New Cytotoxic Cyclic Peptide from the Marine Sponge-Associated Nocardiopsis sp. UR67
Abstract
1. Introduction
2. Results and Discussion
2.1. Metabolomic Analysis
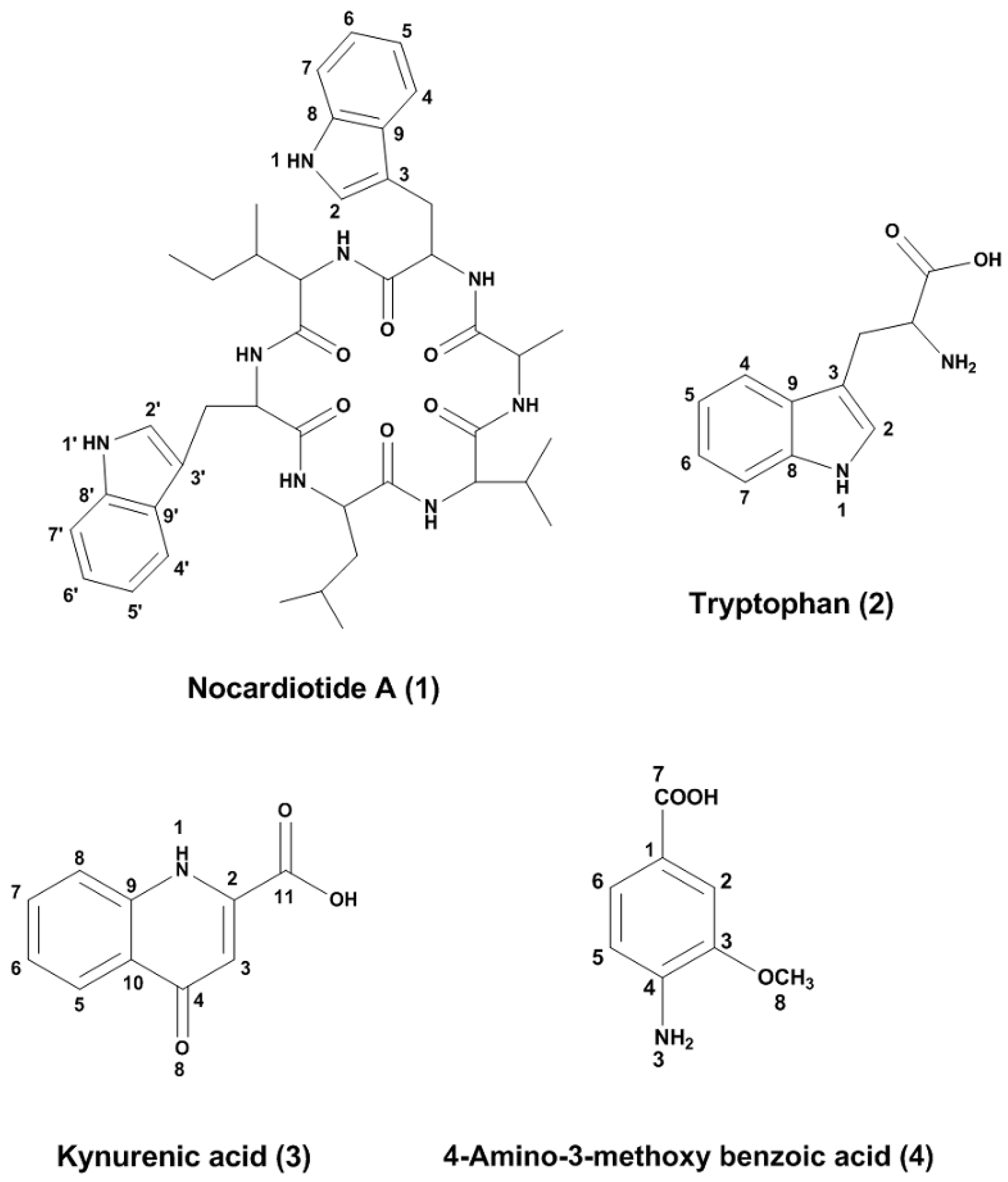
2.2. Structure Elucidation
2.3. Biological Activities of the Isolated Compounds
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Sponge Collection
3.3. Isolation, Fermentation, and Extract Preparation of Nocardiopsis sp. UR67
3.4. LC-HR/MS Analysis
3.5. Metabolites Isolation
3.6. Marfey’s Analysis
3.7. Cytotoxic Activity
3.8. Compounds Characterization
3.8.1. (Nocardiotide A) (1)
3.8.2. (Tryptophan) (2)
3.8.3. (Kynurenicacid) (3)
3.8.4. (4-Amino3-methoxy benzoic acid) (4)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yoshida, A.; Seo, Y.; Suzuki, S.; Nishino, T.; Kobayashi, T.; Hamada-Sato, N.; Kogure, K.; Imada, C. Actinomycetal community structures in seawater and freshwater examined by DGGE analysis of 16S rRNA gene fragments. Mar. Biotechnol. 2008, 10, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Klenk, H.-P.; Clément, C.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, physiology, and natural products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Abdelmohsen, U.R.; Balasubramanian, S.; Oelschlaeger, T.A.; Grkovic, T.; Pham, N.B.; Quinn, R.J.; Hentschel, U. Potential of marine natural products against drug-resistant fungal, viral, and parasitic infections. Lancet Infect. Dis. 2017, 17, e30–e41. [Google Scholar] [CrossRef]
- Manivasagan, P.; Kang, K.-H.; Sivakumar, K.; Li-Chan, E.C.; Oh, H.-M.; Kim, S.-K. Marine actinobacteria: An important source of bioactive natural products. Environ. Toxicol. Pharmacol. 2014, 38, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Manivasagan, P.; Venkatesan, J.; Sivakumar, K.; Kim, S.-K. Pharmaceutically active secondary metabolites of marine actinobacteria. Microbiol. Res. 2014, 169, 262–278. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.-H.; Vederas, J.C. Drug discovery and natural products: End of an era or an endless frontier? Science 2009, 325, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J. Nocardiopsis, a new genus of the order Actinomycetales. Int. J. Syst. Evol. Microbiol. 1976, 26, 487–493. [Google Scholar] [CrossRef]
