Cyanobacteria as Nanogold Factories: Chemical and Anti-Myocardial Infarction Properties of Gold Nanoparticles Synthesized by Lyngbya majuscula
Abstract
1. Introduction
2. Materials and Methods
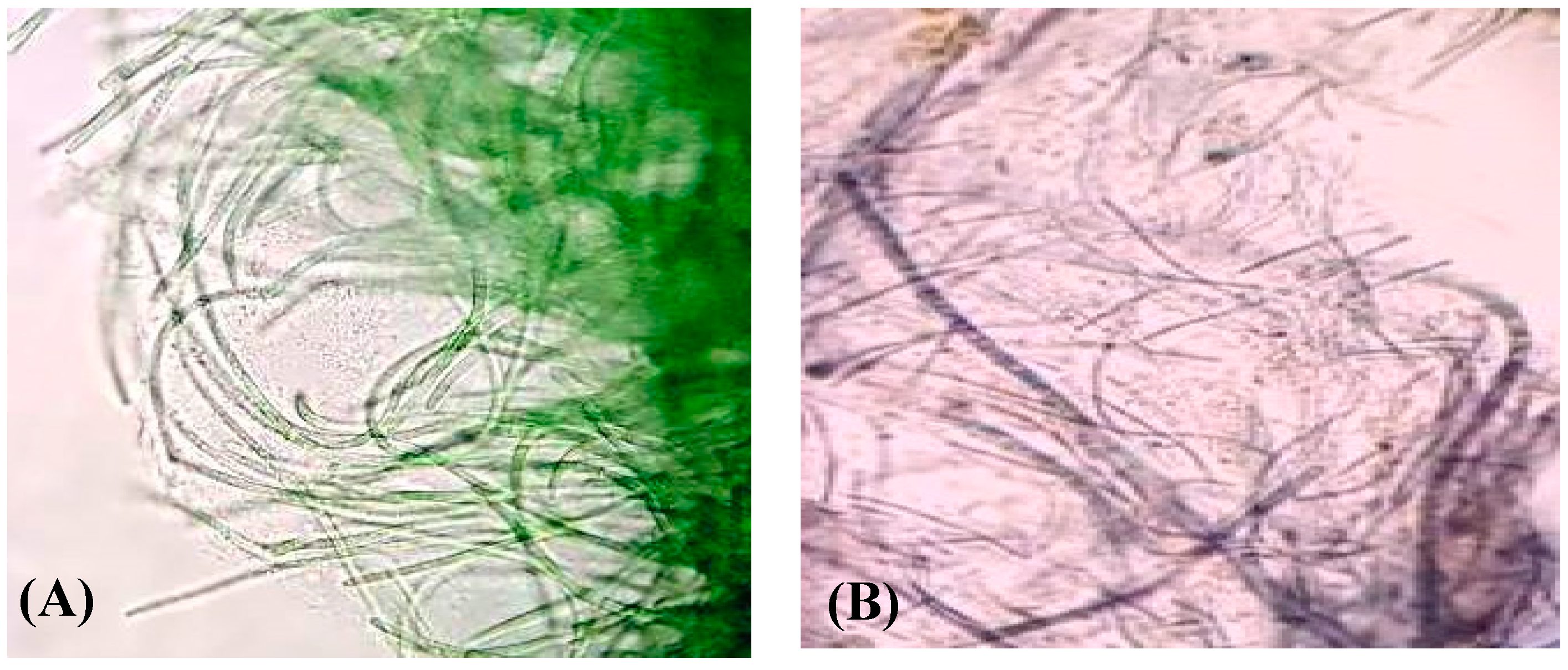
2.1. Isolation and Characterization of the Cyanobacterium Lyngbya majuscula from the Arabian Gulf Region
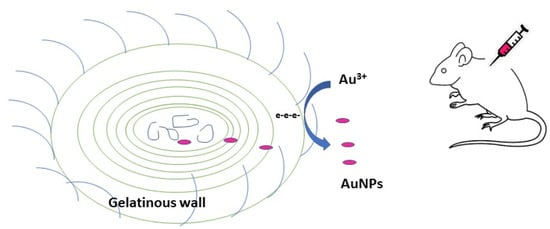
2.2. Synthesis of Gold Nanoparticles by the Isolated Lyngbya majuscula
2.3. Characterization of Gold Nanoparticles Produced
2.3.1. Scanning Electron Microscopy (SEM)
2.3.2. FTIR Analysis
2.3.3. Cyclic Voltammetry
2.4. Determination of the Anti-Myocardial Infarction Activity
2.4.1. Materials
2.4.2. Extraction of the Cyanobacteria Lyngbya majuscula
2.4.3. Preparation of Gold Nanoparticle Solution for Animal Application
2.4.4. Animals
2.4.5. Experimental Design
2.4.6. Electrocardiogram (ECG) and Blood Pressure (BP) Recording and Measurement
2.4.7. Tissue Handling and Biochemical Estimation
2.4.8. Determination of Cardiac Marker Enzymes
2.4.9. Estimation of Antioxidant Activity
2.5. Statistical Analysis
3. Results
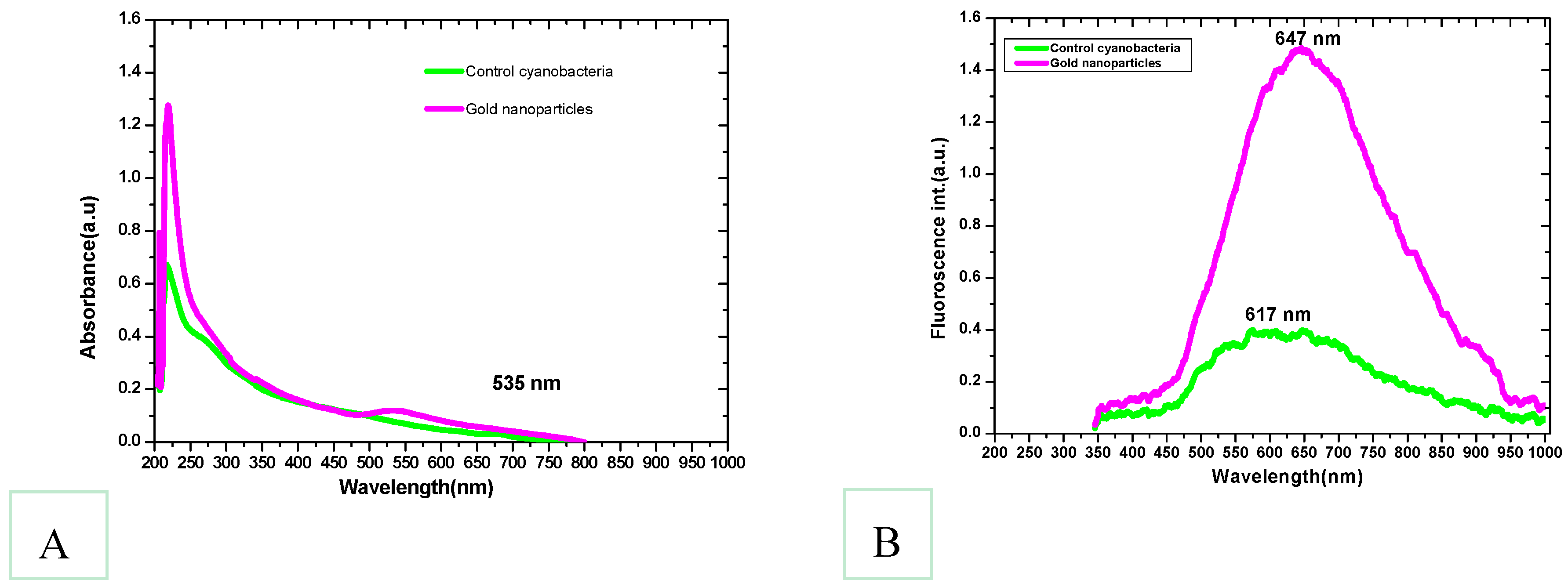
3.1. Synthesis of Gold Nanoparticles by the Isolated Lyngbya majuscula
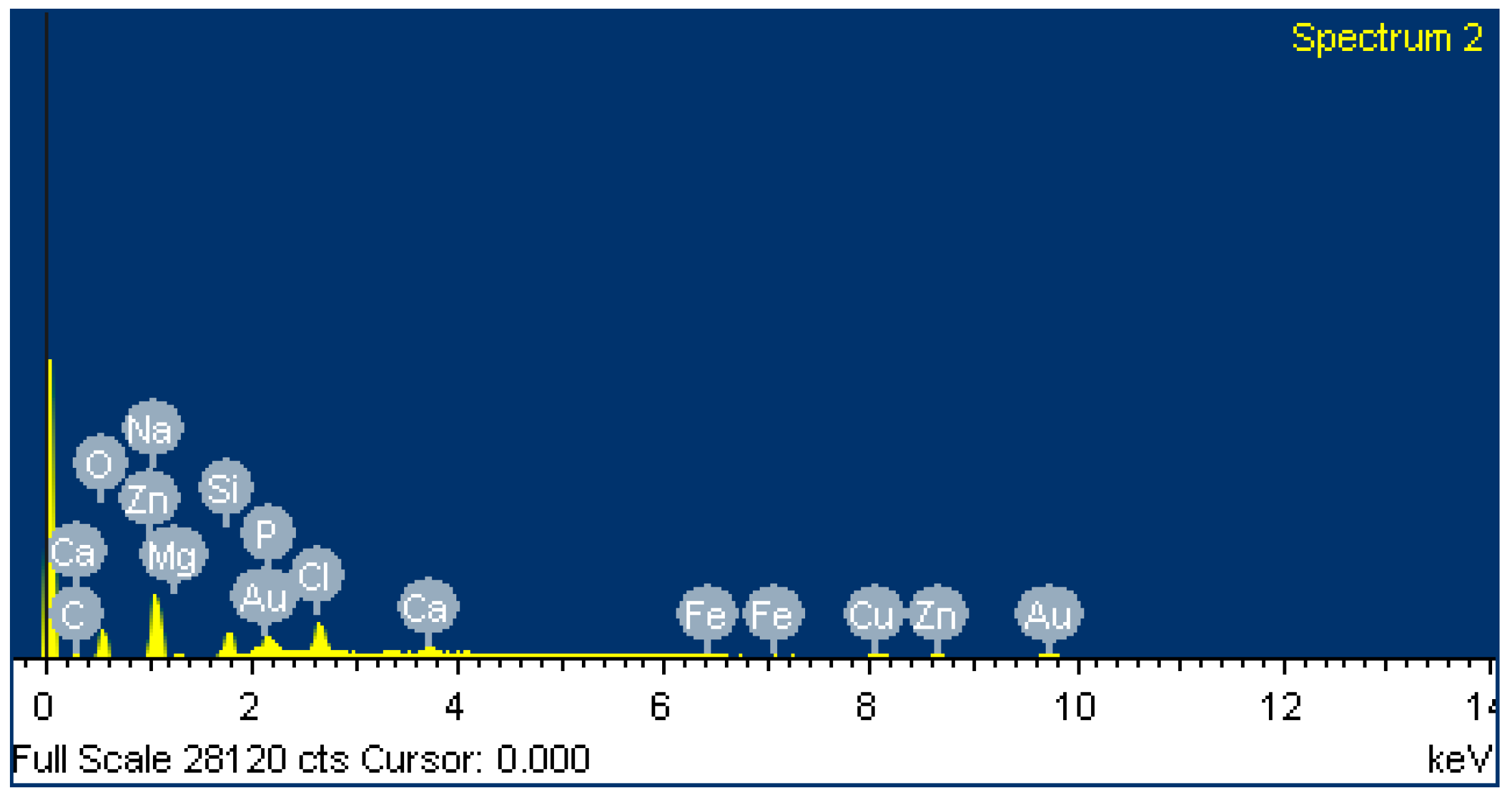
3.2. Energy-Dispersive X-ray Spectroscopy (EDX Images)
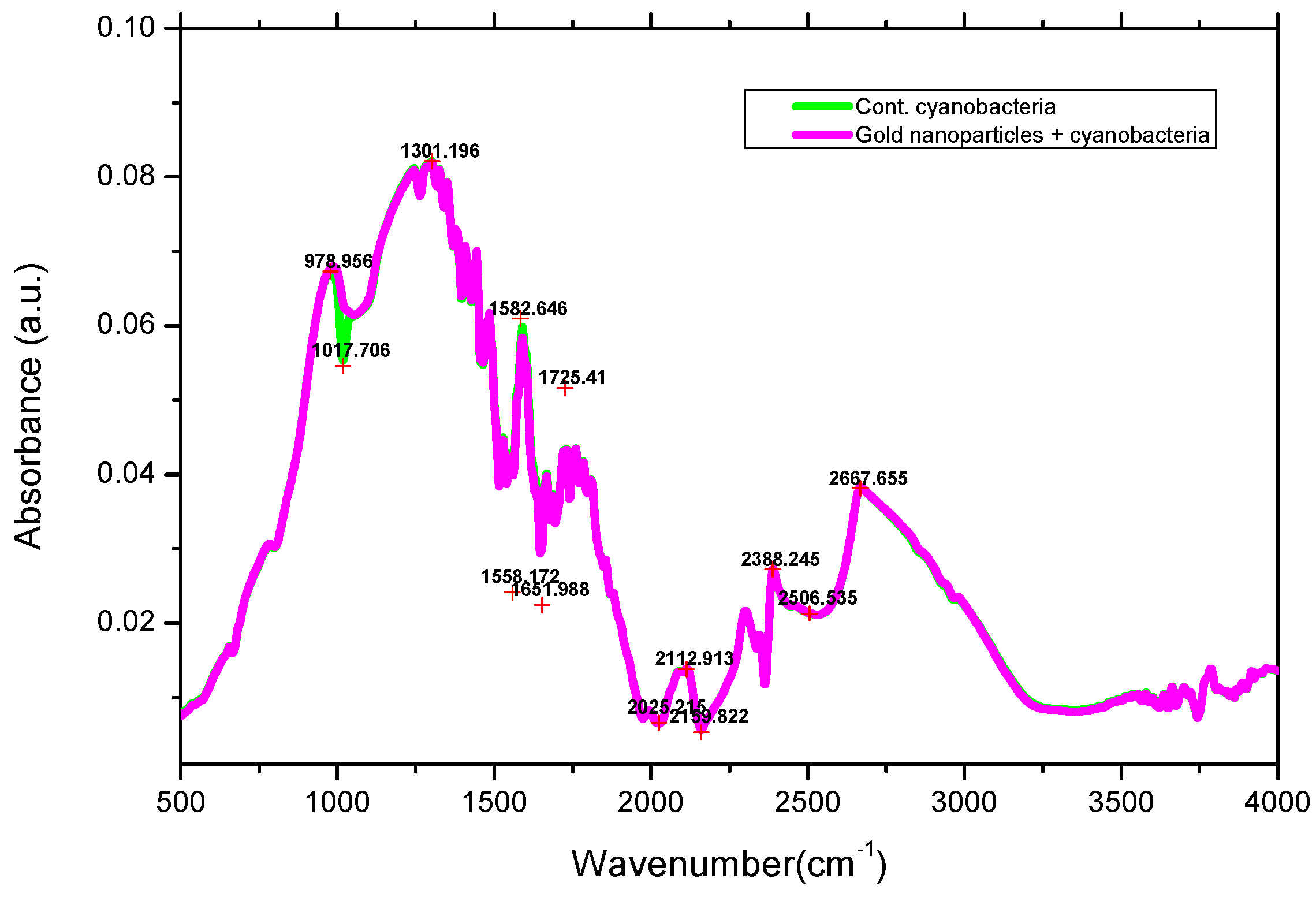
3.3. FTIR Spectra
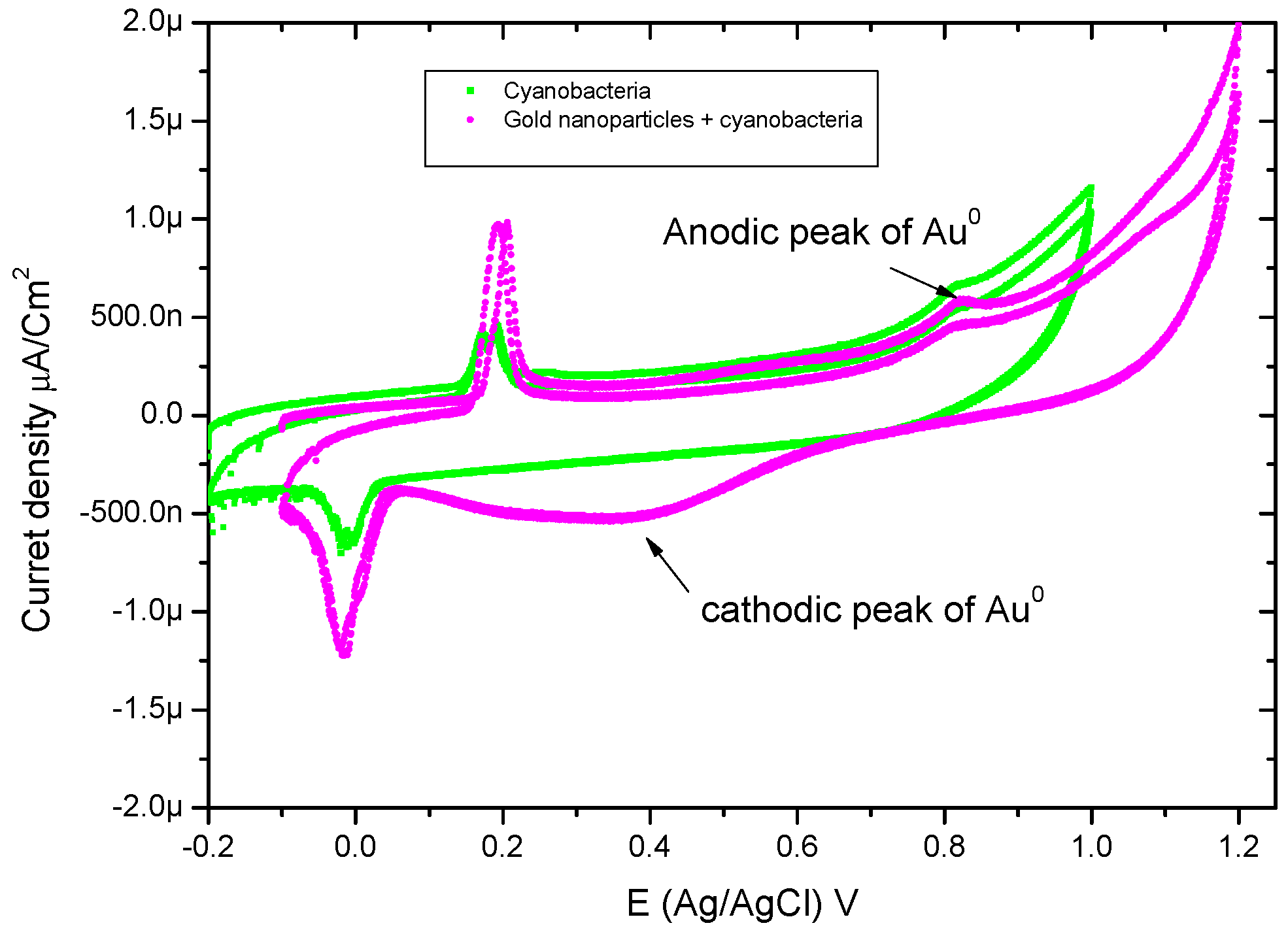
3.4. Cyclic-Voltammetry
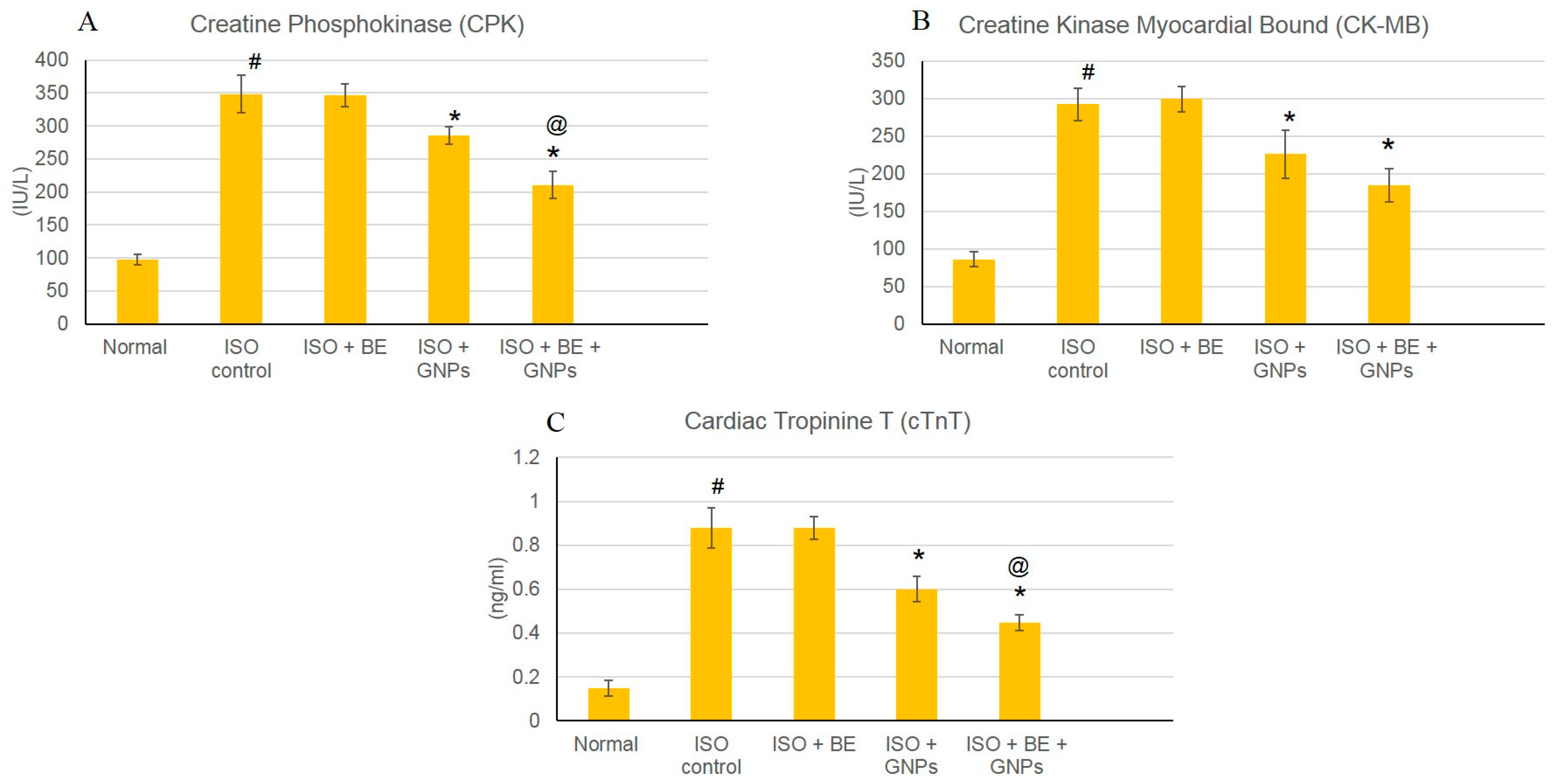
3.5. Effect of Cyanobacterial Extract, GNPs and their Combination on Cardiac Marker Enzymes
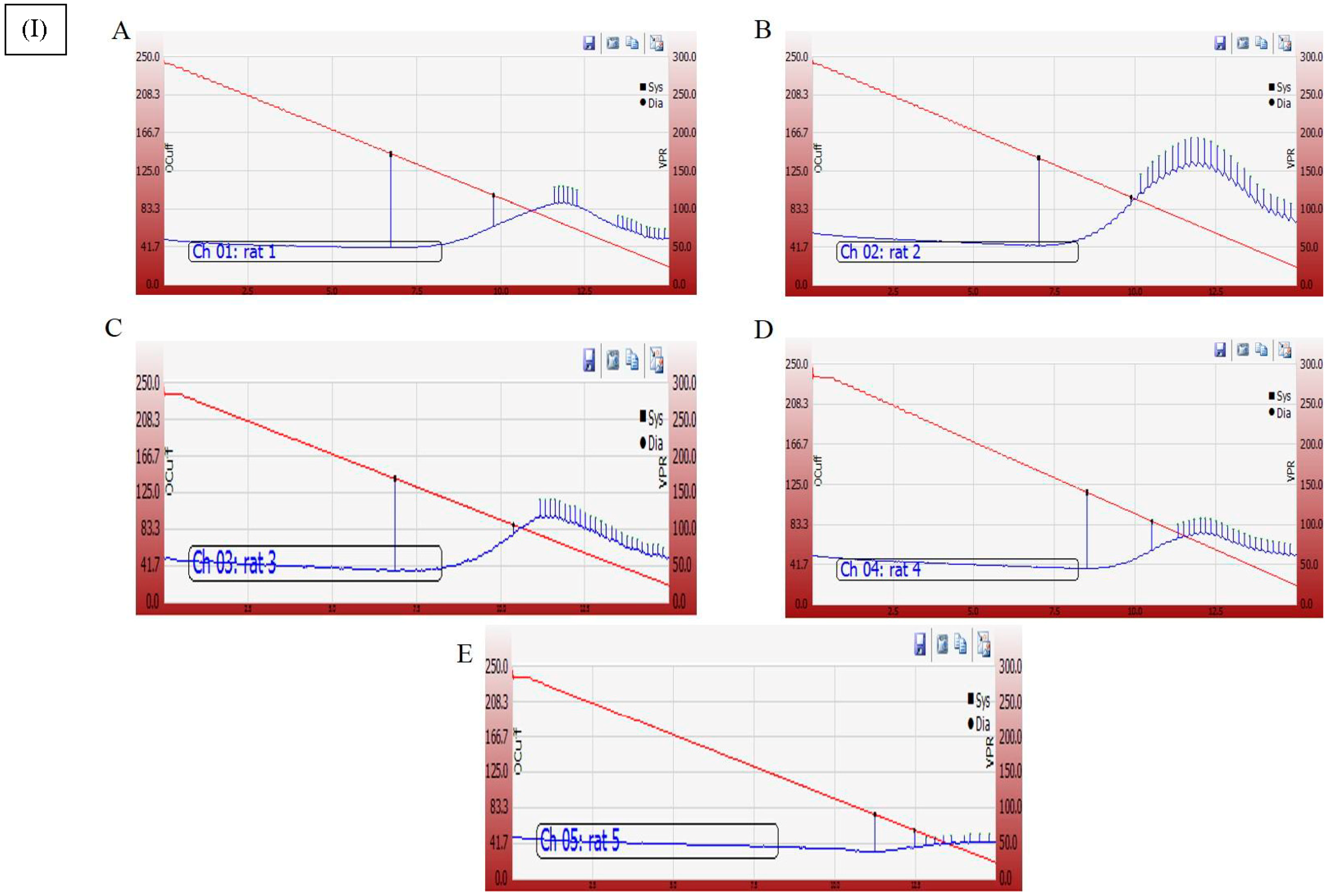
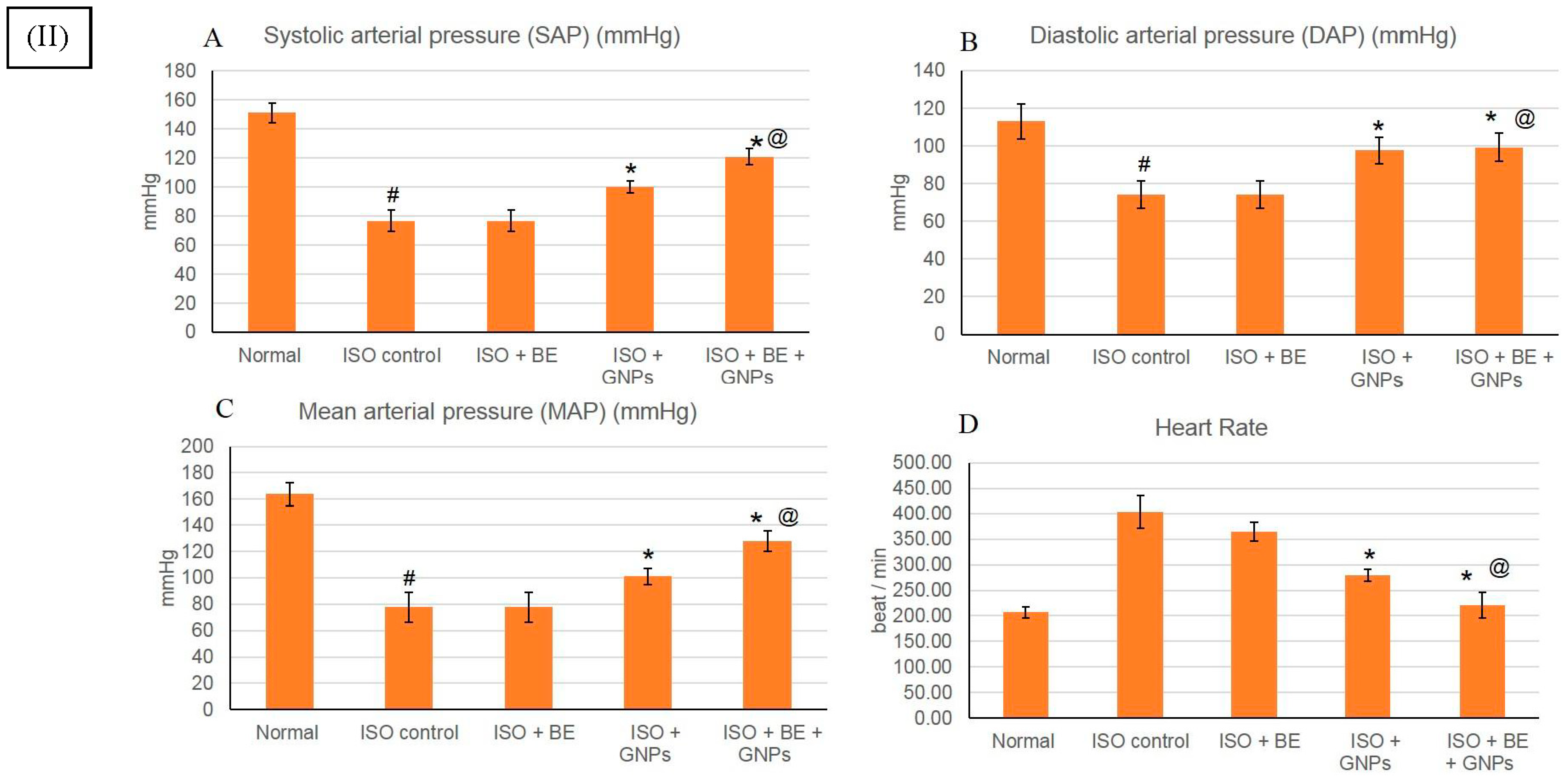
3.6. Effect of Cyanobacterial Extract, GNPs and Their Combination on Heart Rate and Blood Pressure Indices Recording and Measurement
3.7. Effect of Cyanobacterial Extract, GNPs and Their Combination on Electrocardiographic Trace Recording and Measurement
