Anti-Obesity Effect of Chitosan Oligosaccharide Capsules (COSCs) in Obese Rats by Ameliorating Leptin Resistance and Adipogenesis
Abstract
1. Introduction
2. Results
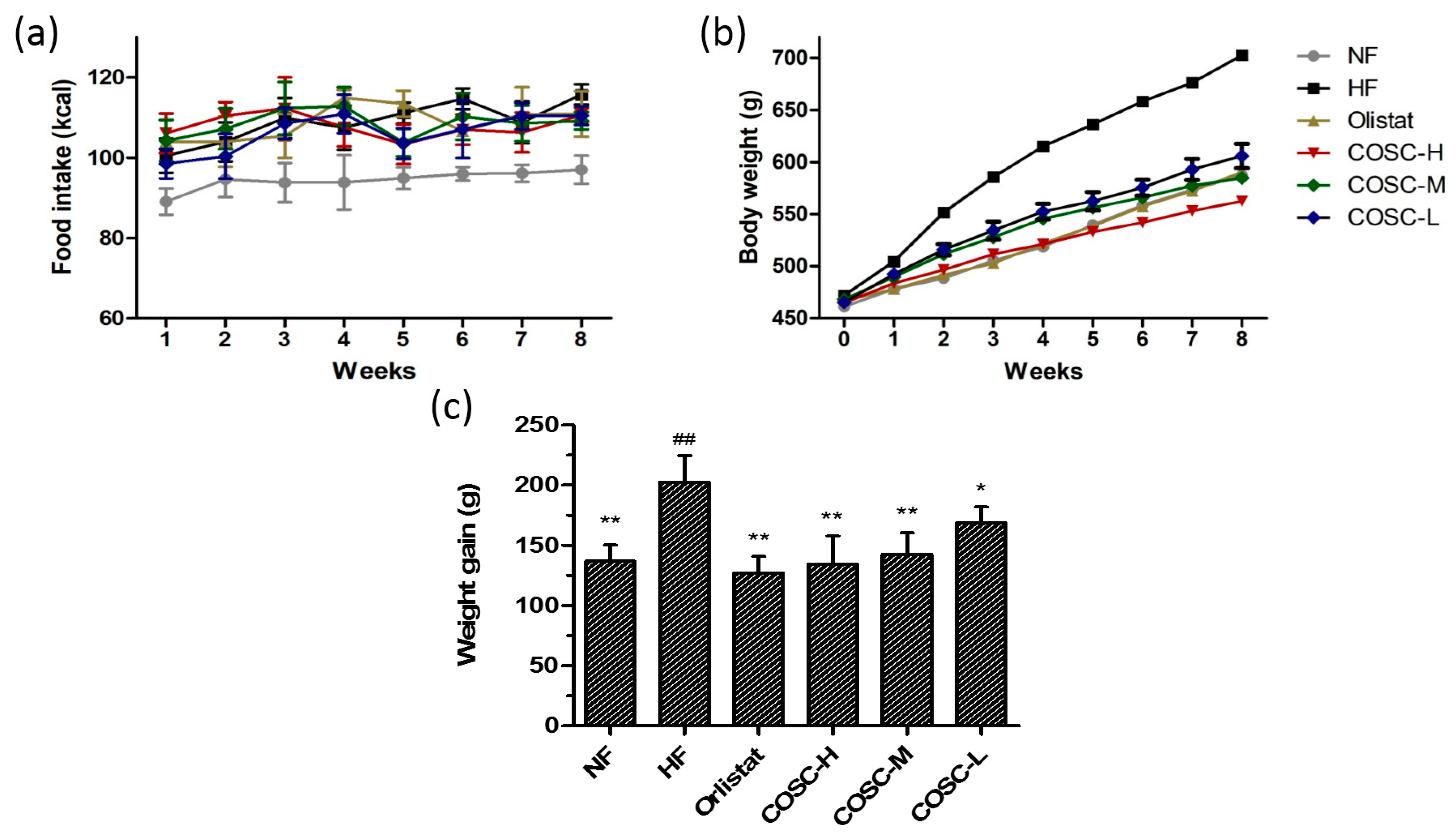
2.1. Food Intake, Body Weight, and Body Weight Gain
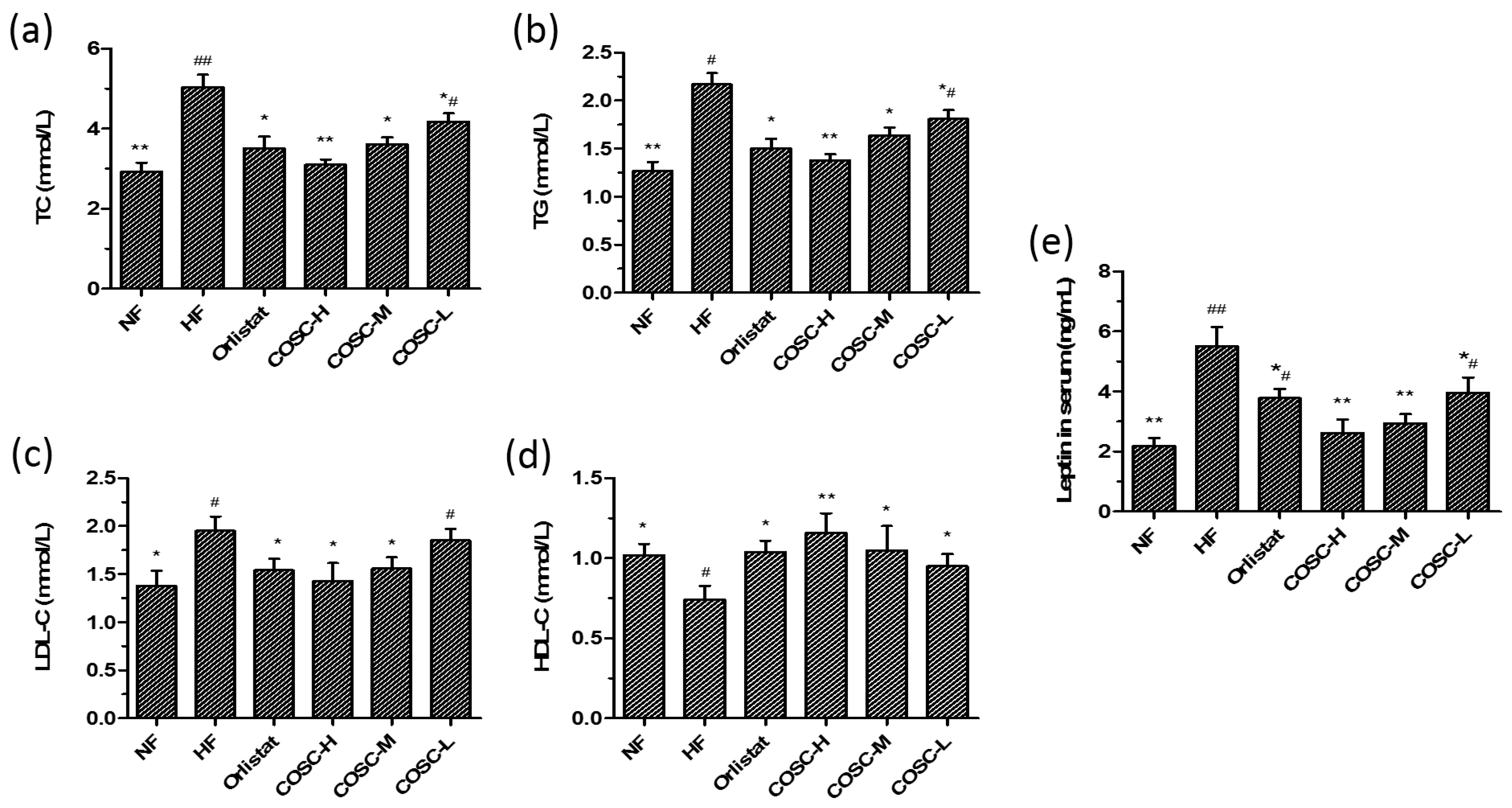
2.2. Serum Lipids and Leptin
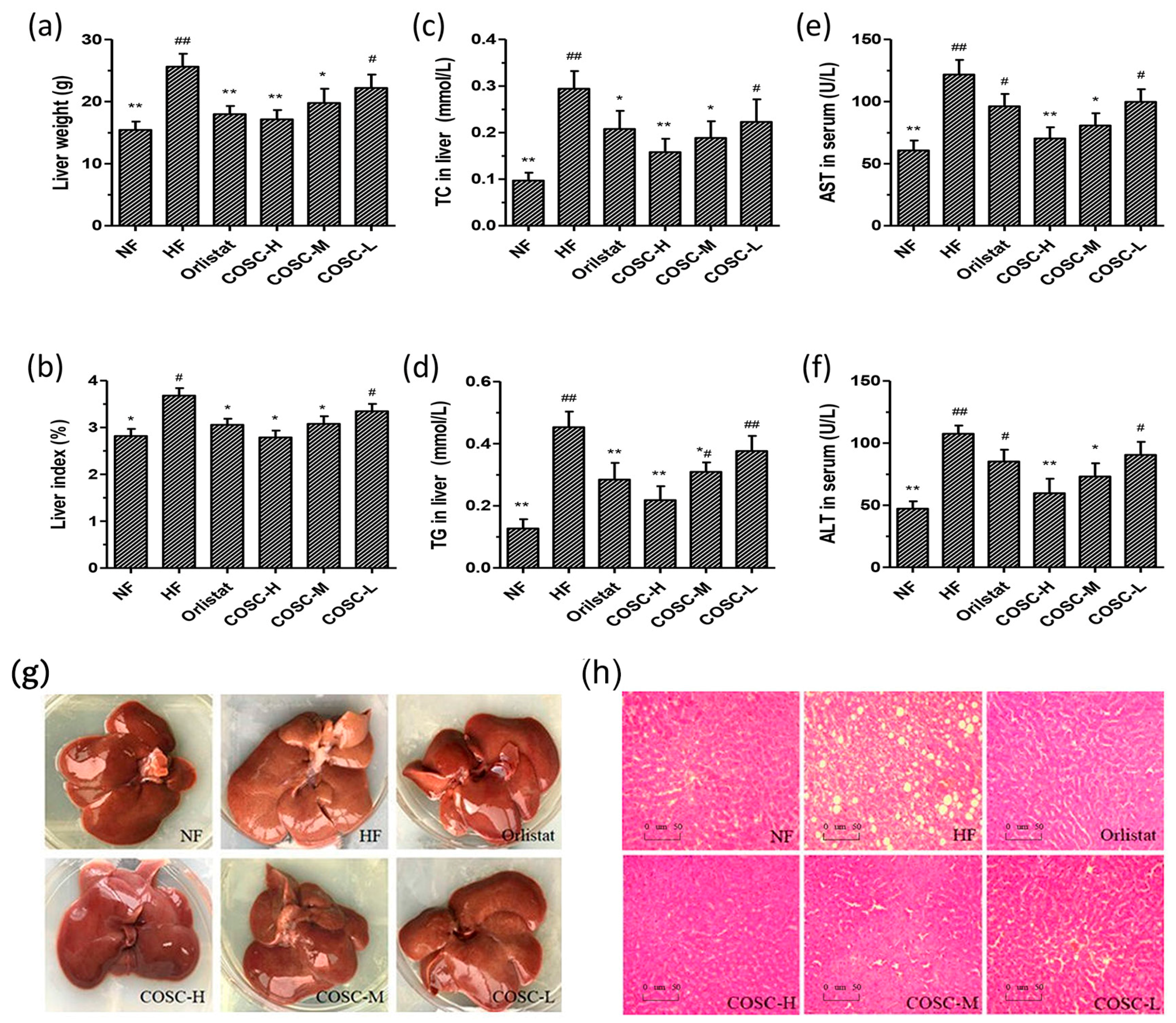
2.3. COSCs Facilitate Hepatoprotective Effects
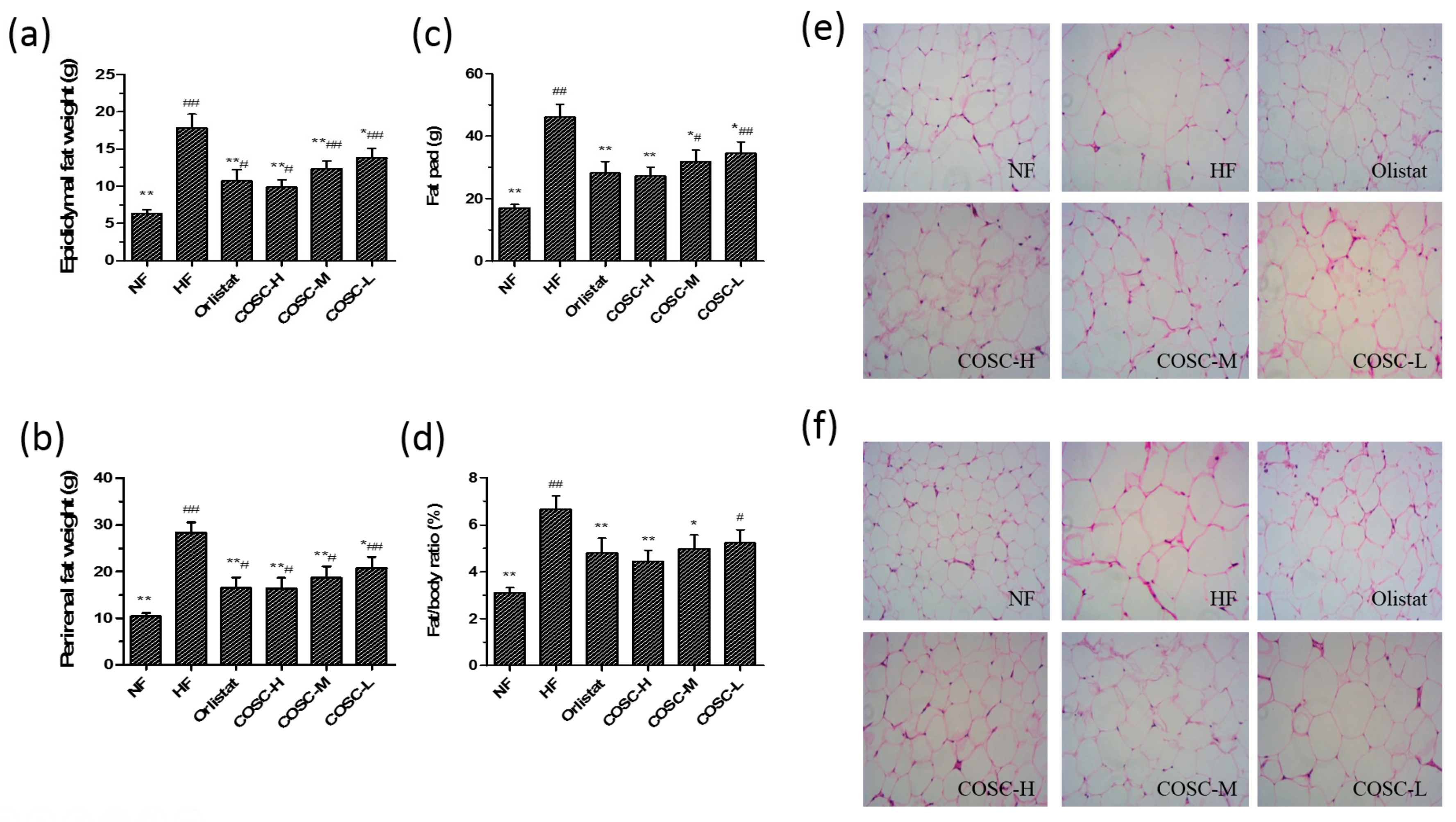
2.4. Fat Pad and Fat/Body Ratio
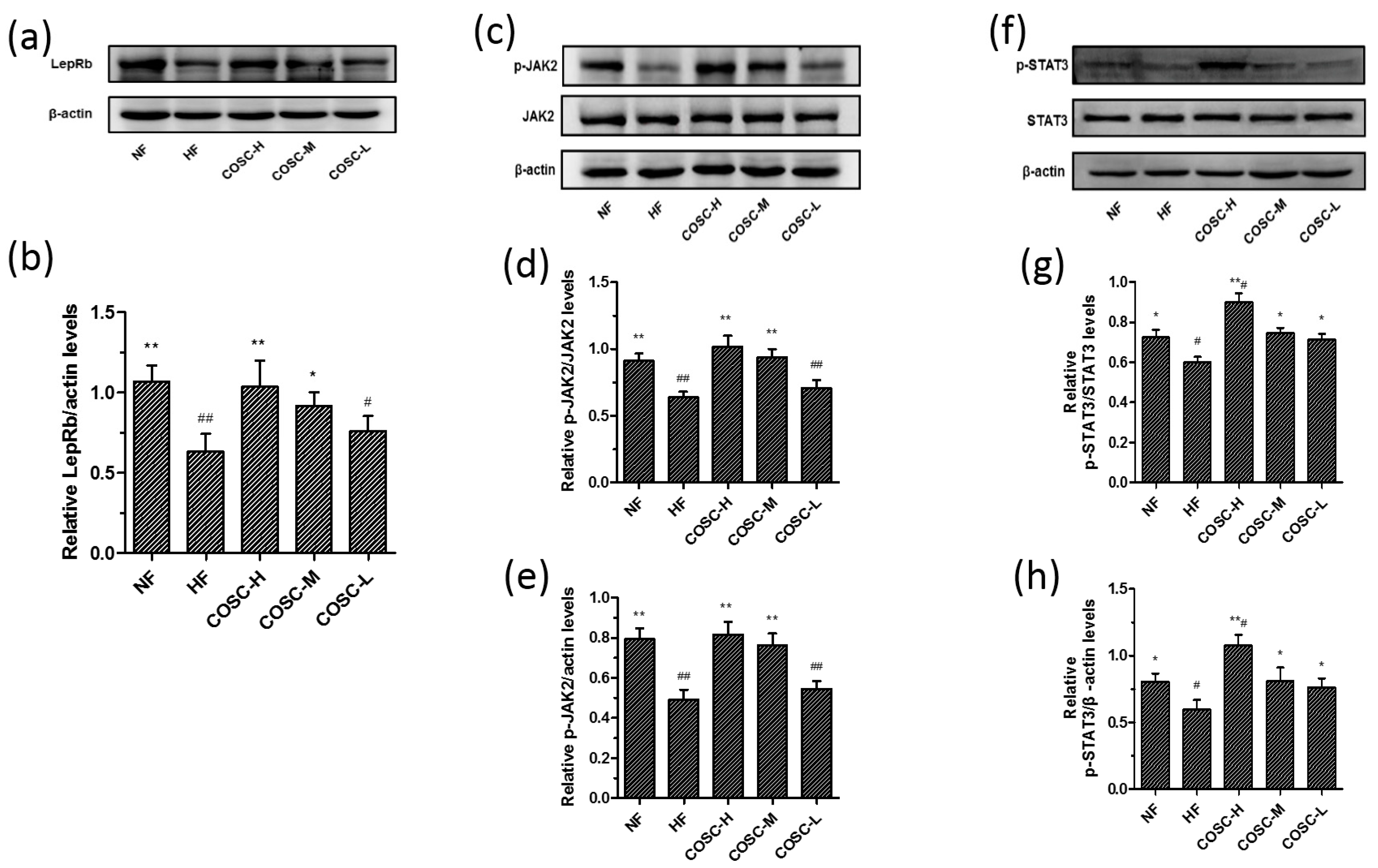
2.5. COSCs Activate Leptin Signaling Transduction
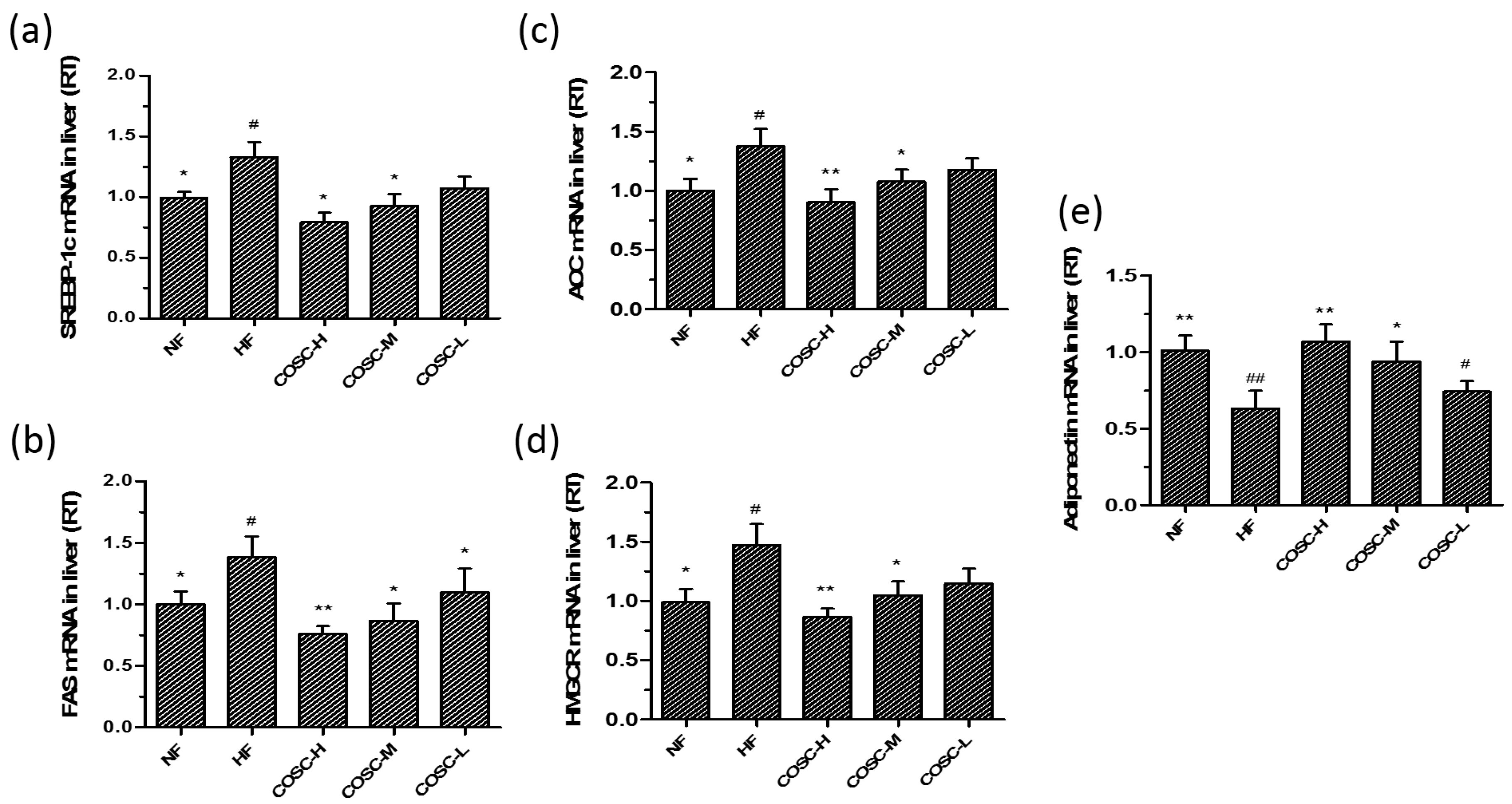
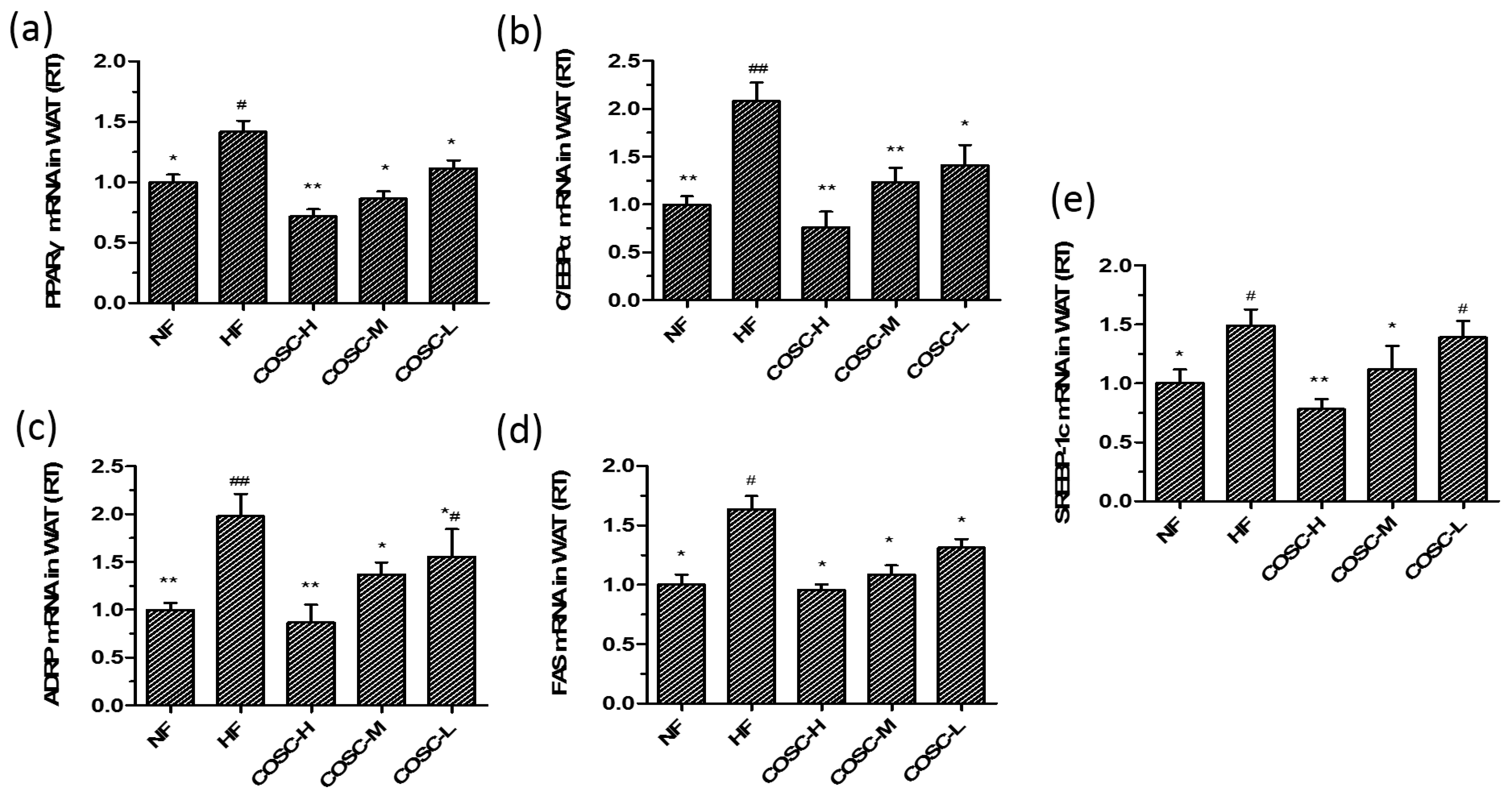
2.6. COSCs Regulate the Expression of Lipogenesis-Related Genes
3. Discussion
4. Materials and Methods
4.1. Materials and Supplies
4.2. COSCs Preparation
4.3. Animals and Diets
4.4. Experimental Design
4.4.1. Determination of Food Intake and Weight Gain
4.4.2. Determination of Serum Lipid and Leptin Levels
4.4.3. Determination of Fat Pad, Fat/Body Ratio, and Visceral Index
4.4.4. Determination of Hepatic Lipids, AST, and ALT
4.5. Histology of the Different Tissues
4.6. Western Blotting Assays
4.7. Reverse Transcription-Polymerase Chain Reaction (RT-PCR) Analysis
4.8. Statistical Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ye, Z.J.; Liu, G.H.; Guo, J.; Su, Z.Q. Hypothalamic endoplasmic reticulum stress as a key mediator of obesity-induced leptin resistance. Obes. Rev. 2018, 19, 770–785. [Google Scholar] [CrossRef] [PubMed]
- Franks, P.W.; McCarthy, M.I. Exposing the exposures responsible for type 2 diabetes and obesity. Science 2016, 354, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Onakpoya, I.J.; Heneghan, C.J.; Aronson, J.K. Post-marketing withdrawal of anti-obesity medicinal products because of adverse drug reactions: A systematic review. BMC Med. 2016, 14, 191. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, G.; Apovian, C. Future pharmacotherapy for obesity: New anti-obesity drugs on the horizon. Curr. Obes. Rep. 2018, 7, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Gallwitz, B. Novel oral anti-obesity agents: New perspectives with lorcaserin? Drugs 2013, 73, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Zhong, X.B.; Tao, Y.; Wu, S.H.; Su, Z.Q. Effects of chitosan and water-soluble chitosan micro- and nanoparticles in obese rats fed a high-fat diet. Int. J. Nanomed. 2012, 7, 4069–4076. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.R.; Gao, B.; Tao, Y.; Guo, J.; Su, Z.Q. Antiobese effects of capsaicin-chitosan microsphere (CCMS) in obese rats induced by high fat diet. J. Agric. Food Chem. 2014, 62, 1866–1874. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Yuan, X.; Cheng, G.; Jiao, S.; Feng, C.; Zhao, X.; Yin, H.; Du, Y.; Liu, H. Chitosan oligosaccharides improve the disturbance in glucose metabolism and reverse the dysbiosis of gut microbiota in diabetic mice. Carbohydr. Polym. 2018, 190, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.L.; Chen, J.; Cao, P.Q.; Pan, H.T.; Ding, C.; Xiao, T.C.; Zhang, P.F.; Guo, J.; Su, Z.Q. Anti-obesity effect of glucosamine and chitosan oligosaccharide in high-fat diet-induced obese rats. Mar. Drugs 2015, 13, 2732–2756. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.T.; Yang, Q.Y.; Huang, G.D.; Ding, C.; Cao, P.Q.; Huang, L.L.; Xiao, T.C.; Guo, J.; Su, Z.Q. Hypolipidemic effects of chitosan and its derivatives in hyperlipidemic rats induced by a high-fat diet. Food Nutr. Res. 2016, 60, 31137. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, J.; Chen, L.; Wu, Q.; Yu, C. Chitosan oligosaccharides enhance lipid droplets via down-regulation of PCSK9 gene expression in HepG2 cells. Exp. Cell Res. 2018, 366, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.C.; Sereno, J.; Garrido, P.; Parada, B.; Cunha, M.F.X.; Reis, F.; Pintado, M.E.; Santos-Silva, A. Inhibition of bladder tumor growth by chitooligosaccharides in an experimental carcinogenesis model. Mar. Drugs 2012, 10, 2661–2675. [Google Scholar] [CrossRef] [PubMed]
- Muanprasat, C.; Chatsudthipong, V. Chitosan oligosaccharide: Biological activities and potential therapeutic applications. Pharmacol. Therapeut. 2017, 170, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.H.; Jiang, Y.; Guo, J.; Su, Z.Q. Natural products with anti-obesity effects and different mechanisms of action. J. Agric. Food Chem. 2016, 64, 9571–9585. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.H.; Guo, J.; Su, Z.Q. The anti-obesity effect of chitosan oligosaccharide capsules in a high-fat diet-induced obese rat model. Basic Clin. Pharmacol. 2017, 121, 12. [Google Scholar]
- Chiu, C.Y.; Feng, S.A.; Liu, S.H.; Chiang, M.T. Functional comparison for lipid metabolism and intestinal and fecal microflora enzyme activities between low molecular weight chitosan and chitosan oligosaccharide in high-fat-diet-fed rats. Mar. Drugs 2017, 15, 234. [Google Scholar] [CrossRef] [PubMed]
- Ruud, J.; Bruning, J.C. METABOLISM Light on leptin link to lipolysis. Nature 2015, 527, 43–44. [Google Scholar] [CrossRef] [PubMed]
- Schneeberger, M.; Dietrich, M.O.; Sebastian, D.; Imbernon, M.; Castano, C.; Garcia, A.; Esteban, Y.; Gonzalez-Franquesa, A.; Rodriguez, I.C.; Bortolozzi, A.; et al. Mitofusin 2 in POMC neurons connects ER stress with leptin resistance and energy imbalance. Cell 2013, 155, 172–187. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.T.; Guo, J.; Su, Z.Q. Advances in understanding the interrelations between leptin resistance and obesity. Physiol. Behav. 2014, 130, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.; Kim, K.W.; Kim, M.S. Leptin signalling pathways in hypothalamic neurons. Cell. Mol. Life Sci. 2016, 73, 1457–1477. [Google Scholar] [CrossRef] [PubMed]
- Peelman, F.; Zabeau, L.; Moharana, K.; Savvides, S.N.; Tavernier, J. 20 YEARS OF LEPTIN Insights into signaling assemblies of the leptin receptor. J. Endocrinol. 2014, 223, T9–T23. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Ogawa, W.; Ozaki, M.; Haga, S.; Matsumoto, M.; Furukawa, K.; Hashimoto, N.; Kido, Y.; Mori, T.; Sakaue, H.; et al. Role of STAT-3 in regulation of hepatic gluconeogenic genes and carbohydrate metabolism in vivo. Nat. Med. 2004, 10, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.Y.; Martin, R.G.; Duncan, R.E.; Choi, D.; Lu, S.Y.; Schroer, S.A.; Cai, E.P.; Luk, C.T.; Hopperton, K.E.; Domenichiello, A.F.; et al. Hepatocyte-specific deletion of Janus Kinase 2 (JAK2) Protects against diet-induced steatohepatitis and glucose intolerance. J. Biol. Chem. 2012, 287, 10277–10288. [Google Scholar] [CrossRef] [PubMed]
- Adolph, T.E.; Grander, C.; Grabherr, F.; Tilg, H. Adipokines and non-alcoholic fatty liver disease: Multiple interactions. Int. J. Mol. Sci. 2017, 18, 1649. [Google Scholar] [CrossRef] [PubMed]
- Ghadge, A.A.; Khaire, A.A.; Kuvalekar, A.A. Adiponectin: A potential therapeutic target for metabolic syndrome. Cytokine Growth Factor Rev. 2018, 39, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Mantzoros, C.S. Adiponectin as a target for the treatment of nonalcoholic steatohepatitis with thiazolidinediones: A systematic review. Metabolism 2016, 65, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Linden, A.G.; Li, S.L.; Choi, H.Y.; Fang, F.; Fukasawa, M.; Uyeda, K.; Hammer, R.E.; Horton, J.D.; Engelking, L.J.; Liang, G.S. Interplay between ChREBP and SREBP-1c coordinates postprandial glycolysis and lipogenesis in livers of mice. J. Lipid Res. 2018, 59, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Ferre, P.; Foufelle, F. Hepatic steatosis: A role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes. Metab. 2010, 12 (Suppl. 2), 83–92. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.S.; Lee, W.C.; Lin, Y.E.; Ho, C.T.; Lu, K.H.; Lin, S.H.; Panyod, S.; Chu, Y.L.; Sheen, L.Y. Ginger essential oil ameliorates hepatic injury and lipid accumulation in high fat diet-induced nonalcoholic fatty liver disease. J. Agric. Food Chem. 2016, 64, 2062–2071. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.M.; Yuan, R.S.; Zhuang, W.Y.; Sun, J.H.; Wu, J.Y.; Li, H.; Chen, J.G. Schisandra polysaccharide inhibits hepatic lipid accumulation by downregulating expression of SREBPs in NAFLD mice. Lipids Health Dis. 2016, 15, 195. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, T.T.; Li, J.; Wang, S.J.; Qiu, F.; Yu, H.Y.; Zhang, Y.; Wang, T. Effects of natural products on fructose-induced nonalcoholic fatty liver disease (NAFLD). Nutrients 2017, 9, 96. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.D.; Gong, J.J.; Zhao, Y.Y.; Zhuang, X.Y.; Ye, Y.; Lin, W.T. Sulfated polysaccharides from Enteromorpha prolifera suppress SREBP-2 and HMG-CoA reductase expression and attenuate non-alcoholic fatty liver disease induced by a high-fat diet. Food Funct. 2017, 8, 1899–1904. [Google Scholar] [CrossRef] [PubMed]
