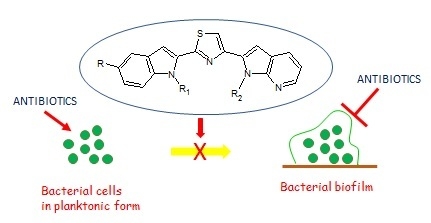
New Thiazole Nortopsentin Analogues Inhibit Bacterial Biofilm Formation
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biology
3. Materials and Methods
3.1. Chemistry
3.1.1. General
3.1.2. General Procedure for the Synthesis of 1H-Indole-3-carbonitriles (4a–d)
3.1.3. General Procedure for the Synthesis of Tert-Butyl [2-(3-cyano-1H-indol-1-yl)ethyl]carbamates (5a–d)
3.1.4. General Procedure for the Synthesis of Tert-Butyl [2-(3-carbamothioyl-1H-indol-1-yl)ethyl]carbamate (6a–d)
3.1.5. General Procedure for the Synthesis of Thiazoles (1a–h)
3.1.6. General Procedure for the Synthesis of Thiazoles (1i–p)
3.2. Biology
3.2.1. MICs Determination
3.2.2. Inhibition of Biofilm Formation (Crystal Violet Method).
3.2.3. Antibiofilm Activity (Crystal Violet Method)
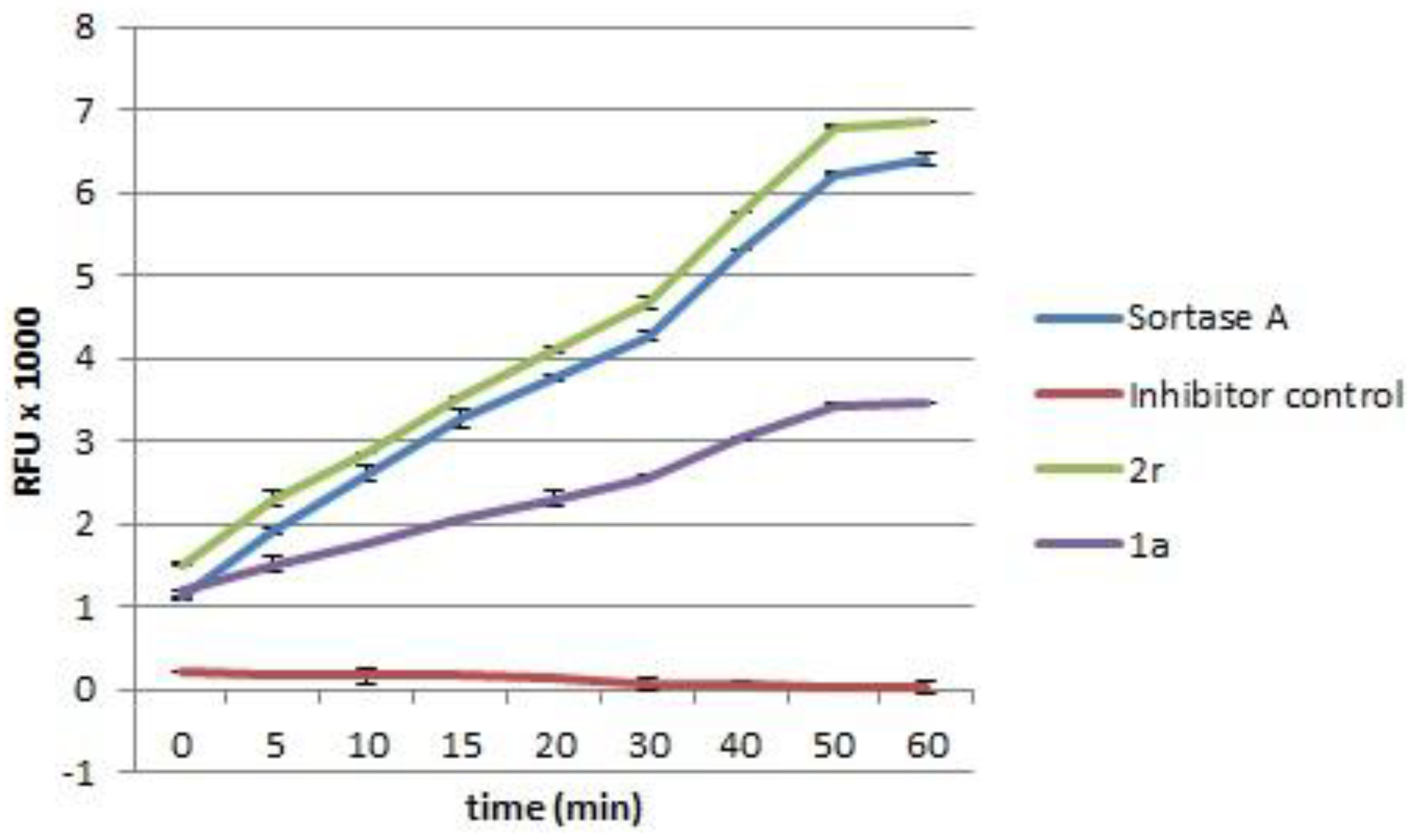
3.2.4. Screening as Sortase A (SrtA) Inhibitors
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Schillaci, D.; Spanò, V.; Parrino, B.; Carbone, A.; Montalbano, A.; Barraja, P.; Diana, P.; Cirrincione, G.; Cascioferro, S. Pharmaceutical approaches to target antibiotic resistance mechanisms. J. Med. Chem. 2017, 80, 8268–8297. [Google Scholar] [CrossRef] [PubMed]
- RömLing, U.; Balsalobre, C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J. Intern. Med. 2012, 272, 541–561. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef] [PubMed]
- Schillaci, D.; Spinello, A.; Cusimano, M.G.; Cascioferro, S.; Barone, G.; Vitale, M.; Arizza, V. A peptide from human β thymosin as a platform for the development of new anti-biofilm agents for Staphylococcus spp. and Pseudomonas aeruginosa. World J. Microbiol. Biotechnol. 2016, 32, 124. [Google Scholar] [CrossRef] [PubMed]
- Cascioferro, S.; Maggio, B.; Raffa, D.; Raimondi, M.V.; Cusimano, M.G.; Schillaci, D.; Manachini, B.; Leonchiks, A.; Daidone, G. A new class of phenylhydrazinylidene derivatives as inhibitors of Staphylococcus aureus biofilm formation. Med. Chem. Res. 2016, 25, 870–878. [Google Scholar] [CrossRef]
- Cascioferro, S.; Maggio, B.; Raffa, D.; Raimondi, M.V.; Cusimano, M.G.; Schillaci, D.; Manachini, B.; Plescia, F.; Daidone, G. Synthesis and biofilm formation reduction of pyrazole-4-carboxamide derivatives in some Staphylococcus aureus strains. Eur. J. Med. Chem. 2016, 123, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef] [PubMed]
