A Novel Peptide, Nicotinyl–Isoleucine–Valine–Histidine (NA–IVH), Promotes Antioxidant Gene Expression and Wound Healing in HaCaT Cells
Abstract
1. Introduction
2. Results
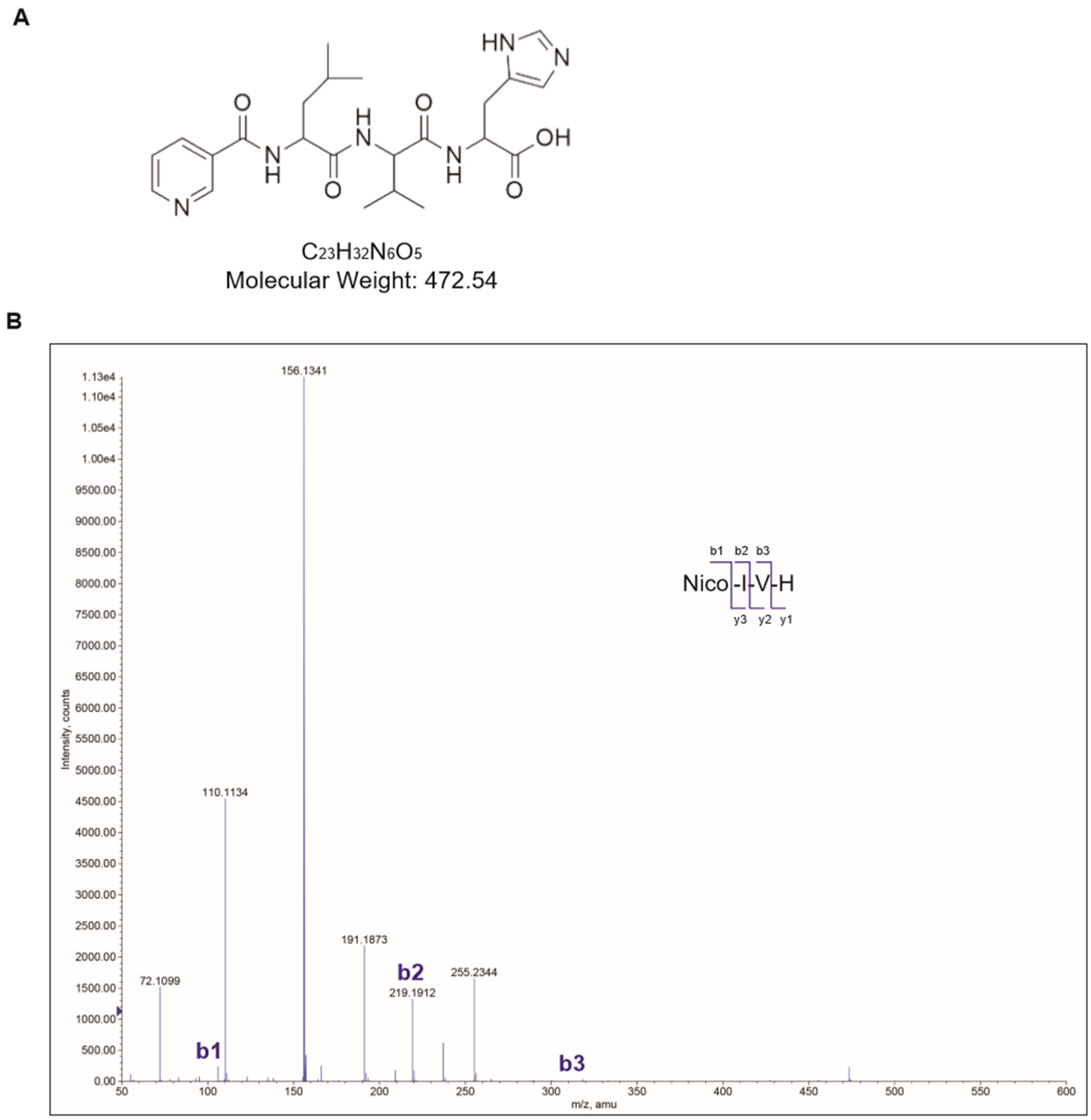
2.1. Synthesis of Nicotinyl–Isoleucine–Valine–Histidine (NA–IVH)
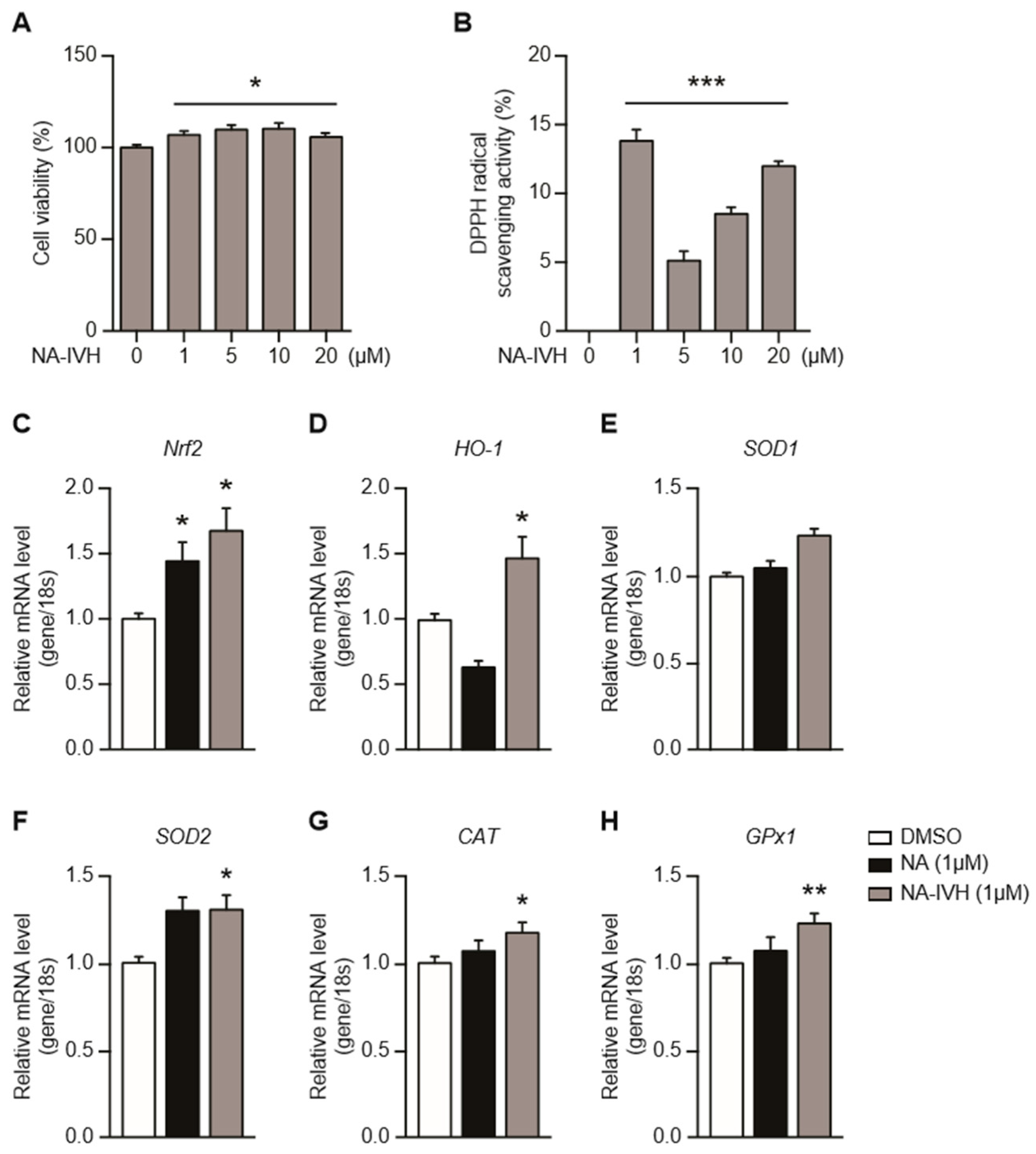
2.2. Cytotoxicity and Antioxidant Activity of NA–IVH
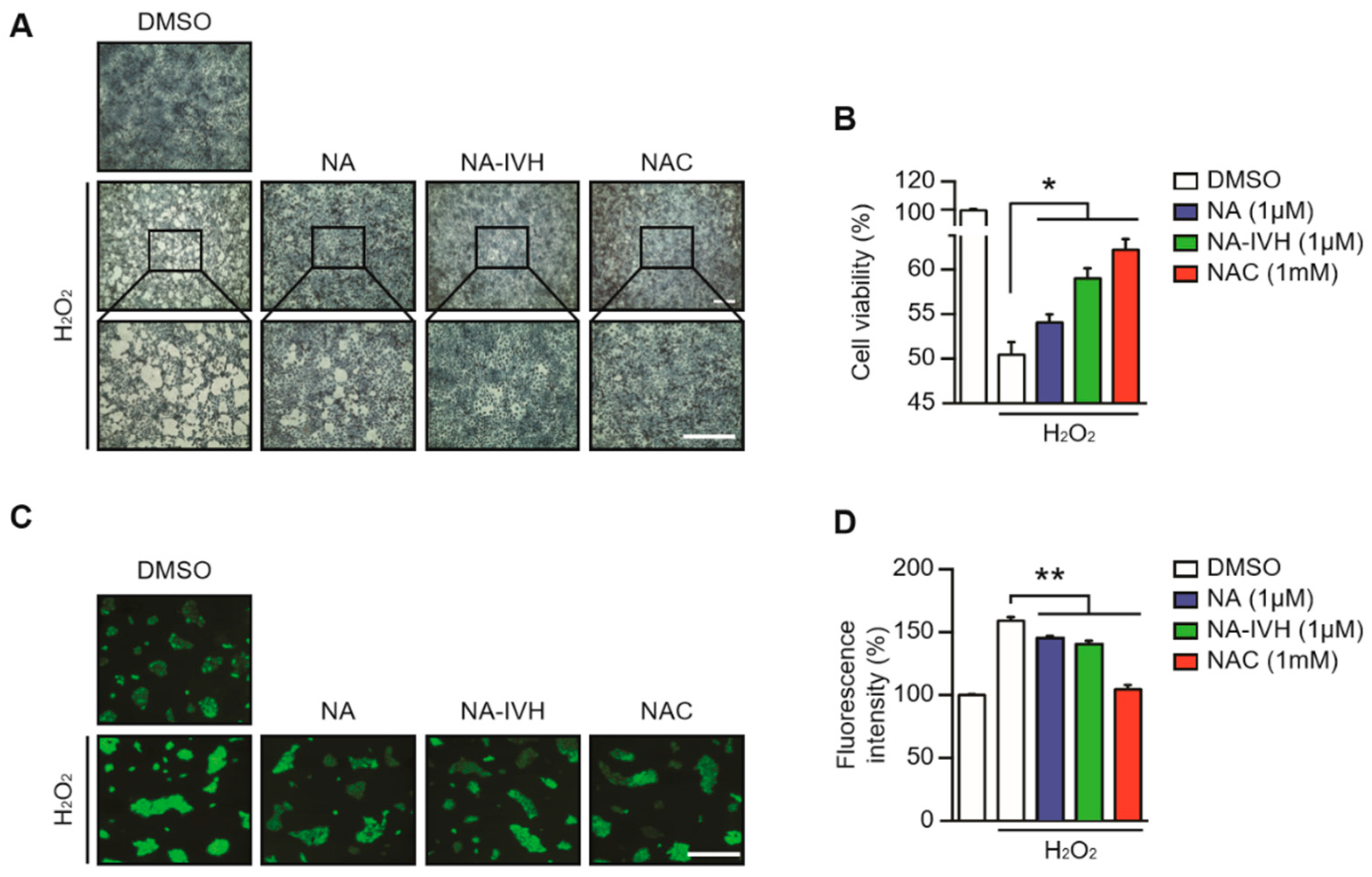
2.3. Protective Role of NA–IVH on ROS-Induced Cytotoxicity
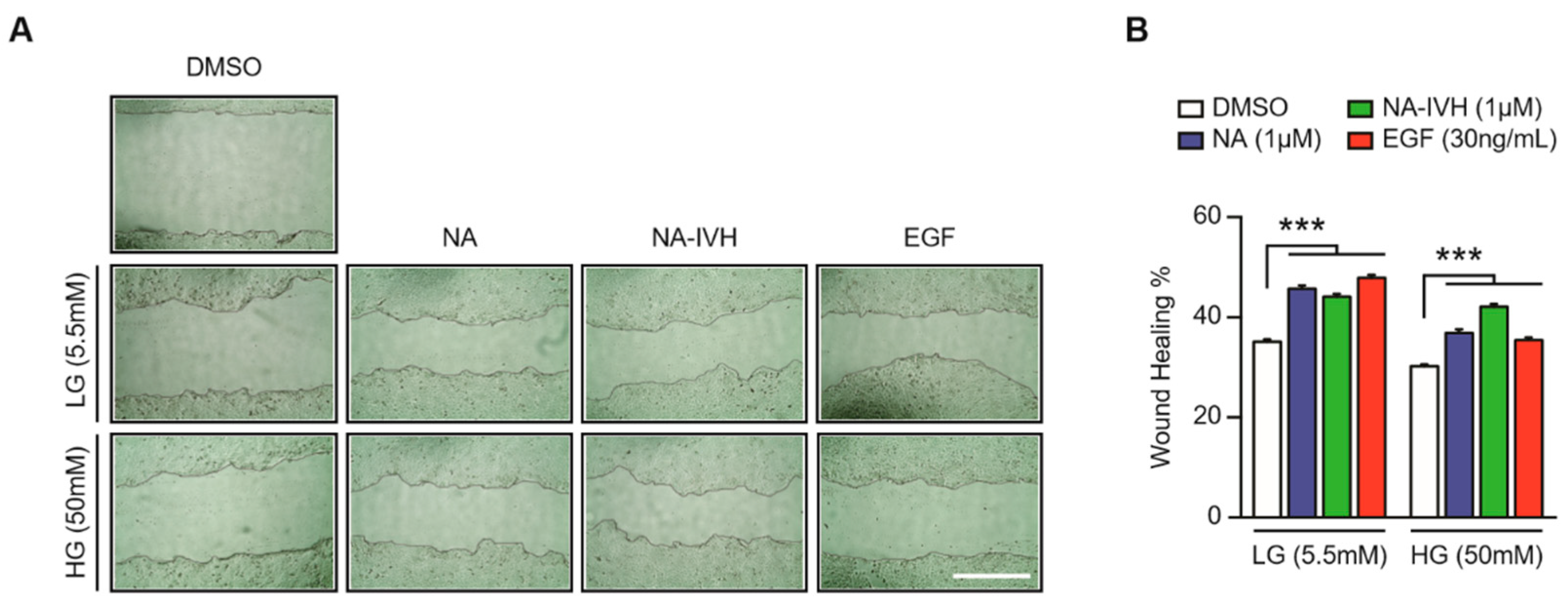
2.4. Improved Wound-Healing Effect of NA–IVH in High Glucose Condition
3. Discussion
4. Materials and Methods
4.1. Synthesis and Purification of Nicotinic Acid–IVH
4.2. Cell Culture and Reagents
4.3. Cell Viability Assay
4.4. DPPH Radical Scavenging Assay
4.5. ROS Scavenging Assay
4.6. In Vitro Wound-Healing Assay
4.7. Quantitative Real-Time PCR
5. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stadelmann, W.K.; Digenis, A.G.; Tobin, G.R. Physiology and healing dynamics of chronic cutaneous wounds. Am. J. Surg. 1998, 176, 26S–38S. [Google Scholar] [CrossRef]
- Koh, T.J.; DiPietro, L.A. Inflammation and wound healing: The role of the macrophage. Expert Rev. Mol. Med. 2011, 13, e23. [Google Scholar] [CrossRef] [PubMed]
- Landen, N.X.; Li, D.; Stahle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.; Werner, S. Oxidative stress in normal and impaired wound repair. Pharmacol. Res. 2008, 58, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Cappiello, F.; Di Grazia, A.; Segev-Zarko, L.A.; Scali, S.; Ferrera, L.; Galietta, L.; Pini, A.; Shai, Y.; Di, Y.P.; Mangoni, M.L. Esculentin-1a-derived peptides promote clearance of pseudomonas aeruginosa internalized in bronchial cells of cystic fibrosis patients and lung cell migration: Biochemical properties and a plausible mode of action. Antimicrob. Agents Chemother. 2016, 60, 7252–7262. [Google Scholar] [PubMed]