- Rainey, F.A.; Ward-Rainey, N.; Kroppenstedt, R.M.; Stackebrandt, E. The genus Nocardiopsis represents a phylogenetically coherent taxon and a distinct actinomycete lineage: Proposal of Nocardiopsaceae fam. nov. Int. J. Syst. Evol. Microbiol. 1996, 46, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Bennur, T.; Ravi Kumar, A.; Zinjarde, S.; Javdekar, V. Nocardiopsis species: A potential source of bioactive compounds. J. Appl. Microbiol. 2015, 120, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.H.; Desoukey, S.Y.; Fouad, M.A.; Kamel, M.S.; Gulder, T.A.; Abdelmohsen, U.R. Natural Product Potential of the Genus Nocardiopsis. Mar. Drugs 2018, 16, 147. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, K.; Degnes, K.F.; Kemmler, M.; Bredholt, H.; Fjærvik, E.; Klinkenberg, G.; Sletta, H.; Ellingsen, T.E.; Zotchev, S.B. Production of a new thiopeptide antibiotic, TP-1161, by a marine Nocardiopsis species. Appl. Environ. Microbiol. 2010, 76, 4969–4976. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Seo, Y.; Lee, H.-S.; Rho, J.-R.; Mo, S.J. A new cyclic peptide from a marine-derived bacterium of the genus Nocardiopsis. J. Nat. Prod. 2003, 66, 883–884. [Google Scholar] [CrossRef] [PubMed]
- Raju, R.; Piggott, A.M.; Quezada, M.; Capon, R.J. Nocardiopsins C and D and nocardiopyroneA: Newpolyketides from an Australian marine-derived Nocardiopsis sp. Tetrahedron 2013, 69, 692–698. [Google Scholar] [CrossRef]
- Dashti, Y.; Grkovic, T.; Abdelmohsen, U.R.; Hentschel, U.; Quinn, R.J. Actinomycete Metabolome Induction/Suppression with N-Acetylglucosamine. J. Nat. Prod. 2017, 80, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Raju, R.; Piggott, A.M.; Conte, M.; Tnimov, Z.; Alexandrov, K.; Capon, R.J. Nocardiopsins: New FKBP12-Binding Macrolide Polyketides from an Australian Marine-Derived Actinomycete, Nocardiopsis sp. Chem. Eur. J. 2010, 16, 3194–3200. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Yang, Y.; Liu, K.; Xiong, Z.; Xu, L.; Zhao, L. Antimicrobial metabolites from a novel halophilic actinomycete Nocardiopsis terrae YIM 90022. Nat. Prod. Res. 2014, 28, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Raju, R.; Piggott, A.M.; Huang, X.-C.; Capon, R.J. Nocardioazines: A novel bridged diketopiperazine scaffold from a marine-derived bacterium inhibits P-glycoprotein. Org. Lett. 2011, 13, 2770–2773. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Liu, P.; Qu, H.; Wang, Y.; Chen, D.; Wang, H.; Li, J.; Zhu, W. α-Pyrones and diketopiperazine derivatives from the marine-derived actinomycete Nocardiopsis dassonvillei HR10-5. J. Nat. Prod. 2011, 74, 2219–2223. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Ogura, H.; Akasaka, K.; Oikawa, T.; Matsuura, N.; Imada, C.; Yasuda, H.; Igarashi, Y. Nocapyrones: α-and γ-Pyrones from a Marine-Derived Nocardiopsis sp. Mar. Drugs 2014, 12, 4110–4125. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.C.; Kwon, O.-W.; Park, J.-S.; Kim, S.Y.; Kwon, H.C. Nocapyrones, H–J, 3, 6-disubstituted α-pyrones from the marine actinomycete Nocardiopsis sp. KMF-001. Chem. Pharm. Bull. 2013, 61, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.-G.; Zhao, J.-Y.; Li, M.-G.; Huang, R.; Li, Q.-M.; Cui, X.-L.; Zhu, H.-J.; Wen, M.-L. Griseusins F and G spiro-naphthoquinones from a tin mine tailings-derived alkalophilic Nocardiopsis species. J. Nat. Prod. 2012, 75, 1994–1998. [Google Scholar] [PubMed]
- Gao, X.; Lu, Y.; Xing, Y.; Ma, Y.; Lu, J.; Bao, W.; Wang, Y.; Xi, T. A novel anticancer and antifungusphenazine derivative from a marine actinomycete BM-17. Microbiol. Res. 2012, 167, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Li, Y.; Wang, H.; Wang, B.; Shen, Y. A new phenoxazine derivative isolated from marine sediment actinomycetes, Nocardiopsis sp. 236. Drug Discov. Ther. 2013, 7, 101–104. [Google Scholar] [PubMed]
- He, J.; Roemer, E.; Lange, C.; Huang, X.; Maier, A.; Kelter, G.; Jiang, Y.; Xu, L.-H.; Menzel, K.-D.; Grabley, S.; et al. Structure, derivatization, and antitumor activity of new griseusins from Nocardiopsis sp. J. Med. Chem. 2007, 50, 5168–5175. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.-Z.; Pu, X.; Luo, G.; Zhao, L.-X.; Xu, L.-H.; Li, W.-J.; Luo, Y. Isolation and characterization of new p-terphenyls with antifungal, antibacterial, and antioxidant activities from halophilic actinomycete Nocardiopsis gilva YIM 90087. J. Agric. Food Chem. 2013, 61, 3006–3012. [Google Scholar] [CrossRef] [PubMed]
- Kase, H.; Iwahashi, K.; Matsuda, Y. K-252a, a potent inhibitor of protein kinase C from microbial origin. J. Antibiot. 1986, 39, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Grkovic, T.; Abdelmohsen, U.R.; Othman, E.M.; Stopper, H.; Edrada-Ebel, R.; Hentschel, U.; Quinn, R.J. Two new antioxidant actinosporin analogues from the calcium alginate beads culture of sponge-associated Actinokineospora sp. strain EG49. Bioorg. Med. Chem. Lett. 2014, 24, 5089–5092. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Williams, P.G.; Kwon, H.C.; Jensen, P.R.; Fenical, W. Lucentamycins A–D, cytotoxic peptides from the marine-derived actinomycete Nocardiopsis lucentensis. J. Nat. Prod. 2007, 70, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Sun, C.; Wang, N.; Gao, R.; Bai, S.; Zheng, H.; You, X.; Li, R. Preliminary report on the biological effects of space flight on the producing strain of a new immunosuppressant, Kanglemycin C. J. Ind. Microbiol. Biotechnol. 2006, 33, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Poumale, H.M.; Ngadjui, B.T.; Helmke, E.; Laatscha, H. New anthraquinones from a marine Streptomyces sp.—isolation, structure determination and biological activities. Z. Naturforsch. B 2006, 61, 1450–1454. [Google Scholar] [CrossRef]
- Zhou, X.; Fenical, W. The unique chemistry and biology of the piericidins. J. Antibiot. (Tokyo) 2016, 69, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, K.; Furihata, K.; Shimazu, A.; Hayakawa, Y.; Seto, H. Isolation and characterization of atramycin A and atramycin B, new isotetracenone type antitumor antibiotics. J. Antibiot. (Tokyo) 1991, 44, 1025–1028. [Google Scholar] [CrossRef]
- Urakawa, A.; Sasaki, T.; Yoshida, K.; Otani, T.; Lei, Y.; Yun, W. IT-143-A and B, novel piericidin-group antibiotics produced by Streptomyces sp. J. Antibiot. (Tokyo) 1996, 49, 1052–1055. [Google Scholar] [CrossRef] [PubMed]
- Harada, S. Studies on lankacidin-group (T-2636) antibiotics. VI. Chemical structures of lankacidin-group antibiotics. II. Chem. Pharm. Bull. (Tokyo) 1975, 23, 2201–2210. [Google Scholar] [CrossRef] [PubMed]
- Patton, S.M.; Cropp, T.A.; Reynolds, K.A. A novel delta(3),delta(2)-enoyl-CoA isomerase involved in the biosynthesis of the cyclohexanecarboxylic acid-derived moiety of the Polyketideans atrienin A. Biochemistry 2000, 39, 7595–7604. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.C.; Cao, S.; Raveh, A.; MacArthur, R.; Dranchak, P.; Chlipala, G.; Okoneski, M.T.; Guha, R.; Eastman, R.T.; Yuan, J.; et al. Actinoramide A Identified as a Potent Antimalarial from Titration-Based Screening of Marine Natural Product Extracts. J. Nat. Prod. 2015, 78, 2411–2422. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wang, Y.; Huang, T.; Tao, M.; Deng, Z.; Lin, S. Identification and characterization of the biosynthetic gene cluster of polyoxypeptin A, a potent apoptosis inducer. BMC Microbiol. 