Chitosan-Based Composite Materials for Prospective Hemostatic Applications
Abstract
1. Introduction
2. Hemostatic Mechanisms of Chitosan (CS)
2.1. Aggregation of Red Blood Cells
2.2. Stimulation of Platelets
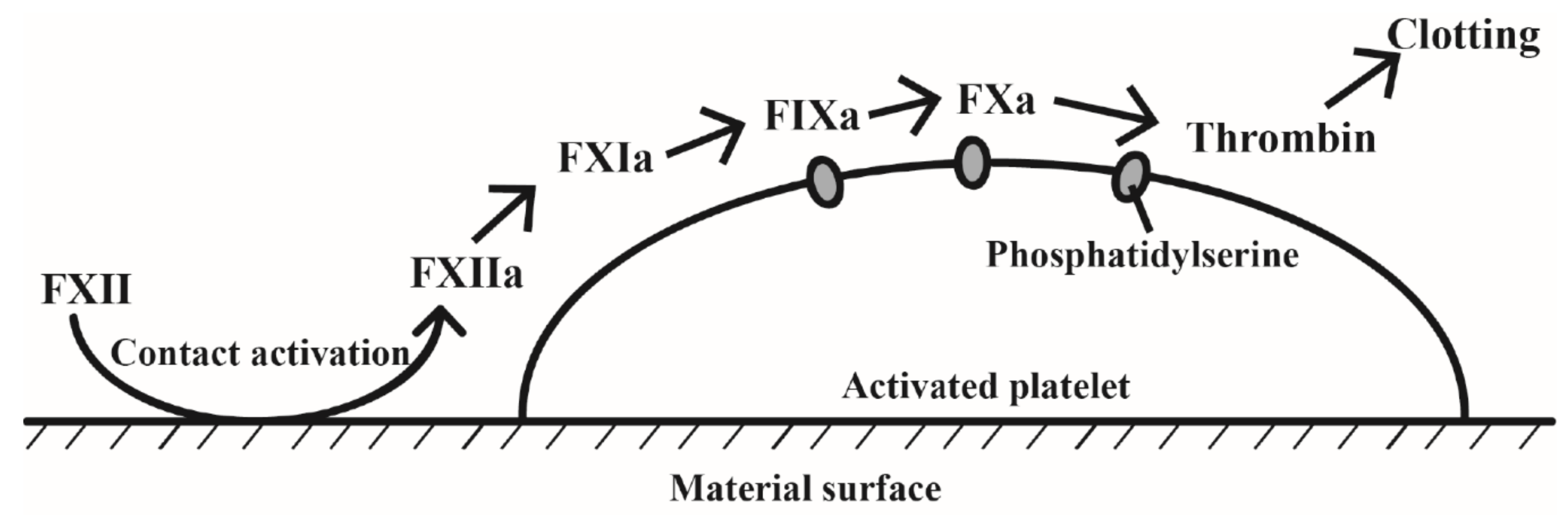
2.3. Contact System Activation
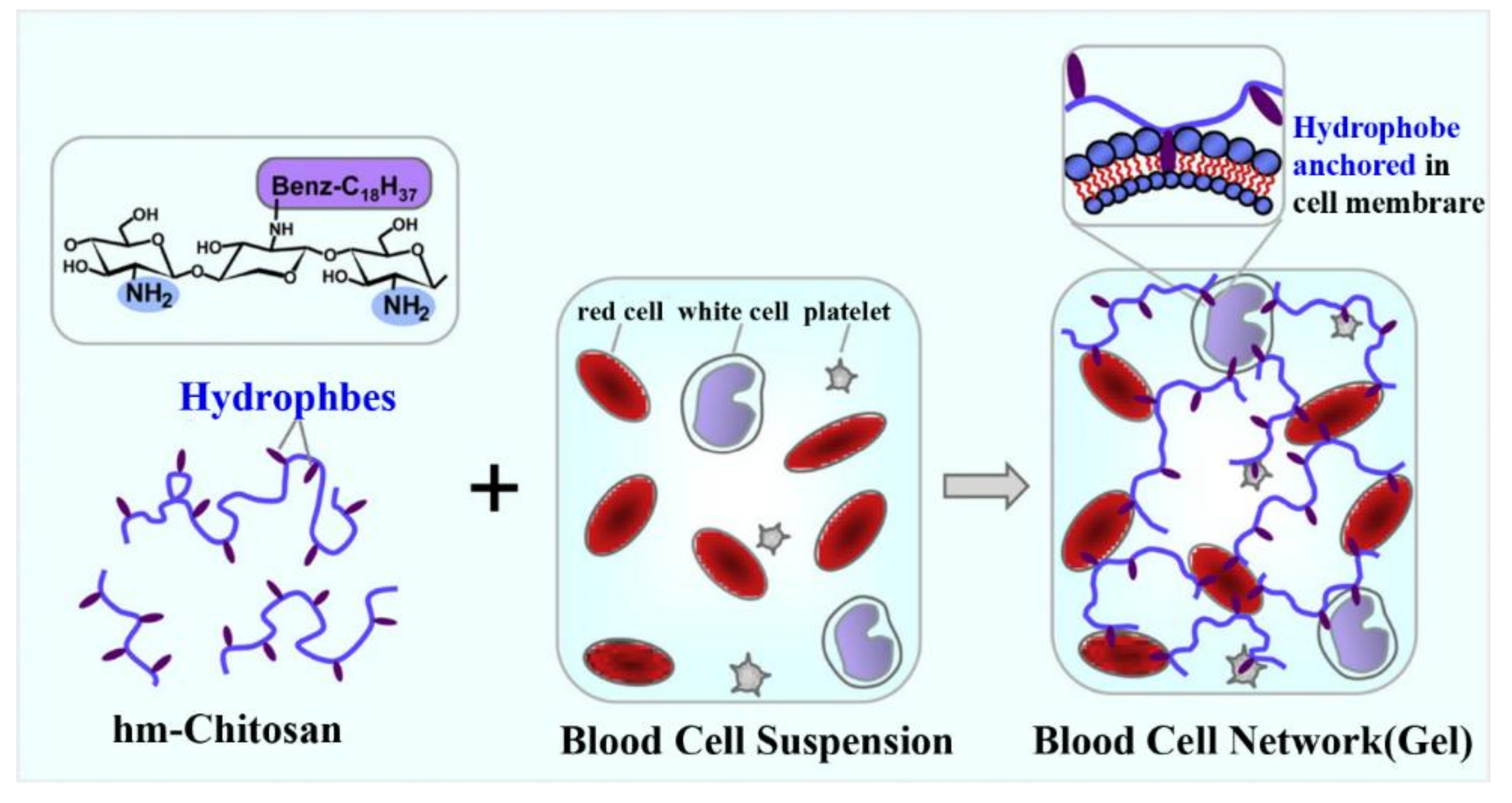
2.4. Formation of Spatial Network Structure
3. CS-Based Composite Hemostatic Materials
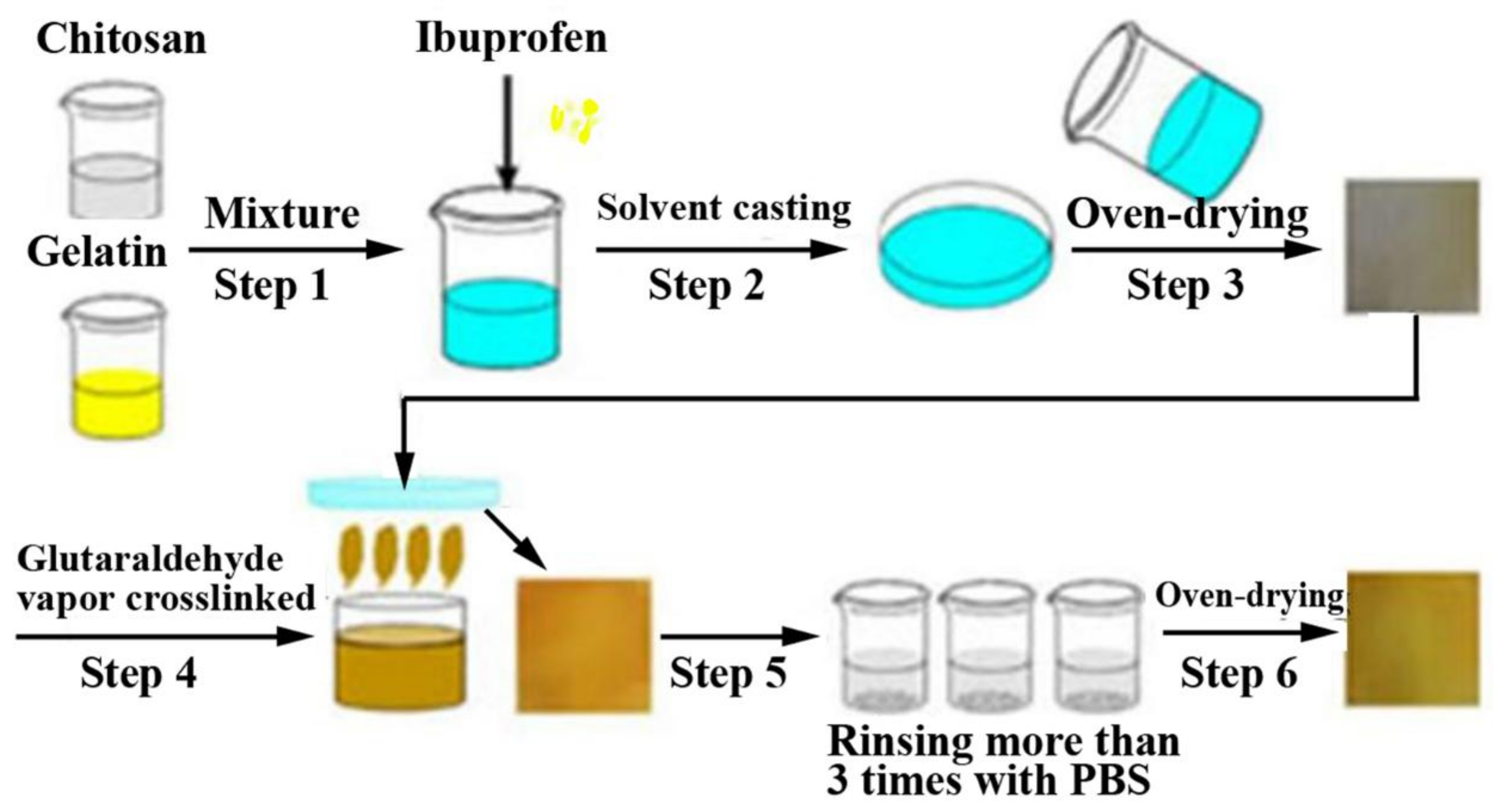
3.1. CS-Based Composite Hemostatic Films
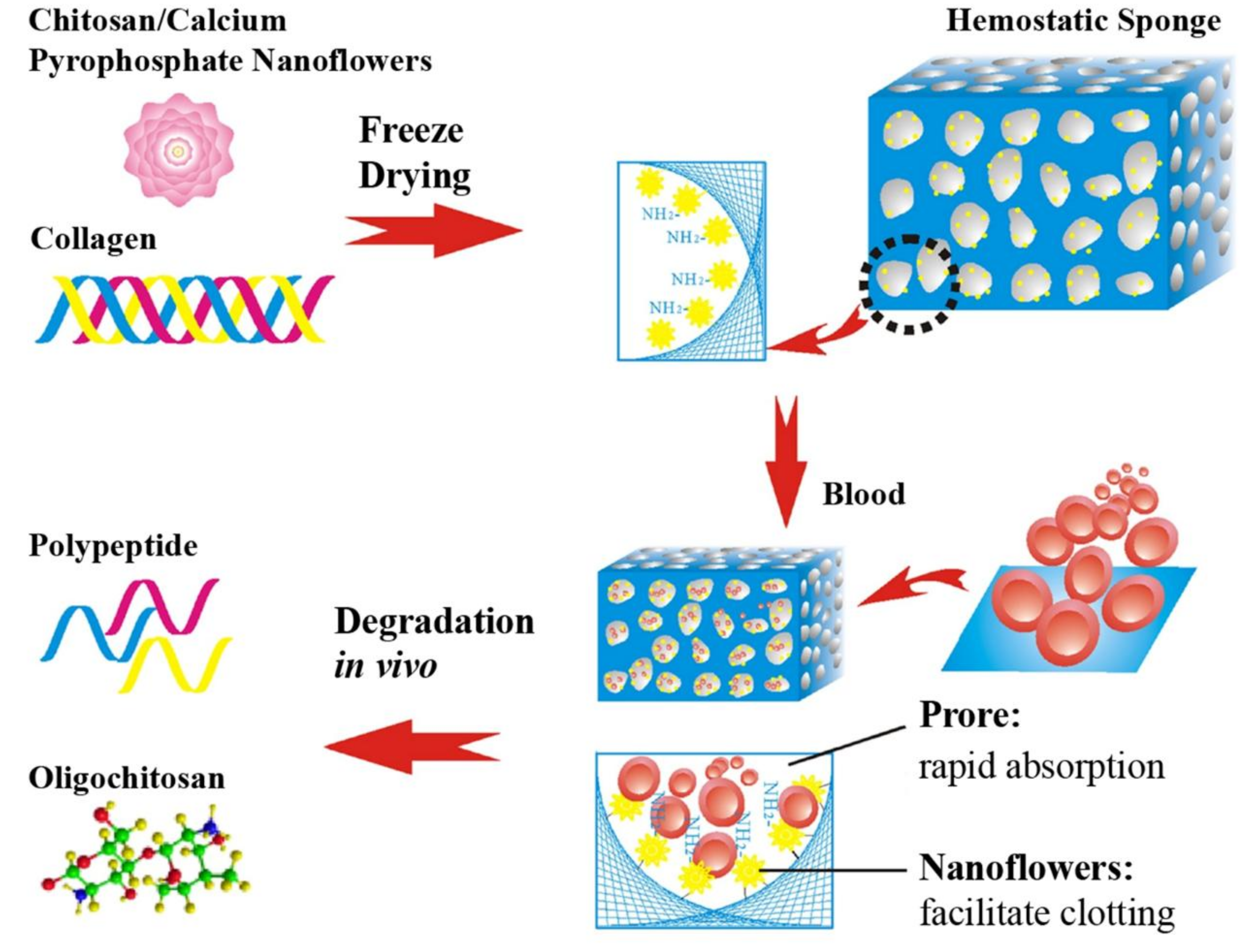
3.2. CS-Based Composite Hemostatic Sponges
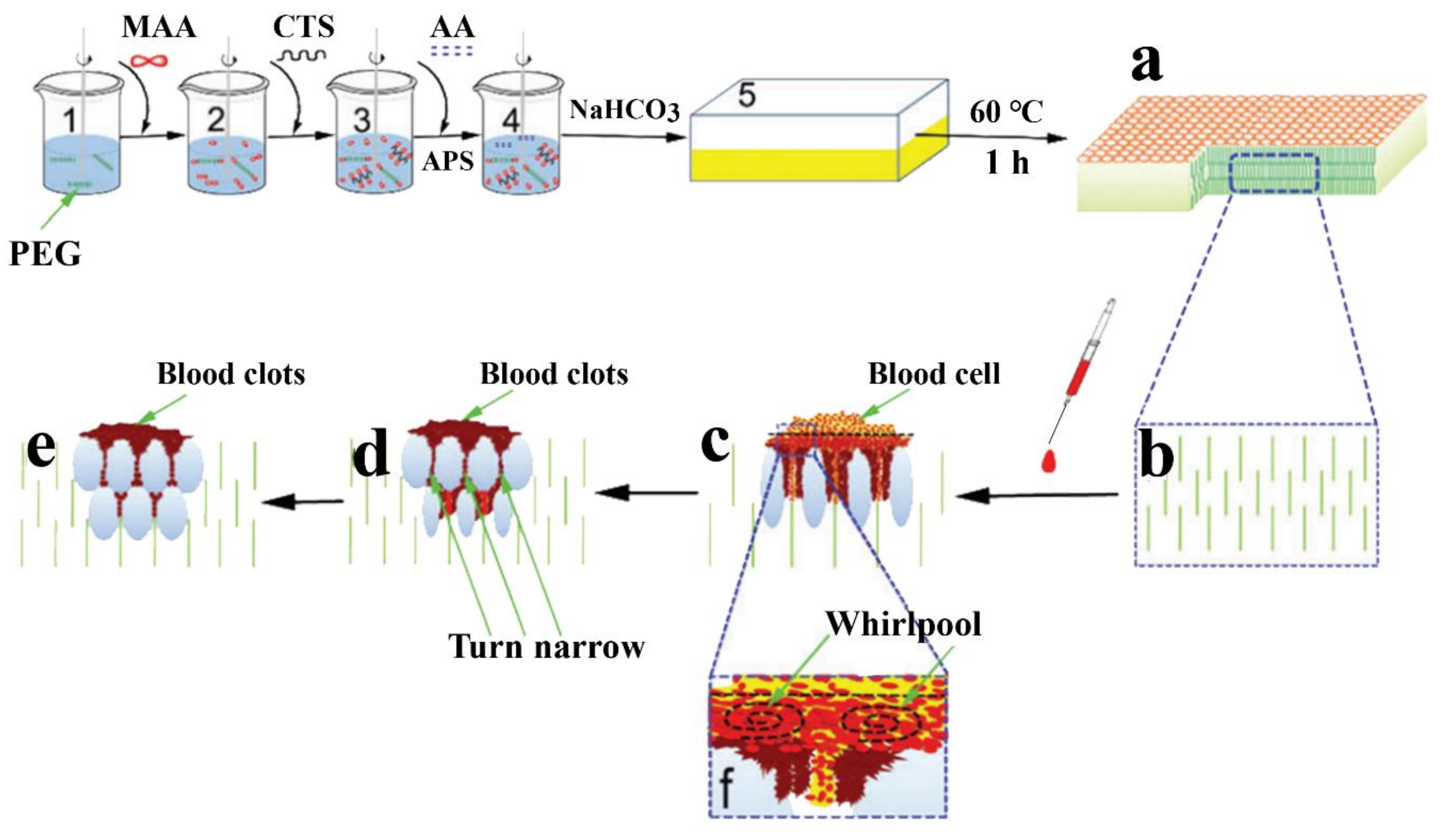
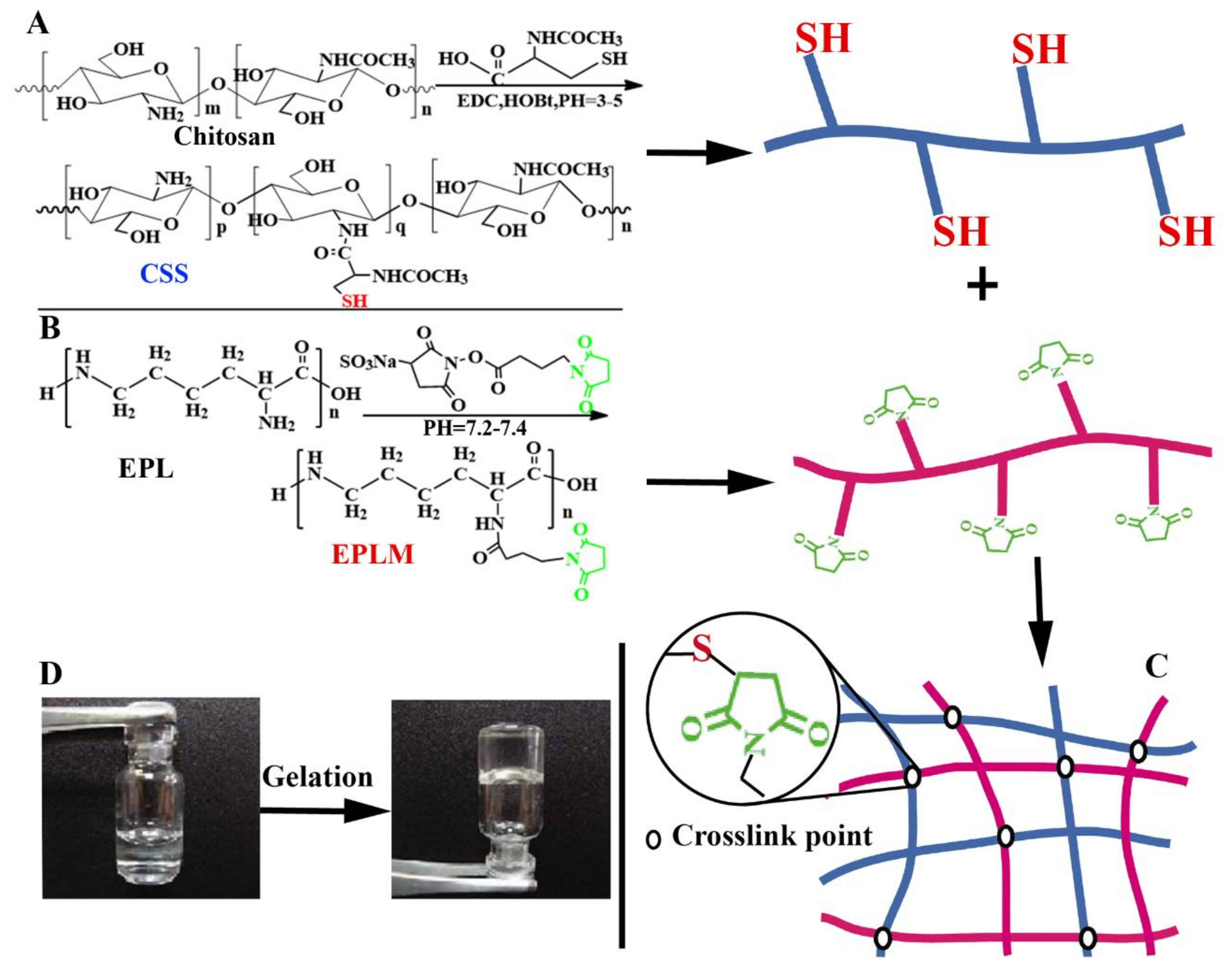
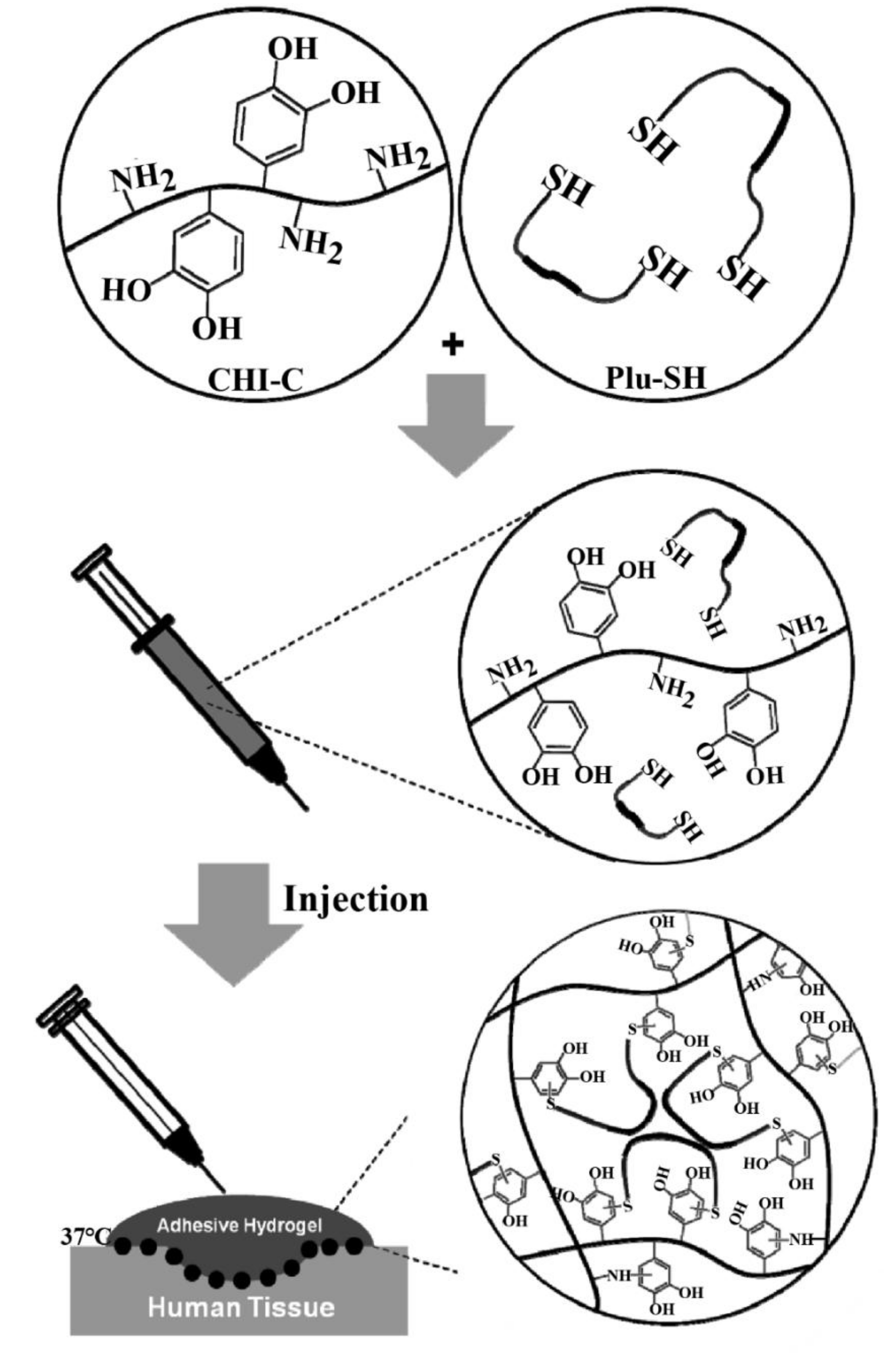
3.3. CS-Based Composite Hemostatic Hydrogels
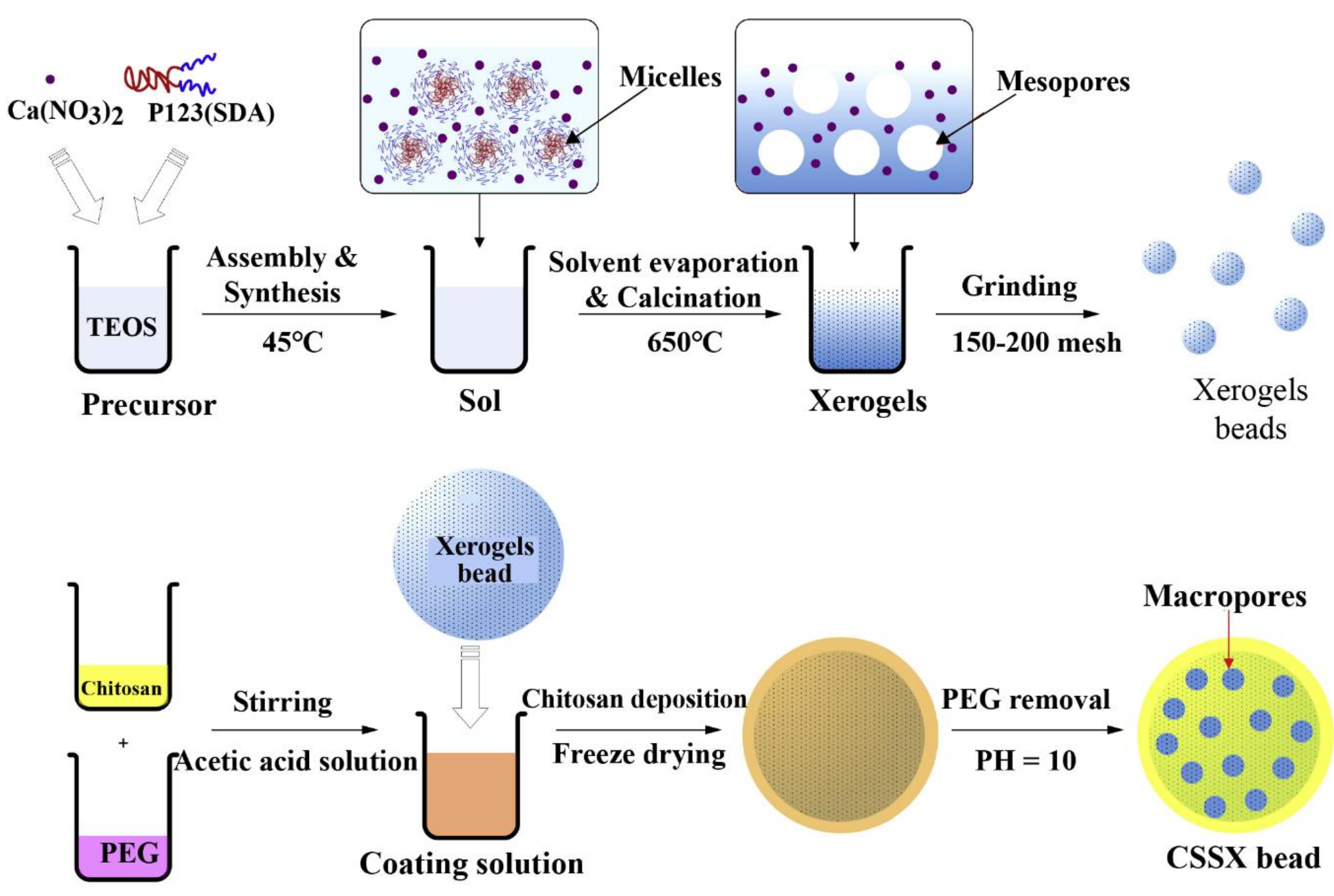
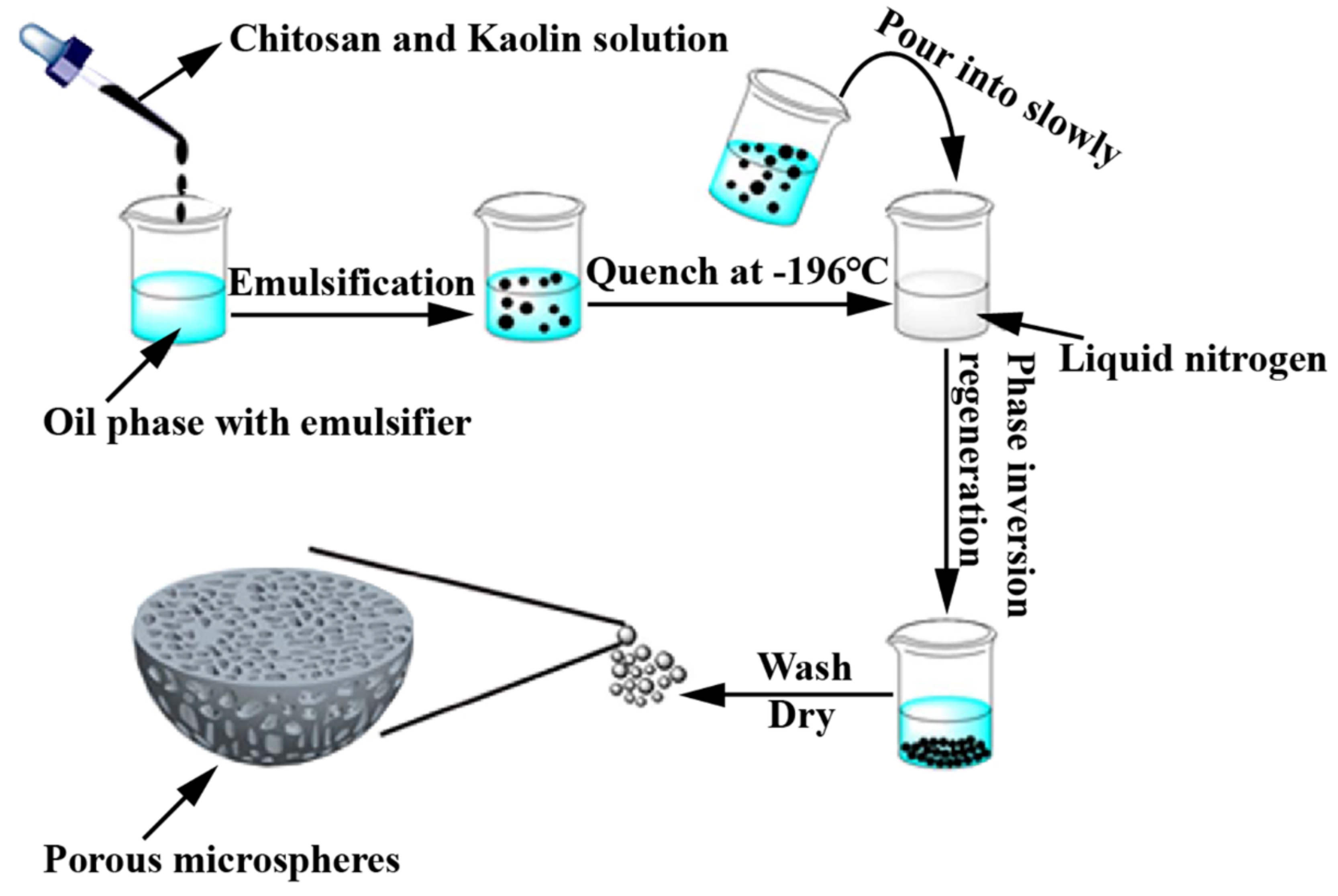
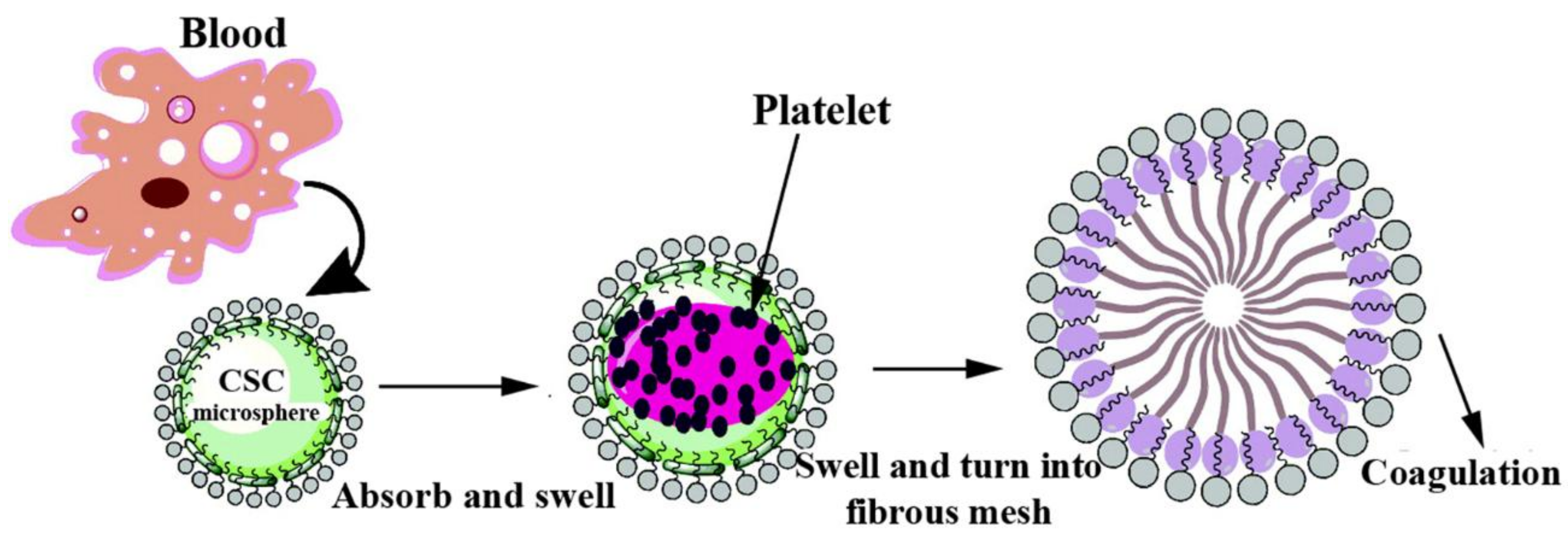
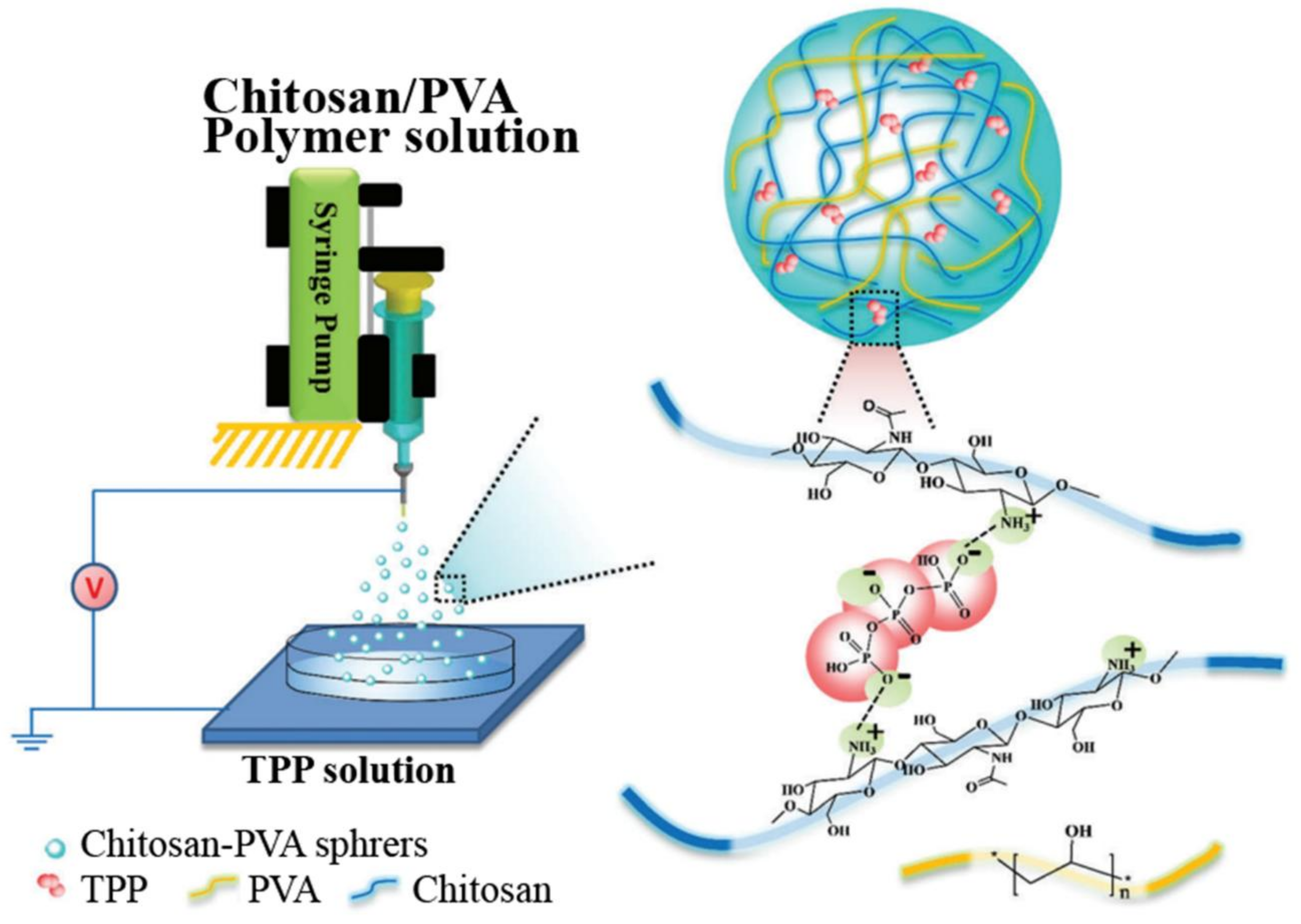
3.4. CS-Based Composite Hemostatic Particles
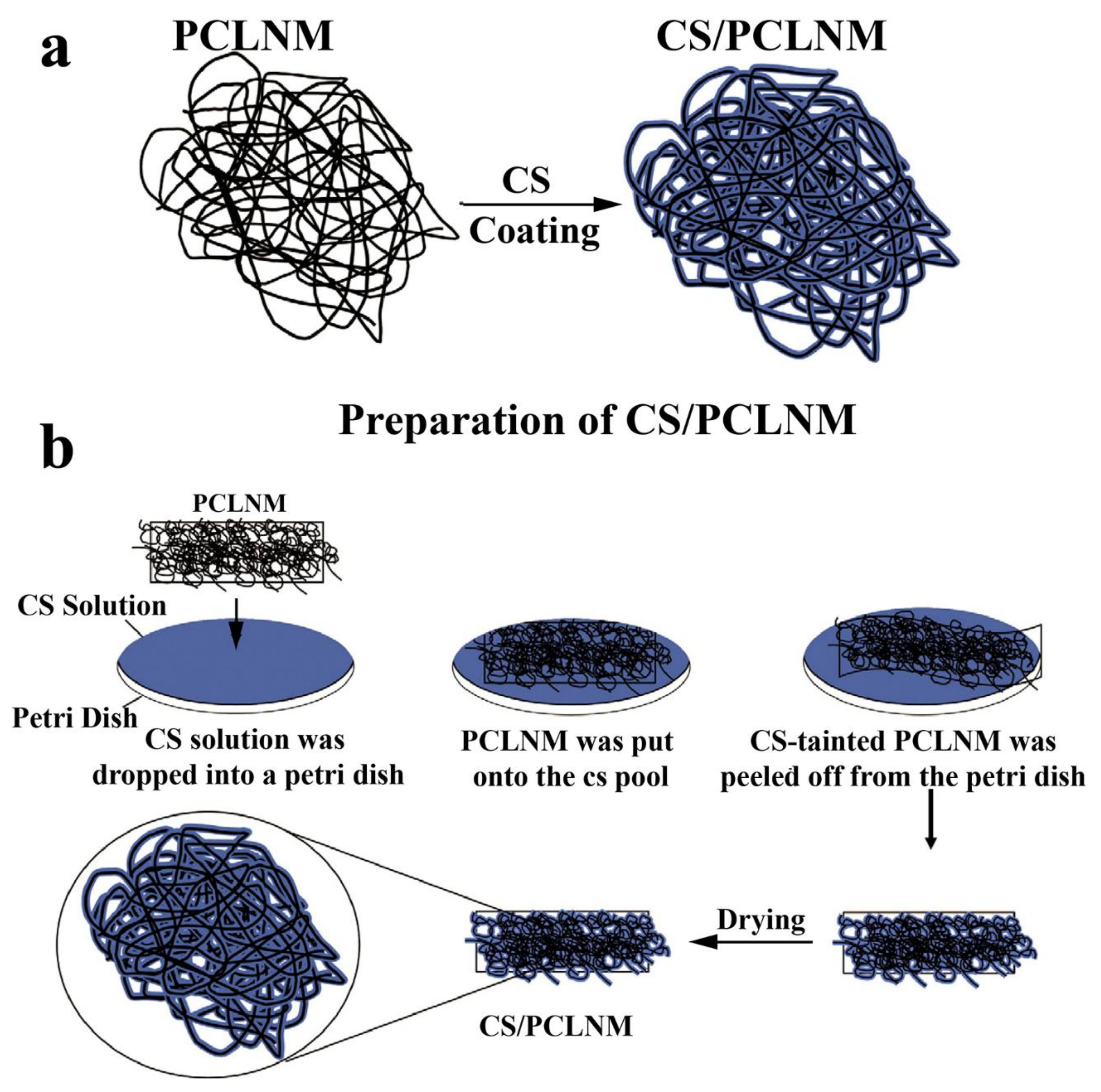
3.5. CS-Based Fibrous Hemostatic Materials
3.6. Other CS-Based Composite Hemostatic Materials
4. Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AG | Alginate |
| ChiPP | Chitosan-polyphosphate |
| CMCS | Carboxymethyl chitosan |
| Col | Collagen |
| CS | Chitosan |
| CS-C | Catechol-functionalized chitosan |
| CSS | Thiol functionalized chitosan |
| EPLM | Maleimide group modified ε-polylysine |
| GE | Gelatin |
| HBC | Hydroxybutyl chitosan |
| HLC | Human-like collagen |
| HM-CS | Hydrophobically modified chitosan |
| MBG | Mesoporous bioactive glass |
| MSX | Mesoporous silica xerogels |
| ONFC | Oxidized nanofibrillar cellulose |
| ORC | Oxidized regenerated cellulose |
| PAA | Poly (acrylic acid) |
| PCL | Polycaprolactone |
| PEG | Polyethylene glycol |
| Plu-SH | Thiol-terminated Pluronic |
| PVA | Polyvinyl alcohol |
| PVM/MA | Poly-(methyl vinyl ether-co-maleic anhydride) |
| rBat | Recombinant batroxobin |
| RBC | Red blood cell |
| SIP | Squid ink polysaccharide |
| SPA | Sodium polyacrylate |
References
- Kauvar, D.S.; Lefering, R.; Wade, C.E. Impact of hemorrhage on trauma outcome: An overview of epidemiology, clinical presentations, and therapeutic considerations. J. Trauma. 2006, 60, S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Seyednejad, H.; Imani, M.; Jamieson, T.; Seifalian, A.M. Topical haemostatic agents. Br. J. Surg. 2008, 95, 1197–1225. [Google Scholar] [CrossRef] [PubMed]
- Kunio, N.R.; Riha, G.M.; Watson, K.M.; Differding, J.A.; Schreiber, M.A.; Watters, J.M. Chitosan based advanced hemostatic dressing is associated with decreased blood loss in a swine uncontrolled hemorrhage model. Am. J. Surg. 2013, 205, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.B.; Lee, O.J.; Sultan, M.T.; Lee, J.M.; Park, Y.R.; Yeon, Y.K.; Lee, J.S.; Lee, Y.J.; Kim, S.H.; Park, C.H. In vitro and in vivo evaluation of the duck’s feet collagen sponge for hemostatic applications. J. Biomater. Appl. 2017, 32, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Kind, G.M.; Bines, S.D.; Staren, E.D.; Templeton, A.J.; Economou, S.G. Chitosan: Evaluation of a new hemostatic agent. Curr. Surg. 1990, 47, 37–39. [Google Scholar] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.B.; Sharma, C.P. Use of chitosan as a biomaterial: Studies on its safety and hemostatic potential. J. Biomed. Mater. Res. 1997, 34, 21–28. [Google Scholar] [CrossRef]
- Benesch, J.; Tengvall, P. Blood protein adsorption onto chitosan. Biomaterials 2002, 23, 2561–2568. [Google Scholar] [CrossRef]
- Thatte, H.S.; Zagarins, S.; Khuri, S.F.; Fischer, T.H. Mechanisms of poly-N-acetyl glucosamine polymermediated hemostasis: Platelet interactions. J. Trauma 2004, 57, S13–S21. [Google Scholar] [CrossRef] [PubMed]
- Bennett, B.L.; Littlejohn, L. Review of new topical hemostatic dressings for combat casualty care. Mil. Med. 2014, 179, 497–514. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Feng, L.; Zhang, Y.; He, L.; Wang, C.; Xu, J.; Wu, J.; Kirk, T.B.; Guo, R.; Xue, W. Hemostasis mechanism and applications of N-alkylated chitosan sponge. Polym. Adv. Technol. 2017, 28, 1107–1114. [Google Scholar] [CrossRef]
