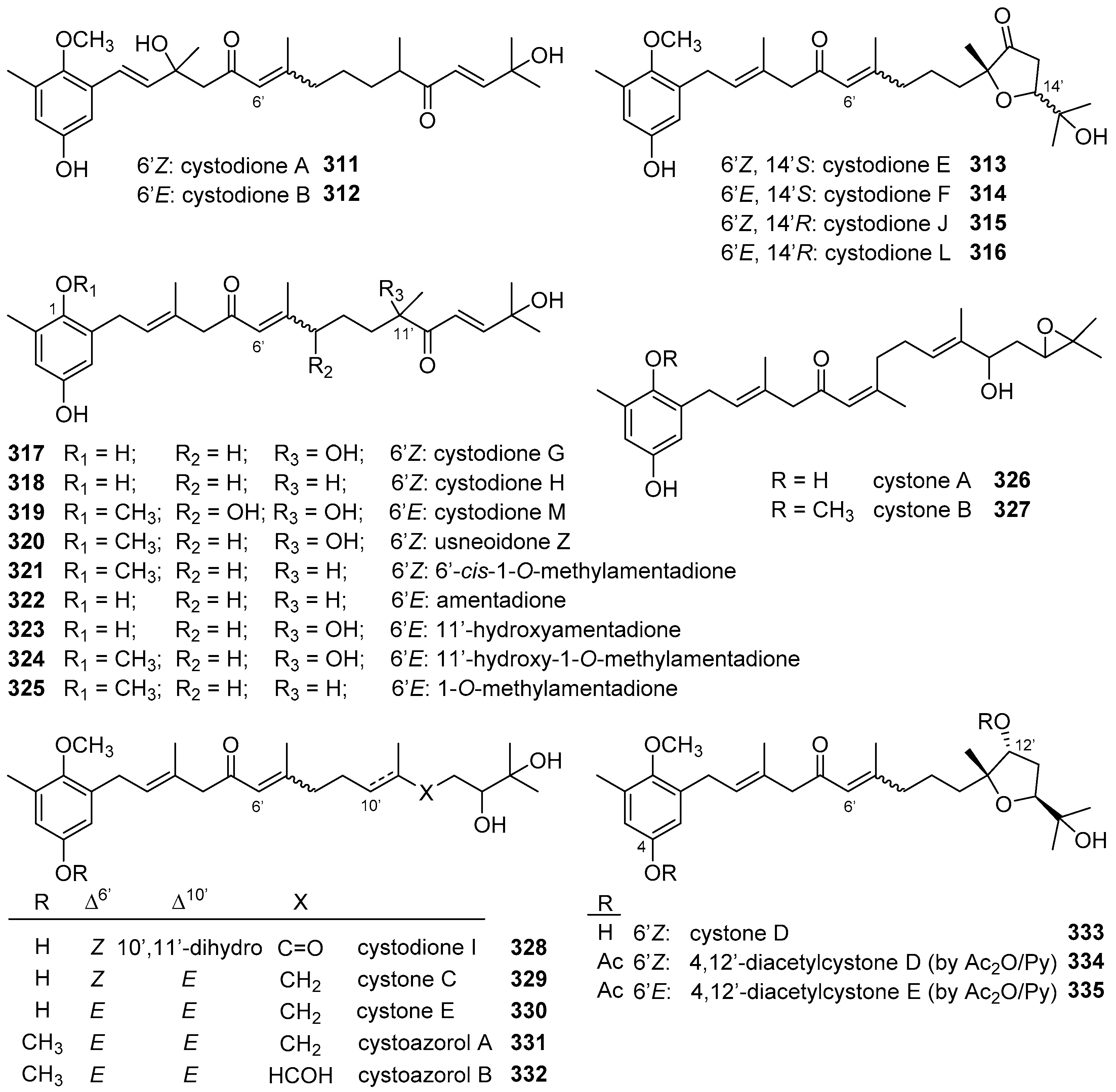
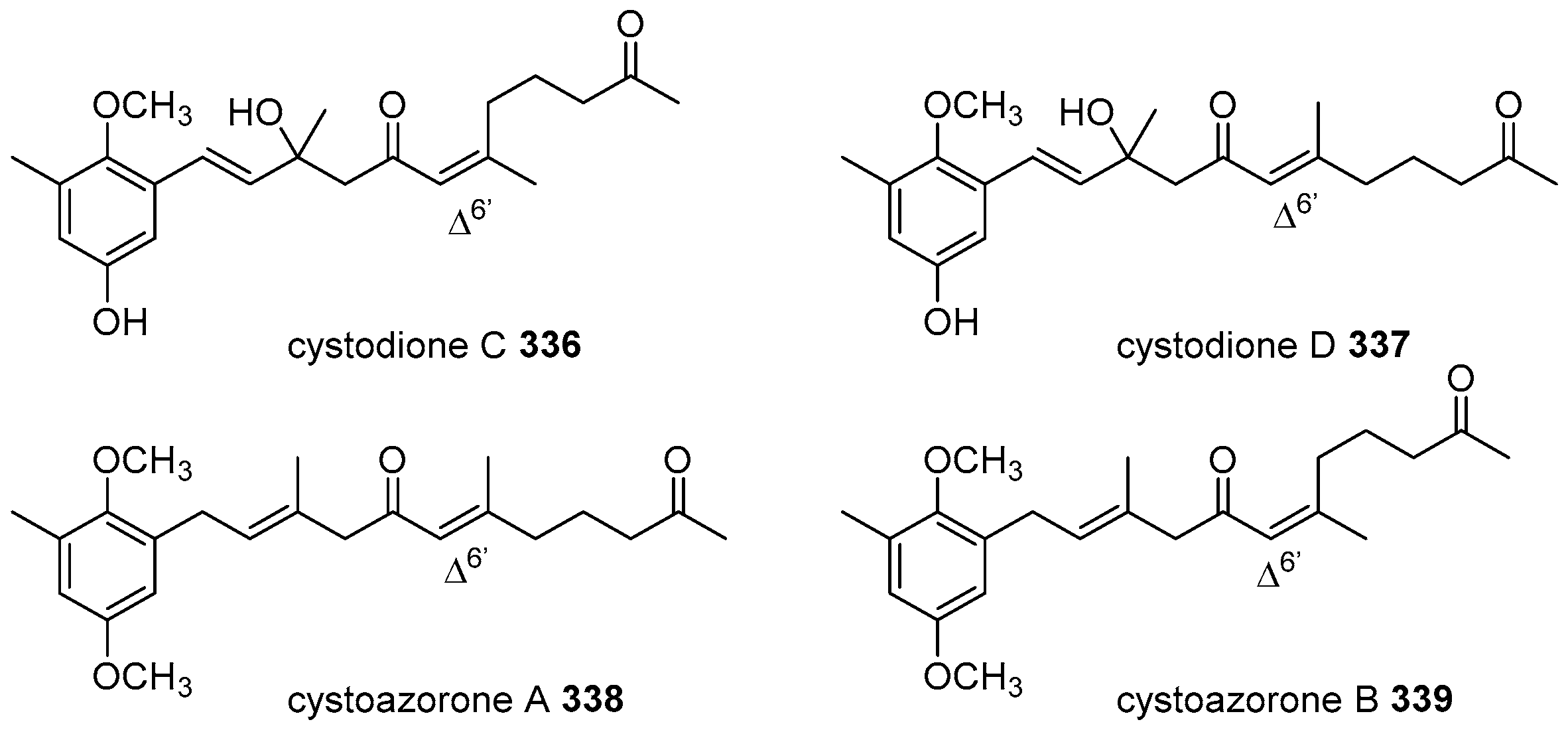
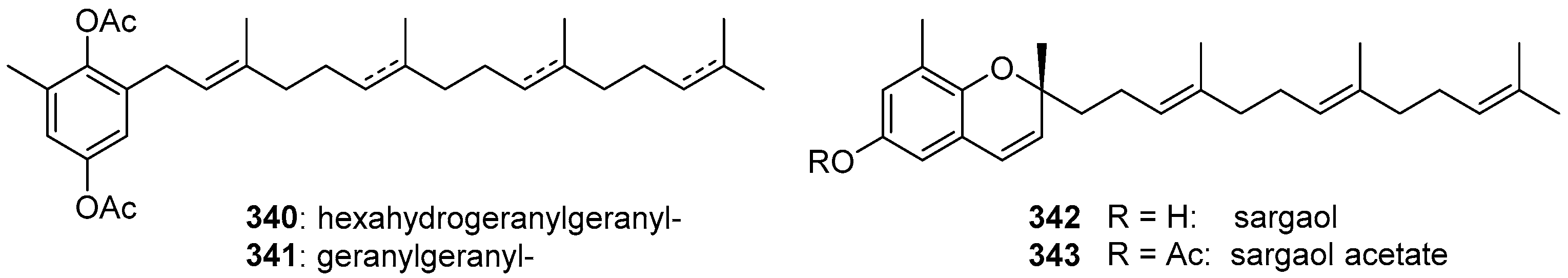
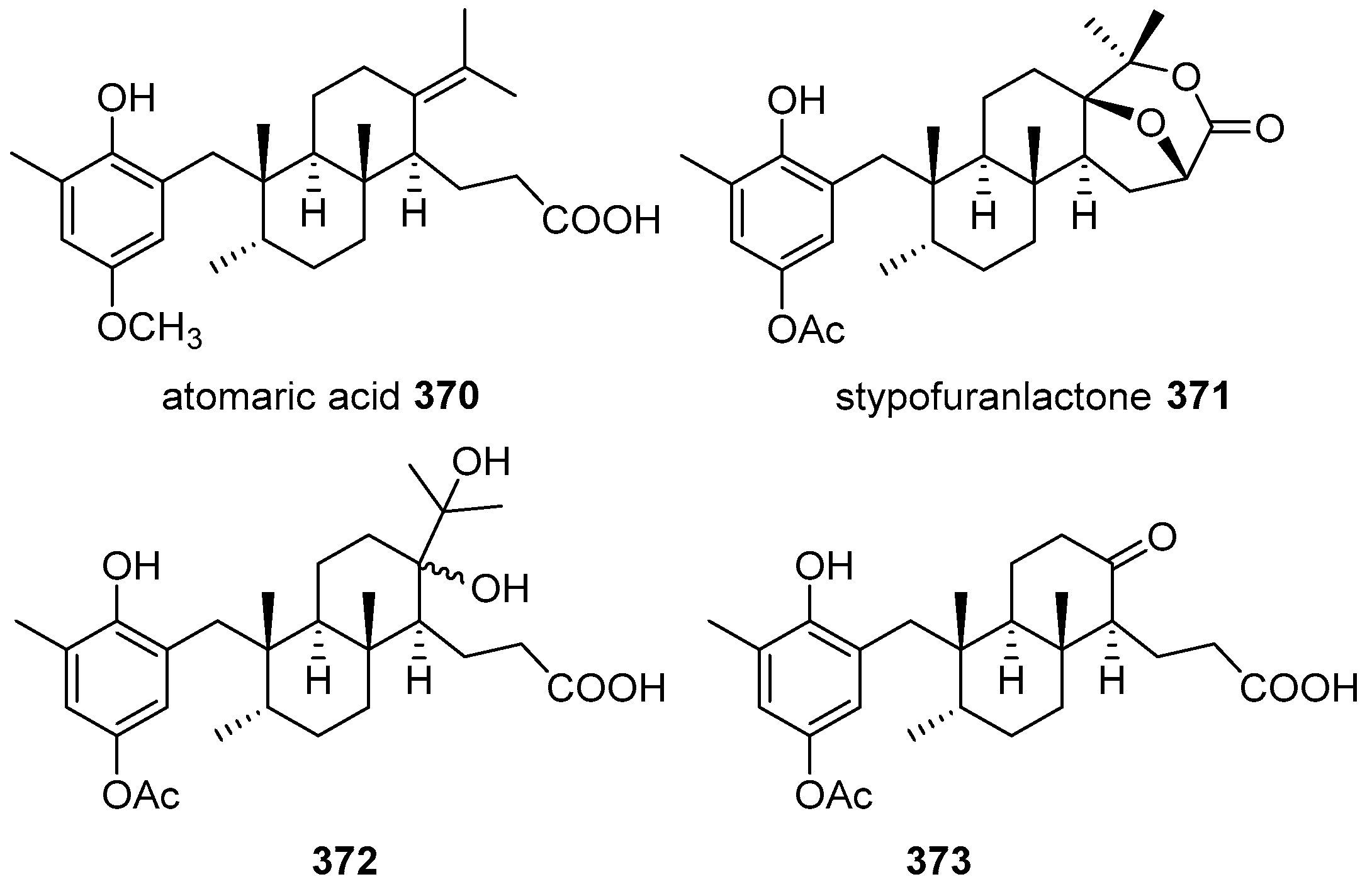
5. Meroterpenoids with Open-Chain and Cyclic Diterpenoids
5.1. Open-Chain Diterpenoids
5.2. Monocyclic Diterpenoids
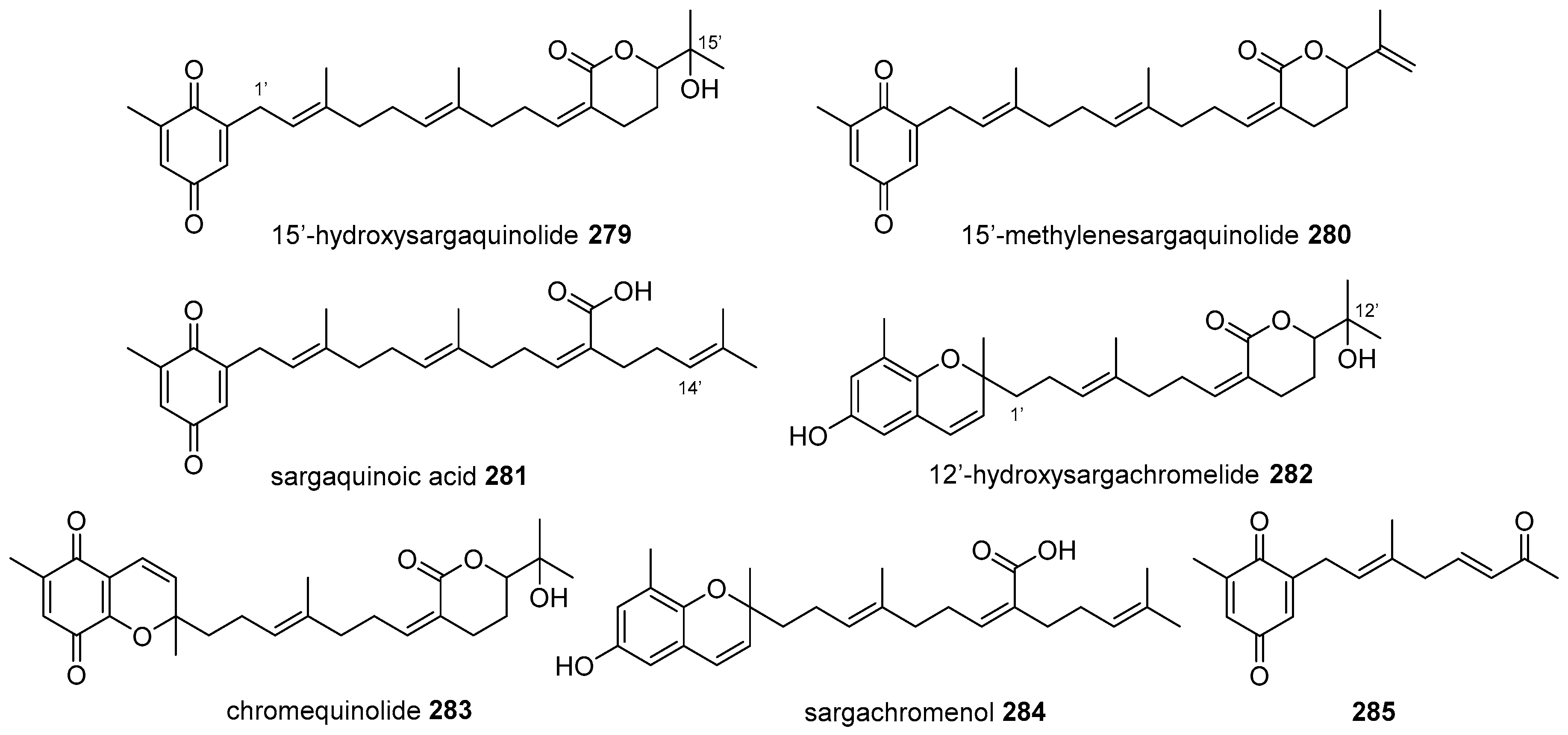
5.3. Bicyclic Diterpenoids
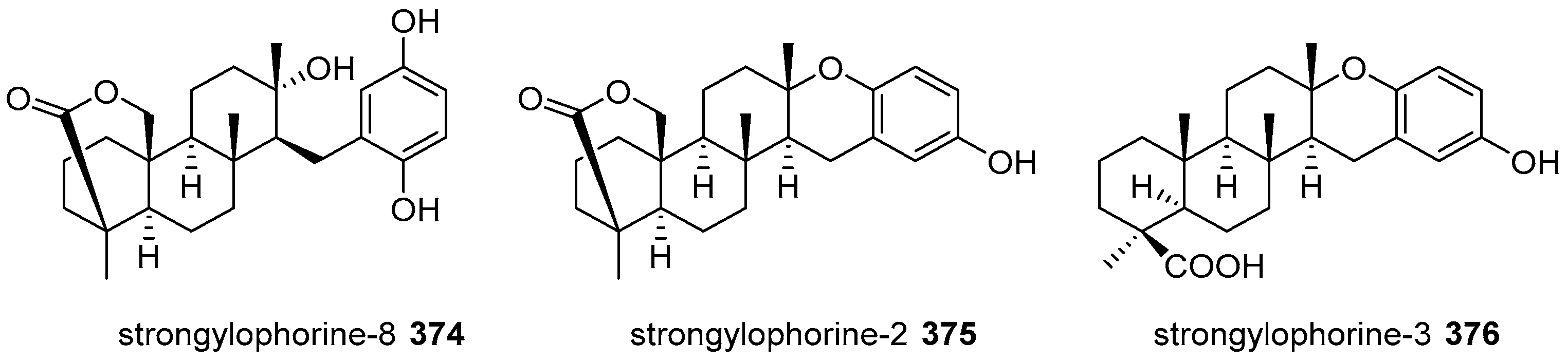
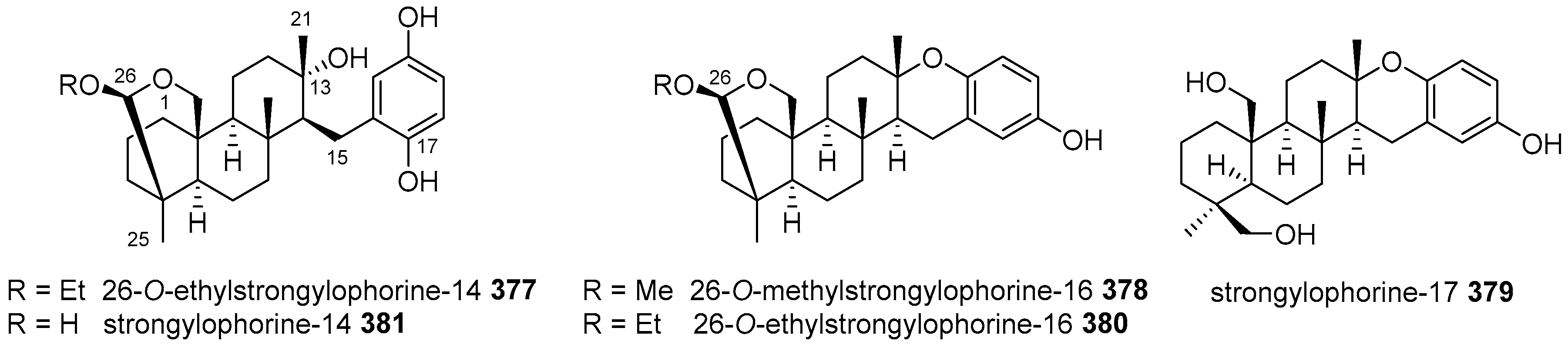
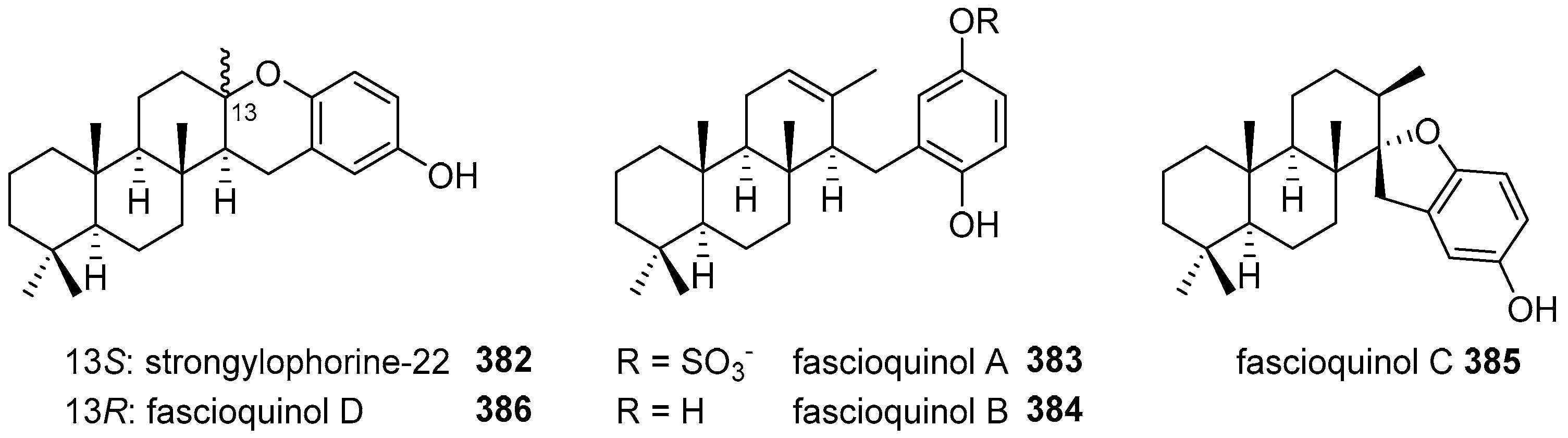
5.4. Tricyclic and Tetracyclic Diterpenoids
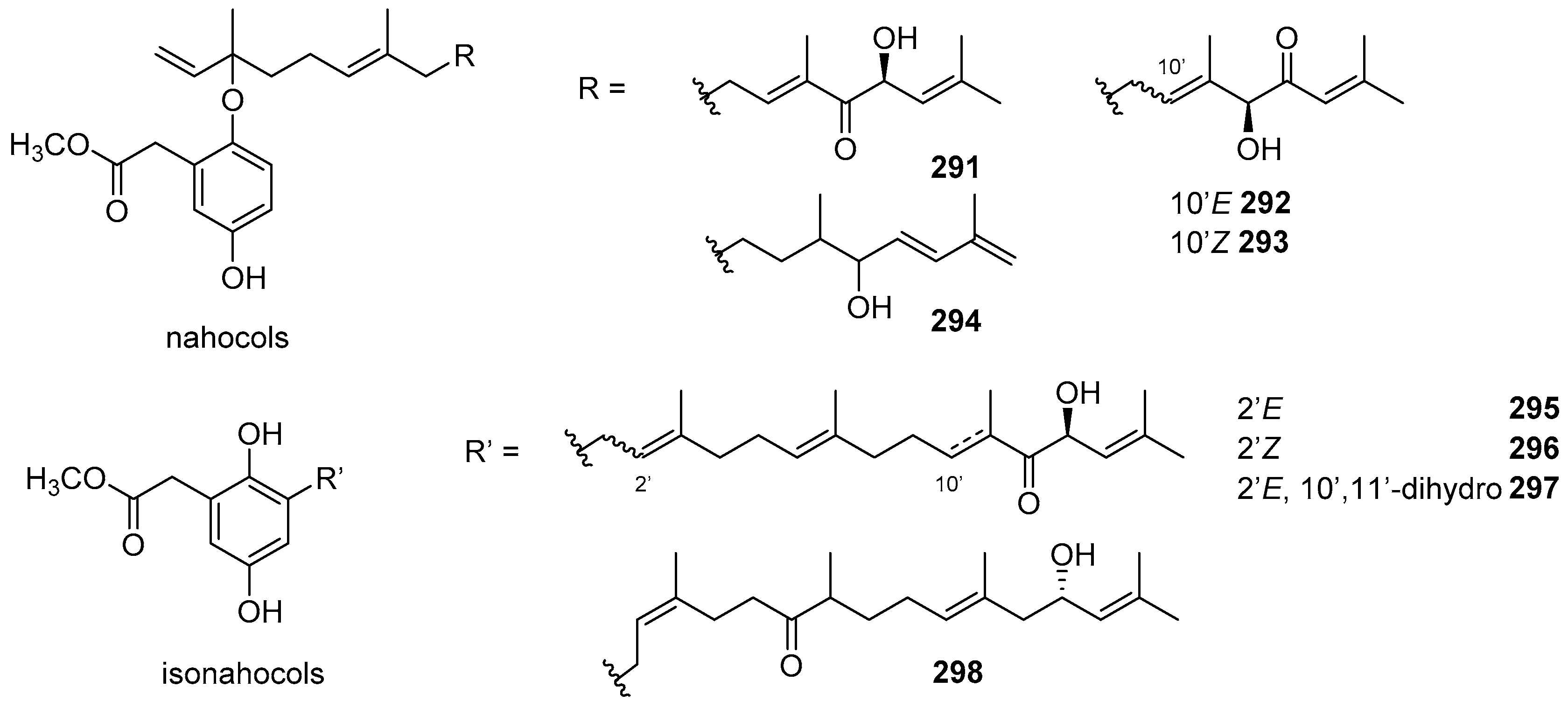
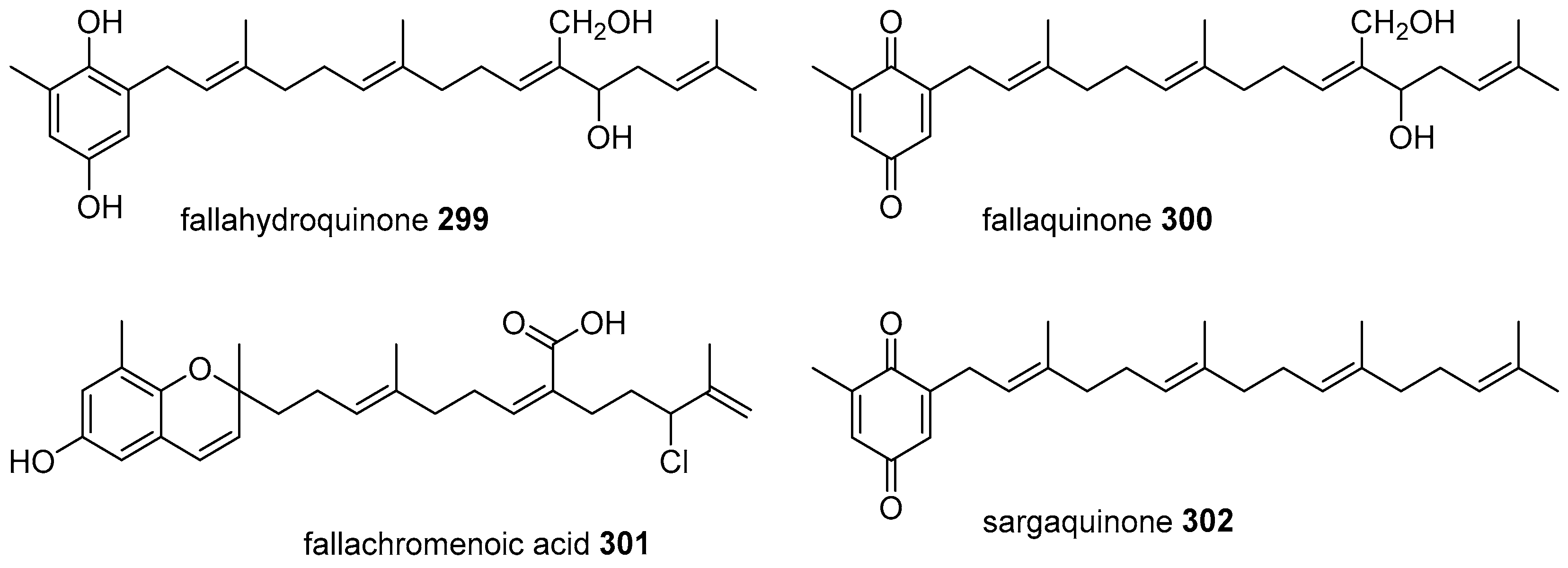
6. Meroterpenoids with Larger Terpenoids
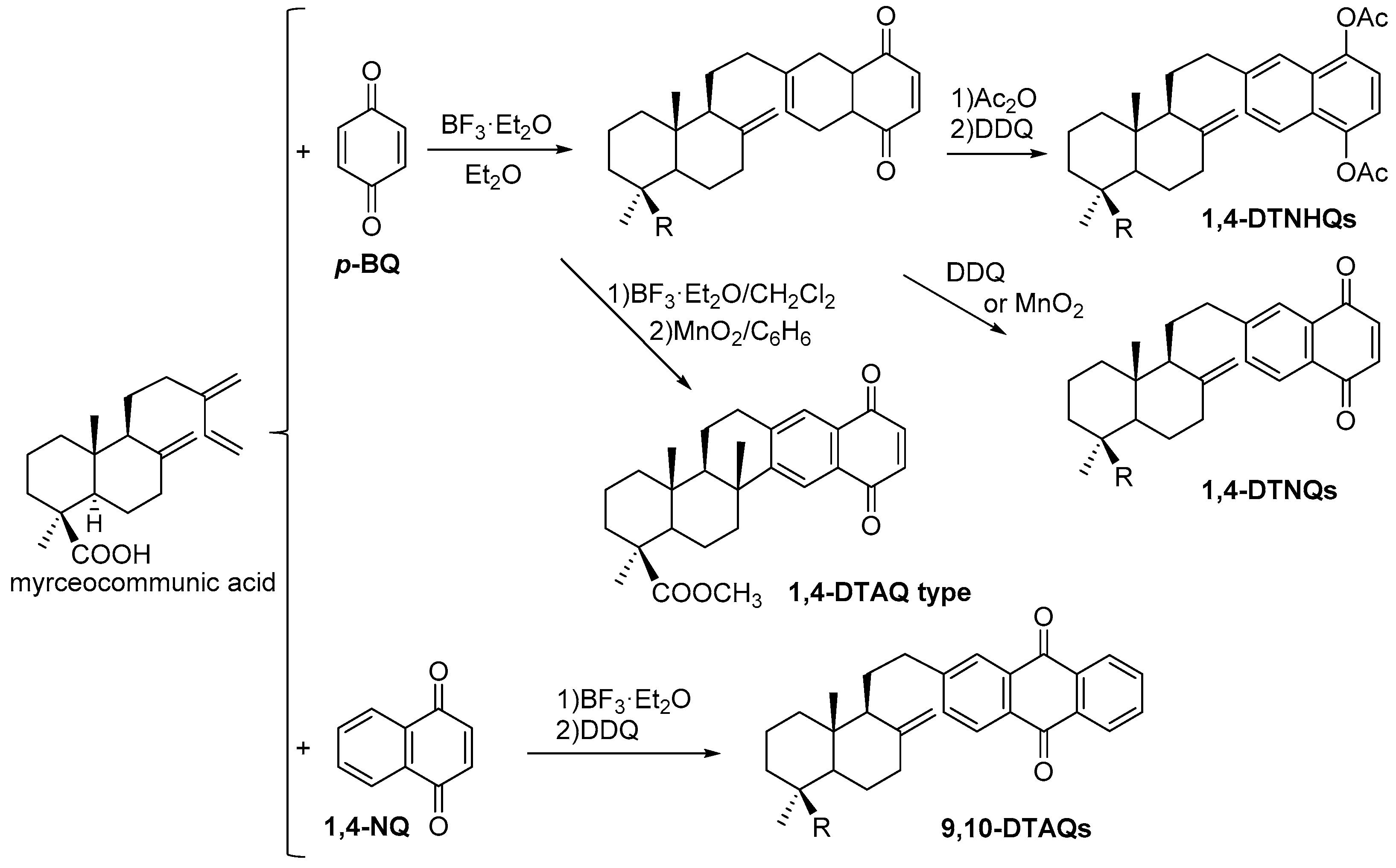
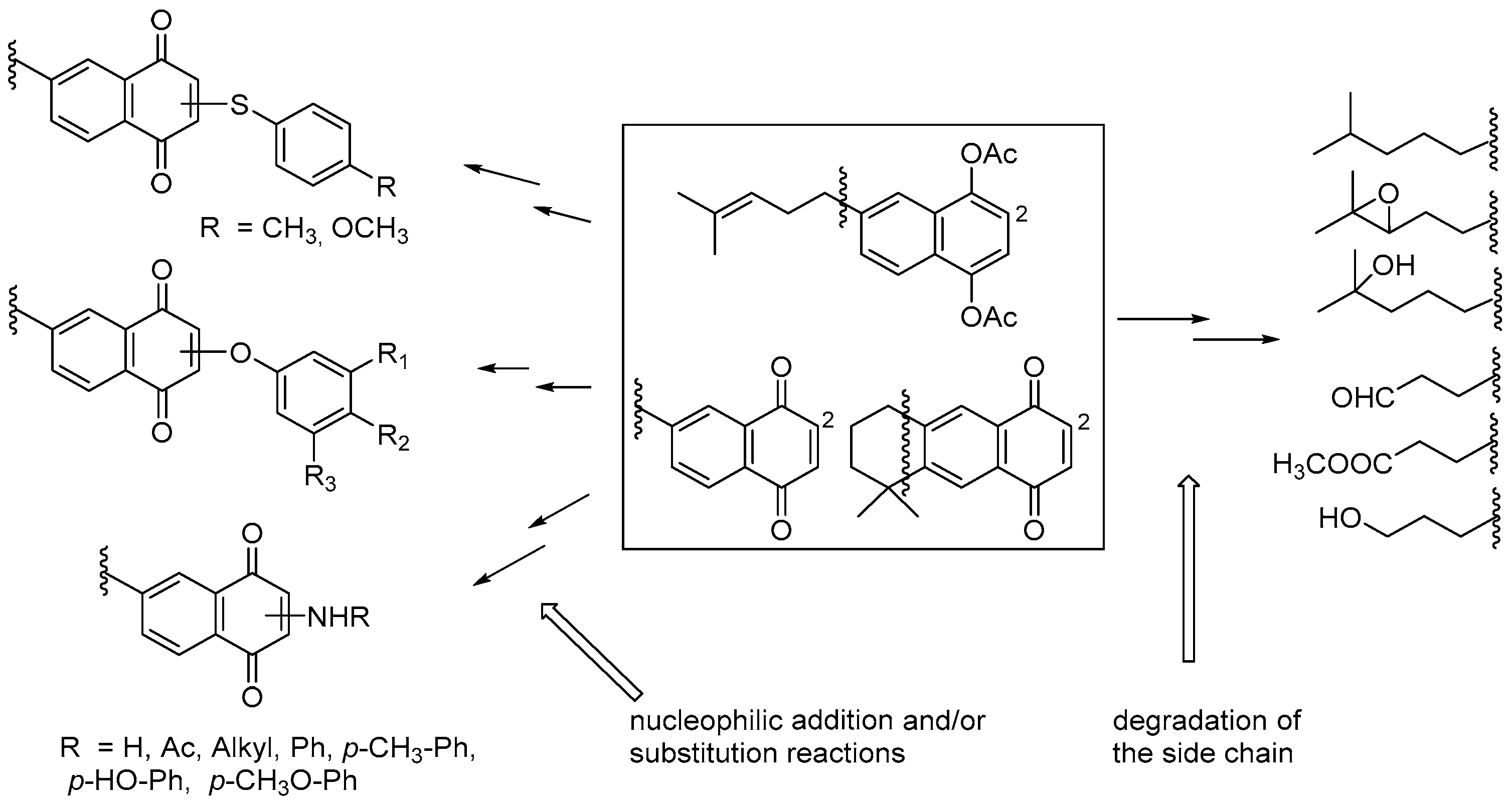
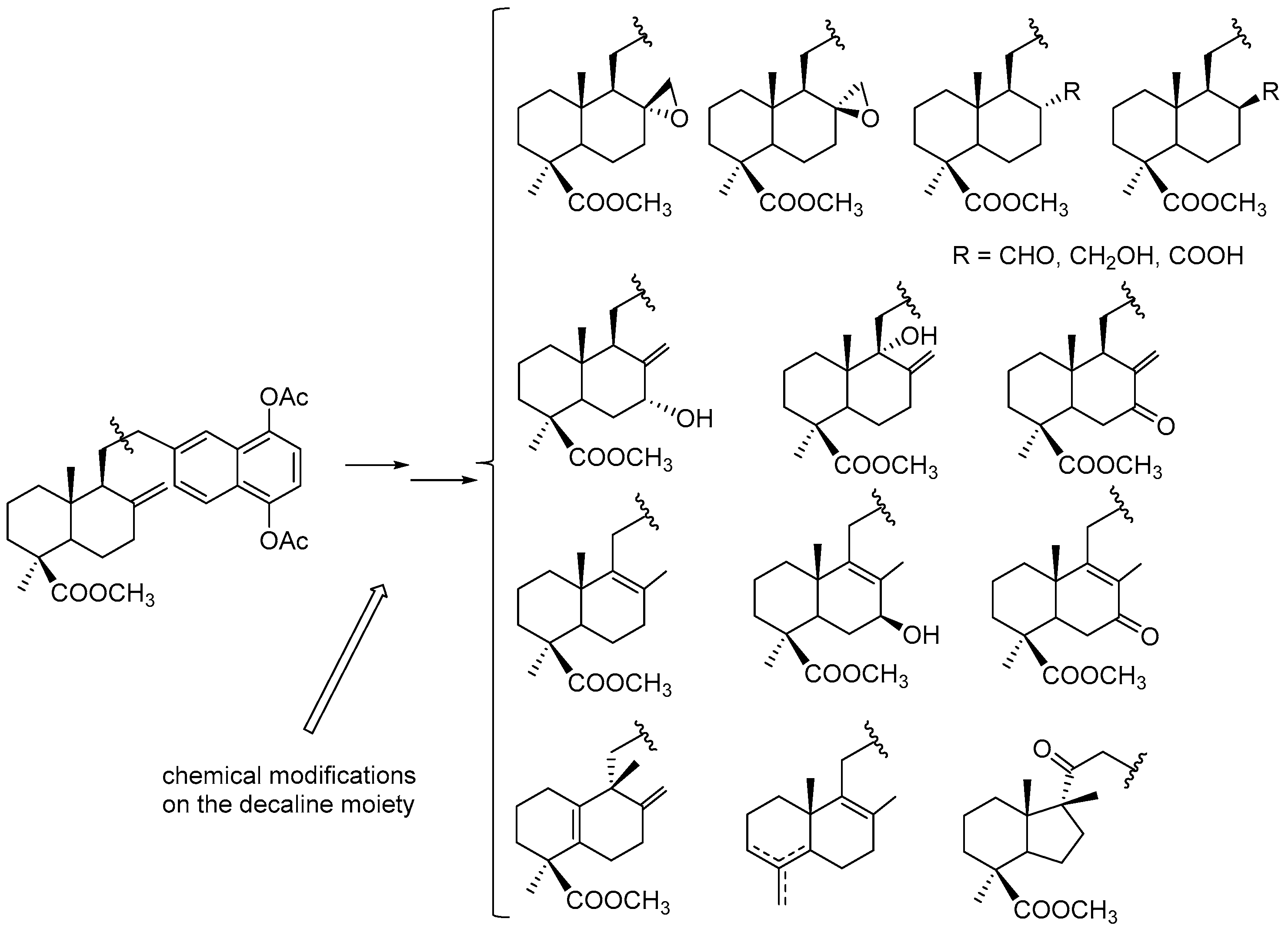
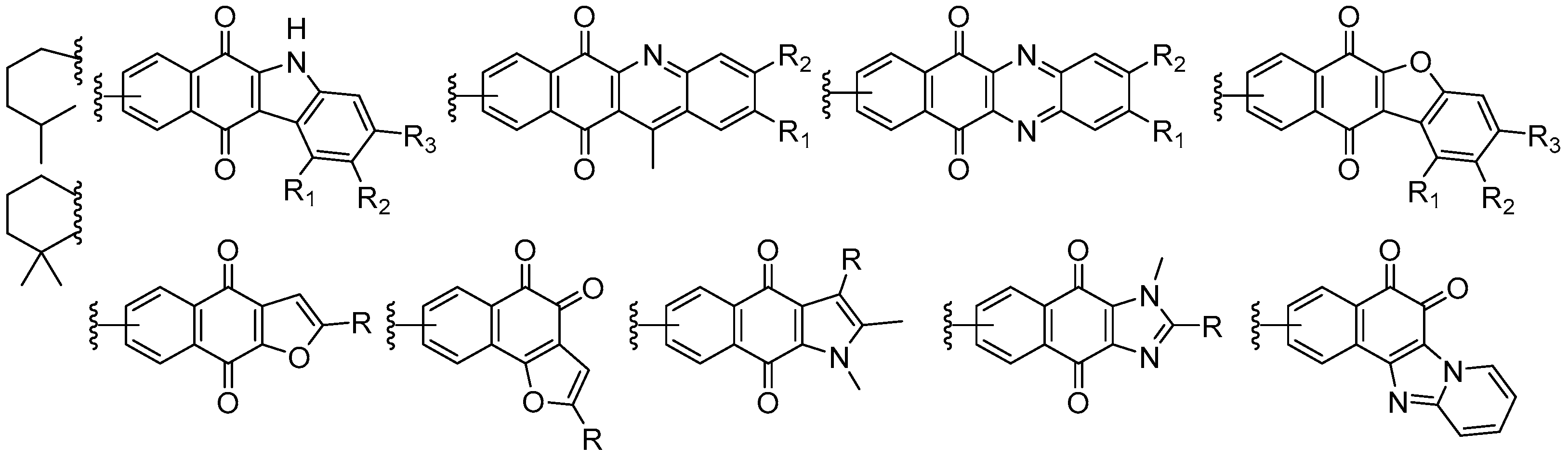
7. Preparation of Bioactive TQs/HQs from Inactive Terpenoids
8. Concluding Remarks and Perspectives
Author Contributions
Acknowledgments
Conflicts of Interest
References
- De Rosa, S.; Tommonaro, G. Bioactive marine prenylated quinones/quinols. In Bioactive Natural Products; UrRahman, A., Ed.; Elsevier: New York, NY, USA, 2012; Volume 36, pp. 163–218. [Google Scholar]
- Menna, M.; Imperatore, C.; D’Aniello, F.; Aiello, A. Meroterpenes from marine invertebrates: Structures, occurrence and ecological implications. Mar. Drugs 2013, 11, 1602–1643. [Google Scholar] [CrossRef] [PubMed]
- Sunassee, S.N.; Davies-Coleman, M.T. Cytotoxic and antioxidant marine prenylated quinones and hydroquinones. Nat. Prod. Rep. 2012, 29, 513–535. [Google Scholar] [CrossRef] [PubMed]
- Gordaliza, M. Cytotoxic terpene quinones from marine sponges. Mar. Drugs 2010, 8, 2849–2870. [Google Scholar] [CrossRef] [PubMed]
- Bertanha, C.S.; Januario, A.H.; Alvarenga, T.A.; Pimenta, L.P.; Silva, M.; Cunha, W.R.; Pauletti, P.M. Quinone and hydroquinone metabolites from the ascidians of the genus Aplidium. Mar. Drugs 2014, 12, 3608–3633. [Google Scholar] [CrossRef] [PubMed]
- Marcos, I.S.; Conde, A.; Moro, R.F.; Basabe, P.; Diez, D.; Urones, J.G. Quinone/hydroquinone sesquiterpenes. Mini-Rev. Org. Chem. 2010, 7, 230–254. [Google Scholar] [CrossRef]
- Howard, B.M.; Clarkson, K.; Bernstein, R.L. Simple prenylated hydroquinone derivatives from the marine urochordate Aplidium californicum. Natural anti-cancer and anti-mutagenic agents. Tetrahedron Lett. 1979, 20, 4449–4452. [Google Scholar] [CrossRef]
- Appleton, D.R.; Chuen, C.S.; Berridge, M.V.; Webb, V.L.; Copp, B.R. Rossinones A and B, biologically active meroterpenoids from the Antarctic ascidian, Aplidium species. J. Org. Chem. 2009, 74, 9195–9198. [Google Scholar] [CrossRef] [PubMed]
- Ovenden, S.P.B.; Nielson, J.L.; Liptrot, C.H.; Willis, R.H.; Wright, A.D.; Motti, C.A.; Tapiolas, D.M. Comosusols A-D and comosone A: Cytotoxic compounds from the brown alga Sporochnus comosus. J. Nat. Prod. 2011, 74, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Coombes, C.L.; Moody, C.J. First syntheses of 2,2-dimethyl-7-(2′-methylbut-3′-en-2′-yl)-2H-chromen-6-ol and 2-(3′-methylbut-2′-enyl)-5-(2′-methylbut-3′-en-2′-yl)-1,4-benzoquinone, novel prenylated quinone derivatives from the New Zealand brown alga Perithalia capillaris. J. Org. Chem. 2008, 73, 6758–6762. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, S.; Li, J.; Chen, Y.; Saurav, K.; Zhang, Q.; Zhang, H.; Zhang, W.; Zhang, W.; Zhang, S.; et al. Antibacterial and cytotoxic new napyradiomycins from the marine-derived Streptomyces sp. SCSIO 10428. Mar. Drugs 2013, 11, 2113–2125. [Google Scholar] [CrossRef] [PubMed]
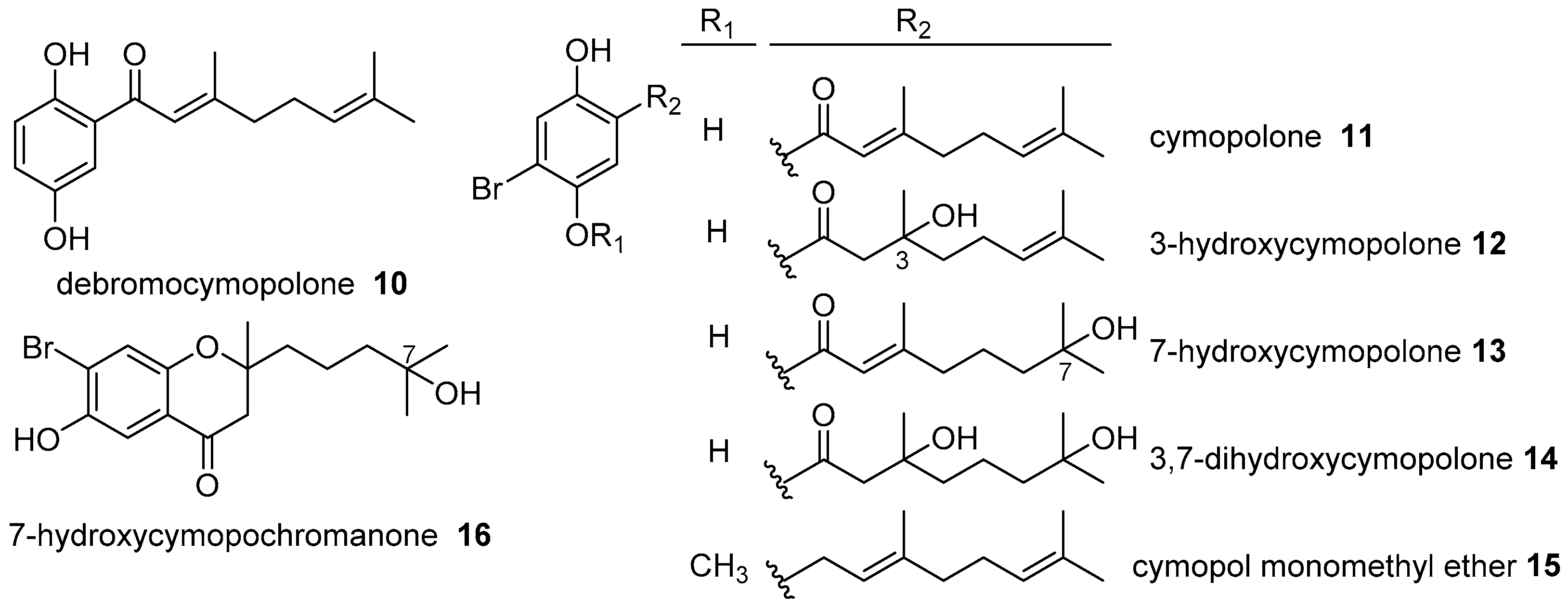
- Gallimore, W.A.; Sambo, T.; Campbell, T. Debromocymopolone from the green alga, Cymopolia barbata. J. Chem. Res. 2009. [Google Scholar] [CrossRef]
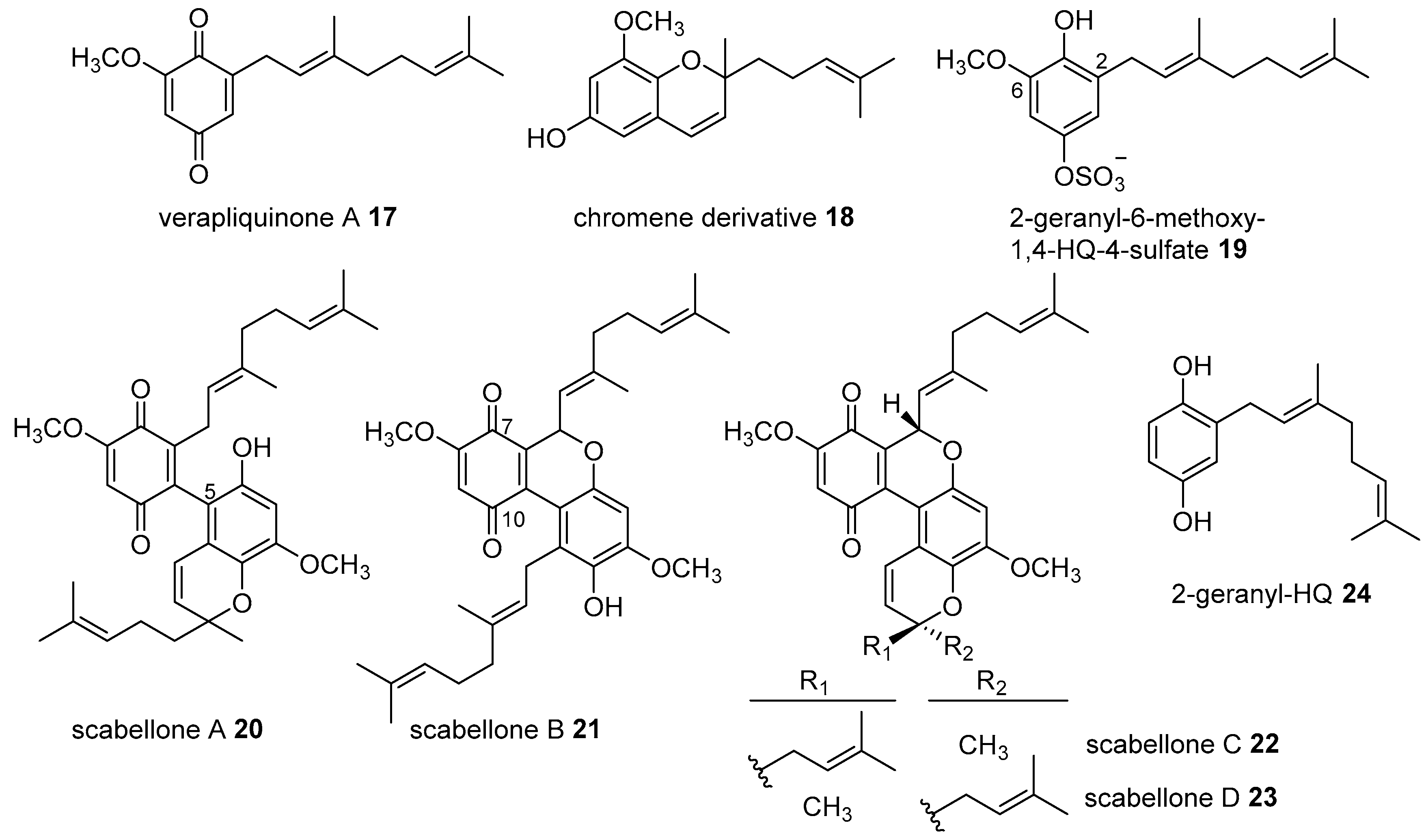
- Chan, S.T.S.; Pearce, A.N.; Januario, A.H.; Page, M.J.; Kaiser, M.; McLaughlin, R.J.; Harper, J.L.; Webb, V.L.; Barker, D.; Copp, B.R. Anti-inflammatory and antimalarial meroterpenoids from the New Zealand ascidian Aplidium scabellum. J. Org. Chem. 2011, 76, 9151–9156. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.T.S.; Pullar, M.A.; Khalil, I.M.; Allouche, E.; Barker, D.; Copp, B.R. Bio-inspired dimerisation of prenylated quinones directed towards the synthesis of the meroterpenoid natural products, the scabellones. Tetrahedron Lett. 2015, 56, 1486–1488. [Google Scholar] [CrossRef]
- Aiello, A.; Fattorusso, E.; Luciano, P.; Macho, A.; Menna, M.; Munoz, E. Antitumor effects of two novel naturally occurring terpene quinones isolated from the Mediterranean ascidian Aplidium conicum. J. Med. Chem. 2005, 48, 3410–3416. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Lucas, C.L.; Moody, C.J. Biomimetic synthesis of the apoptosis-inducing thiazinoquinone thiaplidiaquinone A. J. Org. Chem. 2012, 77, 9179–9189. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.M.; Barker, D.; Copp, B.R. Biomimetic synthesis of thiaplidiaquinones A and B. J. Nat. Prod. 2012, 75, 2256–2260. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.L.; Khalil, I.M.; Shaw, L.; Bourguet-Kondracki, M.L.; Dubois, J.; Valentin, A.; Barker, D.; Copp, B.R. Structure-activity relationships of the bioactive thiazinoquinone marine natural products thiaplidiaquinones A and B. Mar. Drugs 2015, 13, 5102–5110. [Google Scholar] [CrossRef] [PubMed]
- Cadelis, M.M.; Bourguet-Kondracki, M.-L.; Dubois, J.; Kaiser, M.; Brunel, J.M.; Barker, D.; Copp, B.R. Structure-activity relationship studies on thiaplidiaquinones A and B as novel inhibitors of Plasmodium falciparum and farnesyltransferase. Bioorg. Med. Chem. 2017, 25, 4433–4443. [Google Scholar] [CrossRef] [PubMed]
- Menna, M.; Aiello, A.; D’Aniello, F.; Imperatore, C.; Luciano, P.; Vitalone, R.; Irace, C.; Santamaria, R. Conithiaquinones A and B, tetracyclic cytotoxic meroterpenes from the Mediterranean ascidian Aplidium conicum. Eur. J. Org. Chem. 2013, 3241–3246. [Google Scholar] [CrossRef]
- Imperatore, C.; Cimino, P.; Cebrian-Torrejon, G.; Persico, M.; Aiello, A.; Senese, M.; Fattorusso, C.; Menna, M.; Domenech-Carbo, A. Insight into the mechanism of action of marine cytotoxic thiazinoquinones. Mar. Drugs 2017, 15, 335. [Google Scholar] [CrossRef] [PubMed]
- Imperatore, C.; Persico, M.; Aiello, A.; Luciano, P.; Guiso, M.; Sanasi, M.F.; Taramelli, D.; Parapini, S.; Cebrian-Torrejon, G.; Domenech-Carbo, A.; et al. Marine inspired antiplasmodial thiazinoquinones: Synthesis, computational studies and electrochemical assays. RSC Adv. 2015, 5, 70698–70702. [Google Scholar] [CrossRef]
- Simon-Levert, A.; Arrault, A.; Bontemps-Subielos, N.; Canal, C.; Banaigs, B. Meroterpenes from the ascidian Aplidium aff. densum. J. Nat. Prod. 2005, 68, 1412–1415. [Google Scholar] [CrossRef] [PubMed]