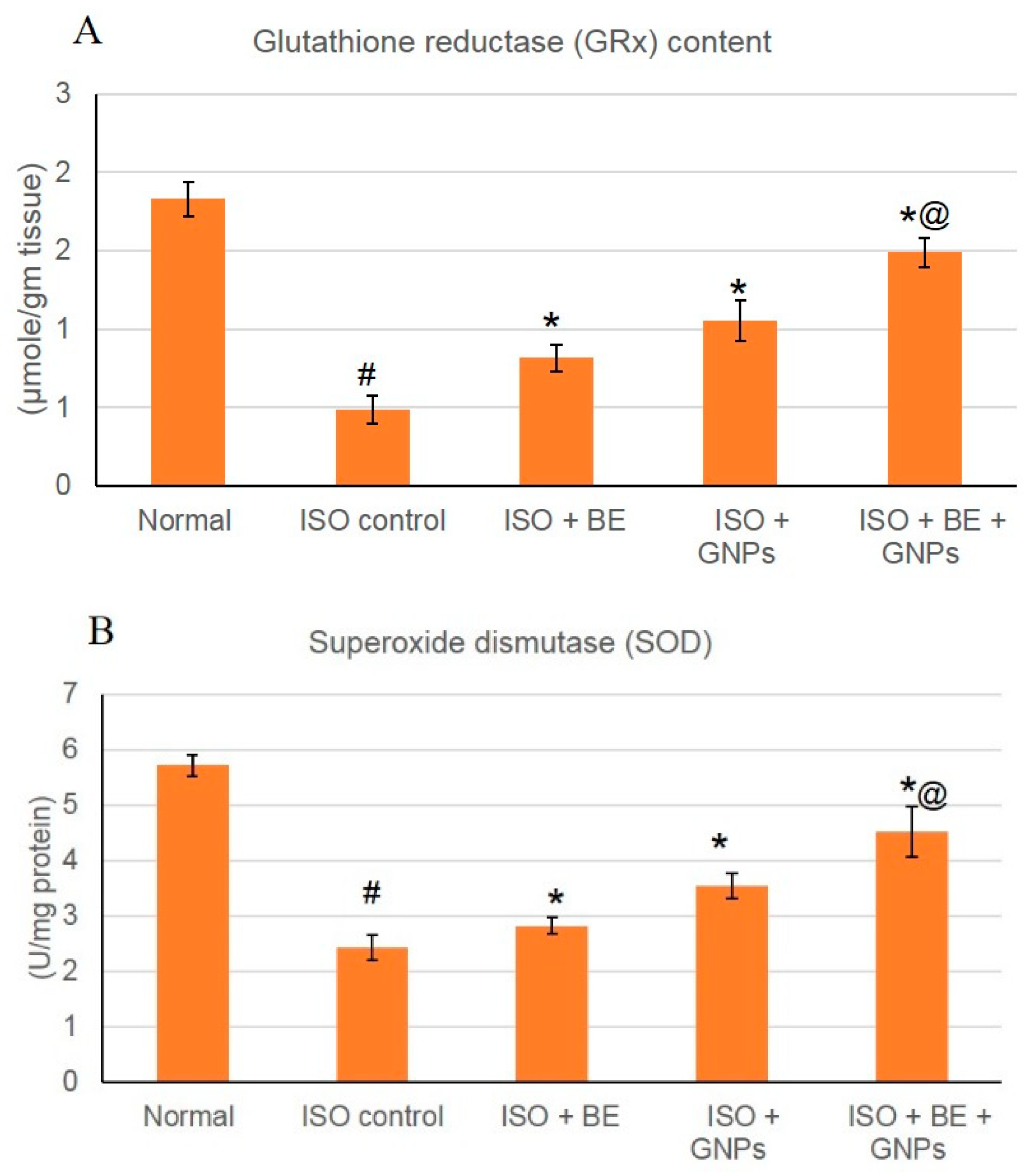
3.8. Effect of Cyanobacterial Extract, GNPs and Their Combination on Lipid Peroxidation and the Activities of Antioxidant Enzymes in ISO-Induced MI in Rats
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Leliaert, F.; Smith, D.R.; Moreau, H.; Herron, M.D.; Verbruggen, H.; Delwiche, C.F.; De Clerck, O. Phylogeny and molecular evolution of the green algae. Crit. Rev. Plant Sci. 2012, 31, 1–46. [Google Scholar] [CrossRef]
- Al-Fredan, M.A.; Fathi, A.A. Preliminary survey of edaphic algae in al-hasa region, Saudi Arabia. Pak. J. Biol. Sci. 2007, 10, 3210–3214. [Google Scholar] [PubMed]
- Gadd, G.M.; Pan, X. Biomineralization, Bioremediation and Biorecovery of Toxic Metals and Radionuclides; Taylor & Francis: Abingdon, UK, 2016. [Google Scholar]
- Lengke, M.F.; Ravel, B.; Fleet, M.E.; Wanger, G.; Gordon, R.A.; Southam, G. Mechanisms of gold bioaccumulation by filamentous cyanobacteria from gold (III)- chloride complex. Environ. Sci. Technol. 2006, 40, 6304–6309. [Google Scholar] [CrossRef] [PubMed]
- Lengke, M.F.; Fleet, M.E.; Southam, G. Biosynthesis of silver nanoparticles by filamentous cyanobacteria from a silver(I) nitrate complex. Langmuir 2007, 23, 2694–2699. [Google Scholar] [CrossRef] [PubMed]
- Vahabi, K.; Mansoori, G.A.; Karimi, S. Biosynthesis of silver nanoparticles by fungus Trichoderma reesei (a route for large-scale production of AgNPs). Insciences J. 2011, 1, 65–79. [Google Scholar] [CrossRef]
- Parial, D.; Patra, H.K.; Roychoudhury, P.; Dasgupta, A.K.; Pal, R. Gold nanorod production by cyanobacteria—A green chemistry approach. J. Appl. Phycol. 2012, 24, 55–60. [Google Scholar] [CrossRef]
- Omajali, J.B.; Mikheenko, I.P.; Merroun, M.L.; Wood, J.; Macaskie, L.E. Characterization of intracellular palladium nanoparticles synthesized by Desulfovibrio desulfuricans and Bacillus benzeovorans. J. Nanopart. Res. 2015, 17, 264. [Google Scholar] [CrossRef] [PubMed]