- Rane, R.A.; Karpoormath, R.; Naphade, S.S.; Bangalore, P.; Shaikh, M.; Hampannavar, G. Novel synthetic organic compounds inspired from antifeedant marine alkaloids as potent bacterial biofilm inhibitors. Bioorg. Chem. 2015, 61, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Hodnik, Z.; Los, J.M.; Zula, A.; Zidar, N.; Jakopin, Z.; Los, M.; Dolenc, M.S.; Ilas, J.; Wegrzyn, G.; Masic, L.P.; et al. Inhibition of biofilm formation by conformationally constrained indole-based analogues of the marine oroidin. Bioorg. Med. Chem. Lett. 2014, 24, 2530–2534. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.H.; Chung, S.-C.; Shin, J.; Lee, S.-H.; Kim, T.-I.; Lee, H.-S.; Oh, K.-B. Aaptamines as sortase A inhibitors from the tropical sponge Aaptos aaptos. Bioorg. Med. Chem. Lett. 2005, 15, 4927–4931. [Google Scholar] [CrossRef] [PubMed]
- Cascioferro, S.; Totsika, M.; Schillaci, D. Sortase A: An Ideal Target for Anti-Virulence Drug Development. Microb. Pathog. 2014, 77C, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Cascioferro, S.; Raffa, D.; Maggio, B.; Raimondi, M.V.; Schillaci, D.; Daidone, G. Sortase A Inhibitors: Recent Advances and Future Perspectives. J. Med. Chem. 2015, 58, 9108–9123. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.-B.; Mar, W.; Kim, S.; Kim, J.-Y.; Oh, M.-N.; Kim, J.-G.; Shin, D.; Sim, C.J.; Shin, J. Bis(indole)alkaloids as sortase A inhibitors from the sponge Spongosorites sp. Bioorg. Med. Chem. Lett. 2005, 15, 4927–4931. [Google Scholar] [CrossRef] [PubMed]
- Montalbano, A.; Parrino, B.; Diana, P.; Barraja, P.; Carbone, A.; Spanò, V.; Cirrincione, G. Synthesis of the new oligopeptide pyrrole derivative isonetropsin and its one pyrrole unit analogue. Tetrahedron 2013, 69, 2550–2554. [Google Scholar] [CrossRef]
- Barraja, P.; Caracausi, L.; Diana, P.; Spanò, V.; Montalbano, A.; Carbone, A.; Parrino, B.; Cirrincione, G. Synthesis and Antiproliferative Activity of the Ring System [1,2]Oxazolo[4,5-g]indole. ChemMedChem 2012, 7, 1901–1904. [Google Scholar] [CrossRef] [PubMed]
- Parrino, B.; Ullo, S.; Attanzio, A.; Spanò, V.; Cascioferro, S.; Montalbano, A.; Barraja, P.; Tesoriere, L.; Diana, P.; Cirrincione, G. New tripentone analogs with antiproliferative activity. Molecules 2017, 22, 1–13. [Google Scholar]
- Diana, P.; Stagno, A.; Barraja, P.; Montalbano, A.; Carbone, A.; Parrino, B.; Cirrincione, G. Synthesis of the new ring system pyrrolizino[2,3-b]indol-4(5H)-one. Tetrahedron 2011, 67, 3374–3379. [Google Scholar] [CrossRef]