- Simon-Levert, A.; Menniti, C.; Soulere, L.; Geneviere, A.M.; Barthomeuf, C.; Banaigs, B.; Witczak, A. Marine natural meroterpenes: Synthesis and antiproliferative activity. Mar. Drugs 2010, 8, 347–358. [Google Scholar] [CrossRef] [PubMed]
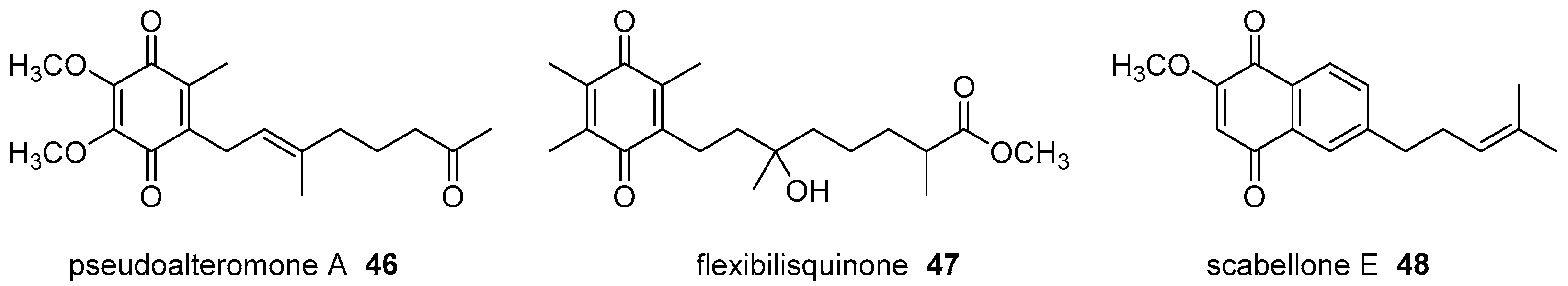
- Chen, Y.H.; Lu, M.C.; Chang, Y.C.; Hwang, T.L.; Wang, W.H.; Weng, C.F.; Kuo, J.M.; Sung, P.J. Pseudoalteromone A: A novel bioactive ubiquinone from a marine bacterium Pseudoalteromonas sp. CGH2XX (Pseudoalteromonadaceae). Tetrahedron Lett. 2012, 53, 1675–1677. [Google Scholar] [CrossRef]
- Lin, Y.F.; Kuo, C.Y.; Wen, Z.H.; Lin, Y.Y.; Wang, W.H.; Su, J.H.; Sheu, J.H.; Sung, P.J. Flexibilisquinone, a new anti-inflammatory quinone from the cultured soft coral Sinularia flexibilis. Molecules 2013, 18, 8160–8167. [Google Scholar] [CrossRef] [PubMed]
- Martucci, H.; Campit, S.E.; Gee, S.R.; Bray, W.M.; Gokey, T.; Cada, A.K.; Yen, T.Y.; Minoura, K.; Guliaev, A.B.; Lokey, R.S.; et al. Naphthablins B and C, meroterpenoids identified from the marine sediment-derived Streptomyces sp. CP26-58 using HeLa cell-based cytological profiling. J. Nat. Prod. 2017, 80, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Kwon, H.C. New naphthoquinone terpenoids from marine actinobacterium, Streptomyces sp. CNQ-509. Mar. Drugs 2018, 16, 90. [Google Scholar] [CrossRef] [PubMed]
- Motohashi, K.; Irie, K.; Toda, T.; Matsuo, Y.; Kasai, H.; Sue, M.; Furihata, K.; Seto, H. Studies on terpenoids produced by actinomycetes. 5-Dimethylallylindole-3-carboxylic acid and A80915G-8′′-acid produced by marine-derived Streptomyces sp. MS239. J. Antibiot. 2008, 61, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Miles, Z.D.; Diethelm, S.; Pepper, H.P.; Huang, D.M.; George, J.H.; Moore, B.S. A unifying paradigm for naphthoquinone-based meroterpenoid (bio)synthesis. Nat. Chem. 2017, 9, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.S. Asymmetric alkene and arene halofunctionalization reactions in meroterpenoid biosynthesis. Synlett 2018, 29, 401–409. [Google Scholar] [CrossRef]
- Lacret, R.; Perez-Victoria, I.; Oves-Costales, D.; de la Cruz, M.; Domingo, E.; Martin, J.; Diaz, C.; Vicente, F.; Genilloud, O.; Reyes, F. MDN-0170, a new napyradiomycin from Streptomyces sp. strain CA-271078. Mar. Drugs 2016, 14, 188. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.B.; Jensen, P.R.; Fenical, W. Cytotoxic and antimicrobial napyradiomycins from two marine-derived Streptomyces strains. Eur. J. Org. Chem. 2013, 3751–3757. [Google Scholar] [CrossRef] [PubMed]
- Farnaes, L.; Coufal, N.G.; Kauffman, C.A.; Rheingold, A.L.; DiPasquale, A.G.; Jensen, P.R.; Fenical, W. Napyradiomycin derivatives, produced by a marine-derived actinomycete, illustrate cytotoxicity by induction of apoptosis. J. Nat. Prod. 2014, 77, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Haste, N.M.; Farnaes, L.; Perera, V.R.; Fenical, W.; Nizet, V.; Hensler, M.E. Bactericidal kinetics of marine-derived napyradiomycins against contemporary methicillin-resistant Staphylococcus aureus. Mar. Drugs 2011, 9, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yang, A.G.; Wu, C.M.; Guo, P.; Proksch, P.; Lin, W.H. Lipid-lowering effects of farnesylquinone and related analogues from the marine-derived Streptomyces nitrosporeus. Bioorg. Med. Chem. Lett. 2014, 24, 5288–5293. [Google Scholar] [CrossRef] [PubMed]
- He, W.J.; Zhou, X.J.; Qin, X.C.; Mai, Y.X.; Lin, X.P.; Liao, S.R.; Yang, B.; Zhang, T.Y.; Tu, Z.C.; Wang, J.F.; et al. Quinone/hydroquinone meroterpenoids with antitubercular and cytotoxic activities produced by the sponge-derived fungus Gliomastix sp. ZSDS1-F7. Nat. Prod. Res. 2017, 31, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.Y.; Chokkalingam, U.; Chiou, S.F.; Hwang, T.L.; Chen, S.L.; Wang, W.L.; Sheu, J.H. Bioactive chemical constituents from the brown alga Homoeostrichus formosana. Int. J. Mol. Sci. 2015, 16, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Gothel, Q.; Kock, M. New sesquiterpene hydroquinones from the Caribbean sponge Aka coralliphagum. Beilstein J. Org. Chem. 2014, 10, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Kaysser, L.; Bernhardt, P.; Nam, S.J.; Loesgen, S.; Ruby, J.G.; Skewes-Cox, P.; Jensen, P.R.; Fenical, W.; Moore, B.S. Merochlorins A-D, cyclic meroterpenoid antibiotics biosynthesized in divergent pathways with vanadium-dependent chloroperoxidases. J. Am. Chem. Soc. 2012, 134, 11988–11991. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, V.L.M.; Seca, A.M.L.; Barreto, M.C.; Neto, A.I.; Kijjoa, A.; Silva, A.M.S. Cytotoxic meroterpenoids from the macroalga Cystoseira abies-marina. Phytochem. Lett. 2013, 6, 593–597. [Google Scholar] [CrossRef]
- De los Reyes, C.; Zbakh, H.; Motilva, V.; Zubia, E. Antioxidant and anti-inflammatory meroterpenoids from the brown alga Cystoseira usneoides. J. Nat. Prod. 2013, 76, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Ovenden, S.P.B.; Nielson, J.L.; Liptrot, C.H.; Willis, R.H.; Tapiolas, D.M.; Wright, A.D.; Motti, C.A. Metachromins U-W: Cytotoxic merosesquiterpenoids from an Australian specimen of the sponge Thorecta reticulata. J. Nat. Prod. 2011, 74, 1335–1338. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.X.; Huang, H.B.; Chen, Y.C.; Ding, J.; Zhang, Y.; Sun, A.J.; Zhang, W.M.; Ju, J.H. Cytotoxic and antibacterial marfuraquinocins from the deep South China Sea-derived Streptomyces niveus SCSIO 3406. J. Nat. Prod. 2013, 76, 2263–2268. [Google Scholar] [CrossRef] [PubMed]
- Peña-Lopez, M.; Martinez, M.M.; Sarandeses, L.A.; Pérez-Sestelo, J. Total synthesis of (+)-neomarinone. Chem. Eur. J. 2009, 15, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.Y.; Huang, K.J.; Wang, S.K.; Duh, C.Y. Capilloquinol: A novel farnesyl quinol from the Dongsha Atoll soft coral Sinularia capillosa. Mar. Drugs 2011, 9, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Shang, Z.; Li, X.M.; Li, C.S.; Wang, B.G. Diverse secondary metabolites produced by marine-derived fungus Nigrospora sp. MA75 on various culture media. Chem. Biodivers. 2012, 9, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.H.; Huang, X.J.; Yang, J.S.; Yang, F.; Piao, S.J.; Gao, H.; Li, J.; Ye, W.C.; Yao, X.S.; Chen, W.S.; et al. Dysidavarones A-D, new sesquiterpene quinones from the marine sponge Dysidea avara. Org. Lett. 2012, 14, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.W.L.; Jankam, A.; Hooper, J.N.A.; Suksamrarn, A.; Garson, M.J. Stereochemical evaluation of sesquiterpene quinones from two sponges of the genus Dactylospongia and the implication for enantioselective processes in marine terpene biosynthesis. Tetrahedron 2008, 64, 6341–6348. [Google Scholar] [CrossRef]
- Mori, K.; Komatsu, M. Synthesis and absolute-configuration of zonarol, a fungitoxic hydroquinone from the brown seaweed Dictyopteris zonarioides. Bull. Soc. Chim. Belg. 1986, 95, 771–781. [Google Scholar] [CrossRef]