- Edwards, J.V.; Howley, P.; Prevost, N.; Condon, B.; Arnold, J.; Diegelmann, R. Positively and negatively charged ionic modifications to cellulose assessed as cotton-based protease-lowering and hemostatic wound agents. Cellulose 2009, 16, 911–921. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, X.; Zhou, J.; Li, L. An investigation of chitosan and its derivatives on red blood cell agglutination. RSC Adv. 2017, 7, 12247–12254. [Google Scholar] [CrossRef]
- Hattori, H.; Ishihara, M. Changes in blood aggregation with differences in molecular weight and degree of deacetylation of chitosan. Biomed. Mater. 2015, 10, 015014. [Google Scholar] [CrossRef] [PubMed]
- Klokkevold, P.R.; Fukayama, H.; Sung, E.C.; Bertolami, C.N. The effect of chitosan (poly-N-acetyl glucosamine) on lingual hemostasis in heparinized rabbits. J. Oral Maxil Surg. 1999, 57, 49–52. [Google Scholar] [CrossRef]
- Hattori, H.; Amano, Y.; Nogami, Y.; Takase, B.; Ishihara, M. Hemostasis for severe hemorrhage with photocrosslinkable chitosan hydrogel and calcium alginate. Ann. Biomed. Eng. 2010, 38, 3724–3732. [Google Scholar] [CrossRef] [PubMed]
- Sagnella, S.; Kwok, J.; Marchant, R.E.; Kottke-Marchant, K. Shear-induced platelet activation and adhesion on human pulmonary artery endothelial cells seeded onto hydrophilic polymers. J. Biomed. Mater. Res. 2001, 57, 419–431. [Google Scholar] [CrossRef]
- Chou, T.C.; Fu, E.; Wu, C.J.; Yeh, J.H. Chitosan enhances platelet adhesion and aggregation. Biochem. Biophys. Res. Commun. 2003, 302, 480–483. [Google Scholar] [CrossRef]
- Sagnella, S.; Mai-Ngam, K. Chitosan based surfactant polymers designed to improve blood compatibility on biomaterials. Colloid. Surface. B. 2005, 42, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Sperling, C.; Fischer, M.; Maitz, M.F.; Werner, C. Blood coagulation on biomaterials requires the combination of distinct activation processes. Biomaterials 2009, 30, 4447–4456. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Gong, K.; Ao, Q.; Ma, T.; Yan, Y.; Gong, Y.; Zhang, X. Positive charge of chitosan retards blood coagulation on chitosan films. J. Biomater. Appl. 2013, 27, 1032–1045. [Google Scholar] [CrossRef] [PubMed]
- Pugnaloni, A.; Lai, G.; Ravaglia, F.; Santori, M.; Piciotti, D.; Sprovieri, G.; Muzzarelli, R.A.A.; Emanuelli, M.; Sapelli, P.; Baldassarre, V. Evaluation of chitosan effects on the hemostatic process. Clin. Lab. 1986, 10, 151–154. [Google Scholar]
- Fischer, T.H.; Connolly, R.; Thatte, H.S.; Schwaitzberg, S.S. Comparison of structural and hemostatic properties of the poly-N-acetyl glucosamine Syvek Patch with products containing chitosan. Microsc. Res. Tech. 2004, 63, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Dowling, M.B.; Kumar, R.; Keibler, M.A.; Hess, J.R.; Bochicchio, G.V.; Raghavan, S.R. A self-assembling hydrophobically modified chitosan capable of reversible hemostatic action. Biomaterials 2011, 32, 3351–3357. [Google Scholar] [CrossRef] [PubMed]
- Hardiansyah, A.; Tanadi, H.; Yang, M.C.; Liu, T.Y. Electrospinning and antibacterial activity of chitosan-blended poly (lactic acid) nanofibers. J. Polym. Res. 2015, 22, 1–10. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Morganti, P.; Morganti, G.; Palombo, P.; Palombo, M.; Biagini, G.; Belmonte, M.M.; Giantomassi, F.; Orlandi, F.; Muzzarelli, C. Chitin nanofibrils/chitosan glycolate composites as wound medicaments. Carbohydr. Polym. 2007, 70, 274–284. [Google Scholar] [CrossRef]
- Nandi, S.K.; Kundu, B.; Basu, D. Protein growth factors loaded highly porous chitosan scaffold: A comparison of bone healing properties. Mat. Sci. Eng. C 2013, 33, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cheng, F.; Gao, S.; Wu, Z.; Dong, L.; Lin, S.; Luo, Z.; Li, X. Preparation, characterization, antibacterial properties, and hemostatic evaluation of ibuprofen-loaded chitosan/gelatin composite films. J. Appl. Polym. Sci. 2017, 134, 1–9. [Google Scholar] [CrossRef]
- Lu, B.; Hu, S.; Zhu, X.; Zhang, L.T.; Zhang, Z.L. Experimental study of hemostasis effect of chitosan/sodium-alginate-yunnanbaiyao composite film. Health Res. 2011, 31, 94–96. [Google Scholar]
- Jia, T.B.; Chen, J.Y.; Feng, X.X. Fabrication and characterization of chitosan/mesoporous bioactive glasses porous films. J. Clin. Rehabilitative Tissue Eng. Res. 2011, 15, 7877–7880. [Google Scholar]