- Mangoni, M.L.; McDermott, A.M.; Zasloff, M. Antimicrobial peptides and wound healing: Biological and therapeutic considerations. Exp. Dermatol. 2016, 25, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Di Grazia, A.; Cappiello, F.; Imanishi, A.; Mastrofrancesco, A.; Picardo, M.; Paus, R.; Mangoni, M.L. The frog skin-derived antimicrobial peptide esculentin-1a(1-21)NH2 promotes the migration of human HaCaT keratinocytes in an EGF receptor-dependent manner: A novel promoter of human skin wound healing? PLoS ONE 2015, 10, e0128663. [Google Scholar] [CrossRef] [PubMed]
- Schreml, S.; Szeimies, R.M.; Prantl, L.; Karrer, S.; Landthaler, M.; Babilas, P. Oxygen in acute and chronic wound healing. Br. J. Dermatol. 2010, 163, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Lu, W.; Ogasawara, M.A.; Nilsa, R.D.; Huang, P. Redox regulation of cell survival. Antioxid. Redox Signal. 2008, 10, 1343–1374. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Jhun, B.S.; Yoon, Y. High-glucose stimulation increases reactive oxygen species production through the calcium and mitogen-activated protein kinase-mediated activation of mitochondrial fission. Antioxid. Redox Signal. 2011, 14, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Nouvong, A.; Ambrus, A.M.; Zhang, E.R.; Hultman, L.; Coller, H.A. Reactive oxygen species and bacterial biofilms in diabetic wound healing. Physiol. Genomics 2016, 48, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Jurkiewicz, B.A.; Buettner, G.R. EPR detection of free radicals in UV-irradiated skin: Mouse versus human. Photochem. Photobiol. 1996, 64, 918–922. [Google Scholar] [CrossRef] [PubMed]