2014, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Wiese, J.; Abdelmohsen, U.R.; Motiei, A.; Humeida, U.H.; Imhoff, J.F. Bacicyclin, a new antibacterial cyclic hexapeptide from Bacillus sp. strain BC028 isolated from Mytilusedulis. Bioorg. Med. Chem. Lett. 2018, 28, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.; Edrada-Ebel, R.; Mohamed, G.A.; Youssef, D.T.; Wray, V.; Proksch, P. Callyaerin G, a new cytotoxic cyclic peptide from the marine sponge Callyspongia aerizusa. Arkivoc 2008, 2008, 164. [Google Scholar]
- Wu, Z.-C.; Li, S.; Nam, S.-J.; Liu, Z.; Zhang, C. Nocardiamides A and B, two cyclohexapeptides from the marine-derived actinomycete Nocardiopsis sp. CNX037. J. Nat. Prod. 2013, 76, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.-W.; Chang, F.-R.; Wu, C.-C.; Wu, K.-Y.; Li, C.-M.; Chen, S.-L.; Wu, Y.-C. New cytotoxic cyclic peptides and dianthramide from Dianthus superbus. J. Nat. Prod. 2004, 67, 1522–1527. [Google Scholar] [CrossRef] [PubMed]
- Al-Khalil, S.; Alkofahi, A.; El-Eisawi, D.; Al-Shibib, A. Transitorine, a new quinoline alkaloid from Ephedra transitoria. J. Nat. Prod. 1998, 61, 262–263, Correction in 1999, 62, 1214. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, Y.; Refaat, J.; Abdelmohsen, U.R.; Ahmed, S.; Fouad, M.A. Rhodozepinone, a new antitrypanosomal azepino-diindole alkaloid from the marine sponge-derived bacterium Rhodococcus sp. UA13. Med. Chem. Res. 2017, 26, 2751–2760. [Google Scholar] [CrossRef]
- Webster, N.S.; Wilson, K.J.; Blackall, L.L.; Hill, R.T. Phylogenetic diversity of bacteria associated with the marine sponge Rhopaloeidesod orabile. Appl. Environ. Microbiol. 2001, 67, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Tawfike, A.F.; Tate, R.; Abbott, G.; Young, L.; Viegelmann, C.; Schumacher, M.; Diederich, M.; Edrada-Ebel, R. Metabolomic Tools to Assess the Chemistry and Bioactivity of Endophytic Aspergillus Strain. Chem. Biodivers. 2017, 14, e1700040. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, R.; Brückner, H. Marfey’s reagent for chiral amino acid analysis: A review. Amino Acids 2004, 27, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Kochhar, S.; Christen, P. Amino acid analysis by high-performance liquid chromatography after derivatization with 1-fluoro-2, 4-dinitrophenyl-5-l-alanine amide. Anal. Biochem. 1989, 178, 17–21. [Google Scholar] [CrossRef]
- Marfey, P. Determination of d-amino acids. II. Use of a bifunctional reagent, 1,5-difluoro-2,4-dinitrobenzene. Carlsberg Res. Commun. 1984, 49, 591. [Google Scholar] [CrossRef]