- Barraja, P.; Spanò, V.; Giallombardo, D.; Diana, P.; Montalbano, A.; Carbone, A.; Parrino, B.; Cirrincione, G. Synthesis of [1,2]oxazolo[5,4-e]indazoles as antitumour agents. Tetrahedron 2013, 69, 6474–6477. [Google Scholar] [CrossRef]
- Spanò, V.; Montalbano, A.; Carbone, A.; Parrino, B.; Diana, P.; Cirrincione, G.; Castagliuolo, I.; Brun, P.; Issinger, O.-G.; Tisi, S.; et al. Synthesis of a new class of pyrrolo[3,4-h]quinazolines with antimitotic activity. Eur. J. Med. Chem. 2014, 74, 340–357. [Google Scholar]
- Parrino, B.; Carbone, A.; Muscarella, M.; Spanò, V.; Montalbano, A.; Barraja, P.; Salvador, A.; Vedaldi, D.; Cirrincione, G.; Diana, P. 11H-Pyrido[3′,2′:4,5]pyrrolo[3,2-c]cinnoline and pyrido[3′,2′:4,5]pyrrolo[1,2-c][1,2,3]benzotriazine: Two new ring systems with antitumor activity. J. Med. Chem. 2014, 57, 9495–9511. [Google Scholar] [CrossRef] [PubMed]
- Parrino, B.; Carbone, A.; Ciancimino, C.; Spanò, V.; Montalbano, A.; Barraja, P.; Cirrincione, G.; Diana, P.; Sissi, C.; Palumbo, M.; et al. Water-soluble isoindolo[2,1-a]quinoxalin-6-imines: In vitro antiproliferative activity and molecular mechanism(s) of action. Eur. J. Med. Chem. 2015, 94, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Parrino, B.; Carbone, A.; Spanò, V.; Montalbano, A.; Giallombardo, D.; Barraja, P.; Attanzio, A.; Tesoriere, L.; Palumbo, M.; Sissi, C.; et al. Aza-isoindolo and isoindolo-azaquinoxaline derivatives with antiproliferative activity. Eur. J. Med. Chem. 2015, 94, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Spanò, V.; Parrino, B.; Carbone, A.; Montalbano, A.; Salvador, A.; Brun, P.; Vedaldi, D.; Diana, P.; Cirrincione, G.; Barraja, P. Pyrazolo[3,4-h]quinolines promising photosensitizing agents in the treatment of cancer. Eur. J. Med. Chem. 2015, 102, 334–351. [Google Scholar] [CrossRef] [PubMed]
- Diana, P.; Stagno, A.; Barraja, P.; Carbone, A.; Parrino, B.; Dall’Acqua, F.; Vedaldi, D.; Salvador, A.; Brun, P.; Castagliuolo, I.; et al. Synthesis of triazeno-azaindoles a new class of triazenes with antitumor activity. ChemMedChem 2011, 6, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Spanò, V.; Pennati, M.; Parrino, B.; Carbone, A.; Montalbano, A.; Cilibrasi, V.; Zuco, V.; Lopergolo, A.; Cominetti, D.; Diana, P.; et al. Preclinical activity of new [1,2]oxazolo[5,4-e]isoindole derivatives in diffuse malignant peritoneal mesothelioma. J. Med. Chem. 2016, 59, 7223–7238. [Google Scholar]