- Masaki, H.; Okano, Y.; Sakurai, H. Generation of active oxygen species from advanced glycation end-products (ages) during ultraviolet light a (UVA) irradiation and a possible mechanism for cell damaging. Biochim. Biophys. Acta 1999, 1428, 45–56. [Google Scholar] [CrossRef]
- Masaki, H.; Atsumi, T.; Sakurai, H. Detection of hydrogen peroxide and hydroxyl radicals in murine skin fibroblasts under UVB irradiation. Biochem. Biophys. Res. Commun. 1995, 206, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.C.; Martin, A.J.; Damian, D.L. Nicotinamide for skin-cancer chemoprevention. N. Engl. J. Med. 2016, 374, 790. [Google Scholar] [PubMed]
- Damian, D.L.; Patterson, C.R.; Stapelberg, M.; Park, J.; Barnetson, R.S.; Halliday, G.M. UV radiation-induced immunosuppression is greater in men and prevented by topical nicotinamide. J. Investig. Dermatol. 2008, 128, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Yiasemides, E.; Sivapirabu, G.; Halliday, G.M.; Park, J.; Damian, D.L. Oral nicotinamide protects against ultraviolet radiation-induced immunosuppression in humans. Carcinogenesis 2009, 30, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Gensler, H.L.; Williams, T.; Huang, A.C.; Jacobson, E.L. Oral niacin prevents photocarcinogenesis and photoimmunosuppression in mice. Nutr. Cancer 1999, 34, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Gille, A.; Bodor, E.T.; Ahmed, K.; Offermanns, S. Nicotinic acid: Pharmacological effects and mechanisms of action. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 79–106. [Google Scholar] [CrossRef] [PubMed]