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef] [PubMed]
- Springer, T.; Chadtova Song, X.; Ermini, M.L.; Lamacova, J.; Homola, J. Functional gold nanoparticles for optical affinity biosensing. Anal. Bioanal. Chem. 2017, 409, 4087–4097. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Chang, Y.; Zhou, B.; Wang, X.; Liu, L. Gold nanoparticles-based electrochemical method for the detection of protein kinase with a peptide-like inhibitor as the bioreceptor. Int. J. Nanomed. 2017, 12, 1905–1915. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Zheng, Z.; Xu, Y.; Lin, J.; Chen, G.; Weng, C.; Lin, D.; Qiu, S.; Cheng, M.; Huang, Z.; et al. A noninvasive cancer detection strategy based on gold nanoparticle surface-enhanced raman spectroscopy of urinary modified nucleosides isolated by affinity chromatography. Biosens. Bioelectron. 2017, 91, 616–622. [Google Scholar] [CrossRef] [PubMed]
- De la Escosura-Muniz, A.; Plichta, Z.; Horak, D.; Merkoci, A. Alzheimer’s disease biomarkers detection in human samples by efficient capturing through porous magnetic microspheres and labelling with electrocatalytic gold nanoparticles. Biosens. Bioelectron. 2015, 67, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bai, X.; Wen, W.; Zhang, X.; Wang, S. Ultrasensitive Electrochemical Biosensor for HIV Gene Detection Based on Graphene Stabilized Gold Nanoclusters with Exonuclease Amplification. ACS Appl. Mater. Interfaces 2015, 7, 18872–18879. [Google Scholar] [CrossRef] [PubMed]
- De la Escosura-Muniz, A.; Maltez-da Costa, M.; Sanchez-Espinel, C.; Diaz-Freitas, B.; Fernandez-Suarez, J.; Gonzalez-Fernandez, A.; Merkoci, A. Gold nanoparticle-based electrochemical magnetoimmunosensor for rapid detection of anti-hepatitis B virus antibodies in human serum. Biosens. Bioelectron. 2010, 26, 1710–1714. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Kim, E.B.; Lee, S.W.; Cheon, S.A.; Kim, H.J.; Lee, J.; Lee, M.K.; Ko, S.; Park, T.J. An easy and sensitive sandwich assay for detection of Mycobacterium tuberculosis Ag85B antigen using quantum dots and gold nanorods. Biosens. Bioelectron. 2017, 87, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Krishnan, S. An electrochemical mass sensor for diagnosing diabetes in human serum. Analyst 2014, 139, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Zhou, Q.; Zhao, K.; Chi, Y.; Liu, B.; Min, X.; Shi, Z.; Zou, B.; Cui, L. Detection of influenza viruses by coupling multiplex reverse-transcription loop-mediated isothermal amplification with cascade invasive reaction using nanoparticles as a sensor. Int. J. Nanomed. 2017, 12, 2645–2656. [Google Scholar] [CrossRef] [PubMed]
- Ashokkumar, T.; Prabhu, D.; Geetha, R.; Govindaraju, K.; Manikandan, R.; Arulvasu, C.; Singaravelu, G. Apoptosis in liver cancer (HepG2) cells induced by functionalized gold nanoparticles. Colloids Surf. B Biointerfaces 2014, 123, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.G.; Poornachandra, Y.; Chandrasekhar, C. Green synthesis of bacterial mediated anti-proliferative gold nanoparticles: Inducing mitotic arrest (G2/M phase) and apoptosis (intrinsic pathway). Nanoscale 2015, 7, 18738–18750. [Google Scholar] [CrossRef] [PubMed]
- Shahriari, S.; Bakhshi, M.; Shahverdi, A.R.; Berahmeh, A.; Safavifar, F.; Khorramizadeh, M.R. Targeted Intracellular Heat Transfer in Cancer Therapy: Assessment of Asparagine-laminated Gold Nanoparticles in Cell Model of T cell Leukemia. Iran. J. Public Health 2017, 46, 357–367. [Google Scholar] [PubMed]
- Costa Lima, S.A.; Reis, S. Temperature-responsive polymeric nanospheres containing methotrexate and gold nanoparticles: A multi-drug system for theranostic in rheumatoid arthritis. Colloids Surf. B Biointerfaces 2015, 133, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, S.M.; Park, K.H.; Mun, C.H.; Park, Y.B.; Yoo, K.H. Drug-loaded