- Barraja, P.; Diana, P.; Spanò, V.; Montalbano, A.; Carbone, A.; Parrino, B.; Cirrincione, G. An efficient synthesis of pyrrolo[3′,2′:4,5]thiopyrano[3,2-b]pyridin-2-one: A new ring system of pharmaceutical interest. Tetrahedron 2012, 68, 5087–5094. [Google Scholar] [CrossRef]
- Spanò, V.; Frasson, I.; Giallombardo, D.; Doria, F.; Parrino, B.; Carbone, A.; Montalbano, A.; Nadai, M.; Diana, P.; Cirrincione, G.; et al. Synthesis and antiproliferative mechanism of action of pyrrole[3′,2′:6,7]cyclohepta[1,2-d]pyrimidin-2-amines as singlet oxygen photosensitizers. Eur. J. Med. Chem. 2016, 123, 447–461. [Google Scholar]
- Parrino, B.; Ciancimino, C.; Carbone, A.; Spanò, V.; Montalbano, A.; Barraja, P.; Cirrincione, G.; Diana, P. Synthesis of isoindolo[1,4]benzoxazinone and isoindolo[1,5]benzoxazepine: Two new ring systems of pharmaceutical interest. Tetrahedron 2015, 71, 7332–7338. [Google Scholar] [CrossRef]
- Spanò, V.; Pennati, M.; Parrino, B.; Carbone, A.; Montalbano, A.; Lopergolo, A.; Zuco, V.; Cominetti, D.; Diana, P.; Cirrincione, G.; et al. [1,2]oxazolo[5,4-e]isoindoles as promising tubulin polymerization inhibitors. Eur. J. Med. Chem. 2016, 124, 840–851. [Google Scholar]
- Spanò, V.; Montalbano, A.; Carbone, A.; Parrino, B.; Barraja, P.; Diana, P.; Cirrincione, G. Convenient synthesis of pyrrolo[3,4-g]indazole. Tetrahedron 2013, 69, 9839–9847. [Google Scholar] [CrossRef]
- Spanò, V.; Giallombardo, D.; Cilibrasi, V.; Parrino, B.; Carbone, A.; Montalbano, A.; Frasson, I.; Salvador, A.; Richter, S.N.; Doria, F.; et al. Pyrrolo[3’,2’:6,7]cyclohepta[1,2-b]pyridines with potent photo-antiproliferative activity. Eur. J. Med. Chem. 2017, 128, 300–318. [Google Scholar]
- Carbone, A.; Parrino, B.; Barraja, P.; Spanò, V.; Cirrincione, G.; Diana, P.; Maier, A.; Kelter, G.; Fiebig, H.-H. Synthesis and antiproliferative activity of 2,5-bis(3′-indolyl)pyrroles, analogues of the marine alkaloid Nortopsentin. Mar. Drugs 2013, 11, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Pennati, M.; Barraja, P.; Montalbano, A.; Parrino, B.; Spanò, V.; Lopergolo, A.; Sbarra, S.; Doldi, V.; Zaffaroni, N.; et al. Synthesis and antiproliferative activity of substituted 3[2-(1H-indol-3-yl)-1,3-thiazol-4-yl]-1H-pyrrolo[3,2-b]piridine, marine alkaloid nortopsentin analogues. Curr. Med. Chem. 2014, 21, 1654–1666. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Parrino, B.; Di Vita, G.; Attanzio, A.; Spanò, V.; Montalbano, A.; Barraja, P.; Tesoriere, L.; Livrea, M.A.; Diana, P.; Cirrincione, G. Synthesis and antiproliferative activity of thiazolyl-bis-pyrrolo[2,3-b]pyridines and indolyl-thiazolyl-pyrrolo[2,3-c]pyridines, nortopsentin analogues. Mar. Drugs 2015, 13, 460–492. [Google Scholar] [CrossRef] [PubMed]