- Ganji, S.H.; Qin, S.; Zhang, L.; Kamanna, V.S.; Kashyap, M.L. Niacin inhibits vascular oxidative stress, redox-sensitive genes, and monocyte adhesion to human aortic endothelial cells. Atherosclerosis 2009, 202, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Bissett, D.L.; Oblong, J.E.; Berge, C.A. Niacinamide: A b vitamin that improves aging facial skin appearance. Dermatol. Surg. 2005, 31, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.C.; Damian, D.L. Nicotinamide and the skin. Australas. J. Dermatol. 2014, 55, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Wohlrab, J.; Kreft, D. Niacinamide—Mechanisms of action and its topical use in dermatology. Skin Pharmacol. Physiol. 2014, 27, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Lecci, R.M.; Durante, M.; Meli, F.; Piraino, S. The bright side of gelatinous blooms: Nutraceutical value and antioxidant properties of three mediterranean jellyfish (scyphozoa). Mar. Drugs 2015, 13, 4654–4681. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yu, H.; Xing, R.; Liu, S.; Qing, Y.; Li, K.; Li, B.; Meng, X.; Cui, J.; Li, P. Isolation, identification and characterization of a novel antioxidant protein from the nematocyst of the jellyfish Stomolophus meleagris. Int. J. Biol. Macromol. 2012, 51, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Munawir, A.; Cha, M.; Sohn, E.T.; Lee, H.; Kim, J.S.; Yoon, W.D.; Lim, D.; Kim, E. Cytotoxicity and hemolytic activity of jellyfish Nemopilema nomurai (Scyphozoa: Rhizostomeae) venom. Comp. Biochem. Physiol. C 2009, 150, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Liu, X.; Dong, X.; Li, C.; Xing, R.; Liu, S.; Li, P. Insecticidal activity of proteinous venom from tentacle of jellyfish Rhopilema esculentum Kishinouye. Bioorg. Med. Chem. Lett. 2005, 15, 4949–4952. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, S.; Isbister, G.K.; Seymour, J.E.; Hodgson, W.C. Pharmacologically distinct cardiovascular effects of box jellyfish (Chironex fleckeri) venom and a tentacle-only extract in rats. Toxicol. Lett. 2005, 155, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Liu, X.; Xing, R.; Liu, S.; Li, C.; Li, P. Radical scavenging activity of protein from tentacles of jellyfish Rhopilema esculentum. Bioorg. Med. Chem. Lett. 2005, 15, 2659–2664. [Google Scholar] [CrossRef] [PubMed]
- Ovchinnikova, T.V.; Balandin, S.V.; Aleshina, G.M.; Tagaev, A.A.; Leonova, Y.F.; Krasnodembsky, E.D.; Men’shenin, A.V.; Kokryakov, V.N. Aurelin, a novel antimicrobial peptide from jellyfish Aurelia aurita with structural features of defensins and channel-blocking toxins. Biochem. Biophys. Res. Commun. 2006, 348, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Han, J.G.; Kulkarni, A.; Jeon, J.Y.; Kim, B.R.; Song, M.Y.; Jeong, H.Y.; Yang, D.J.; You, S.H.; Kim, K.W.; Moh, S.H.; et al. A novel tripeptide derived from chlorella vulgaris regulates skin homeostasis through antioxidant activity. Sci. Adv. Mater. 2015, 7, 2476–2480. [Google Scholar] [CrossRef]
- Amblard, M.; Fehrentz, J.A.; Martinez, J.; Subra, G. Methods and protocols of modern solid phase peptide synthesis. Mol. Biotechnol. 2006, 33, 239–254. [Google Scholar] [CrossRef]
- Long, M.; Rojo de la Vega, M.; Wen, Q.; Bharara, M.; Jiang, T.; Zhang, R.; Zhou, S.; Wong, P.K.; Wondrak, G.T.; Zheng, H.; et al. An essential role of Nrf2 in diabetic wound healing. Diabetes 2016, 65, 780–793. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kim, J.E.; Lee, S.M.; Lee, S.H.; Lee, J.W.; Kim, M.K.; Lee, K.J.; Kim, H.; Lee, J.D.; Choi, K.Y. N-nicotinoyl dopamine, a novel niacinamide derivative, retains high antioxidant activity and inhibits skin pigmentation. Exp. Dermatol. 2011, 20, 950–952. [Google Scholar] [CrossRef] [PubMed]
