Physicochemical Properties and Biocompatibility Evaluation of Collagen from the Skin of Giant Croaker (Nibea japonica)
Abstract
:1. Introduction
2. Results and Discussion
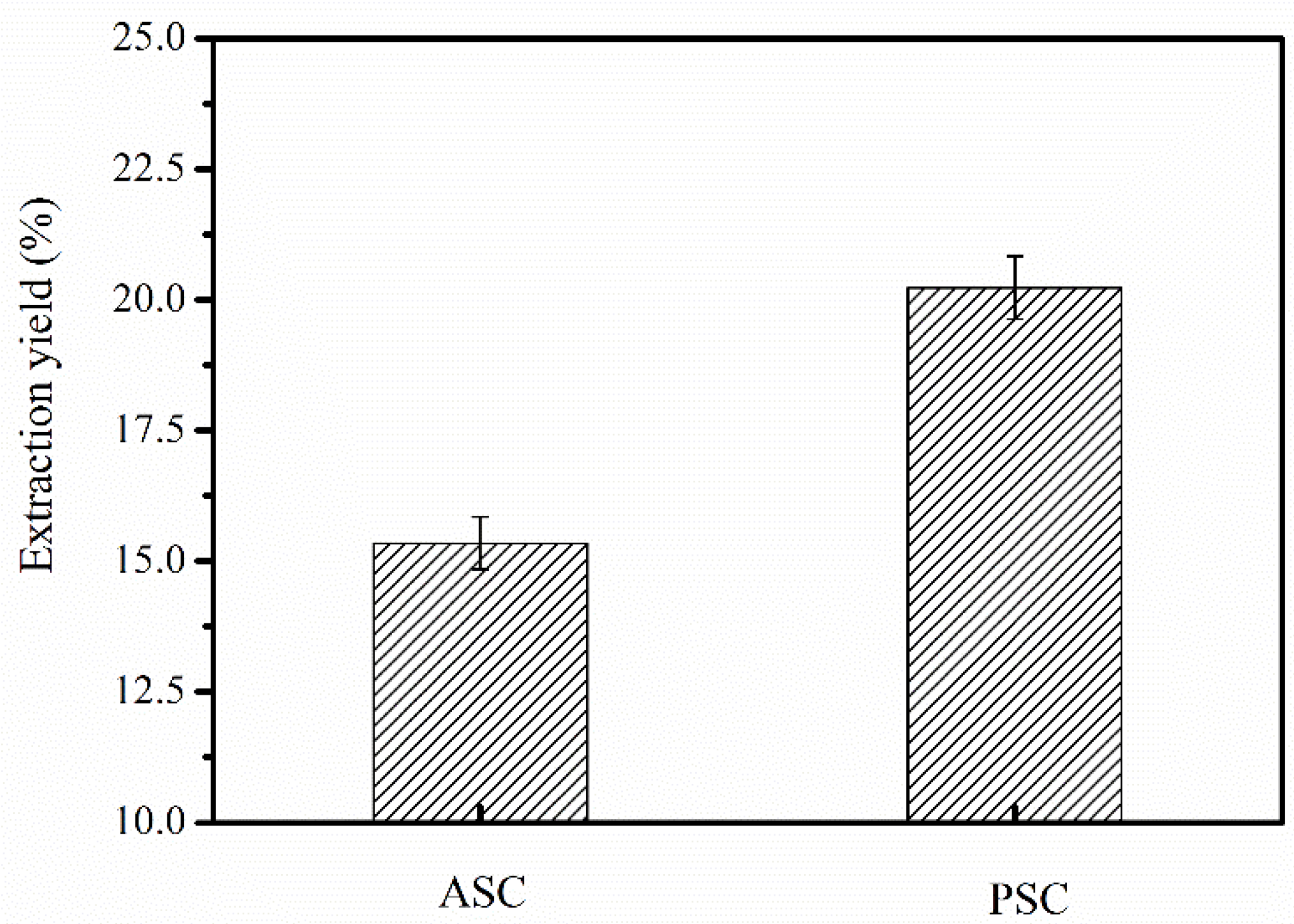
2.1. ASC and PSC Yields from Nibea japonica Skin
2.2. Amino Acid Composition
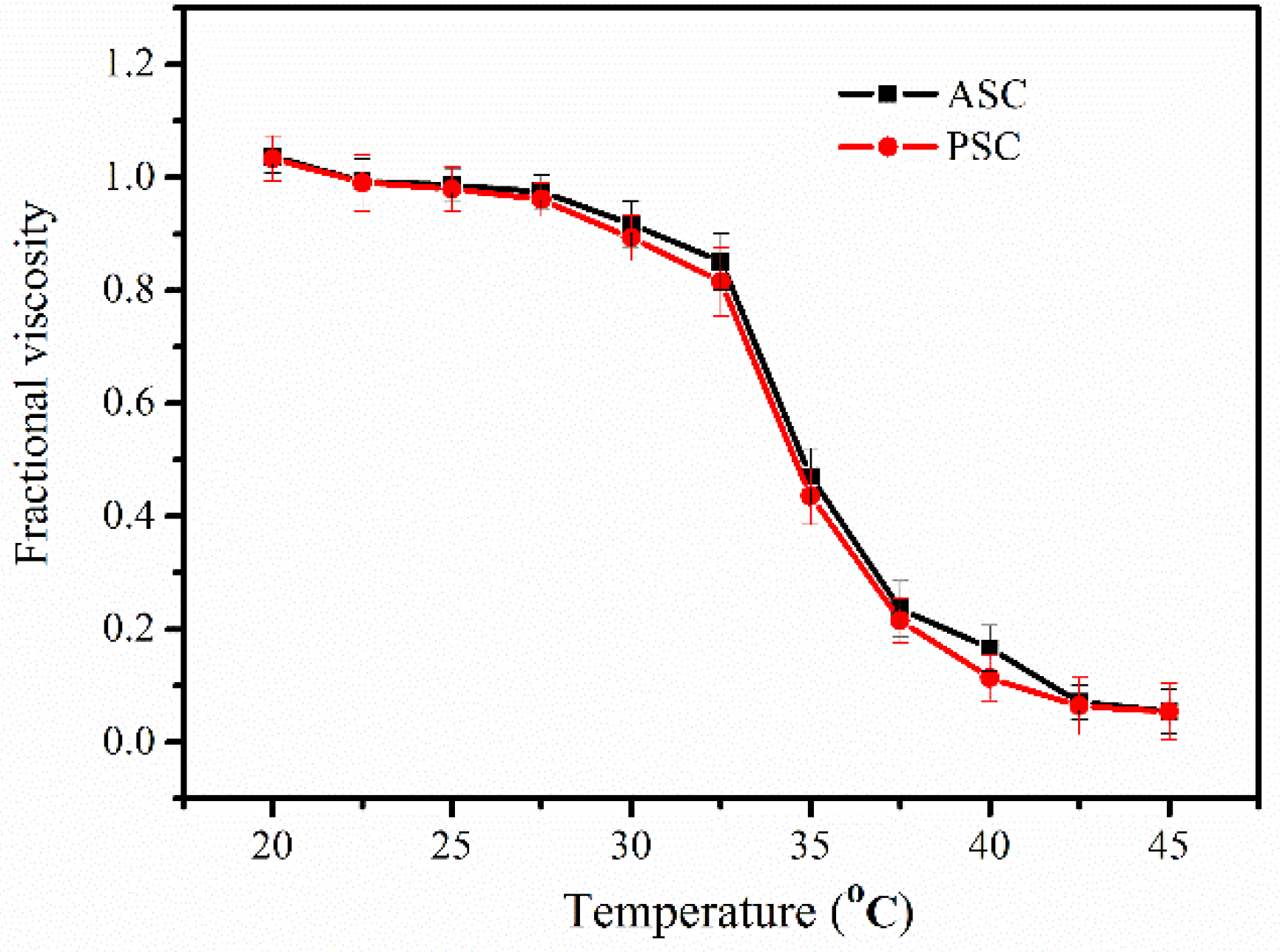
2.3. Determination of Denaturation Temperature (Td)
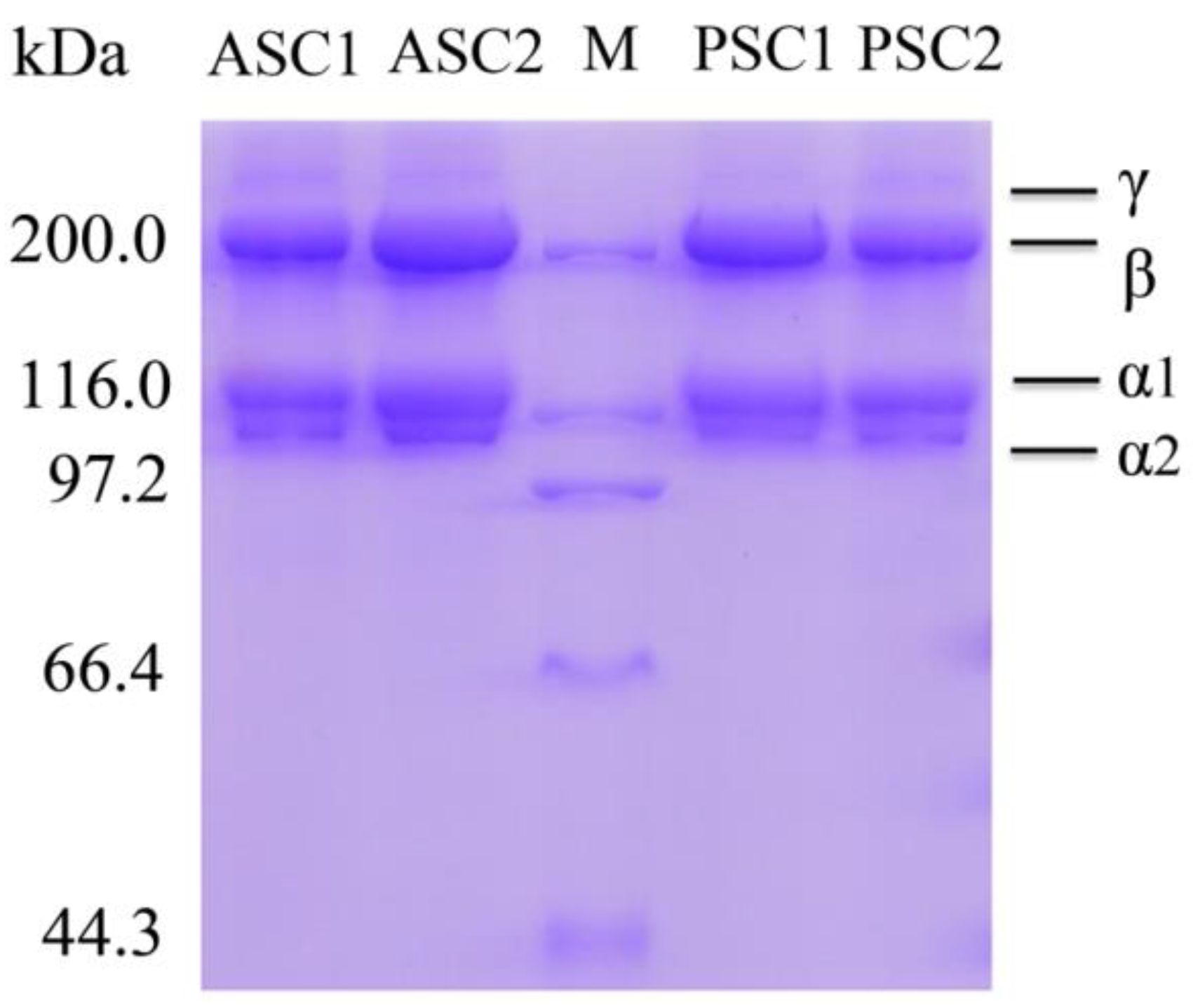
2.4. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) Analysis
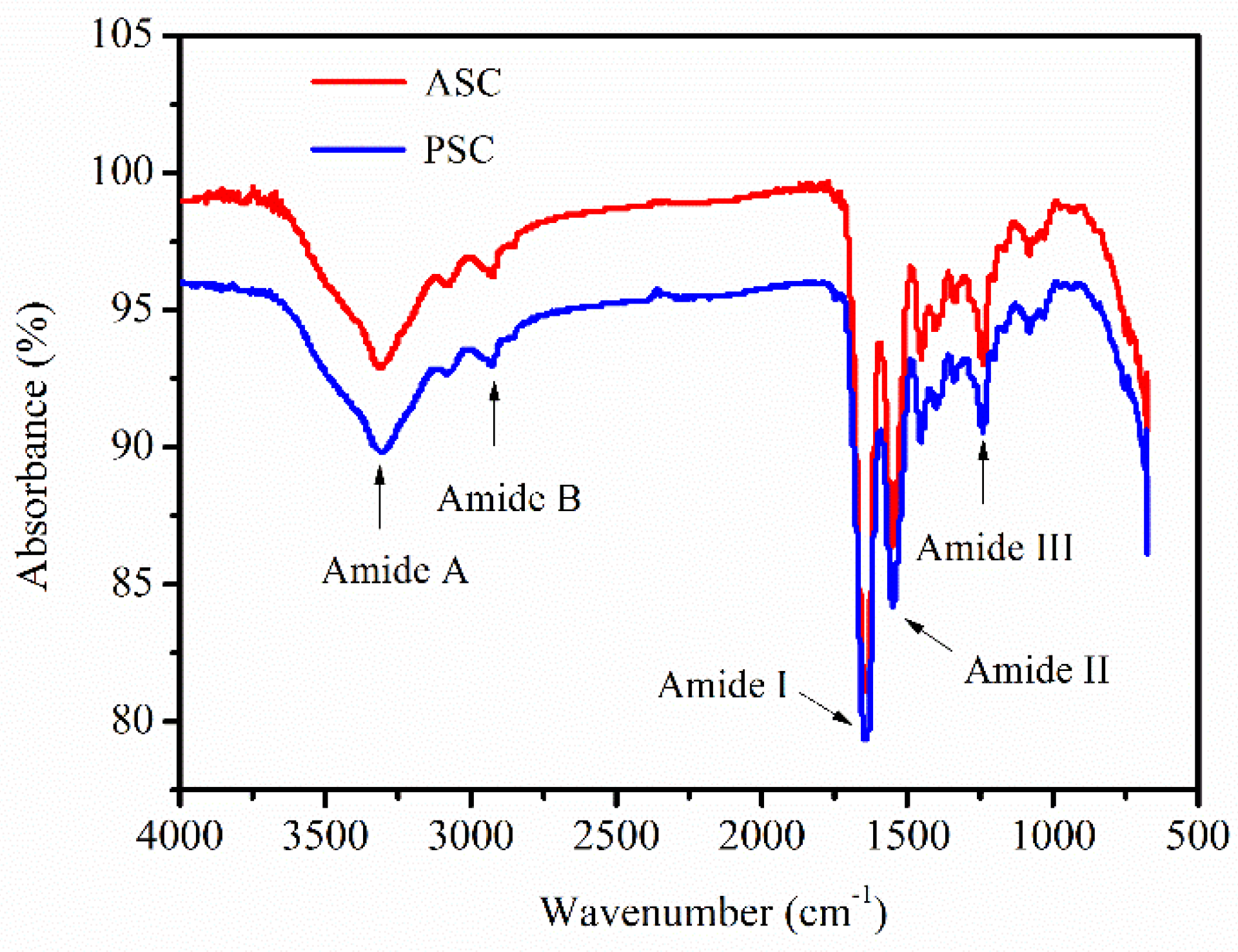
2.5. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.6. Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
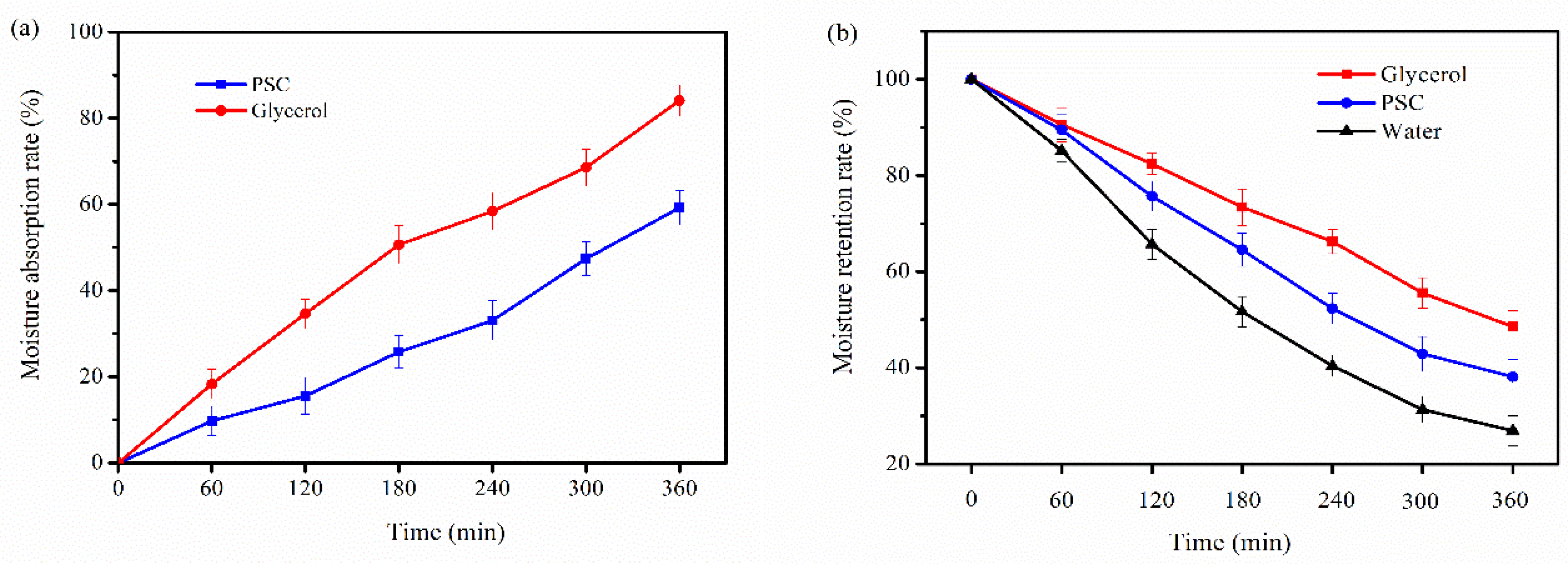
2.7. Moisture Absorption and Retention Properties
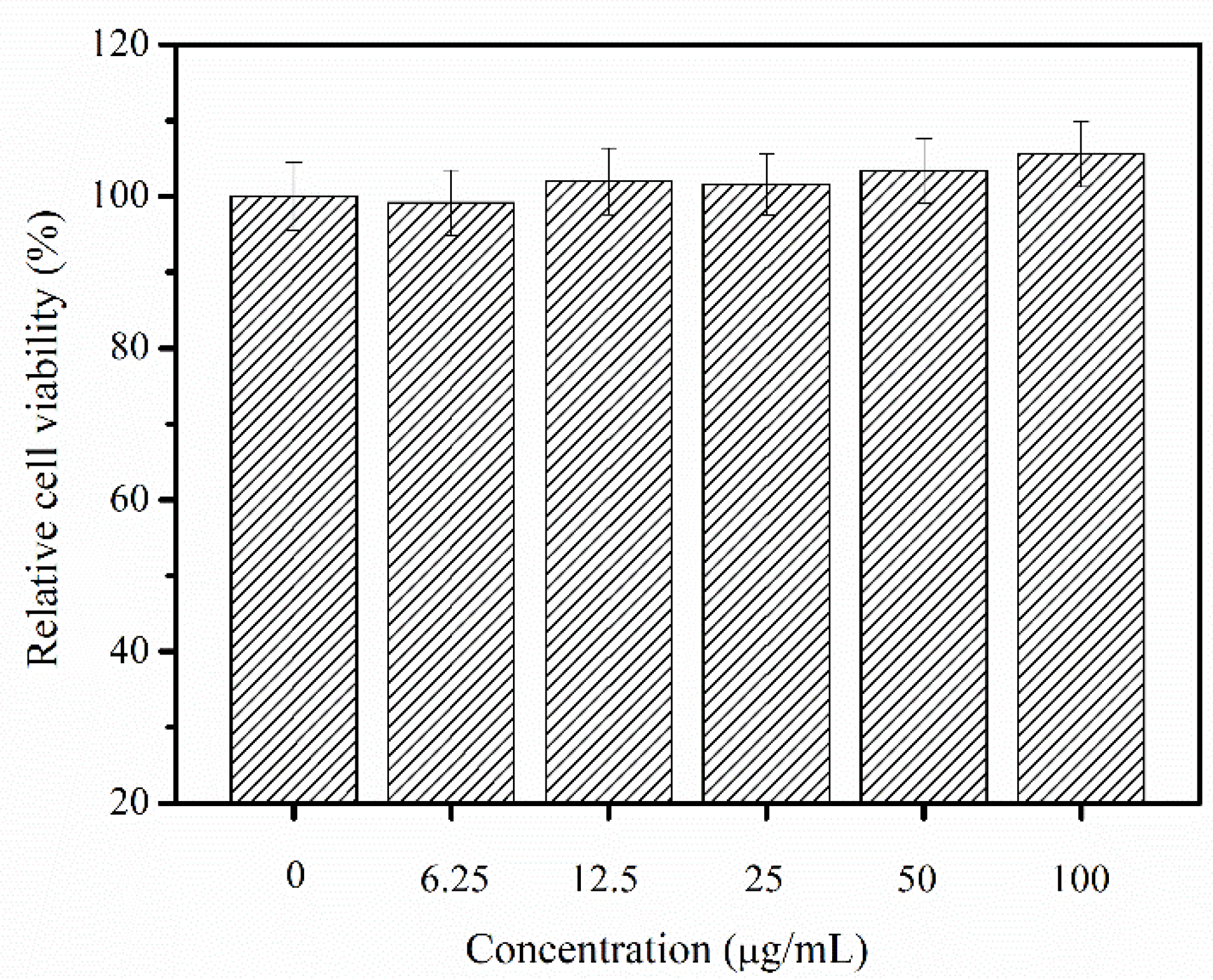
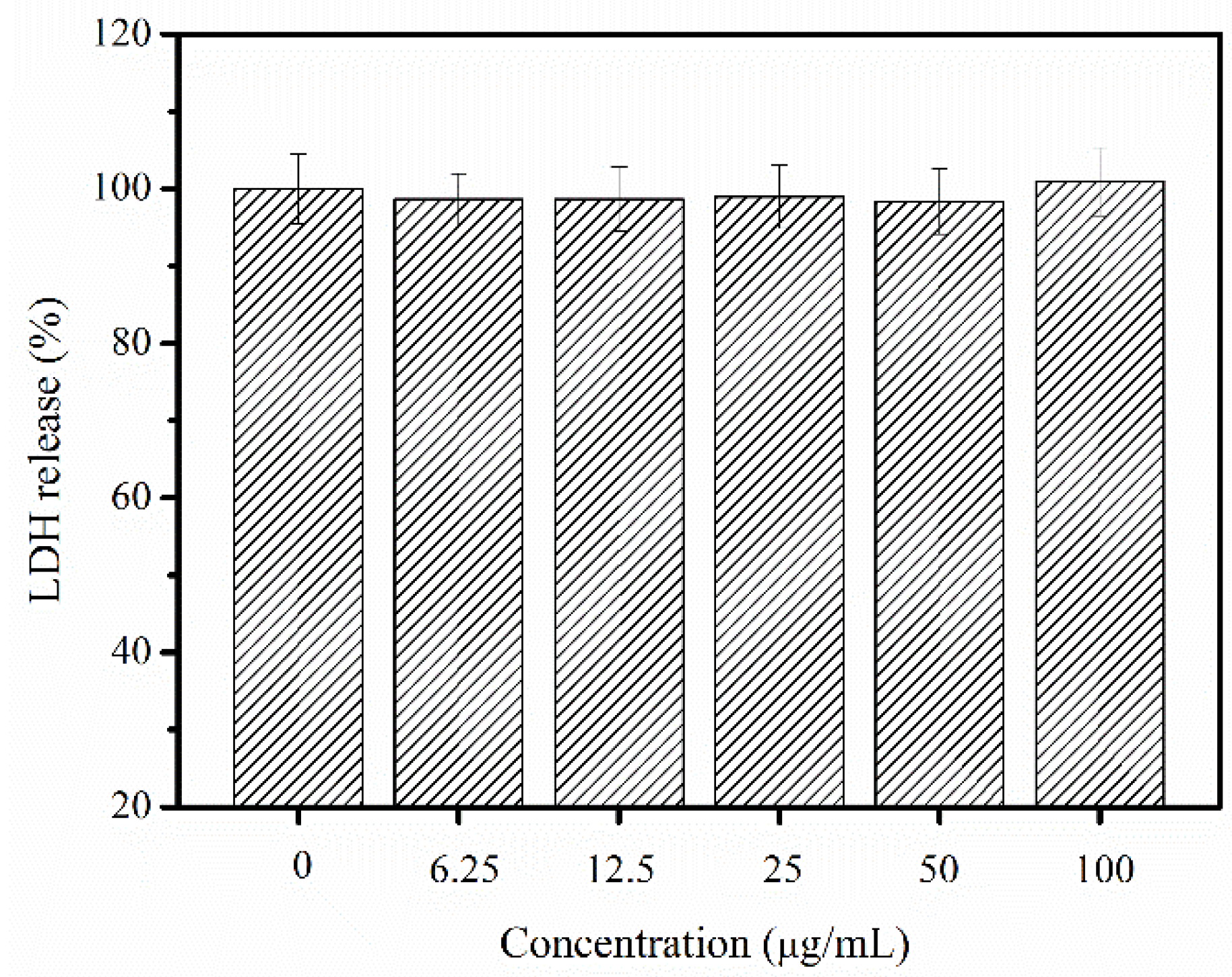
2.8. Cytotoxic and Allergenic Possibility Test
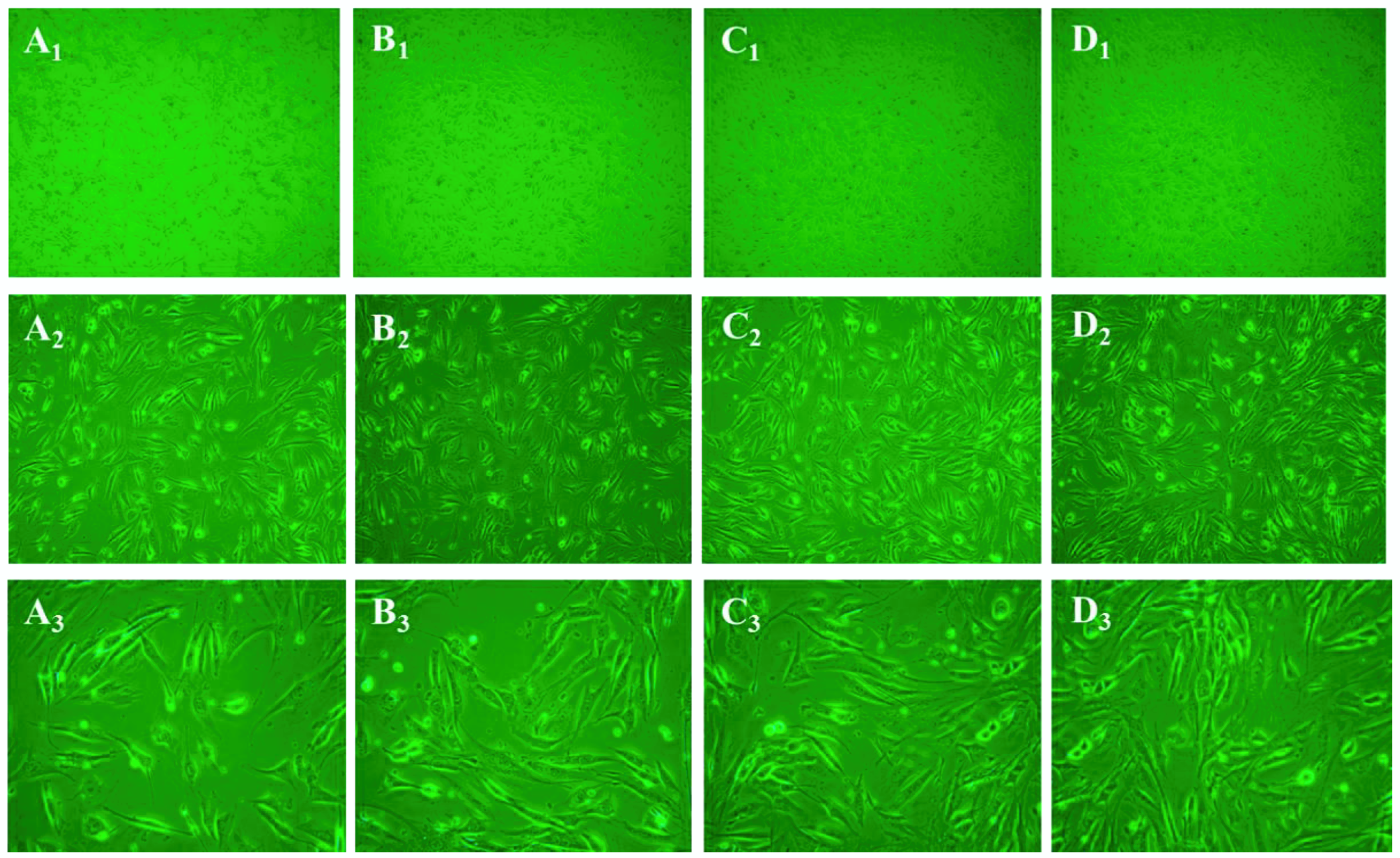
2.9. Morphological Examination
3. Materials and Methods
3.1. Raw Materials
3.2. Extraction of ASC and PSC
3.3. Amino Acid Analysis
3.4. Determination of Td Values
3.5. SDS-PAGE Analysis
3.6. FTIR Analysis
3.7. ICP-MS Analysis
3.8. Moisture Absorption and Retention Properties
3.9. Cytotoxic and Allergenic Possibility of Nibea japonica Skin Collagen
3.10. Morphological Changes of Cells Treated with PSC from Nibea japonica Skin