- Cheng, C.; Othman, E.M.; Stopper, H.; Edrada-Ebel, R.; Hentschel, U.; Abdelmohsen, U.R. Isolation of Petrocidin A, a New Cytotoxic Cyclic Dipeptide from the Marine Sponge-Derived Bacterium Streptomyces sp. SBT348. Mar. Drugs 2017, 15, 383. [Google Scholar] [CrossRef] [PubMed]
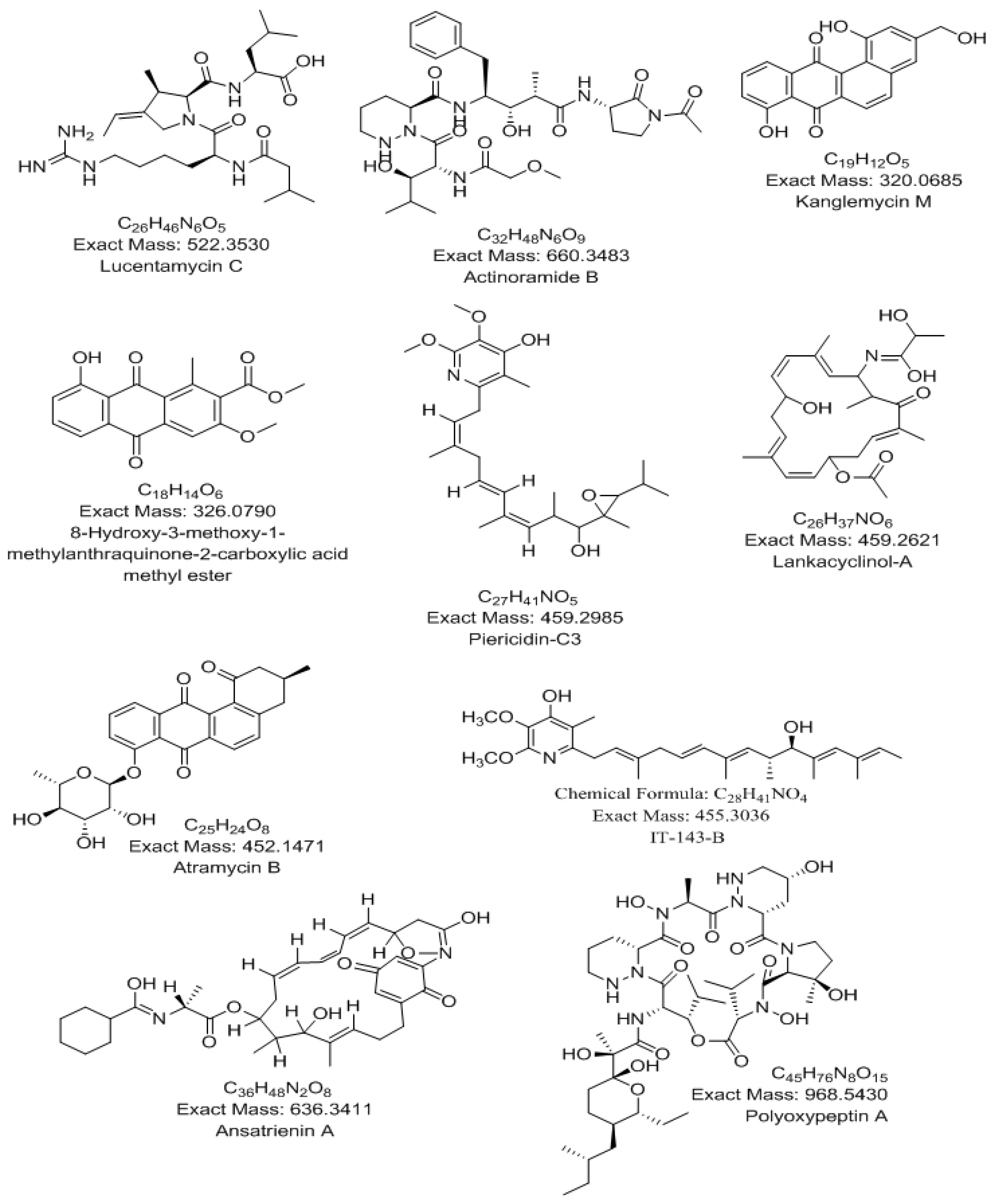


| Polarity | m/z | Rt (min.) | Formula | Name | Source |
|---|---|---|---|---|---|
| [M + H]+ | 327.0866 | 2.91 | C18H14O6 | 8-Hydroxy-3-methoxy-1-methylanthraquinone-2-carboxylic acid | Streptomyces sp. |
| [M − H]− | 967.5399 | 3.53 | C45H76N8O15 | Polyoxypeptin A | Streptomyces sp. MK498-98 F14 |
| [M − H]− | 635.3316 | 3.81 | C36H48N2O8 | Ansatrienin A | Streptomyces collinus |
| [M + H]+ | 321.0760 | 4.6 | C19H12O5 | Kanglemycin M | Nocardiamediterranei var. kanglensis 1747-64 |
| [M + H]+ | 661.3568 | 6.41 | C32H48N6O9 | Actinoramide B | Streptomyces ballenaensis and Streptomyces bangulaensis |
| [M + H]+ | 460.2697 | 6.95 | C26H37NO6 | Lankacyclinol-A | Streptomyces rochei var. volubilis |
| [M − H]− | 451.1392 | 7.24 | C25H24O8 | Atramycin B | Streptomyces atratus |
| [M − H]− | 458.2906 | 7.56 | C27H41NO5 | Piericidin-C3 | Streptomyces pactum |
| [M + H]+ | 456.3108 | 7.98 | C28H41NO4 | IT-143-B | Streptomyces species |
| [M + H]+ | 523.3601 | 11.23 | C26H46N6O5 | Lucentamycin C | Nocardiopsis lucentensis |
| Aminoacids | δC | δH, mult (J in Hz) | COSY | HMBC | NOESY |
|---|---|---|---|---|---|
| Ile | |||||
| CO | 173.42 | ||||
| α | 58.94 | 4.20, d (4.6) | β | CO, β, γ′, δ | β-Trp2 |
| β | 36.93 | 2.03, m | α, γ, γ′ | γ′, δ | |
| γ | 12.14 | 0.84, d (7.0) | γ′, β | ||
| γ′ | 27.49 | 1.28, m | β, γ, δ | α, β, γ, δ | |
| δ | 14.65 | 0.85, d (7.4) | γ′ | α, β, γ′ | |
| Trp1 | |||||
| CO | 179.06 | ||||
| α | 56.90 | 4.37, dd (3.8, 9.9) | β, β′ | CO, β/β′, C-3, Ala-CO | |
| β | 29.19 | 3.05, dd (14.7, 9.9) | α | CO, α, C-2, C-3, C-8, C-9 | |
| β′ | 29.19 | 3.30, dd (14.7, 3.8) | α | ||
| 2 | 124.65 | 7.03 (s) | β/β′, C-3, C-8, C-9 | α-Ileu | |
| 3 | 112.29 | ||||
| 4 | 119.55 | 7.55, dt (7.84, 0.9) | H-5, H-6, H-7 | C-3, C-6, C-8, C-9 | |
| 5 | 119.44 | 6.88 m | H-4, H-6, H-7 | C-7, C-9 | |
| 6 | 122.05 | 6.94 (m) | H-4, H-5, H-7 | C-4, C-9 | |
| 7 | 112.04 | 7.18, dt (8.11, 0.9) | H-4, H-5, H-6 | C-5, C-9 | |
| 8 | 137.98 | ||||
| 9 | 128.92 | ||||
| Ala | |||||
| CO | 173.93 | ||||
| α | 49.77 | 4.31, q, (7.2) | β | CO, β | α-Val |