gold/iron/gold plasmonic nanoparticles for magnetic targeted chemo-photothermal treatment of rheumatoid arthritis. Biomaterials 2015, 61, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Fatima, F.; Bajpai, P.; Pathak, N.; Singh, S.; Priya, S.; Verma, S.R. Antimicrobial and immunomodulatory efficacy of extracellularly synthesized silver and gold nanoparticles by a novel phosphate solubilizing fungus Bipolaris tetramera. BMC Microbiol. 2015, 15, 52. [Google Scholar] [CrossRef] [PubMed]
- Penders, J.; Stolzoff, M.; Hickey, D.J.; Andersson, M.; Webster, T.J. Shape-dependent antibacterial effects of non-cytotoxic gold nanoparticles. Int. J. Nanomed. 2017, 12, 2457–2468. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Leejae, S.; Jaiswal, L.; Voravuthikunchai, S.P. Metallic nanoparticles augmented the antibacterial potency of Rhodomyrtus tomentosa acetone extract against Escherichia coli. Microb. Pathog. 2017, 107, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Dykman, L.; Khlebtsov, N. Gold nanoparticles in biomedical applications: Recent advances and perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.G.; Poornachandra, Y.; Mamidyala, S.K. Green synthesis of bacterial gold nanoparticles conjugated to resveratrol as delivery vehicles. Colloids Surf. B Biointerfaces 2014, 123, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Pissuwan, D.; Niidome, T.; Cortie, M.B. The forthcoming applications of gold nanoparticles in drug and gene delivery systems. J. Controll. Release 2011, 149, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Ingle, A.P.; Gupta, I.; Brandelli, A. Bioactivity of noble metal nanoparticles decorated with biopolymers and their application in drug delivery. Int. J. Pharm. 2015, 496, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.; Abdelrahman, S.A.; Salama, A.E. Efficacy of gold nanoparticles against isoproterenol induced acute myocardial infarction in adult male albino rats. Ultrastruct. Pathol. 2017, 41, 168–185. [Google Scholar] [CrossRef] [PubMed]