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Corinaldesi, C.; Barone, G.; Marcellini, F.; Dell’Anno, A.; Danovaro, R. Marine microbial-derived molecules and their potential use in cosmeceutical and cosmetic products. Mar. Drugs 2017, 15, 118. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wu, X.; Wang, Q.; Cai, N.; Zhang, H.; Jiang, Z.; Wan, M.; Oda, T. Immunomodulatory effects of alginate oligosaccharides on murine Macrophage RAW264.7 cells and their structure-activity relationships. J. Agric. Food Chem. 2012, 62, 3168–3176. [Google Scholar] [CrossRef] [PubMed]
- Sable, R.; Parajuli, P.; Jois, S. Peptides, Peptidomimetics, and polypeptides from marine sources: A wealth of natural sources for pharmaceutical applications. Mar. Drugs 2017, 15, 124. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Therkildsen, M.; Aluko, R.E.; Lametsch, R.; Fu, Y.; Therkildsen, M.; Aluko, R.E.; Lametsch, R. Exploration of collagen recovered from animal by-products as a precursor of bioactive peptides: Successes and challenges. Crit. Rev. Food Sci. Nutr. 2018, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Anil, S.; Kim, S.K.; Min, S.S. Marine fish proteins and peptides for cosmeceuticals: A review. Mar. Drugs 2017, 15, 143. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.R.; Wang, B.; Chi, C.F.; Zhang, Q.H.; Gong, Y.D.; Tang, J.J.; Luo, H.Y.; Ding, G.F. Isolation and characterization of acid soluble collagens and pepsin soluble collagens from the skin and bone of Spanish mackerel (Scomberomorous niphonius). Food Hydrocoll. 2013, 31, 103–113. [Google Scholar] [CrossRef]
- Ahmad, M.; Benjakul, S. Extraction and characterisation of pepsin-solubilised collagen from the skin of unicorn leatherjacket (Aluterus monocerous). Food Chem. 2010, 120, 817–824. [Google Scholar] [CrossRef]