- Kannan, S.; Jaiswal, A.K. Low and high dose UVB regulation of transcription factor NF-E2-related factor 2. Cancer Res. 2006, 66, 8421–8429. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.; Werner, S. Nrf2—A regulator of keratinocyte redox signaling. Free Radic. Biol. Med. 2015, 88, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Sulik, K.K.; Chen, S.Y. Nrf2-mediated transcriptional induction of antioxidant response in mouse embryos exposed to ethanol in vivo: Implications for the prevention of fetal alcohol spectrum disorders. Antioxid. Redox Signal. 2008, 10, 2023–2033. [Google Scholar] [CrossRef] [PubMed]
- Dreger, H.; Westphal, K.; Weller, A.; Baumann, G.; Stangl, V.; Meiners, S.; Stangl, K. Nrf2-dependent upregulation of antioxidative enzymes: A novel pathway for proteasome inhibitor-mediated cardioprotection. Cardiovasc. Res. 2009, 83, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Alam, J.; Venkatesan, M.I.; Eiguren-Fernandez, A.; Schmitz, D.; Di Stefano, E.; Slaughter, N.; Killeen, E.; Wang, X.; Huang, A.; et al. Nrf2 is a key transcription factor that regulates antioxidant defense in macrophages and epithelial cells: Protecting against the proinflammatory and oxidizing effects of diesel exhaust chemicals. J. Immunol. 2004, 173, 3467–3481. [Google Scholar] [CrossRef] [PubMed]
- Jindam, A.; Yerra, V.G.; Kumar, A. Nrf2: A promising trove for diabetic wound healing. Ann. Transl. Med. 2017, 5, 469. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, T.X.; Leethanakul, C.; Patel, V.; Mitola, D.; Lund, L.R.; Dano, K.; Johnsen, M.; Gutkind, J.S.; Bugge, T.H. Laser capture microdissection-based in vivo genomic profiling of wound keratinocytes identifies similarities and differences to squamous cell carcinoma. Oncogene 2003, 22, 3964–3976. [Google Scholar] [CrossRef] [PubMed]