- Pourshahrestani, S.; Zeimaran, E.; Kadri, N.A.; Gargiulo, N.; Jindal, H.M.; Naveen, S.V.; Sekaran, S.D.; Kamarul, T.; Towler, M.R. Potency and cytotoxicity of a novel gallium-containing mesoporous bioactive glass/chitosan composite scaffold as hemostatic agents. ACS Appl. Mater. Inter. 2017, 9, 31381–31392. [Google Scholar] [CrossRef] [PubMed]
- Lan, G.; Lu, B.; Wang, T.; Wang, L.; Chen, J.; Yu, K.; Liu, J.; Dai, F.; Wu, D. Chitosan/gelatin composite sponge is an absorbable surgical hemostatic agent. Colloid. Surface. B. 2015, 136, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Lin, J.; Li, S.; Deng, Y.; Kong, S.; Hong, P.; Yang, P.; Liao, M.; Hu, Z. Preparation and evaluation of squid ink polysaccharide-chitosan as a wound-healing sponge. Mat. Sci. Eng. C 2018, 82, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sun, Y.L.; Chen, D.H. Effects of chitin and sepia ink hybrid hemostatic sponge on the blood parameters of mice. Mar. Drugs 2014, 12, 2269–2281. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Bi, S.; Yan, D.; Zhou, Z.; Sun, G.; Cheng, X.; Chen, X. Preparation of composite hydroxybutyl chitosan sponge and its role in promoting wound healing. Carbohydr. Res. 2018, 184, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Sukul, M.; Ventura, R.D.; Bae, S.H.; Choi, H.J.; Lee, S.Y.; Lee, B.T. Plant-derived oxidized nanofibrillar cellulose-chitosan composite as an absorbable hemostat. Mater. Lett. 2017, 197, 150–155. [Google Scholar] [CrossRef]
- Qian, Z.; Wang, H.; Tuo, X.; Guo, H.; Xu, P.; Liu, D.; Wei, Y.; Liu, H.; Fan, Y.; Guo, X. A porous sodium polyacrylate-grafted chitosan xerogel for severe hemorrhage control synthesized from one-pot reaction. J. Mater. Chem. B. 2017, 5, 4845–4851. [Google Scholar] [CrossRef]
- Yan, T.; Cheng, F.; Wei, X.; Huang, Y.; He, J. Biodegradable collagen sponge reinforced with chitosan/calcium pyrophosphate nanoflowers for rapid hemostasis. Carbohydr. Polym. 2017, 170, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Song, H.F.; Chen, A.Z.; Wang, S.B.; Kang, Y.Q.; Ye, S.F.; Liu, Y.G.; Wu, W.G. Preparation of chitosan-based hemostatic sponges by supercritical fluid technology. Materials 2014, 7, 2459–2473. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; Yuan, X.; Zhao, J.; Zhou, Y.; Bao, H. Rapidly in situ forming chitosan/epsilon-polylysine hydrogels for adhesive sealants and hemostatic materials. Carbohydr. Polym. 2013, 96, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Lee, Y.; Kong, W.H.; Kim, T.G.; Park, T.G.; Lee, H. Catechol-functionalized chitosan/pluronic hydrogels for tissue adhesives and hemostatic materials. Biomacromolecules 2011, 12, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Fan, D.; Cao, W.; Zhu, C.; Duan, Z.; Fu, R.; Li, X.; Ma, X. Preparation and characterization of breathable hemostatic hydrogel dressings and determination of their effects on full-thickness defects. Polymers 2017, 9, 727. [Google Scholar] [CrossRef]
- Fan, L.; Yang, H.; Yang, J.; Peng, M.; Hu, J. Preparation and characterization of chitosan/gelatin/PVA hydrogel for wound dressings. Carbohydr. Polym. 2016, 146, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Liu, C.; Wei, J.; Hong, H.; Zhao, Q. Molecular imprinted macroporous chitosan coated mesoporous silica xerogels for hemorrhage control. Biomaterials 2010, 31, 7620–7630. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Tang, Z.; Pan, M.; Wang, Z.; Yang, H.; Liu, H. Chitosan/kaolin composite porous microspheres with high hemostatic efficacy. Carbohydr. Polym. 2017, 177, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Fang, Q.; Ding, M.; Wu, J.; Ye, F.; Lv, Z.; Jin, J. Microspheres of carboxymethyl chitosan, sodium alginate and collagen for a novel hemostatic in vitro study. J. Biomater. Appl. 2016, 30, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, Y.; Wang, T.; Wu, J.; Zhai, X.; Li, Y.; Lu, W.W.; Pan, H.; Zhao, X. Chitosan-PVA monodisperse millimeter-sized spheres prepared by electrospraying reduce the thromboembolic risk in hemorrhage control. J. Mater. Chem. B 2017, 5, 3686–3696. [Google Scholar] [CrossRef]