- Silva, T.H.; Moreira-Silva, J.; Marques, A.L.P.; Domingues, A.; Bayon, Y.; Reis, R.L. Marine origin collagens and its potential applications. Mar. Drugs 2014, 12, 5881–5901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khong, N.M.H.; Yusoff, F.M.; Jamilah, B.; Basri, M.; Maznah, I.; Chan, K.W.; Armania, N.; Nishikawa, J. Improved collagen extraction from jellyfish (Acromitus hardenbergi) with increased physical-induced solubilization processes. Food Chem. 2018, 251, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Nagai, T.; Tanaka, M. Characterisation of acid-soluble collagen from skin and bone of bigeye snapper (Priacanthus tayenus). Food Chem. 2005, 89, 363–372. [Google Scholar] [CrossRef]
- Sun, L.L.; Hou, H.; Li, B.F.; Zhang, Y. Characterization of acid-and pepsin-soluble collagen extracted from the skin of Nile tilapia (Oreochromis niloticus). Int. J. Biol. Macromol. 2017, 99, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Xhauflaireuhoda, E.; Fontaine, K.; Piérard, G.E. Kinetics of moisturizing and firming effects of cosmetic formulations. Int. J. Cosmet. Sci. 2008, 30, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Coelho, R.C.G.; Marques, A.L.P.; Oliveira, S.M.; Diogo, G.S.; Pirraco, R.P.; Moreira-Silva, J.; Xavier, J.C.; Reis, R.L.; Silva, T.H.; Mano, J.F. Extraction and characterization of collagen from Antarctic and Sub-Antarctic squid and its potential application in hybrid