- Beyer, T.A.; auf dem Keller, U.; Braun, S.; Schafer, M.; Werner, S. Roles and mechanisms of action of the Nrf2 transcription factor in skin morphogenesis, wound repair and skin cancer. Cell Death Differ. 2007, 14, 1250–1254. [Google Scholar] [CrossRef] [PubMed]
- Braun, S.; Hanselmann, C.; Gassmann, M.G.; auf dem Keller, U.; Born-Berclaz, C.; Chan, K.; Kan, Y.W.; Werner, S. Nrf2 transcription factor, a novel target of keratinocyte growth factor action which regulates gene expression and inflammation in the healing skin wound. Mol. Cell Biol. 2002, 22, 5492–5505. [Google Scholar] [CrossRef] [PubMed]
- auf dem Keller, U.; Huber, M.; Beyer, T.A.; Kumin, A.; Siemes, C.; Braun, S.; Bugnon, P.; Mitropoulos, V.; Johnson, D.A.; Johnson, J.A.; et al. Nrf transcription factors in keratinocytes are essential for skin tumor prevention but not for wound healing. Mol. Cell Biol. 2006, 26, 3773–3784. [Google Scholar] [CrossRef] [PubMed]
- Masaki, H. Role of antioxidants in the skin: Anti-aging effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Kruk, J.; Duchnik, E. Oxidative stress and skin diseases: Possible role of physical activity. Asian Pac. J. Cancer Prev. 2014, 15, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Fusenig, N.E.; Boukamp, P. Multiple stages and genetic alterations in immortalization, malignant transformation, and tumor progression of human skin keratinocytes. Mol. Carcinogen. 1998, 23, 144–158. [Google Scholar] [CrossRef]
- Xuan, Y.H.; Huang, B.B.; Tian, H.S.; Chi, L.S.; Duan, Y.M.; Wang, X.; Zhu, Z.X.; Cai, W.H.; Zhu, Y.T.; Wei, T.M.; et al. High-glucose inhibits human fibroblast cell migration in wound healing via repression of bfgf-regulating jnk phosphorylation. PLoS ONE 2014, 9, e108182. [Google Scholar] [CrossRef] [PubMed]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Williams, W.E. Rape versus seduction: Liberty and the role of government. J. Med. Assoc. Ga. 1995, 84, 25–28. [Google Scholar] [PubMed]