- Laube, T.; Bernet, A.; Dahse, H.M.; Jacobsen, I.D.; Seifert, K. Synthesis and pharmacological activities of some sesquiterpene quinones and hydroquinones. Bioorg. Med. Chem. 2009, 17, 1422–1427. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Koyama, T.; Noguchi, H.; Ueda, Y.; Kitsuyama, R.; Shimizu, H.; Tanimoto, A.; Wang, K.-Y.; Nawata, A.; Nakayama, T.; et al. Marine hydroquinone zonarol prevents inflammation and apoptosis in dextran sulfate sodium-induced mice ulcerative colitis. PLoS ONE 2014, 9, e113509. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Koyama, T.; Yamada, S.; Lipton, S.A.; Satoh, T. Zonarol, a sesquiterpene from the brown algae Dictyopteris undulata, provides neuroprotection by activating the Nrf2/ARE pathway. Biochem. Biophys. Res. Commun. 2015, 457, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Lane, A.L.; Mular, L.; Drenkard, E.J.; Shearer, T.L.; Engel, S.; Fredericq, S.; Fairchild, C.R.; Prudhomme, J.; Le Roch, K.; Hay, M.E.; et al. Ecological leads for natural product discovery: Novel sesquiterpene hydroquinones from the red macroalga Peyssonnelia sp. Tetrahedron 2010, 66, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.P.; Zhou, X.F.; Wang, F.Z.; Liu, K.S.; Yang, B.; Yang, X.W.; Peng, Y.; Liu, J.; Ren, Z.; Liu, Y.H. A new cytotoxic sesquiterpene quinone produced by Penicillium sp. F00120 isolated from a deep sea sediment sample. Mar. Drugs 2012, 10, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.M.; Cui, C.B.; Li, C.W.; Wu, C.J.; Zhang, Z.J.; Li, L.; Huang, X.J.; Ye, W.C. Purpurogemutantin and purpurogemutantidin, new drimenyl cyclohexenone derivatives produced by a mutant obtained by diethyl sulfate mutagenesis of a marine-derived Penicillium purpurogenum G59. Mar. Drugs 2012, 10, 1266–1287. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, I.E.; Gross, H.; Pontius, A.; Kehraus, S.; Krick, A.; Kelter, G.; Maier, A.; Fiebig, H.H.; Konig, G.M. Epoxyphomalin A and B, prenylated polyketides with potent cytotoxicity from the marine-derived fungus Phoma sp. Org. Lett. 2009, 11, 5014–5017. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, I.E.; Kehraus, S.; Krick, A.; Konig, G.M.; Kelter, G.; Maier, A.; Fiebig, H.H.; Kalesse, M.; Malek, N.P.; Gross, H. Mode of action of epoxyphomalins A and B and characterization of related metabolites from the marine-derived fungus Paraconiothyrium sp. J. Nat. Prod. 2010, 73, 2053–2056. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Zhou, Y.Q.; Liu, X.X.; Zhang, W.J.; Hu, S.S.; Lin, L.P.; Huo, G.M.; Jiao, R.H.; Tan, R.X.; Ge, H.M. Antimicrobial and anti-inflammatory compounds from a marine fungus Pleosporales sp. Tetrahedron Lett. 2015, 56, 6183–6189. [Google Scholar] [CrossRef]
- Takeda, Y.; Narita, K.; Katoh, T. Total synthesis of marine sesquiterpene quinones (+)-cyclospongiaquinone-1 and (-)-dehydrocyclospongiaquinone-1 with a tetracyclic benzo[a]xanthene skeleton. Eur. J. Org. Chem. 2017, 901–907. [Google Scholar] [CrossRef]
- Kamble, R.M.; Ramana, M.M.V. Diels-Alder reaction of 2-ethenyl-1,3,3-trimethylcyclohexene with 4H-chromen-4-ones: A convergent approach to ABCD tetracyclic core of marine diterpenoids related to puupehenone and kampanols. Helv. Chim. Acta 2011, 94, 261–267. [Google Scholar] [CrossRef]
- Kamble, R.M.; Ramana, M.M.V. First Lewis acid catalyzed [4+2] cycloaddition reaction of 1,3,3-trimethyl-2-vinyl-1-cyclohexene with chromones: A new entry to analogues of the puupehenone group of marine diterpenoids and kampanols. Monatsh. Chem. 2011, 142, 501–506. [Google Scholar] [CrossRef]
- Huang, J.H.; Lei, X.G. A nature-inspired concise synthesis of (+)-ent-chromazonarol. Sci. China-Chem. 2013, 56, 349–353. [Google Scholar] [CrossRef]
- Hagiwara, K.; Hernandez, J.E.G.; Harper, M.K.; Carroll, A.; Motti, C.A.; Awaya, J.; Nguyen, H.Y.; Wright, A.D. Puupehenol, a potent antioxidant antimicrobial meroterpenoid from a Hawaiian deep-water Dactylospongia sp. sponge. J. Nat. Prod. 2015, 78, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Zhou, Y.D.; Nagle, D.G. Inducers of hypoxic response: Marine sesquiterpene quinones activate HIF-1. J. Nat. Prod. 2013, 76, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, H.Y.; Huang, A.M.; Wang, L.; Wang, Q.; Cao, P.Y.; Yang, P.M. Antibacterial meroterpenoids from the South China Sea sponge Dysidea sp. Chem. Pharm. Bull. 2016, 64, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Ushio, M.; Kubota, T.; Yamamoto, S.; Fromont, J.; Kobayashi, J. Nakijiquinones J–R, sesquiterpenoid quinones with an amine residue from Okinawan marine sponges. J. Nat. Prod. 2010, 73, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Prawat, H.; Mahidol, C.; Kaweetripob, W.; Wittayalai, S.; Ruchirawat, S. Iodo-sesquiterpene hydroquinone and brominated indole alkaloids from the Thai sponge Smenospongia sp. Tetrahedron 2012, 68, 6881–6886. [Google Scholar] [CrossRef]
- Kamishima, T.; Kikuchi, T.; Katoh, T. Total synthesis of (+)-strongylin A, a rearranged sesquiterpenoid hydroquinone from a marine sponge. Eur. J. Org. Chem. 2013, 4558–4563. [Google Scholar] [CrossRef]
- Kuan, K.K.W.; Pepper, H.P.; Bloch, W.M.; George, J.H. Total synthesis of (+)-aureol. Org. Lett. 2012, 14, 4710–4713. [Google Scholar] [CrossRef] [PubMed]
- Speck, K.; Magauer, T. Evolution of a polyene cyclization cascade for the total synthesis of (-)-cyclosmenospongine. Chem. Eur. J. 2017, 23, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Boufridi, A.; Lachkar, D.; Erpenbeck, D.; Beniddir, M.A.; Evanno, L.; Petek, S.; Debitus, C.; Poupon, E. Ilimaquinone and 5-epi-ilimaquinone: Beyond a simple diastereomeric ratio, biosynthetic considerations from NMR-based analysis. Aust. J. Chem. 2017, 70, 743–750. [Google Scholar] [CrossRef]
- De Caralt, S.; Bry, D.; Bontemps, N.; Turon, X.; Uriz, M.J.; Banaigs, B. Sources of secondary metabolite variation in Dysidea avara (Porifera: Demospongiae): The importance of having good neighbors. Mar. Drugs 2013, 11, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Minale, L.; Riccio, R.; Sodano, G. Avarol, a novel sesquiterpenoid hydroquinone with a rearranged drimane skeleton from sponge Disidea avara. Tetrahedron Lett. 1974, 15, 3401–3404. [Google Scholar] [CrossRef]
- Jiao, W.H.; Xu, T.T.; Gu, B.B.; Shi, G.H.; Zhu, Y.; Yang, F.; Han, B.N.; Wang, S.P.; Li, Y.S.; Zhang, W.; et al. Bioactive sesquiterpene quinols and quinones from the marine sponge Dysidea avara. RSC Adv. 2015, 5, 87730–87738. [Google Scholar] [CrossRef]
- Jiao, W.H.; Xu, T.T.; Yu, H.B.; Mu, F.R.; Li, J.; Li, Y.S.; Yang, F.; Han, B.N.; Lin, H.W. Dysidaminones A-M, cytotoxic and NF-κB inhibitory sesquiterpene aminoquinones from the South China Sea sponge Dysidea fragilis. RSC Adv. 2014, 4, 9236–9246. [Google Scholar] [CrossRef]