scaffolds for tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 787–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Wang, Y.M.; Chi, C.F.; Luo, H.Y.; Deng, S.G.; Ma, J.Y. Isolation and characterization of collagen and antioxidant collagen peptides from scales of croceine croaker (Pseudosciaena crocea). Mar. Drugs 2013, 11, 4641–4661. [Google Scholar] [CrossRef] [PubMed]
- Schurink, M.; Berkel, W.J.H.V.; Wichers, H.J.; Boeriu, C.G. Novel peptides with tyrosinase inhibitory activity. Peptides 2007, 28, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.M.; Ta, Q.V.; Mendis, E.; Rajapakse, N.; Jung, W.K.; Byun, H.G.; Jeon, Y.J.; Kim, S.K. Phlorotannins in Ecklonia cava extract inhibit matrix metalloproteinase activity. Life Sci. 2006, 79, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Kim, S.K. Bioactive peptides from marine sources as potential anti-inflammatory therapeutics. Curr. Protein Pept. Sci. 2013, 14, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Li, X.Y.; Wang, J.T.; Hu, S.X.; Jiang, Y.D.; Zhong, X.D. Effect of dietary lipid level on growth, feed utilization and body composition of juvenile giant croaker Nibea japonica. Aquaculture 2014, 434, 145–150. [Google Scholar] [CrossRef]
- Yu, F.M.; Zong, C.H.; Jin, S.J.; Zheng, J.W.; Chen, N.; Huang, J.; Chen, Y.; Huang, F.F.; Yang, Z.S.; Tang, Y.P.; et al. Optimization of extraction conditions and characterization of pepsin-solubilised collagen from skin of giant croaker (Nibea japonica). Mar. Drugs 2018, 16, 29. [Google Scholar] [CrossRef] [PubMed]