- Cipolletta, D.; Feuerer, M.; Li, A.; Kamei, N.; Lee, J.; Shoelson, S.E.; Benoist, C.; Mathis, D. PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature 2012, 486, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.X.; Chen, W.; Liu, J.; Xu, T.; Zeng, Y.Q. Study on quantitative expression of PPAR. and ADRP in muscle and its association with intramuscular fat deposition of pig. Springerplus 2016, 5, 1501. [Google Scholar] [CrossRef] [PubMed]
- Imamura, M.; Inoguchi, T.; Ikuyama, S.; Taniguchi, S.; Kobayashi, K.; Nakashima, N.; Nawata, H. ADRP stimulates lipid accumulation and lipid droplet formation in murine fibroblasts. Am. J. Physiol.-Endocrinol. Metab. 2002, 283, E775–E783. [Google Scholar] [CrossRef] [PubMed]
- Scheller, E.L.; Burr, A.A.; MacDougald, O.A.; Cawthorn, W.P. Inside out: Bone marrow adipose tissue as a source of circulating adiponectin. Adipocyte 2016, 5, 251–269. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. A dipose tissue, obesity and non-alcoholic fatty liver disease. Minerva Endocrinol. 2017, 42, 92–108. [Google Scholar] [PubMed]
- Cui, H.X.; Lopez, M.; Rahmouni, K. The cellular and molecular bases of leptin and ghrelin resistance in obesity. Nat. Rev. Endocrinol. 2017, 13, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Khandekar, M.J.; Cohen, P.; Spiegelman, B.M. Molecular mechanisms of cancer development in obesity. Nat. Rev. Cancer 2011, 11, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Ahima, R.S. Digging deeper into obesity. J. Clin. Investig. 2011, 121, 2076–2079. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.G.; Rahman, M.A.; Lee, S.H.; Hwang, H.S.; Kim, H.A.; Yun, J.W. Plasma proteome analysis for anti-obesity and anti-diabetic potentials of chitosan oligosaccharides in ob/ob mice. Proteomics 2009, 9, 2149–2162. [Google Scholar] [CrossRef] [PubMed]
- Lijnen, H.R. Murine models of obesity and hormonal therapy. Thromb. Res. 2011, 127, 17S–20S. [Google Scholar] [CrossRef]