- Parrino, B.; Carbone, A.; Di Vita, G.; Ciancimino, C.; Attanzio, A.; Spanò, V.; Montalbano, A.; Barraja, P.; Tesoriere, L.; Livrea, M.A.; et al. 3-[4-(1H-Indol-3-yl)-1,3-thiazol-2-yl]-1H-pyrrolo[2,3-b]pyridines, nortopsentin analogues with antiproliferative activity. Mar. Drugs 2015, 13, 1901–1924. [Google Scholar] [CrossRef] [PubMed]
- Spanò, V.; Attanzio, A.; Cascioferro, S.; Carbone, A.; Montalbano, A.; Barraja, P.; Tesoriere, L.; Cirrincione, G.; Diana, P.; Parrino, B. Synthesis and antitumor activity of new thiazole nortopsentin analogs. Mar. Drugs 2016, 14, 226–243. [Google Scholar] [CrossRef] [PubMed]
- Parrino, B.; Attanzio, A.; Spanò, V.; Cascioferro, S.; Montalbano, A.; Barraja, P.; Tesoriere, L.; Diana, P.; Cirrincione, G.; Carbone, A. Synthesis, antitumor activity and CDK1 inhibiton of new thiazole nortopsentin analogues. Eur. J. Med. Chem. 2017, 138, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Pennati, M.; Parrino, B.; Lopergolo, A.; Barraja, P.; Montalbano, A.; Spanò, V.; Sbarra, S.; Doldi, V.; De Cesare, M.; et al. Novel 1H-pyrrolo[2,3-b]pyridine derivatives nortopsentin analogues: Synthesis and antitumor activity in peritoneal mesothelioma experimental models. J. Med. Chem. 2013, 56, 7060–7072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wen, Q.; Jin, J.; Wang, C.; Lu, P.; Wang, Y. Cyanation of indoles with benzyl cyanide as the cyanide anion surrogate. Tetrahedron 2013, 69, 4236–4240. [Google Scholar] [CrossRef]
- Luescher, M.U.; Vo, C.-V.T.; Bode, J.W. SnAP reagents for the synthesis of piperazines and morpholines. Org. Lett. 2014, 16, 1236–1239. [Google Scholar] [CrossRef] [PubMed]
- Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Available online: http://agris.fao.org/agris-search/search.do?recordID = US201300683461 (accessed on 2 August 2018).
- Mauro, N.; Schillaci, D.; Varvarà, P.; Cusimano, M.G.; Geraci, D.M.; Giuffrè, M.; Cavallaro, G.; Maida, C.M.; Giammona, G. Branched high molecular weight glycopolypeptide with broad-spectrum antimicrobial activity for the treatment of biofilm related infections. ACS Appl. Mater. Interfaces 2018, 10, 318–331. [Google Scholar] [CrossRef] [PubMed]