- Somasundaram, T.; Anguchamy, V.; Muthuvel, A. Isolation and characterization of acid and pepsin-solubilized collagen from the skin of sailfish (Istiophorus platypterus). Food Res. Int. 2013, 54, 1499–1505. [Google Scholar]
- Jongjareonrak, A.; Benjakul, S.; Visessanguan, W.; Nagai, T.; Tanaka, M. Isolation and characterisation of acid and pepsin-solubilised collagens from the skin of Brownstripe red snapper (Lutjanus vitta). Food Chem. 2005, 93, 475–484. [Google Scholar] [CrossRef]
- Zhang, J.J.; Duan, R. Characterisation of acid-soluble and pepsin-solubilised collagen from frog (Rana nigromaculata) skin. Int. J. Biol. Macromol. 2017, 101, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liang, L.; Regenstein, J.M.; Peng, Z. Extraction and characterisation of pepsin-solubilised collagen from fins, scales, skins, bones and swim bladders of bighead carp (Hypophthalmichthys nobilis). Food Chem. 2012, 133, 1441–1448. [Google Scholar] [CrossRef]
- Myllyharju, J.; Nokelainen, M.; Vuorela, A.; Kivirikko, K.I. Expression of recombinant human type I-III collagens in the yeast Pichia pastoris. Biochem. Soc. Trans. 2000, 28, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Araki, Y.; Suzuki, N. Collagen of the skin of ocellate puffer fish (Takifugu rubripes). Food Chem. 2002, 78, 173–177. [Google Scholar] [CrossRef]
- Liu, H.Y.; Li, D.; Guo, S.D. Studies on collagen from the skin of channel catfish (Ictalurus punctaus). Food Chem. 2007, 101, 621–625. [Google Scholar] [CrossRef]