- Teranishi, S.; Kimura, K.; Nishida, T. Role of formation of an ERK-FAK-paxillin complex in migration of human corneal epithelial cells during wound closure in vitro. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5646–5652. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, D.H.; Yang, D.J.; Sun, J.S.; Kim, S.K.; Kang, N.; Kang, J.Y.; Choi, Y.-H.; Lee, J.H.; Moh, S.H.; Shin, D.M.; et al. A Novel Peptide, Nicotinyl–Isoleucine–Valine–Histidine (NA–IVH), Promotes Antioxidant Gene Expression and Wound Healing in HaCaT Cells. Mar. Drugs 2018, 16, 262. https://doi.org/10.3390/md16080262
Son DH, Yang DJ, Sun JS, Kim SK, Kang N, Kang JY, Choi Y-H, Lee JH, Moh SH, Shin DM, et al. A Novel Peptide, Nicotinyl–Isoleucine–Valine–Histidine (NA–IVH), Promotes Antioxidant Gene Expression and Wound Healing in HaCaT Cells. Marine Drugs. 2018; 16(8):262. https://doi.org/10.3390/md16080262
Chicago/Turabian StyleSon, Dong Hwee, Dong Joo Yang, Ji Su Sun, Seul Ki Kim, Namju Kang, Jung Yun Kang, Yun-Hee Choi, Jeong Hun Lee, Sang Hyun Moh, Dong Min Shin, and et al. 2018. "A Novel Peptide, Nicotinyl–Isoleucine–Valine–Histidine (NA–IVH), Promotes Antioxidant Gene Expression and Wound Healing in HaCaT Cells" Marine Drugs 16, no. 8: 262. https://doi.org/10.3390/md16080262
APA StyleSon, D. H., Yang, D. J., Sun, J. S., Kim, S. K., Kang, N., Kang, J. Y., Choi, Y.-H., Lee, J. H., Moh, S. H., Shin, D. M., & Kim, K. W. (2018). A Novel Peptide, Nicotinyl–Isoleucine–Valine–Histidine (NA–IVH), Promotes Antioxidant Gene Expression and Wound Healing in HaCaT Cells. Marine Drugs, 16(8), 262. https://doi.org/10.3390/md16080262