- Gu, B.K.; Park, S.J.; Kim, M.S.; Lee, Y.J.; Kim, J.I.; Kim, C.H. Gelatin blending and sonication of chitosan nanofiber mats produce synergistic effects on hemostatic functions. Int. J. Biol. Macromol. 2016, 82, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Seon, G.M.; Lee, M.H.; Kwon, B.J.; Kim, M.S.; Koo, M.A.; Seomun, Y.; Kim, J.T.; Kim, T.H.; Park, J.C. Recombinant batroxobin-coated nonwoven chitosan as hemostatic dressing for initial hemorrhage control. Int. J. Biol. Macromol. 2018, 113, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.Y.; Chou, T.C.; Tsai, J.C.; Yang, H.C. Active ingredient-containing chitosan/polycaprolactone nonwoven mats: Characterizations and their functional assays. Mat. Sci. Eng. C 2013, 33, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Kyung, K.H.; Tsukada, K.; Kim, S.H.; Shiratori, S. Biodegradable polycaprolactone nanofibres with β-chitosan and calcium carbonate produce a hemostatic effect. Polymer 2017, 123, 194–202. [Google Scholar] [CrossRef]
- He, J.M.; Wu, Y.D.; Wang, F.W.; Cheng, W.L.; Huang, Y.D.; Fu, B. Hemostatic, antibacterial and degradable performance of the water-soluble chitosan-coated oxidized regenerated cellulose gauze. Fiber. Polym. 2014, 15, 504–509. [Google Scholar] [CrossRef]
- Cheng, F.; He, J.; Yan, T.; Liu, C.; Wei, X.; Li, J.; Huang, Y. Antibacterial and hemostatic composite gauze of N,O-carboxymethyl chitosan/oxidized regenerated cellulose. RSC Adv. 2016, 6, 94429–94436. [Google Scholar] [CrossRef]
- Feng, C.; Li, J.; Wu, G.S.; Mu, Y.Z.; Kong, M.; Jiang, C.Q.; Cheng, X.J.; Liu, Y.; Chen, X.G. Chitosan-coated diatom silica as hemostatic agent for hemorrhage control. ACS Appl. Mater. Inter. 2016, 8, 34234–34243. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.Y.; Wu, J.; Moochhala, S.M.; Tan, M.H.; Lu, J. Development of a chitosan-based wound dressing with improved hemostatic and antimicrobial properties. Biomaterials 2008, 29, 4323–4332. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Y.; Wang, F.; Meng, W.; Yang, X.; Li, P.; Jiang, J.; Tan, H.; Zheng, Y. Preparation of porous carboxymethyl chitosan grafted poly (acrylic acid) superabsorbent by solvent precipitation and its application as a hemostatic wound dressing. Mat. Sci. Eng. C 2016, 63, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Abdelgawad, A.M.; Hudson, S.M.; Rojas, O.J. Antimicrobial wound dressing nanofiber mats from multicomponent (chitosan/silver-NPs/polyvinyl alcohol) systems. Carbohydr. Polym. 2014, 100, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.W.; Kim, C.H.; Wang, X.; Pun, S.H.; White, N.J.; Kim, T.H. PolySTAT-modified chitosan gauzes for improved hemostasis in external hemorrhage. Acta Biomater. 2016, 31, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Dai, N.T.; Fu, K.Y.; Hsieh, P.S.; Hung, Y.M.; Fang, Y.L.; Huang, N.C.; Lu, T.W.; Dai, L.G.; Chen, S.G.; Chen, T.M. A biodegradable hemostatic gelatin/polycaprolactone composite for surgical hemostasis. Ann. Plast. Surg. 2017, 78, S124–S128. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.K.; Park, S.J.; Kim, M.S.; Kang, C.M.; Kim, J.I.; Kim, C.H. Fabrication of sonicated chitosan nanofiber mat with enlarged porosity for use as hemostatic materials. Carbohydr. Polym. 2013, 97, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, Y.; Chen, Y.; Zhao, Y.; Zhang, Q.; Zheng, X.; Chen, L.; Zhang, L. Strong and rapidly self-healing hydrogels: Potential hemostatic materials. Adv. Healthc. Mater. 2016, 5, 2813–2822. [Google Scholar] [CrossRef] [PubMed]
- Pinkas, O.; Zilberman, M. Novel gelatin-alginate surgical sealants loaded with hemostatic agents. Int. J. Polym. Mater. 2017, 66, 378–387. [Google Scholar] [CrossRef]
- Pinkas, O.; Zilberman, M. Effect of hemostatic agents on properties of gelatin-alginate soft tissue adhesives. J. Biomat. Sci-polym. E. 2014, 25, 555–573. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.J.; Rui, R.C.; Mano, J.F. Marine origin polysaccharides in drug delivery systems. Mar. Drugs 2016, 14, 34. [Google Scholar] [CrossRef] [PubMed]
- Ladas, E.J.; Karlik, J.B.; Rooney, D.; Taromina, K.; Ndao, D.H.; Granowetter, L.; Kelly, K.M. Topical Yunnan Baiyao administration as an adjunctive therapy for bleeding complications in adolescents with advanced cancer. Support. Care Cancer 2012, 20, 3379–3383. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Liang, Y.; Hao, H.; Zheng, X.; Xie, L.; Guan, T.; Zhou, Y.; Wang, G. Global detection and identification of components from Yunnan Baiyao based on liquid chromatography hybrid ion trap time-of-flight mass spectrometry. J. Sep. Sci. 2013, 36, 1935–1944. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Chang, J. Preparation, in vitro bioactivity and drug release property of well-ordered mesoporous 58S bioactive glass. J. Non-Cryst. Solids 2008, 354, 1338–1341. [Google Scholar] [CrossRef]