- Sun, L.; Li, B.; Song, W.; Si, L.; Hou, H. Characterization of Pacific cod (Gadus macrocephalus) skin collagen and fabrication of collagen sponge as a good biocompatible biomedical material. Process Biochem. 2017, 63, 229–235. [Google Scholar] [CrossRef]
- Ogawa, M.; Moody, M.W.; Portier, R.J.; Bell, J.; Schexnayder, M.A.; Losso, J.N. Biochemical properties of black drum and sheepshead seabream skin collagen. J. Agric. Food Chem. 2003, 51, 8088–8092. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; An, X.; Yang, F.; Xin, Z.; Zhao, L.; Hu, Q. Isolation and characterisation of collagens from the skin, scale and bone of deep-sea redfish (Sebastes mentella). Food Chem. 2008, 108, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Li, B.; Zhao, X.; Ren, G.; Zhuang, Y.; Hou, H.; Zhang, X.; Chen, L.; Fan, Y. Characterization of acid-soluble collagen from the skin of walleye pollock (Theragra chalcogramma). Food Chem. 2008, 107, 1581–1586. [Google Scholar] [CrossRef]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Fourier transform infrared (FTIR) spectroscopic study of acid soluble collagen and gelatin from skins and bones of young and adult Nile perch (Lates niloticus). Food Chem. 2004, 86, 325–332. [Google Scholar] [CrossRef]
- Liu, H.Y.; Han, J.; Guo, S.D. Characteristics of the gelatin extracted from Channel Catfish (Ictalurus Punctatus) head bones. LWT Food Sci. Technol. 2009, 42, 540–544. [Google Scholar] [CrossRef]