| Compd. | R | R1 | R2 | Compd. | R | R1 | R2 |
|---|---|---|---|---|---|---|---|
| 1a | H | CH2CH2NHBoc | H | 1i | H | CH2CH2NH2 | H |
| 1b | H | CH2CH2NHBoc | Me | 1j | H | CH2CH2NH2 | Me |
| 1c | OMe | CH2CH2NHBoc | H | 1k | OMe | CH2CH2NH2 | H |
| 1d | OMe | CH2CH2NHBoc | Me | 1l | OMe | CH2CH2NH2 | Me |
| 1e | Br | CH2CH2NHBoc | H | 1m | Br | CH2CH2NH2 | H |
| 1f | Br | CH2CH2NHBoc | Me | 1n | Br | CH2CH2NH2 | Me |
| 1g | F | CH2CH2NHBoc | H | 1o | F | CH2CH2NH2 | H |
| 1h | F | CH2CH2NHBoc | Me | 1p | F | CH2CH2NH2 | Me |


| Compd. | R | R1 | R2 | Compd. | R | R1 | R2 |
|---|---|---|---|---|---|---|---|
| 2a | H | CH2CH2OMe | H | 2l | Br | Me | CH2CH2OMe |
| 2b | H | CH2CH2OMe | Me | 2m | F | CH2CH2OMe | H |
| 2c | H | CH2CH2OMe | CH2CH2OMe | 2n | F | CH2CH2OMe | Me |
| 2d | H | Me | CH2CH2OMe | 2o | F | CH2CH2OMe | CH2CH2OMe |
| 2e | OMe | CH2CH2OMe | H | 2p | F | Me | CH2CH2OMe |
| 2f | OMe | CH2CH2OMe | Me | 2q | F | H | CH2CH2OMe |
| 2g | OMe | CH2CH2OMe | CH2CH2OMe | 2r | H | Boc | CH2CH2OMe |
| 2h | OMe | Me | CH2CH2OMe | 2s | Br | Boc | CH2CH2OMe |
| 2i | Br | CH2CH2OMe | H | 2t | H | H | CH2CH2OMe |
| 2j | Br | CH2CH2OMe | Me | 2u | Br | H | CH2CH2OMe |
| 2k | Br | CH2CH2OMe | CH2CH2OMe |
| Compd. | S. aureus ATCC 25923 µg/mL µM | S. aureus ATCC 6538 µg/mL µM | P. aeruginosa ATCC 15442 µg/mL µM | |||
|---|---|---|---|---|---|---|
| 1a | 3.9 ± 0.2 | 8.4 ± 0.4 | 5.2 ± 0.3 | 11.3 ± 0.6 | - | - |
| 1b | 13.8 ± 0.9 | 29.1 ± 1.9 | 19.3 ± 1.1 | 40.7 ± 2.3 | 15.6 ± 1.1 | 32.9 ± 2.3 |
| 1c | 7.1 ± 0.1 | 14.5 ± 0.2 | 11.6 ± 0.9 | 23.6 ± 1.8 | - | - |
| 1d | 14.1 ± 1.0 | 27.9 ± 2.0 | 13.1 ± 0.5 | 26.0 ± 1.0 | - | - |
| 1f | 9.3 ± 0.9 | 16.8 ± 1.6 | 6.5 ± 0.5 | 11.7 ± 0.9 | 37.2 ± 2.5 | 67.3 ± 4.5 |
| 1h | 36.9 ± 1.7 | 75.0 ± 3.4 | 9.3 ± 0.4 | 18.9 ± 0.8 | 13.1 ± 0.8 | 26.6 ± 1.6 |
| 1i | 4.7 ± 0.3 | 13.0 ± 0.8 | 9.7 ± 0.9 | 26.9 ± 2.5 | 22.7 ± 2.1 | 63.1 ± 5.8 |
| 1j | 32.9 ± 3.1 | 88.0 ± 8.3 | 6.2 ± 0.09 | 16.6 ± 0.2 | 56.1 ± 3.2 | 150.2 ± 8.6 |
| 1k | 23.3 ± 1.5 | 59.2 ± 3.8 | 4.8 ± 0.1 | 12.3 ± 0.3 | 4.2 ± 0.1 | 10.7 ± 0.2 |
| 1l | 48.7 ± 2.2 | 120.6 ± 5.4 | 7.2 ± 0.7 | 17.8 ± 1.7 | 24.2 ± 0.8 | 59.9 ± 2.0 |
| 1m | 4.4 ± 0.1 | 10.0 ± 0.2 | 3.3 ± 0.08 | 7.5 ± 0.2 | 7.8 ± 0.09 | 17.7 ± 0.2 |
| 1n | 20.1 ± 0.8 | 44.4 ± 1.8 | 5.4 ± 0.2 | 11.9 ± 0.4 | 4.6 ± 0.1 | 10.1 ± 0.2 |