- Dai, C.; Yuan, Y.; Liu, C.; Wei, J.; Hong, H.; Li, X.; Pan, X. Degradable, antibacterial silver exchanged mesoporous silica spheres for hemorrhage control. Biomaterials 2009, 30, 5364–5375. [Google Scholar] [CrossRef] [PubMed]
- Ostomel, T.A.; Shi, Q.; Tsung, C.K.; Liang, H.; Stucky, G.D. Spherical bioactive glass with enhanced rates of hydroxyapatite deposition and hemostatic activity. Small 2006, 2, 1261–1265. [Google Scholar] [CrossRef] [PubMed]
- Shruti, S.; Salinas, A.J.; Lusvardi, G.; Malavasi, G.; Menabue, L.; Vallet-Regi, M. Mesoporous bioactive scaffolds prepared with cerium-, gallium- and zinc-containing glasses. Acta Biomater. 2013, 9, 4836–4844. [Google Scholar] [CrossRef] [PubMed]
- Pourshahrestani, S.; Zeimaran, E.; Kadri, N.A.; Gargiulo, N.; Samuel, S.; Naveen, S.V.; Kamarul, T.; Towler, M.R. Gallium-containing mesoporous bioactive glass with potent hemostatic activity and antibacterial efficacy. J. Mater. Chem. B. 2016, 4, 71–86. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, Y.; Fan, D.; Zhu, C.; Liu, L.; Duan, Z. A novel human-like collagen hemostatic sponge with uniform morphology, good biodegradability and biocompatibility. J. Biomater. Appl. 2017, 31, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.; Valdés, A.; Beltrán, A.; Garrigós, M.C. Gelatin-based films and coatings for food packaging applications. Coatings 2016, 6, 41. [Google Scholar] [CrossRef]
- Rose, J.B.; Pacelli, S.; Haj, A.J.E.; Dua, H.S.; Hopkinson, A.; White, L.J.; Rose, F.R.A.J. Gelatin-based materials in ocular tissue engineering. Materials 2014, 7, 3106–3135. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Miyoshi, T.; Boki, K. Study on hydrophilic properties of gelatin as a clinical wound dressing. I. Hydrophilic properties of gelatin as a wound dressing. Tokushima J. Exp. Med. 1993, 40, 159–167. [Google Scholar] [PubMed]
- Babaei, Z.; Jahanshahi, M.; Rabiee, S.M. The fabrication of nanocomposites via calcium phosphate formation on gelatin-chitosan network and the gelatin influence on the properties of biphasic composites. Mat. Sci. Eng. C 2013, 33, 370–375. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Li, D.; Liu, Y.; Yao, B.; Zhan, H.; Lian, Q.; Lu, B.; Lv, Y. Biomedical materials preparation of chitosan-gelatin hybrid scaffolds with well-organized microstructures for hepatic tissue engineering. Acta Biomater. 2009, 5, 453–461. [Google Scholar]
- Yang, C.; Xu, L.; Zhou, Y.; Zhang, X.; Huang, X.; Wang, M.; Han, Y.; Zhai, M.; Wei, S.; Li, J. A green fabrication approach of gelatin/CM-chitosan hybrid hydrogel for wound healing. Carbohydr. Polym. 2010, 82, 1297–1305. [Google Scholar] [CrossRef]
- Li, F.; Luo, P.; Liu, H. A potential adjuvant agent of chemotherapy: Sepia ink polysaccharides. Mar. Drugs 2018, 16, 106. [Google Scholar]
- Wang, Q.Q.; Kong, M.; An, Y.; Liu, Y.; Li, J.J.; Zhou, X.; Feng, C.; Li, J.; Jiang, S.Y.; Cheng, X.J. Hydroxybutyl chitosan thermo-sensitive hydrogel: A potential drug delivery system. J. Mater. Sci. 2013, 48, 5614–5623. [Google Scholar] [CrossRef]
- Kalia, S.; Dufresne, A.; Cherian, B.M.; Kaith, B.S.; Averous, L.; Njuguna, J.; Nassiopoulos, E. Cellulose-based bio- and nanocomposites: A. Review. Int. J. Polym. Sci. 2011, 2011, 2341–2348. [Google Scholar] [CrossRef]
- Wu, Y.; He, J.; Cheng, W.; Gu, H.; Guo, Z.; Gao, S.; Huang, Y. Oxidized regenerated cellulose-based hemostat with microscopically gradient structure. Carbohydr. Polym. 2012, 88, 1023–1032. [Google Scholar] [CrossRef]
- Kumari, S.; Singh, R.P. Glycolic acid-g-chitosan-gold nanoflower nanocomposite scaffolds for drug delivery and tissue engineering. Int. J. Biol. Macromol. 2012, 50, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.A.; Husain, Q. Potential applications of enzymes immobilized on/in nano materials: A review. Biotechnol. Adv. 2012, 30, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; He, Y. Fast detection of sodium tripolyphosphate using gold nanoflowers coated with luminol and chitosan as a chemiluminescent probe. Microchim. Acta 2017, 184, 563–568. [Google Scholar] [CrossRef]