- Isobe, T.; Kato, Y.; Okubo, Y.; Koga, M. Evaluation of patch testing in atopic dermatitis using commercially available environmental antigens. Allergol. Int. 2001, 50, 153–162. [Google Scholar] [CrossRef]
- Faruqi, S.; Wilmot, R.; Wright, C.; Morice, A.H. Serum LDH in chronic cough: A potential marker of airway inflammation. Clin. Respir. J. 2012, 6, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.P.; Yu, F.M.; Zhang, G.M.; Yang, Z.S.; Huang, F.F.; Ding, G.F. A purified serine protease from Nereis virens and its impaction of apoptosis on human lung cancer cells. Molecules 2017, 22, 1123. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.D.; Li, L.; Yi, R.Z.; Xu, N.H.; Gao, R.; Hong, B.H. Extraction and characterization of acid-soluble collagen from scales and skin of tilapia (Oreochromis niloticus). LWT Food Sci. Technol. 2016, 66, 453–459. [Google Scholar] [CrossRef]
- Wang, J.; Pei, X.; Liu, H.; Zhou, D. Extraction and characterization of acid-soluble and pepsin-soluble collagen from skin of loach (Misgurnus anguillicaudatus). Int. J. Biol. Macromol. 2018, 106, 544–550. [Google Scholar] [CrossRef] [PubMed]
| Amino Acid | ASC | PSC [19] |
|---|---|---|
| Aspartic acid | 47 | 43 |
| Threonine | 22 | 20 |
| Serine | 33 | 29 |
| Glutamic acid | 75 | 73 |
| Glycine | 351 | 348 |
| Alanine | 130 | 128 |
| Cysteine | 0 | 0 |
| Valine | 22 | 19 |
| Methionine | 13 | 10 |
| Isoleucine | 10 | 9 |
| Leucine | 27 | 25 |
| Tyrosine | 4 | 3 |
| Phenylalanine | 8 | 6 |
| Histidine | 11 | 8 |
| Lysine | 30 | 30 |
| Arginine | 54 | 51 |
| Proline | 119 | 116 |
| Hydroxyproline | 74 | 75 |