- Jiao, W.H.; Cheng, B.H.; Shi, G.H.; Chen, G.D.; Gu, B.B.; Zhou, Y.J.; Hong, L.L.; Yang, F.; Liu, Z.Q.; Qiu, S.Q.; et al. Dysivillosins A-D, unusual anti-allergic meroterpenoids from the marine sponge Dysidea villosa. Sci. Rep. 2017, 7, 8947. [Google Scholar] [CrossRef] [PubMed]
- Utkina, N.K.; Denisenko, V.A.; Krasokhin, V.B. Sesquiterpenoid aminoquinones from the marine sponge Dysidea sp. J. Nat. Prod. 2010, 73, 788–791. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.K.; Woo, J.K.; Kim, S.H.; Cho, E.; Lee, Y.J.; Lee, H.S.; Sim, C.J.; Oh, D.C.; Oh, K.B.; Shin, J. Meroterpenoids from a tropical Dysidea sp. sponge. J. Nat. Prod. 2015, 78, 2814–2821. [Google Scholar] [CrossRef] [PubMed]
- Abdjul, D.B.; Yamazaki, H.; Takahashi, O.; Kirikoshi, R.; Ukai, K.; Namikoshi, M. Sesquiterpene hydroquinones with protein tyrosine phosphatase 1B inhibitory activities from a Dysidea sp. marine sponge collected in Okinawa. J. Nat. Prod. 2016, 79, 1842–1847. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Wang, X.M. A new sesquiterpenoid hydroquinone from the marine sponge Dysidea arenaria. Molecules 2008, 13, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Shigemori, H.; Madono, T.; Sasaki, T.; Mikami, Y.; Kobayashi, J. Nakijiquinone A and nakijiquinone B, new antifungal sesquiterpenoid quinones with an amino acid residue from an Okinawan marine sponge. Tetrahedron 1994, 50, 8347–8354. [Google Scholar] [CrossRef]
- Kobayashi, J.; Madono, T.; Shigemori, H. Nakijiquinone C and nakijiquinone D, new sesquiterpenoid quinones with a hydroxy amino acid residue from a marine sponge inhibiting c-erbB-2 kinase. Tetrahedron 1995, 51, 10867–10874. [Google Scholar] [CrossRef]
- Takahashi, Y.; Kubota, T.; Kobayashi, J. Nakijiquinones E and F, new dimeric sesquiterpenoid quinones from marine sponge. Bioorg. Med. Chem. 2009, 17, 2185–2188. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Kubota, T.; Ito, J.; Mikami, Y.; Fromont, J.; Kobayashi, J. Nakijiquinones G-I, new sesquiterpenoid quinones from marine sponge. Bioorg. Med. Chem. 2008, 16, 7561–7564. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Kubota, T.; Takahashi-Nakaguchi, A.; Fromont, J.; Gonoi, T.; Kobayashi, J. Nakijiquinone S and nakijinol C, new meroterpenoids from a marine sponge of the family Spongiidae. Chem. Pharm. Bull. 2014, 62, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Daletos, G.; de Voogd, N.J.; Muller, W.E.G.; Wray, V.; Lin, W.H.; Feger, D.; Kubbutat, M.; Aly, A.H.; Proksch, P. Cytotoxic and protein kinase inhibiting nakijiquinones and nakijiquinols from the sponge Dactylospongia metachromia. J. Nat. Prod. 2014, 77, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Ovenden, S.P.B.; Nielson, J.L.; Liptrot, C.H.; Willis, R.H.; Tapiolas, D.M.; Wright, A.D.; Motti, C.A. Sesquiterpene benzoxazoles and sesquiterpene quinones from the marine sponge Dactylospongia elegans. J. Nat. Prod. 2011, 74, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.; Kawachi, T.; Sato, H.; Setiawan, A.; Kobayashi, M. Marine spongian sesquiterpene phenols, dictyoceratin-C and smenospondiol, display hypoxia-selective growth inhibition against cancer cells. Bioorg. Med. Chem. Lett. 2014, 24, 3155–3157. [Google Scholar] [CrossRef] [PubMed]
- Sumii, Y.; Kotoku, N.; Fukuda, A.; Kawachi, T.; Arai, M.; Kobayashi, M. Enantioselective synthesis of dictyoceratin-A (smenospondiol) and -C, hypoxia-selective growth inhibitors from marine sponge. Bioorg. Med. Chem. 2015, 23, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.M.; Ito, T.; Win, N.N.; Kodama, T.; Hung, V.Q.; Nguyen, H.T.; Morita, H. New antibacterial sesquiterpene aminoquinones from a Vietnamese marine sponge of Spongia sp. Phytochem. Lett. 2016, 17, 288–292. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Ito, T.; Kurimoto, S.; Ogawa, M.; Win, N.N.; Hung, V.Q.; Nguyen, H.T.; Kubota, T.; Kobayashi, J.; Morita, H. New merosesquiterpenes from a Vietnamese marine sponge of Spongia sp. and their biological activities. Bioorg. Med. Chem. Lett. 2017, 27, 3043–3047. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Nguyen, H.M.; Win, N.N.; Vo, H.Q.; Nguyen, H.T.; Morita, H. Three new sesquiterpene aminoquinones from a Vietnamese Spongia sp. and their biological activities. J. Nat. Med. 2018, 72, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Van Kiem, P.; Huyen, L.T.; Hang, D.T.; Nhiem, N.X.; Tai, B.H.; Anh, H.L.T.; Van Cuong, P.; Quang, T.H.; Van Minh, C.; Van Dau, N.; et al. Sesquiterpene derivatives from marine sponge Smenospongia cerebriformis and their anti-inflammatory activity. Bioorg. Med. Chem. Lett. 2017, 27, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gu, B.B.; Sun, F.; Xu, J.R.; Jiao, W.H.; Yu, H.B.; Han, B.N.; Yang, F.; Zhang, X.C.; Lin, H.W. Sesquiterpene quinones/hydroquinones from the marine sponge Spongia pertusa Esper. J. Nat. Prod. 2017, 80, 1436–1445. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Yun, E.; Hwang, I.H.; Yoon, S.; Kim, D.-E.; Kim, J.S.; Na, M.; Song, G.-Y.; Oh, S. Ilimaquinone and ethylsmenoquinone, marine sponge metabolites, suppress the proliferation of multiple myeloma cells by down-regulating the level of β-catenin. Mar. Drugs 2014, 12, 3231–3244. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.H.; Oh, J.; Zhou, W.; Park, S.; Kim, J.H.; Chittiboyina, A.G.; Ferreira, D.; Song, G.Y.; Oh, S.; Na, M.; et al. Cytotoxic activity of rearranged drimane meroterpenoids against colon cancer cells via down-regulation of β-catenin expression. J. Nat. Prod. 2015, 78, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mu, F.R.; Jiao, W.H.; Huang, J.; Hong, L.L.; Yang, F.; Xu, Y.; Wang, S.P.; Sun, F.; Lin, H.W. Meroterpenoids with protein tyrosine phosphatase 1B inhibitory activity from a Hyrtios sp. marine sponge. J. Nat. Prod. 2017, 80, 2509–2514. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Nakai, K.; Narita, K.; Katoh, T. A novel approach to sesquiterpenoid benzoxazole synthesis from marine sponges: Nakijinols A, B and E-G. Org. Biomol. Chem. 2018, 16, 3639–3647. [Google Scholar] [CrossRef] [PubMed]
- Boufridi, A.; Petek, S.; Evanno, L.; Beniddir, M.A.; Debitus, C.; Buisson, D.; Poupon, E. Biotransformations versus chemical modifications: New cytotoxic analogs of marine sesquiterpene ilimaquinone. Tetrahedron Lett. 2016, 57, 4922–4925. [Google Scholar] [CrossRef]
- Sakurai, J.; Oguchi, T.; Watanabe, K.; Abe, H.; Kanno, S.I.; Ishikawa, M.; Katoh, T. Highly efficient total synthesis of the marine natural products (+)-avarone, (+)-avarol, (-)-neoavarone, (-)-neoavarol and (+)-aureol. Chem. Eur. J. 2008, 14, 829–837. [Google Scholar] [CrossRef] [PubMed]