- Huang, H.; Liu, M.; Wang, X.; Zhang, W.; Yang, D.P.; Cui, L.; Wang, X. Label-free 3D Ag nanoflower-based electrochemical immunosensor for the detection of Escherichia coli O157:H7 pathogens. Nanoscale Res. Lett. 2016, 11, 507. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shi, J.; Li, Z.; Zhang, S.; Wu, H.; Jiang, Z.; Yang, C.; Tian, C. Facile one-pot preparation of chitosan/calcium pyrophosphate hybrid microflowers. Acs Appl. Mater. Inter. 2014, 6, 14522–14532. [Google Scholar] [CrossRef] [PubMed]
- Kankala, R.K.; Zhang, Y.S.; Wang, S.B.; Lee, C.H.; Chen, A.Z. Supercritical fluid technology: An emphasis on drug delivery and related biomedical applications. Adv. Healthc. Mater. 2017, 6, 1700433. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, J.; Hu, X.; Liang, J.; Fan, Y.; Zhang, X. Superabsorbent polysaccharide hydrogels based on pullulan derivate as antibacterial release wound dressing. J. Biomed. Mater. Res. A 2011, 98A, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Ba, Q.; Niu, S.; Guo, Y.; Duan, Y.; Zhao, P.; Lin, C.; Sun, J. In-situ forming biodegradable glycol chitosan-based hydrogels: Synthesis, characterization, and chondrocyte culture. Mat. Sci. Eng. C 2012, 32, 2017–2025. [Google Scholar] [CrossRef]
- Pritchard, C.D.; O'Shea, T.M.; Siegwart, D.J.; Calo, E.; Anderson, D.G.; Reynolds, F.M.; Thomas, J.A.; Slotkin, J.R.; Woodard, E.J.; Langer, R. An injectable thiol-acrylate poly(ethylene glycol) hydrogel for sustained release of methylprednisolone sodium succinate. Biomaterials 2011, 32, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Nakanishi, K.; Ono, K.; Sato, M.; Kikuchi, M.; Saito, Y.; Yura, H.; Matsui, T.; Hattori, H.; Uenoyama, M.; et al. Photocrosslinkable chitosan as a dressing for wound occlusion and accelerator in healing process. Biomaterials 2002, 23, 833–840. [Google Scholar] [CrossRef]
- Otani, Y.; Tabata, Y.; Ikada, Y. Hemostatic capability of rapidly curable glues from gelatin, poly (L-glutamic acid), and carbodiimide. Biomaterials 1998, 19, 2091–2098. [Google Scholar] [CrossRef]
- Song, X.; Zhu, C.; Fan, D.; Mi, Y.; Li, X.; Fu, R.; Duan, Z.; Wang, Y.; Feng, R. A novel human-like collagen hydrogel scaffold with porous structure and sponge-like properties. Polymers 2017, 9, 638. [Google Scholar] [CrossRef]
- Lih, E.; Lee, J.S.; Park, K.M.; Park, K.D. Rapidly curable chitosan-PEG hydrogels as tissue adhesives for hemostasis and wound healing. Acta Biomater. 2012, 8, 3261–3269. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, X.; Wu, Y.; Tang, Z.; Sun, X.; Pan, M.; Chen, Y.; Li, J.; Xiao, R.; Wang, Z.; et al. Porous chitosan microspheres for application as quick in vitro and in vivo hemostat. Mat. Sci. Eng. C 2017, 77, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Rhee, P.; Brown, C.; Martin, M.; Salim, A.; Plurad, D.; Green, D.; Chambers, L.; Demetriades, D.; Velmahos, G.; Alam, H. QuikClot use in trauma for hemorrhage control: Case series of 103 documented uses. J. Trauma. 2008, 64, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.Y.; Wang, C.W.; Niu, H.Y.; Duan, B.; Ma, X.Y.; Hong, H.; Yuan, Y.; Liu, C.S. A novel droplet-fabricated mesoporous silica-based nanohybrid granules for hemorrhage control. J. Biomed. Nanotechnol. 2018, 14, 649–661. [Google Scholar]
- Li, X.S.; Liu, C.S.; Yuan, Y.; Wang, L.J.; Wang, Q.Y. Preparation and hemostatic properties of mesoporous silica-based xerogels. J. Inorg. Mater. 2008, 23, 327–331. [Google Scholar] [CrossRef]
- Hossain, K.M.Z.; Patel, U.; Ahmed, I. Development of microspheres for biomedical applications: A review. Prog. Biomater. 2015, 4, 1–19. [Google Scholar] [CrossRef] [PubMed]
- DeFrates, K.G.; Moore, R.; Borgesi, J.; Lin, G.; Mulderig, T.; Beachley, V.; Hu, X. Protein-based fiber materials in medicine: A review. Nanomaterials 2018, 8, 457. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Lv, Y. Application of collagen scaffold in tissue engineering: Recent advances and new perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef]