- Preston, S.H.; Mehta, N.K.; Stokes, A. Modeling obesity histories in cohort analyses of health and mortality. Epidemiology 2013, 24, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.D.; Perseghin, G. Non-alcoholic fatty liver disease: A risk factor for myocardial dysfunction? J. Hepatol. 2018, 68, 640–642. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.C.; Ness, E.; Kowdley, K.V. Nutritional approaches to achieve weight loss in nonalcoholic fatty liver disease. Adv. Nutr. 2017, 8, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Spiegelman, B.M. PPAR gamma: A nuclear regulator of metabolism, differentiation, and cell growth. J. Biol. Chem. 2001, 276, 37731–37734. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.C.; Gao, A.C.; Lai, C.H.; Hsieh, J.T.; Lin, H. Induction of neuroendocrine differentiation in castration resistant prostate cancer cells by adipocyte differentiation-related protein (ADRP) delivered by exosomes. Cancer Lett. 2017, 391, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, H.; Zhang, Y.Y.; Li, L.L.; Fang, R.P.; Li, Y.H.; Liu, Q.; Zhang, W.Y.; Qiu, L.Y.; Liu, F.B.; et al. Oncoprotein HBXIP modulates abnormal lipid metabolism and growth of breast cancer cells by activating the LXRs/SREBP-1c/FAS signaling cascade. Cancer Res. 2016, 76, 4696–4707. [Google Scholar] [CrossRef] [PubMed]
- Huang, L. Preparation of Chitosan Oligosaccharide Capsules, Chitosan Capsules and Assessment of Their Weight Control Function; Guangdong Pharmaceutical University: Guangzhou, China, 2016. [Google Scholar]
| Sequence ID | Name | Sequences |
|---|---|---|
| NM_144744.3 | Adiponectin | Forward: TGGAATGACAGGAGCGGAAG |
| Reverse: GCGAATGGGAACATTGGGGA | ||
| NM_001276707.1 | SREBP-1c | Forward: ATCCTGGCCACAGTACCACT |
| Reverse: GGAACGGTAGCGCTTCTCA | ||