- De Guzman, F.S.; Copp, B.R.; Mayne, C.L.; Concepcion, G.P.; Mangalindan, G.C.; Barrows, L.R.; Ireland, C.M. Bolinaquinone: A novel cytotoxic sesquiterpene hydroxyquinone from a Philippine Dysidea sponge. J. Org. Chem. 1998, 63, 8042–8044. [Google Scholar] [CrossRef]
- Monti, M.C.; Chini, M.G.; Margarucci, L.; Tosco, A.; Riccio, R.; Bifulco, G.; Casapullo, A. The molecular mechanism of human group IIA phospholipase A2 inactivation by bolinaquinone. J. Mol. Recognit. 2009, 22, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Petronzi, C.; Filosa, R.; Peduto, A.; Monti, M.C.; Margarucci, L.; Massa, A.; Ercolino, S.F.; Bizzarro, V.; Parente, L.; Riccio, R.; et al. Structure-based design, synthesis and preliminary anti-inflammatory activity of bolinaquinone analogues. Eur. J. Med. Chem. 2011, 46, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Shen, X.; Guo, Y.W. A novel sesquiterpene quinone from Hainan sponge Dysidea villosa. Bioorg. Med. Chem. Lett. 2009, 19, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Suna, H.; Arai, M.; Tsubotani, Y.; Hayashi, A.; Setiawan, A.; Kobayashi, M. Dysideamine, a new sesquiterpene aminoquinone, protects hippocampal neuronal cells against iodoacetic acid-induced cell death. Bioorg. Med. Chem. 2009, 17, 3968–3972. [Google Scholar] [CrossRef] [PubMed]
- Mehta, G.; Likhite, N.S.; Kumar, C.S.A. A concise synthesis of the bioactive meroterpenoid natural product (+/-)-liphagal, a potent PI3K inhibitor. Tetrahedron Lett. 2009, 50, 5260–5262. [Google Scholar] [CrossRef]
- VanHeyst, M.D.; Wright, D.L. The frondosins: An unusual synthetic and stereochemical journey. Eur. J. Org. Chem. 2015, 1387–1401. [Google Scholar] [CrossRef]
- Kuan, K.K.W.; Hirschvogel, A.M.C.; George, J.H. A biomimetic synthetic approach to the frondosins. Aust. J. Chem. 2016, 69, 1420–1423. [Google Scholar] [CrossRef]
- Jiao, W.H.; Xu, T.T.; Yu, H.B.; Chen, G.D.; Huang, X.J.; Yang, F.; Li, Y.S.; Han, B.N.; Liu, X.Y.; Lin, H.W. Dysideanones A-C, unusual sesquiterpene quinones from the South China Sea sponge Dysidea avara. J. Nat. Prod. 2014, 77, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.H.; Xu, T.T.; Zhao, F.; Gao, H.; Shi, G.H.; Wang, J.; Hong, L.L.; Yu, H.B.; Li, Y.S.; Yang, F.; et al. Dysifragilones A-C, unusual sesquiterpene aminoquinones and inhibitors of NO production from the South China Sea sponge Dysidea fragilis. Eur. J. Org. Chem. 2015, 960–966. [Google Scholar] [CrossRef]
- Jiao, W.H.; Shi, G.H.; Xu, T.T.; Chen, G.D.; Gu, B.B.; Wang, Z.; Peng, S.; Wang, S.P.; Li, J.; Han, B.N.; et al. Dysiherbols A-C and dysideanone E, cytotoxic and NF-κB inhibitory tetracyclic meroterpenes from a Dysidea sp. marine sponge. J. Nat. Prod. 2016, 79, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Desoubzdanne, D.; Marcourt, L.; Raux, R.; Chevalley, S.; Dorin, D.; Doerig, C.; Valentin, A.; Ausseil, F.; Debitus, C. Alisiaquinones and alisiaquinol, dual inhibitors of Plasmodium falciparum enzyme targets from a New Caledonian deep water sponge. J. Nat. Prod. 2008, 71, 1189–1192. [Google Scholar] [CrossRef] [PubMed]
- Winder, P.L.; Baker, H.L.; Linley, P.; Guzman, E.A.; Pomponi, S.A.; Diaz, M.C.; Reed, J.K.; Wright, A.E. Neopetrosiquinones A and B, sesquiterpene benzoquinones isolated from the deep-water sponge Neopetrosia cf. proxima. Bioorg. Med. Chem. 2011, 19, 6599–6603. [Google Scholar] [CrossRef] [PubMed]
- Chayboun, I.; Boulifa, E.; Ibn Mansour, A.; Rodriguez-Serrano, F.; Carrasco, E.; Alvarez, P.J.; Chahboun, R.; Alvarez-Manzaneda, E. First enantiospecific syntheses of marine merosesquiterpenes neopetrosiquinones A and B: Evaluation of biological activity. J. Nat. Prod. 2015, 78, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Schmalzbauer, B.; Herrmann, J.; Muller, R.; Menche, D. Total synthesis and antibacterial activity of dysidavarone A. Org. Lett. 2013, 15, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Fukui, Y.; Narita, K.; Katoh, T. Enantioselective total synthesis of dysidavarone A, a novel sesquiterpenoid quinone from the marine sponge Dysidea avara. Chem. Eur. J. 2014, 20, 2436–2439. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.H.; Zhang, X.G.; Zhang, J.H.; Shen, Z.W. Total synthesis of dysidavarone A. Tetrahedron 2016, 72, 4337–4345. [Google Scholar] [CrossRef]
- Nuñez-Pons, L.; Carbone, M.; Vazquez, J.; Rodriguez, J.; Nieto, R.M.; Varela, M.M.; Gavagnin, M.; Avila, C. Natural products from Antarctic colonial ascidians of the genera Aplidium and Synoicum: Variability and defensive role. Mar. Drugs 2012, 10, 1741–1764. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Nuñez-Pons, L.; Paone, M.; Castelluccio, F.; Avila, C.; Gavagnin, M. Rossinone-related meroterpenes from the Antarctic ascidian Aplidium fuegiense. Tetrahedron 2012, 68, 3541–3544. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Chen, J.H.; Yang, Z.; Tang, Y.F. Rapid biomimetic total synthesis of (+/-)-rossinone B. Org. Lett. 2010, 12, 5554–5557. [Google Scholar] [CrossRef] [PubMed]
- Horie, S.; Tsutsumi, S.; Takada, Y.; Kimura, J. Antibacterial quinone metabolites from the brown alga, Sargassum sagamianum. Bull. Chem. Soc. Jpn. 2008, 81, 1125–1130. [Google Scholar] [CrossRef]
- Hur, S.; Lee, H.; Kim, Y.; Lee, B.-H.; Shin, J.; Kim, T.-Y. Sargaquinoic acid and sargachromenol, extracts of Sargassum sagamianum, induce apoptosis in HaCaT cells and mice skin: Its potentiation of UVB-induced apoptosis. Eur. J. Pharmacol. 2008, 582, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Iwashima, M.; Tako, N.; Hayakawa, T.; Matsunaga, T.; Mori, J.; Saito, H. New chromane derivatives isolated from the brown alga, Sargassum micracanthum. Chem. Pharm. Bull. 2008, 56, 124–128. [Google Scholar] [CrossRef]
- Jung, M.; Jang, K.H.; Kim, B.; Lee, B.H.; Choi, B.W.; Oh, K.B.; Shin, J. Meroditerpenoids from the brown alga Sargassum siliquastrum. J. Nat. Prod. 2008, 71, 1714–1719. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.; Urban, S. Meroditerpenoids from the southern Australian marine brown alga Sargassum fallax. Phytochemistry 2009, 70, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Brkljaca, R.; Urban, S. Chemical profiling (HPLC-NMR & HPLC-MS), isolation, and identification of bioactive meroditerpenoids from the southern Australian marine brown alga Sargassum paradoxum. Mar. Drugs 2015, 13, 102–127. [Google Scholar]