- Lewis, K.M.; Spazierer, D.; Slezak, P.; Baumgartner, B.; Regenbogen, J.; Gulle, H. Swelling, sealing, and hemostatic ability of a novel biomaterial: A polyethylene glycol-coated collagen pad. J. Biomater. Appl. 2014, 29, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Han, S.S. PVA-based hydrogels for tissue engineering: A review. Int. J. Polym. Mater. 2017, 66, 159–182. [Google Scholar] [CrossRef]
- Yuvaraja, G.; Pathak, J.L.; Zhang, W.; Zhang, Y.; Xu, J. Antibacterial and wound healing properties of chitosan/poly (vinyl alcohol)/zinc oxide beads (CS/PVA/ZnO). Int. J. Biol. Macromol. 2017, 103, 234–241. [Google Scholar]
- Cho, S.Y.; Park, H.H.; Jin, H.J. Controlling pore size of electrospun silk fibroin scaffold for tissue engineering. Polym. Korea 2012, 36, 651–655. [Google Scholar] [CrossRef]
- Dzenis, Y.A. Spinning continues fibers for nanotechnology. Science 2004, 304, 1917–1919. [Google Scholar] [CrossRef] [PubMed]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Yan, D.; Hu, S.; Zhou, Z.; Zeenat, S.; Cheng, F.; Li, Y.; Feng, C.; Cheng, X.; Chen, X. Different chemical groups modification on the surface of chitosan nonwoven dressing and the hemostatic properties. Int. J. Biol. Macromol. 2018, 107, 463–469. [Google Scholar] [CrossRef] [PubMed]
- You, W.K.; Choi, W.S.; Koh, Y.S.; Shin, H.C.; Jang, Y.; Chung, K.H. Functional characterization of recombinant batroxobin, a snake venom thrombin-like enzyme, expressed from Pichia pastoris. FEBS Lett. 2004, 571, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- He, J.; Wang, F.; Wu, Y.; Huang, Y.; Zhang, H. Preparation of the water-soluble chitosan-coated oxidized regenerated cellulose gauze. Cellulose 2011, 18, 1651–1659. [Google Scholar] [CrossRef]
- Baker, S.E.; Sawvel, A.M.; Fan, J.; Shi, Q.; Strandwitz, N.; Stucky, G.D. Blood clot initiation by mesocellular foams: Dependence on nanopore size and enzyme immobilization. Langmuir 2008, 24, 14254–14260. [Google Scholar] [CrossRef] [PubMed]
- Gordon, R.; Losic, D.; Tiffany, M.A.; Nagy, S.S.; Sterrenburg, F.A.S. The glass menagerie: Diatoms for novel applications in nanotechnology. Trends Biotechnol. 2009, 27, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Leonardo, S.; Prieto-Simon, B.; Campas, M. Past, present and future of diatoms in biosensing. Trend. Anal Chem. 2016, 79, 276–285. [Google Scholar] [CrossRef]
- Cicco, S.R.; Vona, D.; De Giglio, E.; Cometa, S.; Mattioli-Belmonte, M.; Palumbo, F.; Ragni, R.; Farinola, G.M. Chemically modified diatoms biosilica for bone cell growth with combined drug-delivery and antioxidant properties. Chempluschem 2015, 80, 1104–1112. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.F.; Tan, H.M. Hydroxyethyl chitosan-g-poly (acrylic acid-co-sodium acrylate) superabsorbent polymers. J. Appl. Polym. Sci. 2010, 117, 2233–2240. [Google Scholar]
- Chen, Y.; Liu, Y.F.; Tan, H.M.; Jiang, J.X. Synthesis and characterization of a novel superabsorbent polymer of N,O-carboxymethyl chitosan graft copolymerized with vinyl monomers. Carbohydr. Polym. 2009, 75, 287–292. [Google Scholar] [CrossRef]
- Chen, Y.; Tan, H.M. Crosslinked carboxymethylchitosan-g-poly (acrylic acid) copolymer as a novel superabsorbent polymer. Carbohydr. Res. 2006, 341, 887–896. [Google Scholar] [CrossRef] [PubMed]
| Materials | Major components | Characteristics | Ref. |
|---|---|---|---|
| Films | CS, gelatin (GE), ibuprofen | High swelling degree, antibacterial activity, no obvious cytotoxicity and haemorrhage reducing. | [28] |
| CS, alginate (AG), Yunnan Baiyao | Good hemostatic performance and producing synergetic effects. | [29] | |
| CS, mesoporous bioactive glass (MBG) | High porosity, good degradability, biocompatibility, water adsorption and non-cytotoxicity. | [30] | |
| CS, Ga-MBG | High hemostatic efficacy, facilitating cell proliferation and excellent antibacterial activity. | [31] | |
| Sponges | CS, GE | Promoting cell proliferation, no significant cytotoxicity, obvious bacteriostatic effects and good biodegradability. | [32] |
| CS, squid ink polysaccharide (SIP) | Strong absorptivity, significant procoagulant effects and promoting wound healing. | [33] | |
| Chitin, SIP | No significant effects on the blood parameters including coagulation, anticoagulation, fibrinolytic and hemorheology. | [34] | |