| NM_017332.1 | FAS | Forward: TCGACTTCAAAGGACCCAGC |
| Reverse: ACTGCACAGAGGTGTTAGGC | ||
| NM_022193.1 | ACC | Forward: GTACCGAAGTGGCATCCGTG |
| Reverse: TCTCTTCCCGAAGGGCGAAT | ||
| NM_013134.2 | HMGCR | Forward: CCTCCATTGAGATCCGGAGGA |
| Reverse: ACAAAGAGGCCATGCATACGG | ||
| NM_001145366.1 | PPARγ | Forward: TGGGGATGTCTCACAATGCC |
| Reverse: AGACTCTGGGTTCAGCTGGT | ||
| NM_001287577.1 | C/EBPα | Forward: AGGCCAAGAAGTCGGTGGATA |
| Reverse: TCACTGGTCAACTCCAACACC | ||
| NM_001007144.1 | ADRP | Forward: GGCAGGTGACATCTACTCGG |
| Reverse: AAAGGGACCTACCAGCCAGT | ||
| NM_031144 | β-actin | Forward: CACCCGCGAGTACAACCTTC |
| Reverse: CCCATACCCACCATCACACC |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, H.; Fu, C.; Huang, L.; Jiang, Y.; Deng, X.; Guo, J.; Su, Z. Anti-Obesity Effect of Chitosan Oligosaccharide Capsules (COSCs) in Obese Rats by Ameliorating Leptin Resistance and Adipogenesis. Mar. Drugs 2018, 16, 198. https://doi.org/10.3390/md16060198
Pan H, Fu C, Huang L, Jiang Y, Deng X, Guo J, Su Z. Anti-Obesity Effect of Chitosan Oligosaccharide Capsules (COSCs) in Obese Rats by Ameliorating Leptin Resistance and Adipogenesis. Marine Drugs. 2018; 16(6):198. https://doi.org/10.3390/md16060198
Chicago/Turabian StylePan, Haitao, Chuhan Fu, Lanlan Huang, Yao Jiang, Xiaoyi Deng, Jiao Guo, and Zhengquan Su. 2018. "Anti-Obesity Effect of Chitosan Oligosaccharide Capsules (COSCs) in Obese Rats by Ameliorating Leptin Resistance and Adipogenesis" Marine Drugs 16, no. 6: 198. https://doi.org/10.3390/md16060198
APA StylePan, H., Fu, C., Huang, L., Jiang, Y., Deng, X., Guo, J., & Su, Z. (2018). Anti-Obesity Effect of Chitosan Oligosaccharide Capsules (COSCs) in Obese Rats by Ameliorating Leptin Resistance and Adipogenesis. Marine Drugs, 16(6), 198. https://doi.org/10.3390/md16060198