- Kim, M.C.; Kwon, H.C.; Kim, S.N.; Kim, H.S.; Um, B.H. Plastoquinones from Sargassum yezoense; chemical structures and effects on the activation of peroxisome proliferator-activated receptor gamma. Chem. Pharm. Bull. 2011, 59, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, V.; Seca, A.M.L.; Carmo Barreto, M.; Pinto, D.C.G.A. Di- and sesquiterpenoids from Cystoseira genus: Structure, intra-molecular transformations and biological activity. Mini-Rev. Med. Chem. 2013, 13, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- De los Reyes, C.; Ortega, M.J.; Zbakh, H.; Motilva, V.; Zubia, E. Cystoseira usneoides: A brown alga rich in antioxidant and anti-inflammatory meroditerpenoids. J. Nat. Prod. 2016, 79, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Areche, C.; San-Martin, A.; Rovirosa, J.; Soto-Delgado, J.; Contreras, R. An unusual halogenated meroditerpenoid from Stypopodium flabelliforme: Studies by NMR spectroscopic and computational methods. Phytochemistry 2009, 70, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Saito, K.; Kato, K.; Nozaki, S.; Sato, K. Selective synthesis of monomethyltocols via η-allylnickel complexes. J. Chem. Soc.-Perkin Trans. 1974, 1, 2097–2101. [Google Scholar] [CrossRef]
- Areche, C.; Benites, J.; Cornejo, A.; Ruiz, L.M.; Garcia-Beltran, O.; Simirgiotis, M.J.; Sepulveda, B. Seco-taondiol, an unusual meroterpenoid from the Chilean seaweed Stypopodium flabelliforme and its gastroprotective effect in mouse model. Mar. Drugs 2015, 13, 1726–1738. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Hwang, H.; Chin, J.; Kim, E.; Lee, J.; Nam, S.J.; Lee, B.C.; Rho, B.J.; Kang, H. Tuberatolides, potent FXR antagonists from the Korean marine tunicate Botryllus tuberatus. J. Nat. Prod. 2011, 74, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Kusumi, T.; Shibata, Y.; Ishitsuka, M.; Kinoshita, T.; Kakisawa, H. Structures of new plastoquinones from the brown alga Sargassum serratifolium. Chem. Lett. 1979, 277–278. [Google Scholar] [CrossRef]
- Segawa, M.; Shirahama, H. New plastoquinones from the brown alga Sargassum sagamianum var. yezoense. Chem. Lett. 1987, 16, 1365–1366. [Google Scholar] [CrossRef]
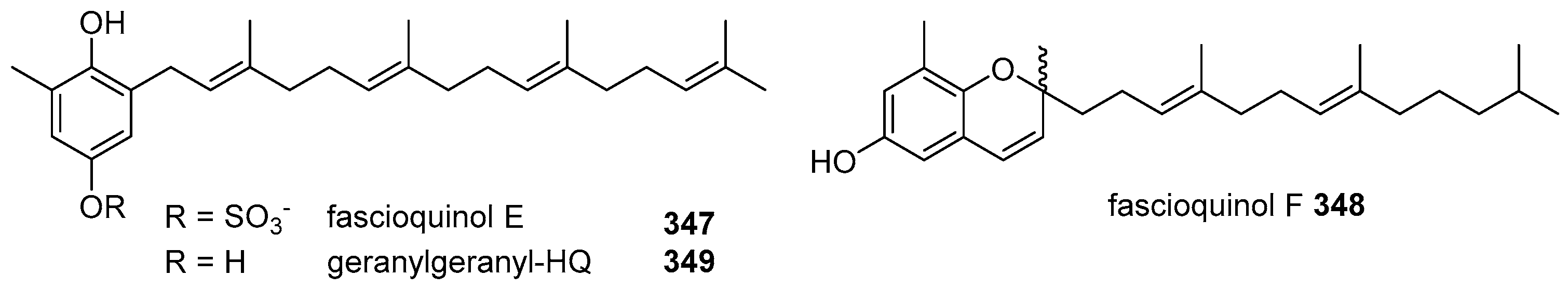
- Zhang, H.; Khalil, Z.G.; Capon, R.J. Fascioquinols A-F: Bioactive meroterpenes from a deep-water southern Australian marine sponge, Fasciospongia sp. Tetrahedron 2011, 67, 2591–2595. [Google Scholar] [CrossRef]
- Wang, C.Y.; Chen, A.N.; Shao, C.L.; Li, L.; Xu, Y.; Qian, P.Y. Chemical constituents of soft coral Sarcophyton infundibuliforme from the South China Sea. Biochem. Syst. Ecol. 2011, 39, 853–856. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Lin, S.T.; Wang, S.K.; Duh, C.Y. α-Tocopherols from the Formosan soft coral Lobophytum crassum. Bull. Chem. Soc. Jpn. 2011, 84, 783–787. [Google Scholar] [CrossRef]
- Jang, K.H.; Lee, B.H.; Choi, B.W.; Lee, H.S.; Shin, J. Chromenes from the brown alga Sargassum siliquastrum. J. Nat. Prod. 2005, 68, 716–723. [Google Scholar] [CrossRef] [PubMed]
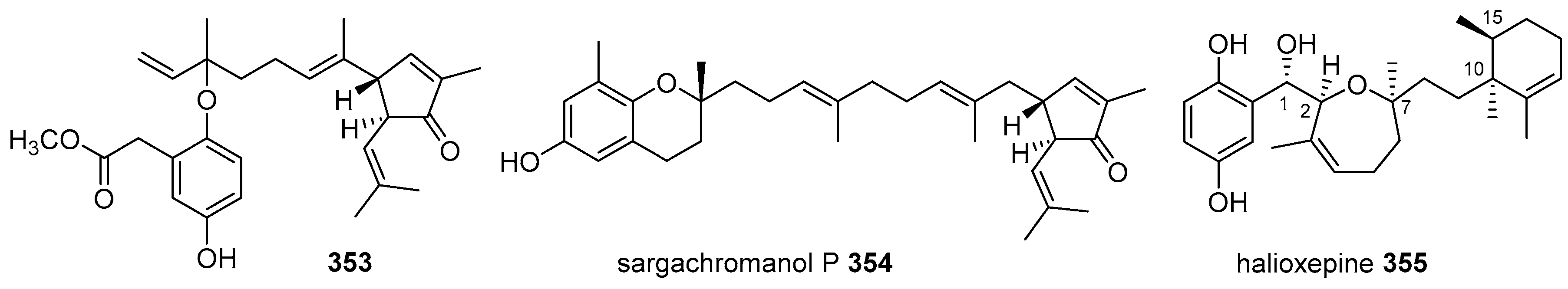
- Trianto, A.; Hermawan, I.; de Voogd, N.J.; Tanaka, J. Halioxepine, a new meroditerpene from an Indonesian sponge Haliclona sp. Chem. Pharm. Bull. 2011, 59, 1311–1313. [Google Scholar] [CrossRef] [PubMed]
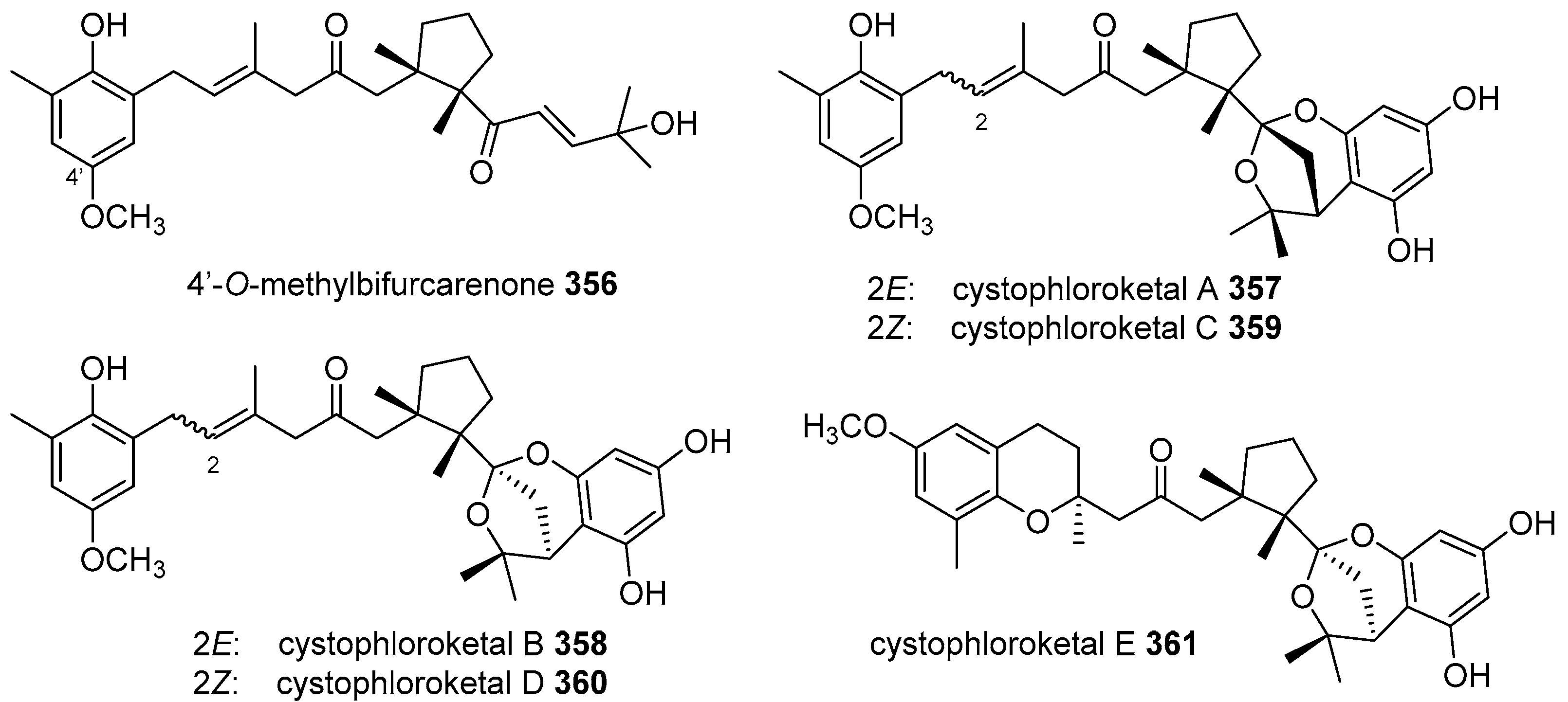
- El Hattab, M.; Genta-Jouve, G.; Bouzidi, N.; Ortalo-Magne, A.; Hellio, C.; Marechal, J.P.; Piovetti, L.; Thomas, O.P.; Culioli, G. Cystophloroketals A-E, unusual phloroglucinol-meroterpenoid hybrids from the brown alga Cystoseira tamariscifolia. J. Nat. Prod. 2015, 78, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.P.; Bharate, S.B. Phloroglucinol compounds of natural origin. Nat. Prod. Rep. 2006, 23, 558–591. [Google Scholar] [CrossRef] [PubMed]
- Mokrini, R.; Ben Mesaoud, M.; Daoudi, M.; Hellio, C.; Marechal, J.P.; El Hattab, M.; Ortalo-Magne, A.; Piovetti, L.; Culioli, G. Meroditerpenoids and derivatives from the brown alga Cystoseira baccata and their antifouling properties. J. Nat. Prod. 2008, 71, 1806–1811. [Google Scholar] [CrossRef] [PubMed]
- Valls, R.; Piovetti, L. The chemistry of the Cystoseiraceae (Fucales: Pheophyceae): Chemotaxonomic relationships. Biochem. Syst. Ecol. 1995, 23, 723–745. [Google Scholar] [CrossRef]
- Amico, V. Marine brown-algae of family Cystoseiraceae: Chemistry and chemotaxonomy. Phytochemistry 1995, 39, 1257–1279. [Google Scholar] [CrossRef]
- Basabe, P.; Lithgow, A.M.; Moro, R.F.; Lopez, M.S.; Araujo, M.E.; Brito Palma, F.M.S. Meroterpenes from Cystoseira baccata and Cystoseira abies-marina. Stud. Chem. 1992, 17, 101–107. [Google Scholar]
- Valls, R.; Piovetti, L.; Banaigs, B.; Praud, A. Secondary metabolites from Morocco brown-algae of the genus Cystoseira. Phytochemistry 1993, 32, 961–966. [Google Scholar] [CrossRef]
- Soares, A.R.; Duarte, H.M.; Tinnoco, L.W.; Pereira, R.C.; Teixeira, V.L. Intraspecific variation of meroditerpenoids in the brown alga Stypopodium zonale guiding the isolation of new compounds. Rev. Bras. Farm.-Braz. J. Pharmacogn. 2015, 25, 627–633. [Google Scholar] [CrossRef]
- Braekman, J.C.; Daloze, D.; Hulot, G.; Tursch, B.; Declercq, J.P.; Germain, G.; Vanmeerssche, M. 3 Novel meroditerpenoids from the sponge Strongylophora durissima. Bull. Soc. Chim. Belg. 1978, 87, 917–926. [Google Scholar] [CrossRef]
- Mohammed, K.A.; Jadulco, R.C.; Bugni, T.S.; Harper, M.K.; Sturdy, M.; Ireland, C.M. Strongylophorines: Natural product inhibitors of hypoxia-inducible factor-1 transcriptional pathway. J. Med. Chem. 2008, 51, 1402–1405. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Abdjul, D.B.; Yamazaki, H.; Takahashi, O.; Kirikoshi, R.; Ukai, K.; Namikoshi, M. Strongylophorines, new protein tyrosine phosphatase 1B inhibitors, from the marine sponge Strongylophora strongilata collected at Iriomote Island. Bioorg. Med. Chem. Lett. 2015, 25, 3900–3902. [Google Scholar] [CrossRef] [PubMed]
- Noda, A.; Sakai, E.; Kato, H.; Losung, F.; Mangindaan, R.E.P.; de Voogd, N.J.; Yokosawa, H.; Tsukamoto, S. Strongylophorines, meroditerpenoids from the marine sponge Petrosia corticata, function as proteasome inhibitors. Bioorg. Med. Chem. Lett. 2015, 25, 2650–2653. [Google Scholar] [CrossRef] [PubMed]
- Penicooke, N.; Walford, K.; Badal, S.; Delgoda, R.; Williams, L.A.D.; Joseph-Nathan, P.; Gordillo-Roman, B.; Gallimore, W. Antiproliferative activity and absolute configuration of zonaquinone acetate from the Jamaican alga Stypopodium zonale. Phytochemistry 2013, 87, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Jeon, J.E.; Lee, Y.J.; Lee, H.S.; Sim, C.J.; Oh, K.B.; Shin, J. Sesterterpenes from the tropical sponge Coscinoderma sp. J. Nat. Prod. 2011, 74, 1805–1811. [Google Scholar] [CrossRef] [PubMed]
- West, L.A.; Faulkner, D.J. Acanthosulfate, a sulfated hydroxyhydroquinone sesterterpenoid from the sponge Acanthodendrilla sp. J. Nat. Prod. 2008, 71, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Watjen, W.; Putz, A.; Chovolou, Y.; Kampkotter, A.; Totzke, F.; Kubbutat, M.H.G.; Proksch, P.; Konuklugil, B. Hexa-, hepta- and nonaprenylhydroquinones isolated from marine sponges Sarcotragus muscarum and Ircinia fasciculata inhibit NF-κB signalling in H4IIE cells. J. Pharm. Pharmacol. 2009, 61, 919–924. [Google Scholar] [PubMed]