- Guillard, R.R.L.; Ryther, J.H. Studies of marine planktonic diatoms: I. Cyclotella nana Hustedt, and Detonula confervacea (Cleve) Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Lenartowicz, M.; Marek, P.H.; Madura, I.D.; Lipok, J. Formation of Variously Shaped Gold Nanoparticles by Anabaena laxa. J. Clust. Sci. 2017, 28, 3035–3055. [Google Scholar] [CrossRef]
- Kannan, V.; Subramanian, D. Effect of removal of molybdenum in two cyanobacteria Tolypothrix tenuis (Kutz) Schmidt em. and Mastigocladus laminosus Cohn. Indian J. Microbiol. 1992, 32, 185–187. [Google Scholar]
- Reddy, M.N.; Srivastava, V.; Patil, V. Effect of cadmium, lead and zinc on growth of some cyanobacteria. J. Ecobiol. 2002, 14, 161–167. [Google Scholar]
- Rahman, S. Size and Concentration Analysis of Gold Nanoparticles with Ultraviolet-Visible Spectroscopy. Undergrad. J. Math. Model. 2016, 7. [Google Scholar] [CrossRef]
- Srinath, B.S.; Vittal Rai, R. Biosynthesis of Gold Nanoparticles Using Extracellular Molecules Produced by Enterobacter Aerogenes and Their Catalytic Study. J. Clut. Sci. 2014, 26, 1483–1494. [Google Scholar] [CrossRef]
- Bansal, V.; Rautaray, D.; Ahmad, A.; Sastry, M. Biosynthesis of zirconia nanoparticles using the fungus Fusarium oxysporum. J. Mater. Chem. 2004, 14, 3303–3305. [Google Scholar] [CrossRef]
- Luo, C.-H.; Shanmugam, V.; Yeh, C.-S. Nanoparticle biosynthesis using unicellular and subcellular supports. NPG Asia Mater. 2015, 7, e209. [Google Scholar] [CrossRef]
- Talbott, C.M. Spectroscopic Characterization of Nanoparticles for Potential Drug Discovery. Available online: https://www.americanpharmaceuticalreview.com/media/28/Document/SpectroscopicCharacterizationNanoparticles.pdf (accessed on 28 February 2018).