| CS, hydroxybutyl chitosan | High porosity, great water absorption, no cytotoxicity excellent antibacterial properties, and making the blood form viscous gels which were conducive to promoting blood coagulation. | [35] | |
| CS, oxidized nanofibrillar cellulose | Superior biocompatibility and biodegradability, fast absorption of blood and non-cytotoxicity. | [36] | |
| CS, sodium polyacrylate (SPA), polyethylene glycol | Good water absorbency, superior hemostatic effects, wound sealing and external pressure application. | [37] | |
| CS, calcium pyrophosphate, Col | Rapid water absorption ability, high specific surface area, activating the intrinsic pathway of coagulation cascade, and complete biodegradation in three weeks. | [38] | |
| CS, poly-(methyl vinyl ether-co-maleic anhydride) | Good porosity, and strong clotting ability. | [39] | |
| Hydrogels | Thiol functionalized chitosan, maleimide group modified ε-polylysine | Non-toxicity, excellent hemostatic property, and high adhesion strength. | [40] |
| Catechol-functionalized chitosan, thiol-terminated Pluronic | Superior mechanical performance and stability, strong adhesiveness, excellent hemostatic properties, injectable and thermosensitive properties | [41] | |
| Poly (vinyl alcohol) (PVA), human-like collagen, carboxymethyl chitosan (CMCS) | Good swelling ability, hemostatic and bacterial barrier activities, biocompatibility and wound healing. | [42] | |
| CS, GE, PVA | Good pH-sensitivity, swelling ability, water evaporation rate and adhesion. | [43] | |
| Particles | CS, mesoporous silica xerogels | Promoting the cell proliferation, no cytotoxicity; great capacity for water absorption, and accelerating the contact activation pathway of coagulation cascade. | [44] |
| CS, Kaolin clay | High amount of pores, no adverse effects, and the synergetic combination mechanisms. | [45] | |
| CMCS, AG, Col | Facilitating platelet adherence, aggregation and activation, high water absorption ability, good biodegradability and non-cytotoxicity. | [46] | |
| CS, PVA | Significant reduction of the hemostatic time and blood loss, narrow size distribution and good biocompatibility. | [47] | |
| Fibers | CS, GE | High porosity and wettability, rapid blood absorption and effective blood clotting. | [48] |
| CS, recombinant batroxobin | Facilitating erythrocyte aggregation, fibrin clot formation and blood coagulation. | [49] | |
| CS, polycaprolactone (PCL) | Possessing multiple pharmacological effects, such as platelet aggregation, anti-bacterial, anti-adhesive and anti-inflammatory activities | [50] | |
| CS, PCL, CaCO3 | High-performance blood coagulation. | [51] | |
| CS, oxidized regenerated cellulose (ORC) | Good antibacterial and degradable properties, forming a gel by absorbing blood and then sealing off the crevasses of the blood vessels to stop bleeding. | [52] | |
| CMCS, ORC | Excellent hemostatic effects and preventing post-operative adhesion. | [53] | |
| Others | CS, natural diatom silica | Favorable biocompatibility, great fluid absorbability, no cytotoxicity and desirable hemostasis effects. | [54] |
| CS, polyphosphate | Strong antibacterial activity, accelerating blood clotting, platelet adhesion and thrombin generation, and decreasing the mortality rate in a wound model. | [55] | |
| CMCS, poly (acrylic acid) | Excellent hemostatic performance, good swelling capacity and non-cytotoxic. | [56] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Z.; Zhang, D.-Y.; Lu, S.-T.; Li, P.-W.; Li, S.-D. Chitosan-Based Composite Materials for Prospective Hemostatic Applications. Mar. Drugs 2018, 16, 273. https://doi.org/10.3390/md16080273
Hu Z, Zhang D-Y, Lu S-T, Li P-W, Li S-D. Chitosan-Based Composite Materials for Prospective Hemostatic Applications. Marine Drugs. 2018; 16(8):273. https://doi.org/10.3390/md16080273
Chicago/Turabian StyleHu, Zhang, Dong-Ying Zhang, Si-Tong Lu, Pu-Wang Li, and Si-Dong Li. 2018. "Chitosan-Based Composite Materials for Prospective Hemostatic Applications" Marine Drugs 16, no. 8: 273. https://doi.org/10.3390/md16080273
APA StyleHu, Z., Zhang, D.-Y., Lu, S.-T., Li, P.-W., & Li, S.-D. (2018). Chitosan-Based Composite Materials for Prospective Hemostatic Applications. Marine Drugs, 16(8), 273. https://doi.org/10.3390/md16080273