| Imino acid | 194 | 191 |
| Collagen | Element | Cotent (mg/kg) a | National Standard of Edible Gelatin (mg/kg) |
|---|---|---|---|
| ASC | As | 0.55 ± 0.03 | ≤0.8 |
| Pb | 0.31 ± 0.03 | ≤50 | |
| Hg | 0.86 ± 0.05 | ≤50 | |
| PSC | As | 0.42 ± 0.03 | ≤0.8 |
| Pb | 0.34 ± 0.02 | ≤50 | |
| Hg | 1.05 ± 0.06 | ≤50 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Jin, S.; Li, X.; Li, X.; Hu, X.; Chen, Y.; Huang, F.; Yang, Z.; Yu, F.; Ding, G. Physicochemical Properties and Biocompatibility Evaluation of Collagen from the Skin of Giant Croaker (Nibea japonica). Mar. Drugs 2018, 16, 222. https://doi.org/10.3390/md16070222
Tang Y, Jin S, Li X, Li X, Hu X, Chen Y, Huang F, Yang Z, Yu F, Ding G. Physicochemical Properties and Biocompatibility Evaluation of Collagen from the Skin of Giant Croaker (Nibea japonica). Marine Drugs. 2018; 16(7):222. https://doi.org/10.3390/md16070222
Chicago/Turabian StyleTang, Yunping, Shujie Jin, Xiaoyan Li, Xiaojuan Li, Xuyang Hu, Yan Chen, Fangfang Huang, Zuisu Yang, Fangmiao Yu, and Guofang Ding. 2018. "Physicochemical Properties and Biocompatibility Evaluation of Collagen from the Skin of Giant Croaker (Nibea japonica)" Marine Drugs 16, no. 7: 222. https://doi.org/10.3390/md16070222
APA StyleTang, Y., Jin, S., Li, X., Li, X., Hu, X., Chen, Y., Huang, F., Yang, Z., Yu, F., & Ding, G. (2018). Physicochemical Properties and Biocompatibility Evaluation of Collagen from the Skin of Giant Croaker (Nibea japonica). Marine Drugs, 16(7), 222. https://doi.org/10.3390/md16070222