- Vishwakarma, R.; Dhar, D.W.; Pabbi, S. Formulation of a minimal nutritional medium for enhanced lipid productivity in Chlorella sp. and Botryococcus sp. using Response Surface Methodology. Water Sci. Technol. 2018, 77, 1660–1672. [Google Scholar] [CrossRef] [PubMed]
- Sathish Kumar, K.; Amutha, R.; Palaniappan, A.; Sheela, B. Synthesis of Gold Nanoparticles: An Ecofriendly Approach Using Hansenula anomala. ACS Appl. Mater. Interfaces 2011, 3, 1418–1425. [Google Scholar]
- Lovley, D.R. Extracellular electron transfer: Wires, capacitors, iron lungs, and more. Geobiology 2008, 6, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Singh, O.V. Bio-Nanoparticles: Biosynthesis and Sustainable Biotechnological Implications; John Wiley & Sons: Abingdon, UK, 2015. [Google Scholar]
- Mandal, D.; Bolander, M.E.; Mukhopadhyay, D.; Sarkar, G.; Mukherjee, P. The use of microorganisms for the formation of metal nanoparticles and their application. Appl. Microbiol. Biotechnol. 2006, 69, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Purnama, A.; Al-Barwani, H.H. Sensitivity of hypersaline Arabian Gulf to seawater desalination plants. Appl. Math. Model. 2007, 31, 2347–2354. [Google Scholar] [CrossRef]
- MubarakAli, D.; Arunkumar, J.; Nag, K.H.; SheikSyedIshack, K.A.; Baldev, E.; Pandiaraj, D.; Thajuddin, N. Gold nanoparticles from Pro and eukaryotic photosynthetic microorganisms—Comparative studies on synthesis and its application on biolabelling. Colloids Surf. B Biointerfaces 2013, 103, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Berthold, D.; Puranik, P.; Gantar, M. Screening of cyanobacteria and microalgae for their ability to synthesize silver nanoparticles with antibacterial activity. Biotechnol. Rep. 2015, 5, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, N.; Banerjee, A.; Lahiri, S.; Panda, A.; Ghosh, A.N.; Pal, R. Biorecovery of gold using cyanobacteria and an eukaryotic alga with special reference to nanogold formation—A novel phenomenon. J. Appl. Phycol. 2009, 21, 145. [Google Scholar] [CrossRef]
- Parial, D.; Pal, R. Green Synthesis of Gold Nanoparticles Using Cyanobacteria and their Characterization. Indian J. Appl. Res. 2014, 4, 69–72. [Google Scholar] [CrossRef]
- Gupta, P.; Diwan, B. Bacterial Exopolysaccharide mediated heavy metal removal: A Review on biosynthesis, mechanism and remediation strategies. Biotechnol. Rep. 2017, 13, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Shim, W.-B.; Kim, K.-Y.; Chung, D.-H. Development and validation of a gold nanoparticle immunochromatographic assay (ICG) for the detection of zearalenone. J. Agric. Food Chem. 2009, 57, 4035–4041. [Google Scholar] [CrossRef] [PubMed]
- Daniel, W.L.; Han, M.S.; Lee, J.-S.; Mirkin, C.A. Colorimetric nitrite and nitrate detection with gold nanoparticle probes and kinetic end points. J. Am. Chem. Soc. 2009, 131, 6362–6363. [Google Scholar] [CrossRef] [PubMed]
- Rassaei, L.; Sillanp, M.; Marken, F. Modified carbon nanoparticle-chitosan film electrodes: Physisorption versus chemisorption. Electrochim. Acta 2008, 53, 5732–5738. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Peng, H.; Huang, W.; Zhou, Y.; Yan, D. Facile preparation and characterization of highly antimicrobial colloid Ag or Au nanoparticles. J. Colloid Interface Sci. 2008, 325, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Shrivastav, A.; Jose, D.A.; Mishra, S.K.; Chandrakanth, C.K.; Mishra, S.; Das, A. Colorimetric sensor for triphosphates and their application as a viable staining agent for prokaryotes and eukaryotes. Anal. Chem. 2008, 80, 5312–5319. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, N.E.-A.; Hussein, M.H.; El-Sawah, A.A. Bio-fabrication of silver nanoparticles by phycocyanin, characterization, in vitro anticancer activity against breast cancer cell line and in vivo cytotxicity. Sci. Rep. 2017, 7, 10844. [Google Scholar] [CrossRef] [PubMed]
- Katritzky, A.R.; Ramsden, C.A.; Scriven, E.F.V.; Taylor, R.J.K. (Eds.) Comprehensive Heterocyclic Chemistry III; Elsevier: Oxford, UK, 2008; pp. 635–754. [Google Scholar]