- Abed, C.; Legrave, N.; Dufies, M.; Robert, G.; Guerineau, V.; Vacelet, J.; Auberger, P.; Amade, P.; Mehiri, M. A new hydroxylated nonaprenylhydroquinone from the Mediterranean marine sponge Sarcotragus spinosulus. Mar. Drugs 2011, 9, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Katoh, T.; Atsumi, S.; Saito, R.; Narita, K. Unified synthesis of the marine sesquiterpene quinones (+)-smenoqualone, (-)-ilimaquinone, (+)-smenospongine, and (+)-isospongiaquinone. Eur. J. Org. Chem. 2017, 3837–3849. [Google Scholar] [CrossRef]
- Hosoi, H.; Kawai, N.; Hagiwara, H.; Suzuki, T.; Nakazaki, A.; Takao, K.; Umezawa, K.; Kobayashi, S. Synthesis and determination of the relative structure of akaterpin, a potent inhibitor of PI-PLC. Tetrahedron Lett. 2011, 52, 4961–4964. [Google Scholar] [CrossRef]
- Gordaliza, M. Synthetic strategies to terpene quinones/hydroquinones. Mar. Drugs 2012, 10, 358–402. [Google Scholar] [CrossRef] [PubMed]
- Dixon, D.D.; Lockner, J.W.; Zhou, Q.H.; Baran, P.S. Scalable, divergent synthesis of meroterpenoids via “Borono-sclareolide”. J. Am. Chem. Soc. 2012, 134, 8432–8435. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Manzaneda, E.; Chahboun, R.; Alvarez, E.; Fernandez, A.; Alvarez-Manzaneda, R.; Haidour, A.; Ramos, J.M.; Akhaouzan, A. First enantiospecific synthesis of marine sesquiterpene quinol akaol A. Chem. Comm. 2012, 48, 606–608. [Google Scholar] [CrossRef] [PubMed]
- Catalan, L.E.; Marin, K.C.; Villegas, A.M.; Altamirano, H.C.; Garcia, J.V.; Fritis, M.C. Synthesis of two new hemisynthetic diterpenylhydroquinones from natural ent-labdanes. Molecules 2009, 14, 2181–2194. [Google Scholar] [CrossRef] [PubMed]
- Marcos, I.S.; Conde, A.; Moro, R.F.; Basabe, P.; Diez, D.; Urones, J.G. Synthesis of quinone/hydroquinone sesquiterpenes. Tetrahedron 2010, 66, 8280–8290. [Google Scholar] [CrossRef]
- Bozic, T.; Novakovic, I.; Gasic, M.J.; Juranic, Z.; Stanojkovic, T.; Tufegdzic, S.; Kljajic, Z.; Sladic, D. Synthesis and biological activity of derivatives of the marine quinone avarone. Eur. J. Med. Chem. 2010, 45, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Miguel del Corral, J.M.; Gordaliza, M.; Castro, M.A.; Mahiques, M.M.; Chamorro, P.; Molinari, A.; Garcia-Gravalos, M.D.; Broughton, H.B.; San Feliciano, A. New selective cytotoxic diterpenylquinones and diterpenylhydroquinones. J. Med. Chem. 2001, 44, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
- Miguel del Corral, J.M.; Gordaliza, M.; Castro, A.; Mahiques, M.M.; San Feliciano, A.; Garcia-Gravalos, M.D. Further antineoplastic terpenylquinones and terpenylhydroquinones. Bioorg. Med. Chem. 1998, 6, 31–41. [Google Scholar] [CrossRef]
- Miguel del Corral, J.M.; Castro, M.A.; Gordaliza, M.; Martin, M.L.; Oliveira, A.B.; Gualberto, S.A.; Garcia-Gravalos, M.D.; San Feliciano, A. Synthesis and biological evaluation of cytotoxic 6-alkyl-2-hydroxy-1,4-naphthoquinones. Arch. Pharm. 2002, 335, 427–437. [Google Scholar] [CrossRef]
- Miguel del Corral, J.M.; Castro, M.A.; Gordaliza, M.; Martin, M.L.; Gualberto, S.A.; Gamito, A.M.; Cuevas, C.; San Feliciano, A. Synthesis and cytotoxicity of new aminoterpenylquinones. Bioorg. Med. Chem. 2005, 13, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.A.; Miguel del Corral, J.M.; Gordaliza, M.; Garcia, P.A.; Gamito, A.M.; Gualberto, S.A.; Batista, R.; San Feliciano, A. A novel synthetic route to cytotoxic 1,4-anthraquinones from 1,4-benzoquinones. Synth.-Stuttg. 2005, 3202–3208. [Google Scholar] [CrossRef]
- Castro, M.A.; Miguel del Corral, J.M.; Rodriguez, M.L.; San Feliciano, A. An easy route to pentacyclic terpenylquinones. Tetrahedron Lett. 2012, 53, 519–521. [Google Scholar] [CrossRef]
- Molinari, A.; Oliva, A.; Aguilera, N.; Miguel del Corral, J.M.; Gordaliza, M.; Castro, A.; Garcia-Gravalos, D.; San Feliciano, A. 2,3-Functionally dialkyl-1,4-benzohydroquinone diacetate derivatives from cleavage of epoxides. Bol. Soc. Chil. Quím. 2001, 46, 33–39. [Google Scholar] [CrossRef]
- Molinari, A.; Oliva, A.; Reinoso, P.; Miguel del Corral, J.M.; Castro, M.A.; Gordaliza, M.; Gupta, M.P.; Solis, P.; San Feliciano, A. Cytotoxic-antineoplastic activity of hydroquinone derivatives. Eur. J. Med. Chem. 2002, 37, 177–182. [Google Scholar] [CrossRef]
- Molinari, A.; Oliva, A.; Ojeda, C.; Miguel del Corral, J.M.; Castro, M.A.; Cuevas, C.; San Feliciano, A. New cytotoxic-antineoplastic prenyl-1,2-naphthohydroquinone derivatives. Bioorg. Med. Chem. 2005, 13, 6645–6650. [Google Scholar] [CrossRef] [PubMed]
- Molinari, A.; Oliva, A.; Ojeda, C.; Miguel del Corral, J.M.; Castro, M.A.; Cuevas, C.; San Feliciano, A. Cytotoxic-antineoplastic derivatives of prenyl-1,2-naphthohydroquinone. Arch. Pharm. 2008, 341, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Molinari, A.; Oliva, A.; Aguilera, N.; Miguel del Corral, J.M.; Castro, M.A.; Gordaliza, M.; Garcia-Gravalos, M.D.; San Feliciano, A. New antineoplastic prenylhydroquinones. Synthesis and evaluation. Bioorg. Med. Chem. 2000, 8, 1027–1032. [Google Scholar] [CrossRef]
- Molinari, A.; Oliva, A.; Ojeda, C.; Escobar, J.; Gallardo, C.; Miguel del Corral, J.M.; Castro, A.; Cuevas, C.; San Feliciano, A. Synthesis, characterisation and cytotoxicity of chloro derivatives of prenylnaphthohydroquinone. Bioorg. Med. Chem. 2005, 13, 3841–3846. [Google Scholar] [CrossRef] [PubMed]
- Miguel del Corral, J.M.; Castro, M.A.; Rodriguez, M.L.; Chamorro, P.; Cuevas, C.; San Feliciano, A. New cytotoxic diterpenylnaphthohydroquinone derivatives obtained from a natural diterpenoid. Bioorg. Med. Chem. 2007, 15, 5760–5774. [Google Scholar] [CrossRef] [PubMed]
- Miguel del Corral, J.M.; Castro, M.A.; Gordaliza, M.; Martin, M.L.; Gamito, A.M.; Cuevas, C.; San Feliciano, A. Synthesis and cytotoxicity of new heterocyclic terpenylnaphthoquinones. Bioorg. Med. Chem. 2006, 14, 2816–2827. [Google Scholar] [CrossRef] [PubMed]
- Miguel del Corral, J.M.; Castro, M.A.; Oliveira, A.B.; Gualberto, S.A.; Cuevas, C.; San Feliciano, A. New cytotoxic furoquinones obtained from terpenyl-1,4-naphthoquinones and 1,4-anthracenediones. Bioorg. Med. Chem. 2006, 14, 7231–7240. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.A.; Gamito, A.M.; Tangarife-Castano, V.; Roa-Linares, V.; Miguel del Corral, J.M.; Mesa-Arango, A.C.; Betancur-Galvis, L.; Francesch, A.M.; San Feliciano, A. New 1,4-anthracenedione derivatives with fused heterocyclic rings: Synthesis and biological evaluation. RSC Adv. 2015, 5, 1244–1261. [Google Scholar] [CrossRef]