| 1o | 1.5 ± 0.1 | 3.9 ± 0.3 | 6.3 ± 0.4 | 16.6 ± 1.0 | 4.5 ± 0.4 | 11.9 ± 1.1 |
| 1p | 0.5 ± 0.02 | 1.27 ± 0.05 | 5.2 ± 0.08 | 13.2 ± 0.2 | 3.9 ± 0.07 | 9.9 ± 0.2 |
| 2d | 7.5 ± 0.2 | 19.3 ± 0.5 | - | - | - | - |
| 2e | 18.6 ± 0.9 | 45.9 ± 2.2 | 25.4 ± 1.7 | 62.7 ± 4.2 | 20.5 ± 1.2 | 50.6 ± 3.0 |
| 2f | 1.2 ± 0.03 | 2.8 ± 0.07 | 11.5 ± 0.7 | 26.7 ± 1.6 | - | - |
| 2g | 7.9 ± 0.6 | 17.0 ± 1.3 | 11.1 ± 0.2 | 23.9 ± 0.4 | 17.7 ± 0.8 | 38.2 ± 1.7 |
| 2i | 0.79 ± 0.009 | 1.7 ± 0.02 | 9.4 ± 0.3 | 20.7 ± 0.7 | 4.4 ± 0.08 | 9.7 ± 0.2 |
| 2j | 0.95 ± 0.01 | 2.03 ± 0.02 | 11.2 ± 1.1 | 23.9 ± 2.3 | 19.1 ± 0.1 | 40.8 ± 0.2 |
| 2k | 2.9 ± 0.02 | 5.6 ± 0.04 | 18.8 ± 1.5 | 36.7 ± 2.9 | - | - |
| 2l | 2.5 ± 0.02 | 5.3 ± 0.04 | - | - | - | - |
| 2m | 13.8 ± 0.7 | 35.1 ± 1.8 | 0.3 ± 0.002 | 0.7 ± 0.005 | - | - |
| 2n | 0.2 ± 0.006 | 0.4 ± 0.012 | 21.0 ± 1.7 | 51.6 ± 4.2 | - | - |
| 2o | 28.5 ± 1.9 | 63.2 ± 4.2 | - | - | - | - |
| 2q | 13.7 ± 1.1 | 34.9 ± 2.8 | 23.1 ± 1.9 | 58.8 ± 4.8 | - | - |
| 2r | 1.8 ± 0.1 | 3.7 ± 0.2 | 6.9 ± 0.1 | 14.5 ± 0.2 | - | - |
| 2t | 12.9 ± 0.5 | 34.4 ± 1.3 | 7.5 ± 0.6 | 20.0 ± 1.6 | 16.3 ± 1.3 | 43.5 ± 3.5 |
| 2u | 13.1 ± 0.8 | 28.8 ± 1.7 | 9.6 ± 0.9 | 21.1 ± 2.0 | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carbone, A.; Parrino, B.; Cusimano, M.G.; Spanò, V.; Montalbano, A.; Barraja, P.; Schillaci, D.; Cirrincione, G.; Diana, P.; Cascioferro, S. New Thiazole Nortopsentin Analogues Inhibit Bacterial Biofilm Formation. Mar. Drugs 2018, 16, 274. https://doi.org/10.3390/md16080274
Carbone A, Parrino B, Cusimano MG, Spanò V, Montalbano A, Barraja P, Schillaci D, Cirrincione G, Diana P, Cascioferro S. New Thiazole Nortopsentin Analogues Inhibit Bacterial Biofilm Formation. Marine Drugs. 2018; 16(8):274. https://doi.org/10.3390/md16080274
Chicago/Turabian StyleCarbone, Anna, Barbara Parrino, Maria Grazia Cusimano, Virginia Spanò, Alessandra Montalbano, Paola Barraja, Domenico Schillaci, Girolamo Cirrincione, Patrizia Diana, and Stella Cascioferro. 2018. "New Thiazole Nortopsentin Analogues Inhibit Bacterial Biofilm Formation" Marine Drugs 16, no. 8: 274. https://doi.org/10.3390/md16080274
APA StyleCarbone, A., Parrino, B., Cusimano, M. G., Spanò, V., Montalbano, A., Barraja, P., Schillaci, D., Cirrincione, G., Diana, P., & Cascioferro, S. (2018). New Thiazole Nortopsentin Analogues Inhibit Bacterial Biofilm Formation. Marine Drugs, 16(8), 274. https://doi.org/10.3390/md16080274