- Taylor, M.S.; Stahl-Timmins, W.; Redshaw, C.H.; Osborne, N.J. Toxic alkaloids in Lyngbya majuscula and related tropical marine cyanobacteria. Harmful Algae 2014, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Davies-Coleman, M.T.; Dzeha, T.M.; Gray, C.A.; Hess, S.; Pannell, L.K.; Hendricks, D.T.; Arendse, C.E. Isolation of homodolastatin 16, a new cyclic depsipeptide from a Kenyan collection of Lyngbya majuscula. J. Nat. Prod. 2003, 66, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Harrigan, G.G.; Luesch, H.; Yoshida, W.Y.; Moore, R.E.; Nagle, D.G.; Biggs, J.; Park, P.U.; Paul, V.J. Tumonoic acids, novel metabolites from a cyanobacterial assemblage of Lyngbya majuscula and Schizothrix calcicola. J. Nat. Prod. 1999, 62, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Luesch, H.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J.; Mooberry, S.L. Isolation, structure determination, and biological activity of Lyngbyabellin A from the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2000, 63, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Moradi-Arzeloo, M.; Farshid, A.A.; Tamaddonfard, E.; Asri-Rezaei, S. Effects of histidine and vitamin C on isoproterenol-induced acute myocardial infarction in rats. Vet. Res. Forum Int. Q. J. 2016, 7, 47–54. [Google Scholar]
- Tripathi, A.; Fang, W.; Leong, D.T.; Tan, L.T. Biochemical Studies of the Lagunamides, Potent Cytotoxic Cyclic Depsipeptides from the Marine Cyanobacterium Lyngbya majuscula. Mar. Drugs 2012, 10, 1126–1137. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Esteve-Adell, I.; Atienzar, P.; Casas, J.A.; Hernandez, P.; Quintana, C. Cucurbit[7]uril-stabilized gold nanoparticles as catalysts of the nitro compound reduction reaction. RSC Adv. 2016, 6, 86309–86315. [Google Scholar] [CrossRef]
- Reimers, J.R.; Ford, M.J.; Halder, A.; Ulstrup, J.; Hush, N.S. Gold surfaces and nanoparticles are protected by Au(0)–thiyl species and are destroyed when Au(I)–thiolates form. Proc. Natl. Acad. Sci. USA 2016, 113, E1424–E1433. [Google Scholar] [CrossRef] [PubMed]
- Spivak, M.Y.; Bubnov, R.V.; Yemets, I.M.; Lazarenko, L.M.; Tymoshok, N.O.; Ulberg, Z.R. Development and testing of gold nanoparticles for drug delivery and treatment of heart failure: A theranostic potential for PPP cardiology. EPMA J. 2013, 4, 20. [Google Scholar] [CrossRef] [PubMed]

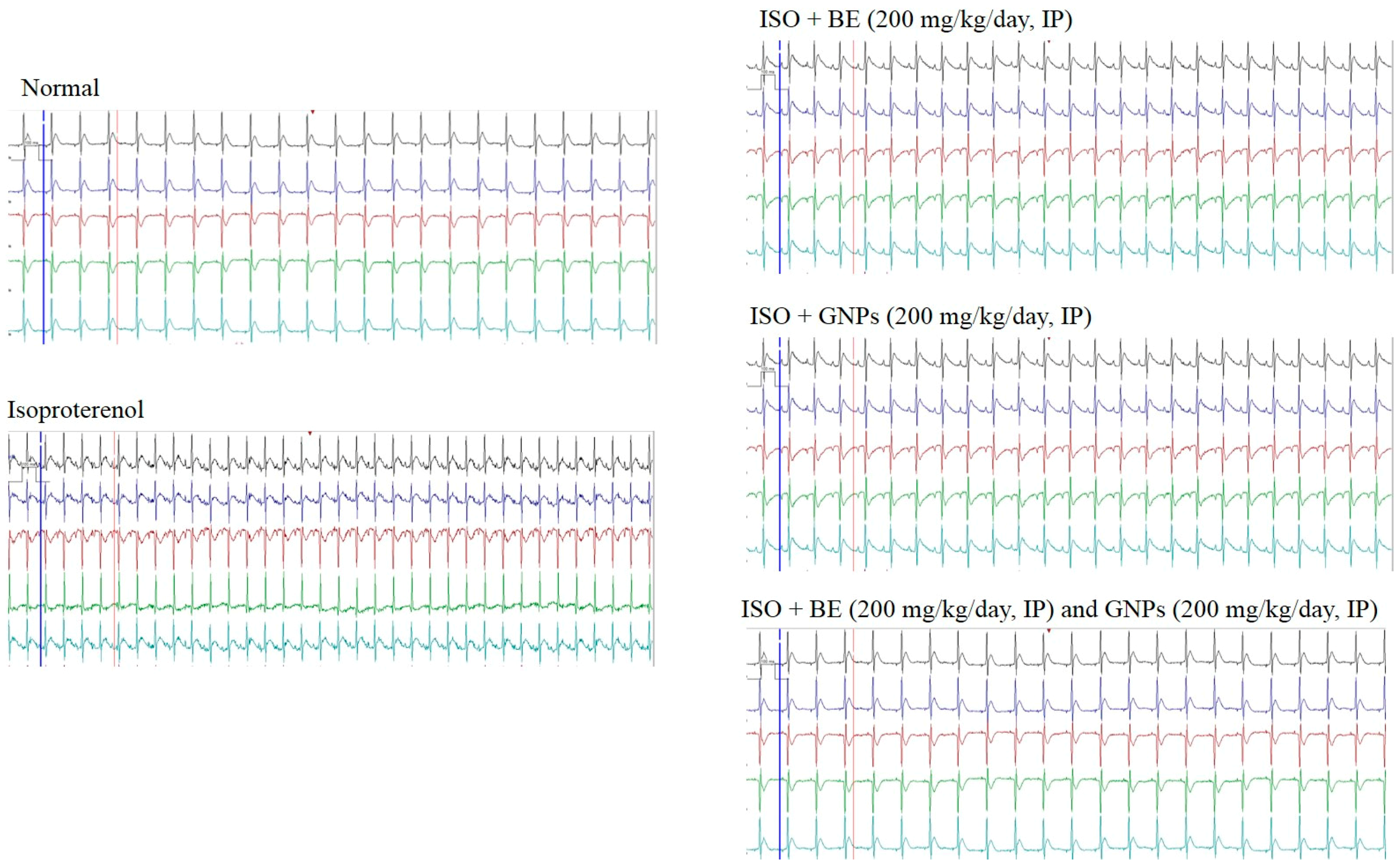
| Normal | ISO Control | ISO + BE | ISO + GNPs | ISO + BE+ GMPs | |
|---|---|---|---|---|---|
| ST elevation (mV) | 0.027 ± 0.002 | 0.184 ± 0.013 # | 0.145 ± 0.029 | 0.064 ± 0.007 * | 0.060 ± 0.009 * |
| QRS complex (s) | 0.042 ± 0.001 | 0.027 ± 0.002 # | 0.027 ± 0.002 | 0.038 ± 0.001 * | 0.042 ± 0.000 * |
| QT interval (s) | 0.045 ± 0.004 | 0.088 ± 0.008 # | 0.069 ± 0.005 | 0.061 ± 0.003 * | 0.061 ± 0.004 * |
| P-R interval (s) | 0.232 ± 0.023 | 0.160 ± 0.003 # | 0.197 ± 0.028 | 0.212 ± 0.019 * | 0.222 ± 0.016 * |
| R-R interval (s) | 0.230 ± 0.031 | 0.151 ± 0.004 # | 0.180 ± 0.003 | 0.199 ± 0.010 * | 0.208 ± 0.014 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakir, E.M.; Younis, N.S.; Mohamed, M.E.; El Semary, N.A. Cyanobacteria as Nanogold Factories: Chemical and Anti-Myocardial Infarction Properties of Gold Nanoparticles Synthesized by Lyngbya majuscula. Mar. Drugs 2018, 16, 217. https://doi.org/10.3390/md16060217
Bakir EM, Younis NS, Mohamed ME, El Semary NA. Cyanobacteria as Nanogold Factories: Chemical and Anti-Myocardial Infarction Properties of Gold Nanoparticles Synthesized by Lyngbya majuscula. Marine Drugs. 2018; 16(6):217. https://doi.org/10.3390/md16060217
Chicago/Turabian StyleBakir, Esam M., Nancy S. Younis, Maged E. Mohamed, and Nermin A. El Semary. 2018. "Cyanobacteria as Nanogold Factories: Chemical and Anti-Myocardial Infarction Properties of Gold Nanoparticles Synthesized by Lyngbya majuscula" Marine Drugs 16, no. 6: 217. https://doi.org/10.3390/md16060217
APA StyleBakir, E. M., Younis, N. S., Mohamed, M. E., & El Semary, N. A. (2018). Cyanobacteria as Nanogold Factories: Chemical and Anti-Myocardial Infarction Properties of Gold Nanoparticles Synthesized by Lyngbya majuscula. Marine Drugs, 16(6), 217. https://doi.org/10.3390/md16060217