| β | 18.05 | 1.17, d, (7.2) | α | CO, α | |
| Val | |||||
| CO | 175.33 | ||||
| α | 60.76 | 4.22, d (7.7) | β | CO, Leu-CO, β, γ′ | α-Leu |
| β | 31.75 | 1.97, m | α, γ, γ′ | CO, α, γ | |
| γ | 19.79 | 0.88, d (3.1) | β | α, β, γ′ | |
| γ′ | 18.95 | 0.9, d (3.1) | β | α, β, γ | |
| Leu | |||||
| CO | 171.56 | ||||
| α | 52.97 | 3.81, t (7.3) | β | CO, β, γ | |
| β | 41.95 | 1.59, m | α, γ | CO, α, γ, δ, δ′ | |
| γ | 25.60 | 1.62, m | β, δ, δ′ | α, β, δ, δ′ | |
| δ | 22.56 | 0.92, d (6.2) | γ | β, γ, δ′ | |
| δ′ | 22.85 | 0.93, d (6.2) | γ | β, γ, δ | |
| Trp2 | |||||
| CO | 173.24 | ||||
| α | 41.56 | 3.35, t (8.04) | β/β’ | CO, β/β′, C-3′ | |
| β/β′ | 26.21 | 2.82, td (8.64, 0.9) | α | α, C-2′, C-3′, C-9′ | α-Ile |
| 2′ | 123.33 | 6.96, s | α, β/β’ | ||
| 3′ | 113.27 | ||||
| 4′ | 119.22 | 7.45, dt (7.92, 0.9) | H-5′, H-6′, H-7′ | C-3′, C-6′, C-8′, C-9′ | |
| 5′ | 119.44 | 6.90, m | H-4′, H-6′, H-7′ | C-7′, C-9′ | |
| 6′ | 122.28 | 6.98, m | H-4′, H-5′, H-7′ | C-4′, C-9′ | |
| 7′ | 112.20 | 7.22, dt (8.17, 0.9) | H-4′, H-’5, H-6′ | C-5′, C-9′ | |
| 8′ | 138.7 | ||||
| 9′ | 128.80 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, A.H.; Attia, E.Z.; Hajjar, D.; Anany, M.A.; Desoukey, S.Y.; Fouad, M.A.; Kamel, M.S.; Wajant, H.; Gulder, T.A.M.; Abdelmohsen, U.R. New Cytotoxic Cyclic Peptide from the Marine Sponge-Associated Nocardiopsis sp. UR67. Mar. Drugs 2018, 16, 290. https://doi.org/10.3390/md16090290
Ibrahim AH, Attia EZ, Hajjar D, Anany MA, Desoukey SY, Fouad MA, Kamel MS, Wajant H, Gulder TAM, Abdelmohsen UR. New Cytotoxic Cyclic Peptide from the Marine Sponge-Associated Nocardiopsis sp. UR67. Marine Drugs. 2018; 16(9):290. https://doi.org/10.3390/md16090290
Chicago/Turabian StyleIbrahim, Alyaa Hatem, Eman Zekry Attia, Dina Hajjar, Mohamed A. Anany, Samar Yehia Desoukey, Mostafa Ahmed Fouad, Mohamed Salah Kamel, Harald Wajant, Tobias A. M. Gulder, and Usama Ramadan Abdelmohsen. 2018. "New Cytotoxic Cyclic Peptide from the Marine Sponge-Associated Nocardiopsis sp. UR67" Marine Drugs 16, no. 9: 290. https://doi.org/10.3390/md16090290
APA StyleIbrahim, A. H., Attia, E. Z., Hajjar, D., Anany, M. A., Desoukey, S. Y., Fouad, M. A., Kamel, M. S., Wajant, H., Gulder, T. A. M., & Abdelmohsen, U. R. (2018). New Cytotoxic Cyclic Peptide from the Marine Sponge-Associated Nocardiopsis sp. UR67. Marine Drugs, 16(9), 290. https://doi.org/10.3390/md16090290