- Castro, M.A.; Gamito, A.M.; Tangarife-Castatno, V.; Zapata, B.; Miguel del Corral, J.M.; Mesa-Arango, A.C.; Betancur-Galvis, L.; San Feliciano, A. Synthesis and antifungal activity of terpenyl-1,4-naphthoquinone and 1,4-anthracenedione derivatives. Eur. J. Med. Chem. 2013, 67, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Molinari, A.; Ojeda, C.; Oliva, A.; Miguel del Corral, J.M.; Castro, M.A.; Garcia, P.A.; Cuevas, C.; San Feliciano, A. Synthesis, characterisation, and antineoplastic cytotoxicity of hybrid naphthohydroquinone-nucleic base mimic derivatives. Med. Chem. Res. 2009, 18, 59–69. [Google Scholar] [CrossRef]
- Molinari, A.; Oliva, A.; Ojeda, C.; Miguel del Corral, J.M.; Castro, M.A.; Cuevas, C.; San Feliciano, A. Synthesis and cytotoxic evaluation of 6-(3-pyrazolylpropyl) derivatives of 1,4-naphthohydroquinone-1,4-diacetate. Arch. Pharm. 2009, 342, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Molinari, A.; Oliva, A.; Ojeda, C.; Miguel del Corral, J.M.; Castro, M.A.; Mollinedo, F.; San Feliciano, A. Synthesis and evaluation as antitumor agents of 1,4-naphthohydroquinone derivatives conjugated with amino acids and purines. Arch. Pharm. 2013, 346, 882–890. [Google Scholar] [CrossRef] [PubMed]
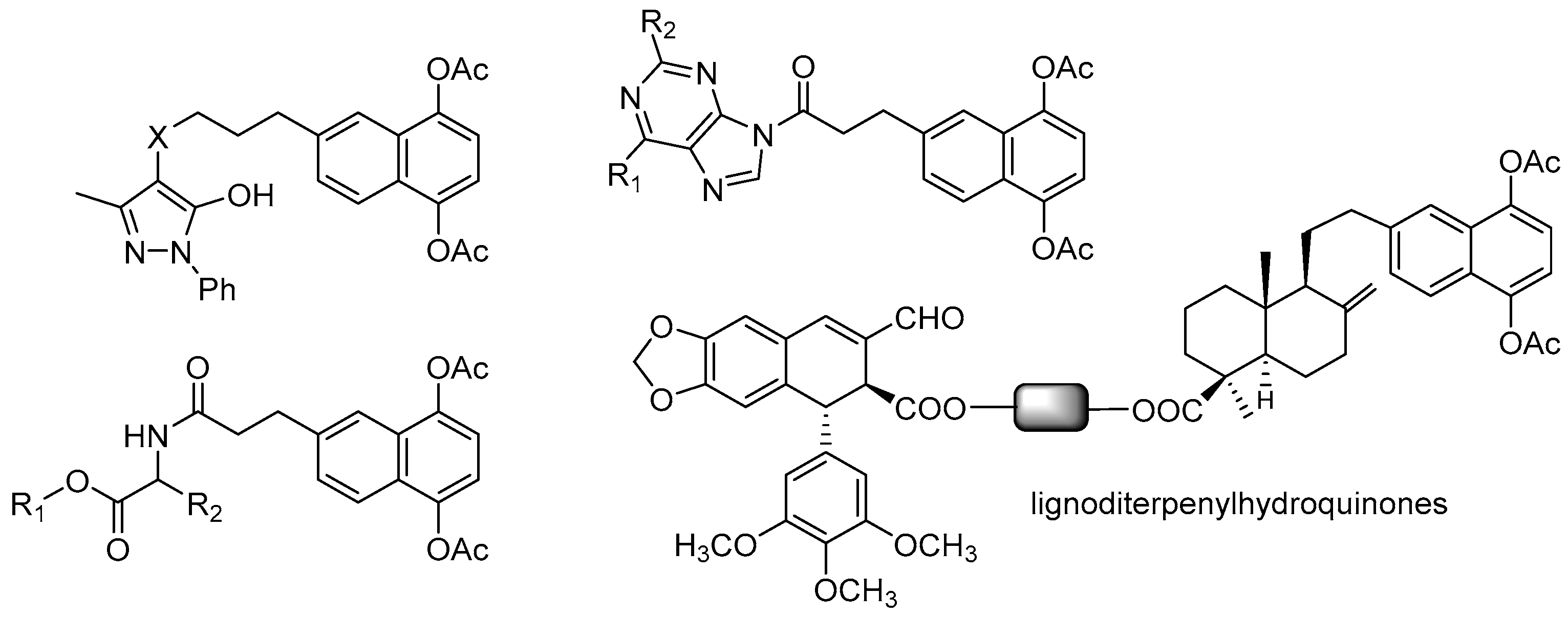
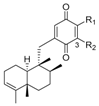
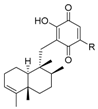
- Hernandez, A.P.; Diez, P.; Garcia, P.A.; Miguel del Corral, J.M.; Perez-Andres, M.; Diez, D.; San Feliciano, A.; Fuentes, M.; Castro, M.A. New hybrids derived from podophyllic aldehyde and diterpenylhydroquinones with selectivity toward osteosarcoma cells. ACS Med. Chem. Lett. 2018, 9, 328–333. [Google Scholar] [CrossRef] [PubMed]


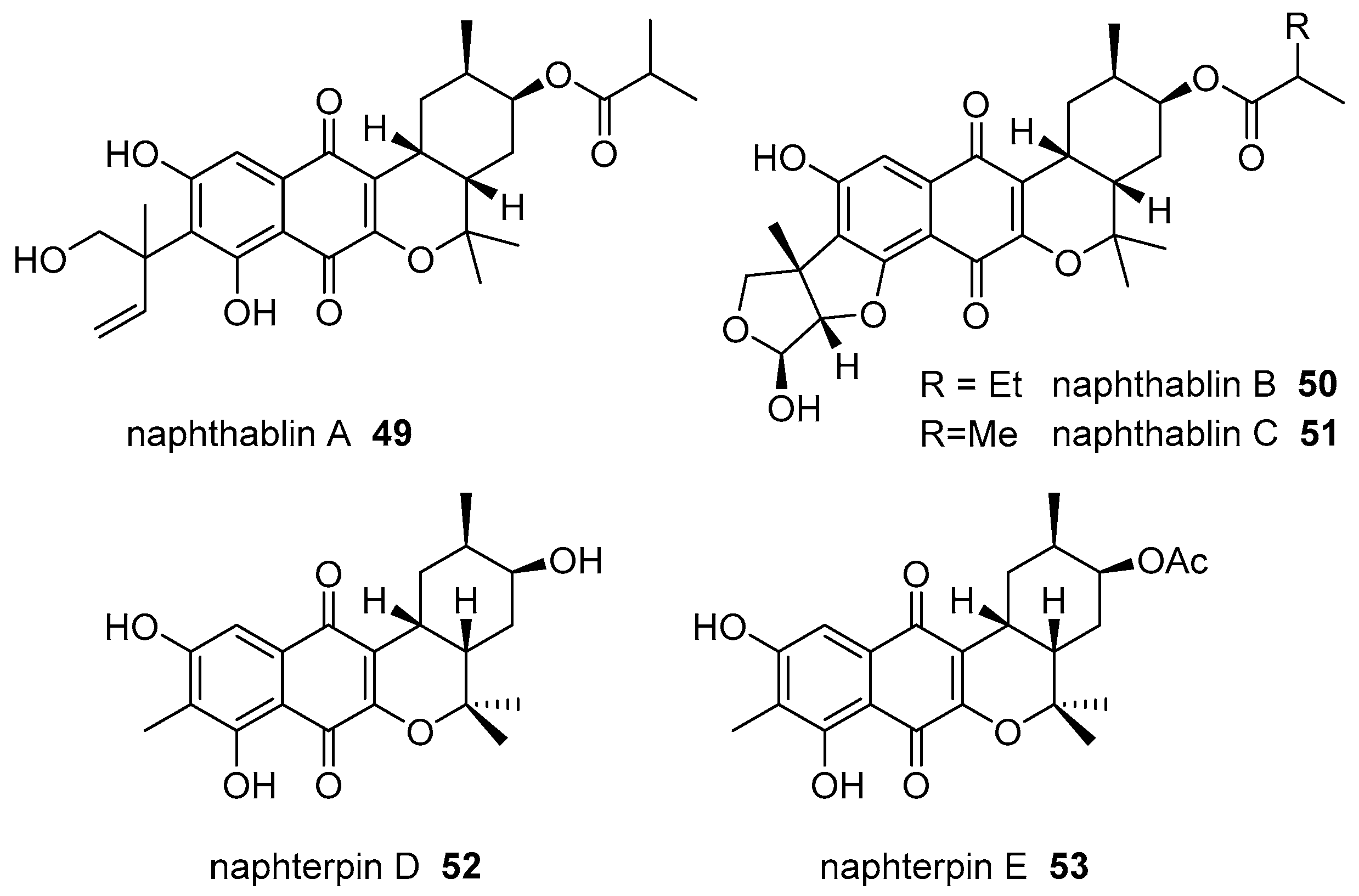
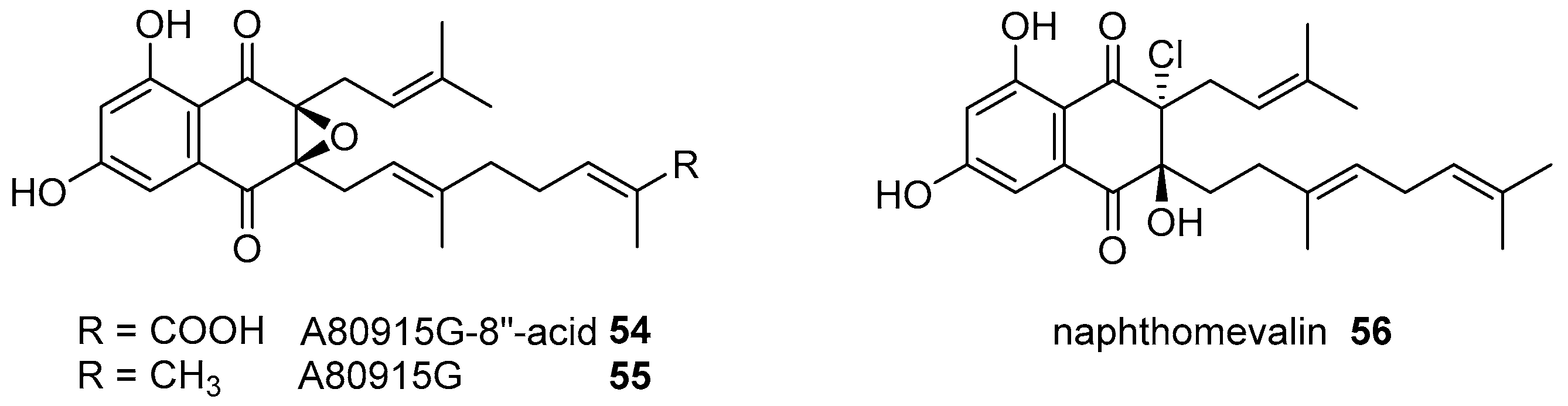
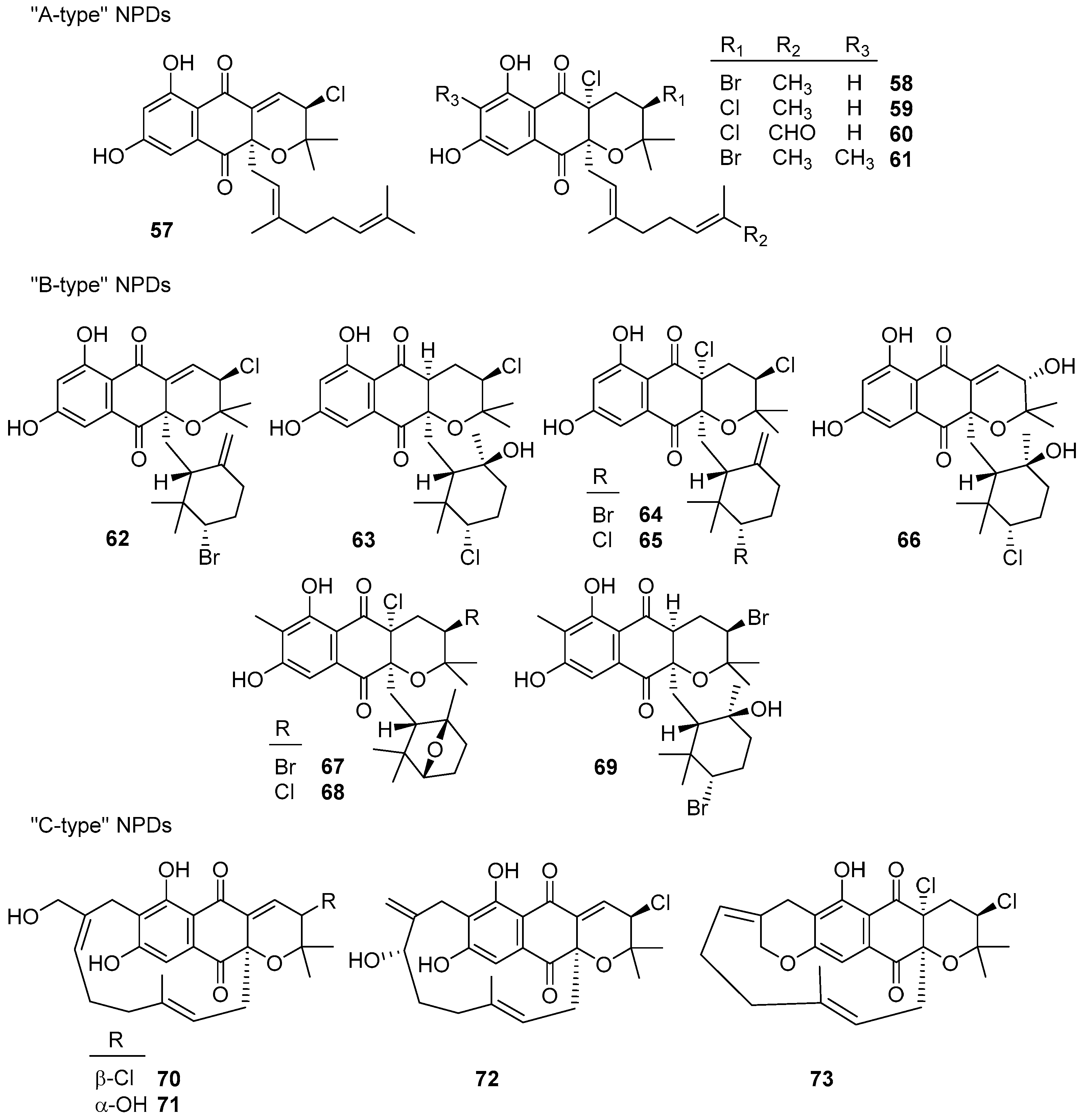
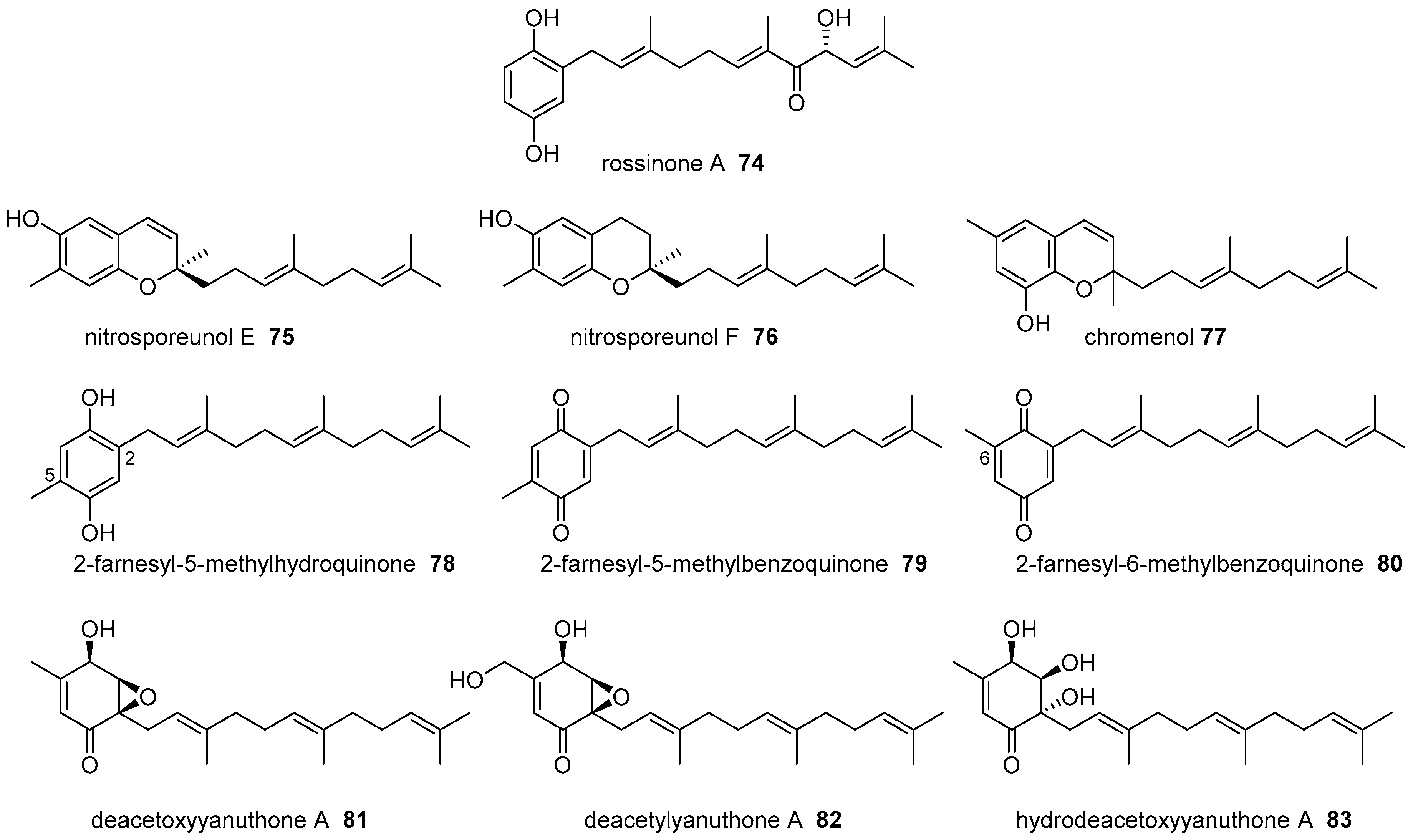


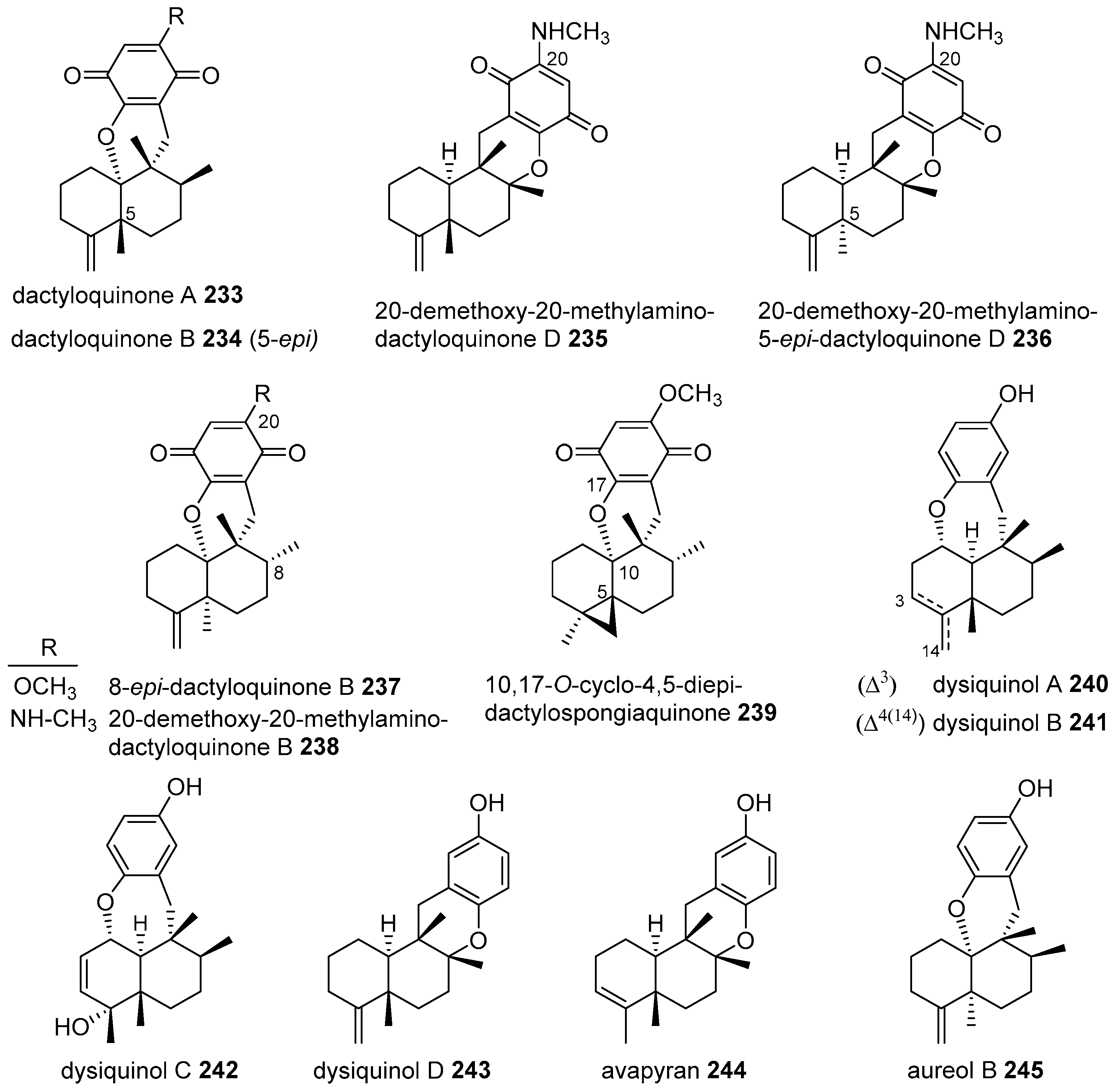
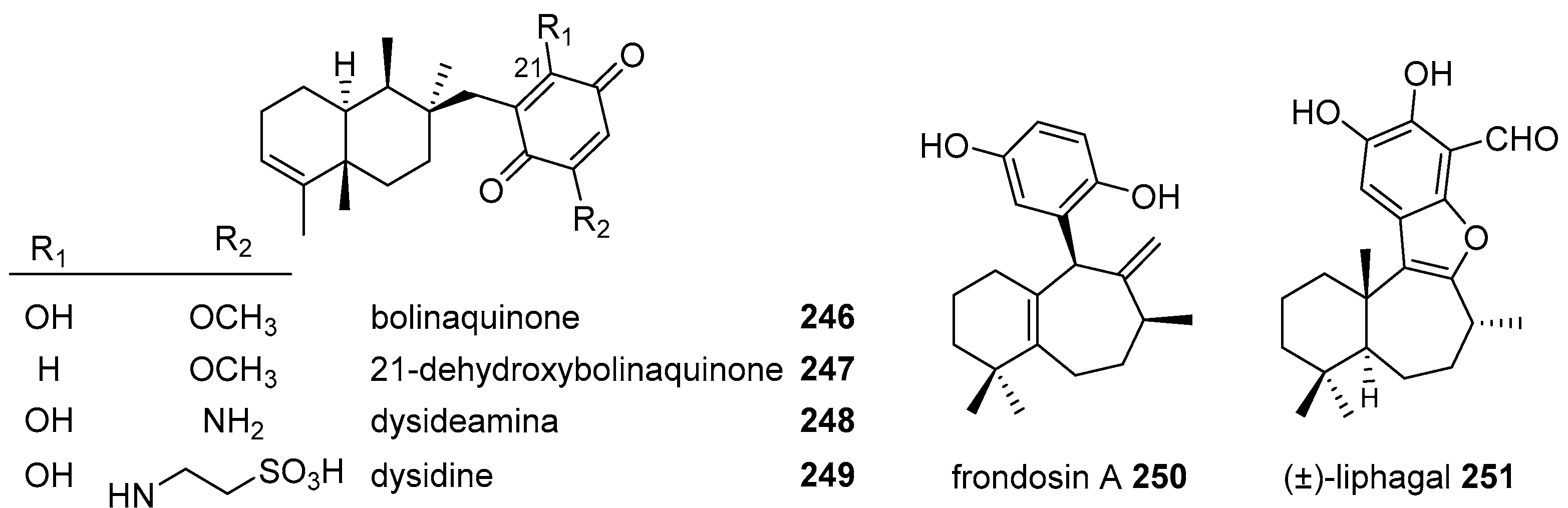
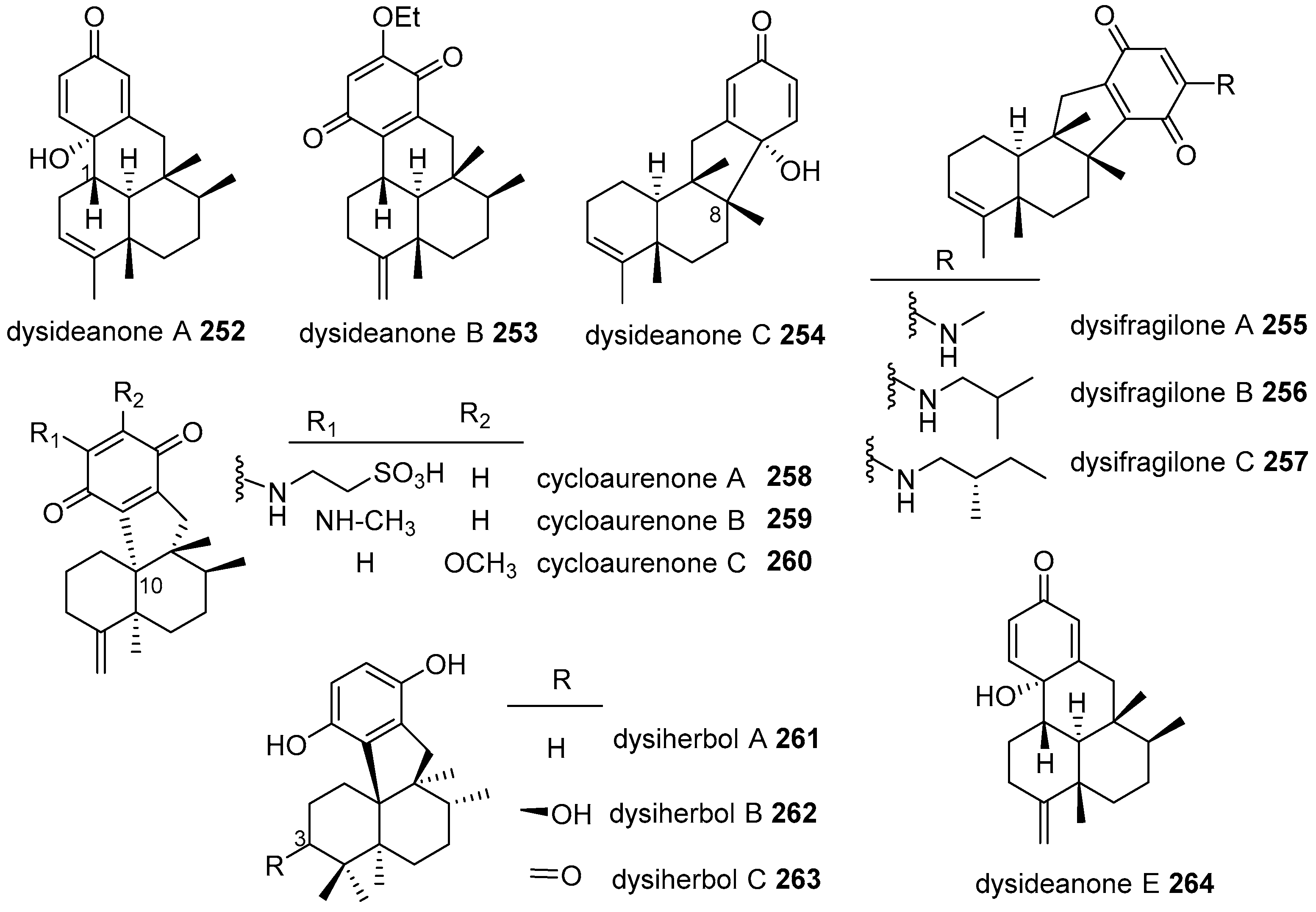
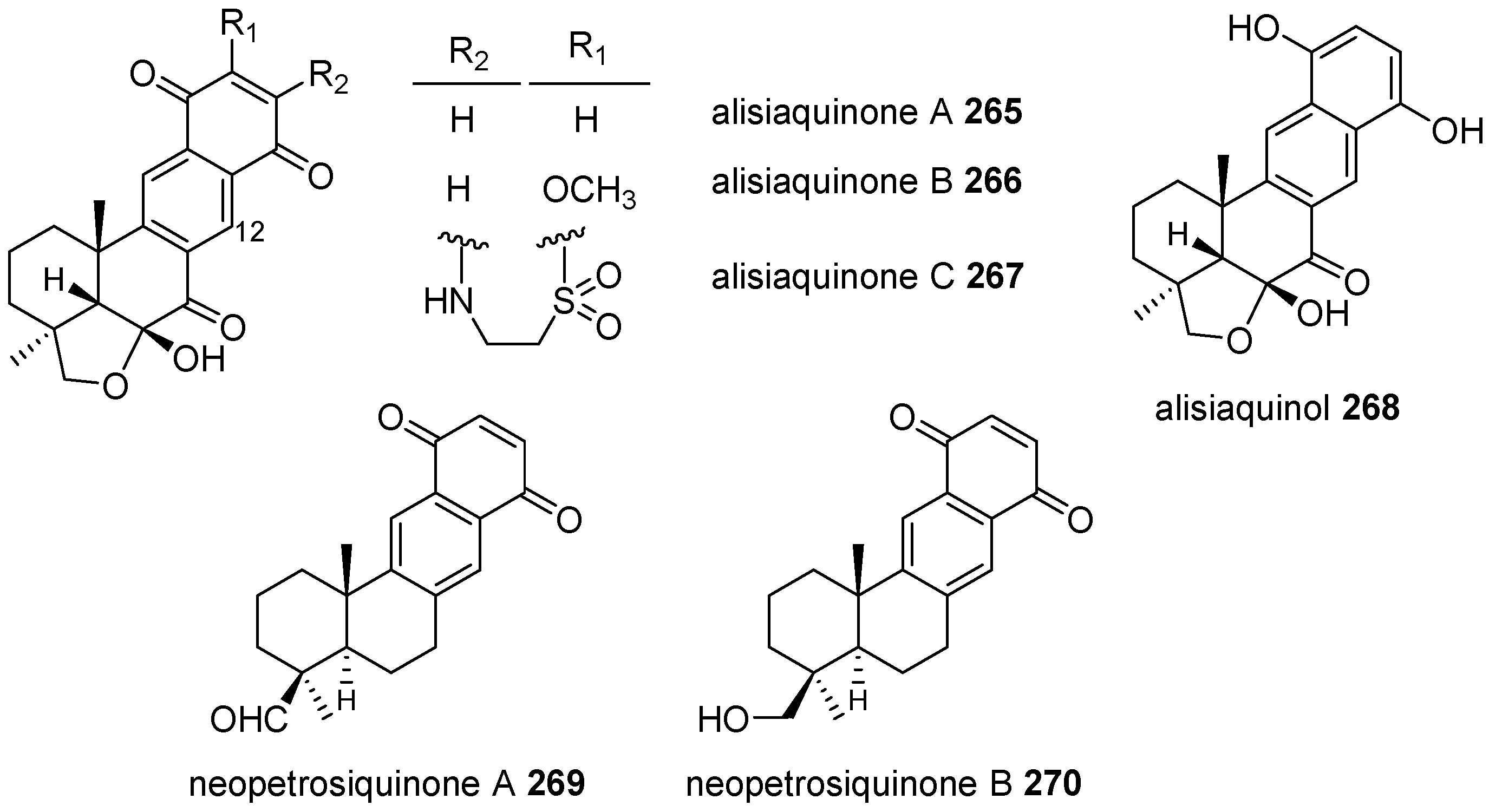





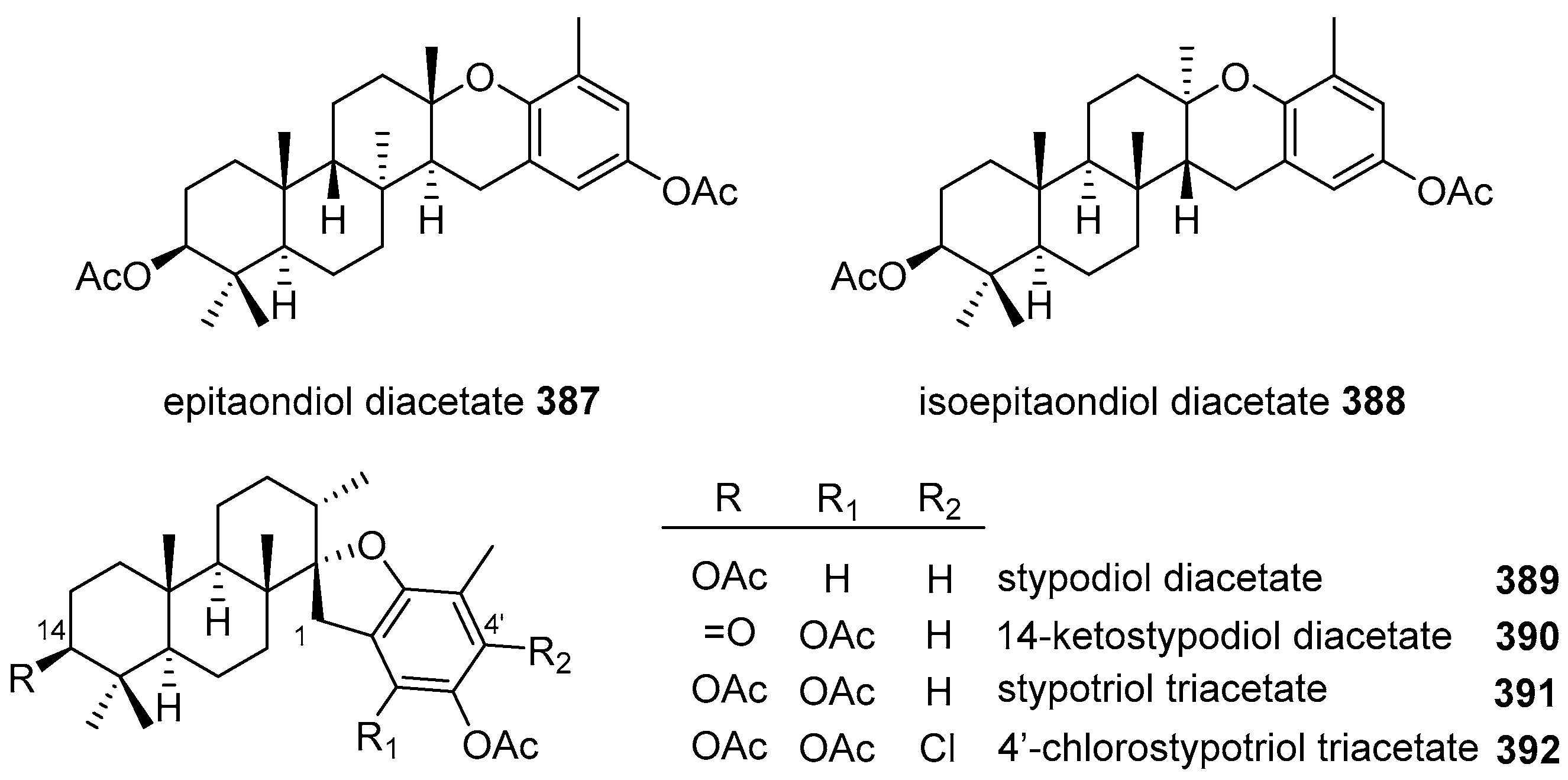
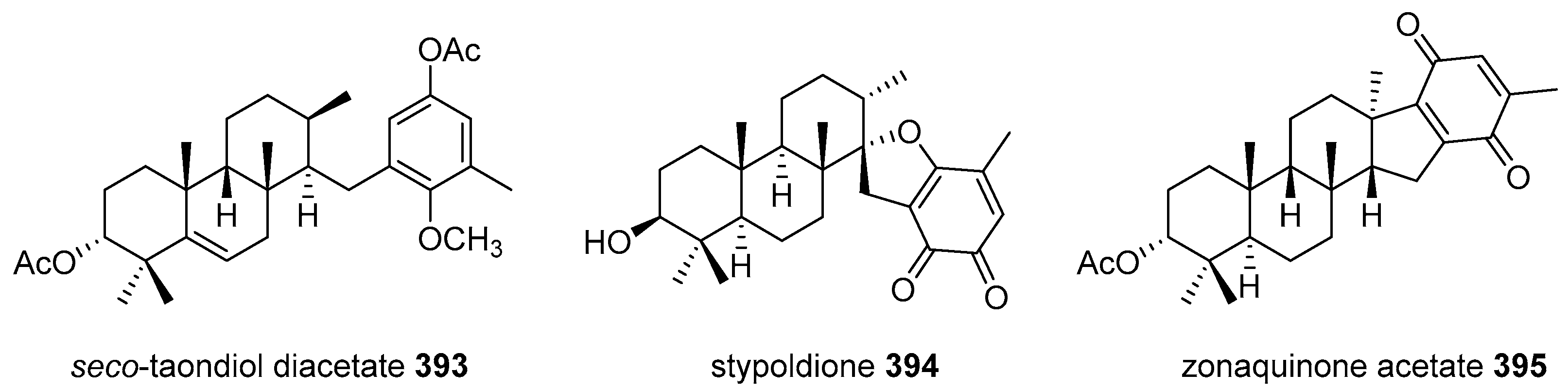
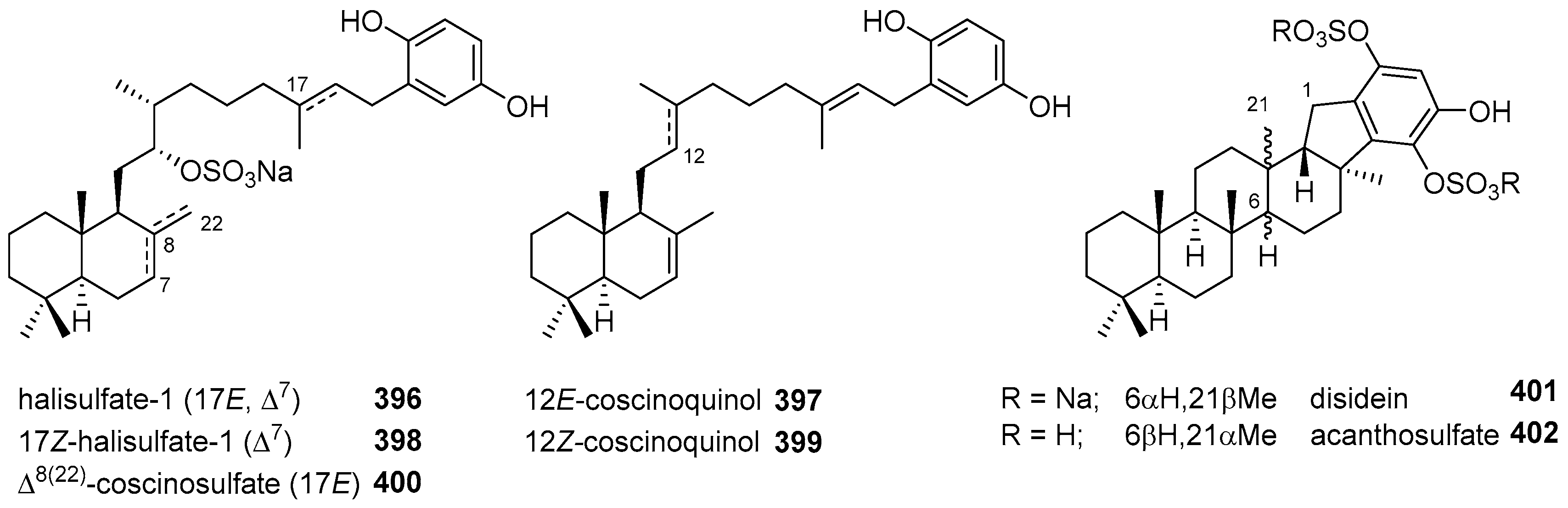
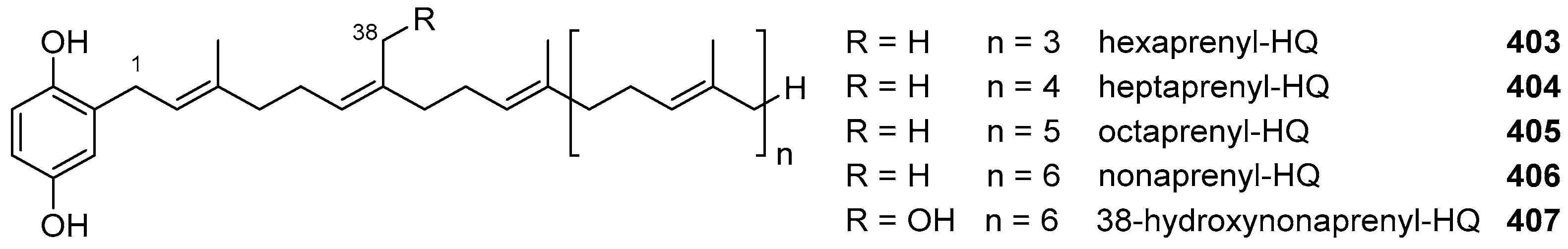


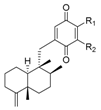
 |  | |||||
| R1 | R2 | Name | R | Name | ||
| H | H | avarone | 139 |  | nakijiquinone S | 148 |
| H | NH2 | 3′-aminoavarone | 140 |  | nakijiquinone K | 149 |
| H | 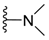 | dysidaminone C | 141 | 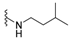 | nakijiquinone N | 150 |
 | H | dysidaminone D | 142 | 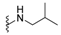 | nakijiquinone O | 151 |
| H |  | dysidaminone E | 143 |  | nakijiquinone Q | 152 |
 | H | dysidaminone A | 144 |  | nakijiquinone G | 153 |
| H |  | dysidaminone B | 145 | 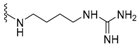 | nakijiquinone H | 154 |
 | H | dysidaminone F | 146 |  | nakijiquinone I | 155 |
 | H | dysidaminone G | 147 |  | nakijiquinone R | 156 |
 | 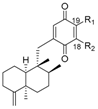 | ||||||
| R1 | R2 | Name | R1 | R2 | Name | ||
| H | H | neoavarone | 157 | H | H | arenarone | 166 |
| H | OEt | 18-ethoxyneoavarone | 158 | H | NH2 | 18-aminoarenarone | 167 |
| OEt | H | 19-ethoxyneoavarone | 159 | NH2 | H | 19-aminoarenarone | 168 |
| H |  | dysidaminone H | 160 | H |  | 18-methylaminoarenarone | 169 |
 | H | dysidaminone I | 161 |  | H | 19-methylaminoarenarone | 170 |
| H |  | dysidaminone J | 162 | H |  | melemeleone C | 171 |
 | H | dysidaminone K | 163 | H |  | melemeleone D | 172 |
 | H | dysidaminone L | 164 | ||||
 | H | dysidaminone M | 165 | ||||
 | |||||
| R | Name | R | Name | ||
| OEt | ethylsmenoquinone | 173 |  | smenospongidine (smenospongiadine) | 180 |
| OCH3 | ilimaquinone | 174 |  | nakijiquinone L | 181 |
 | langcoquinone C | 175 |  | langcoquinone F | 182 |
| NH2 | smenospongine | 176 | 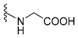 | smenospongine B (glycinylilimaquinone) | 183 |
 | smenospongimine (langcoquinone A) | 177 |  | smenospongine C | 184 |
 | smenospongorine | 178 | 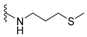 | langcoquinone B | 185 |
 | smenospongiarine | 179 |  | langcoquinone D | 186 |
 | langcoquinone E | 187 | |||
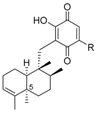 |  | ||||
| R | Name | R | Name | ||
 | 5-epi-nakijiquinone N | 188 | OEt | 5-epi-20-O-ethylsmenoquinone | 193 |
 | 5-epi-nakijiquinone Q | 189 | NH2 | 5-epi-smenospongine | 194 |
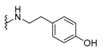 | 5-epi-nakijiquinone S | 190 |  | N-methyl-5-epi-smenospongine | 195 |
 | 5-epi-nakijiquinone U | 191 | |||
 | 5-epi-nakijiquinone T | 192 | |||
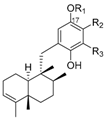 | 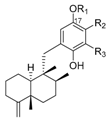 | ||||||||
| R1 | R2 | R3 | Name | R1 | R2 | R3 | Name | ||
| H | H | H | avarol | 196 | H | H | H | neoavarol | 197 |
| Ac | H | H | 17-O-acetylavarol | 198 | Ac | H | H | 17-O-acetylneoavarol | 199 |
| H | H | 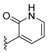 | dysivillosin A | 200 | H | H |  | dysivillosin B | 201 |
| H |  | H | dysivillosin C | 202 | H |  | H | dysivillosin D | 203 |
 | 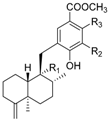 | ||||||||
| R1 | R2 | R3 | Name | R1 | R2 | R3 | Name | ||
| CH3 | OH | OH | nakijinol C | 204 | CH3 | OH | H | dictyoceratin A (smenospondiol) | 210 |
| CH3 | OCH3 | H | polyfibrospongol A | 205 | CH3 | OCH3 | OH | langconol A | 211 |
| CH2OH | OCH3 | H | polyfibrospongol B | 206 | CHO | OCH3 | H | langconol B | 212 |
| CHO | OCH3 | OH | smenohaimien D | 207 | CHO | OCH3 | OH | langconol C | 213 |
| CH2OH | OCH3 | OH | 19-hydroxy-polyfibrospongol B | 208 | |||||
| CH2OH (8-epi-) | OCH3 | OH | smenohaimien E | 209 | |||||
 | 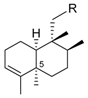 | ||||||
| R1 | R2 | R3 | Name | R | Name | ||
| H | H | H | nakijinol B | 214 |  | 18-hydroxy-5-epi-hyrtiophenol | 219 |
| H | CH3 | CH3 | (−)-nakijinol E (+)-5-epi-nakijinol E | 215 216 | 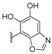 (5-epi-) | nakijinol A | 220 |
| H | CH3 | H | smenohaimien C (nakijinol F) | 217 | 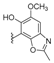 | 5-epi-nakijinol C | 221 |
| H | H | CH3 | nakijinol G | 218 |  | 5-epi-nakijinol D | 222 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García, P.A.; Hernández, Á.P.; San Feliciano, A.; Castro, M.Á. Bioactive Prenyl- and Terpenyl-Quinones/Hydroquinones of Marine Origin †. Mar. Drugs 2018, 16, 292. https://doi.org/10.3390/md16090292
García PA, Hernández ÁP, San Feliciano A, Castro MÁ. Bioactive Prenyl- and Terpenyl-Quinones/Hydroquinones of Marine Origin †. Marine Drugs. 2018; 16(9):292. https://doi.org/10.3390/md16090292
Chicago/Turabian StyleGarcía, Pablo A., Ángela P. Hernández, Arturo San Feliciano, and Mª Ángeles Castro. 2018. "Bioactive Prenyl- and Terpenyl-Quinones/Hydroquinones of Marine Origin †" Marine Drugs 16, no. 9: 292. https://doi.org/10.3390/md16090292
APA StyleGarcía, P. A., Hernández, Á. P., San Feliciano, A., & Castro, M. Á. (2018). Bioactive Prenyl- and Terpenyl-Quinones/Hydroquinones of Marine Origin †. Marine Drugs, 16(9), 292. https://doi.org/10.3390/md16090292







