Glycosaminoglycans from a Sea Snake (Lapemis curtus): Extraction, Structural Characterization and Antioxidant Activity
Abstract
:1. Introduction
2. Results and Discussion
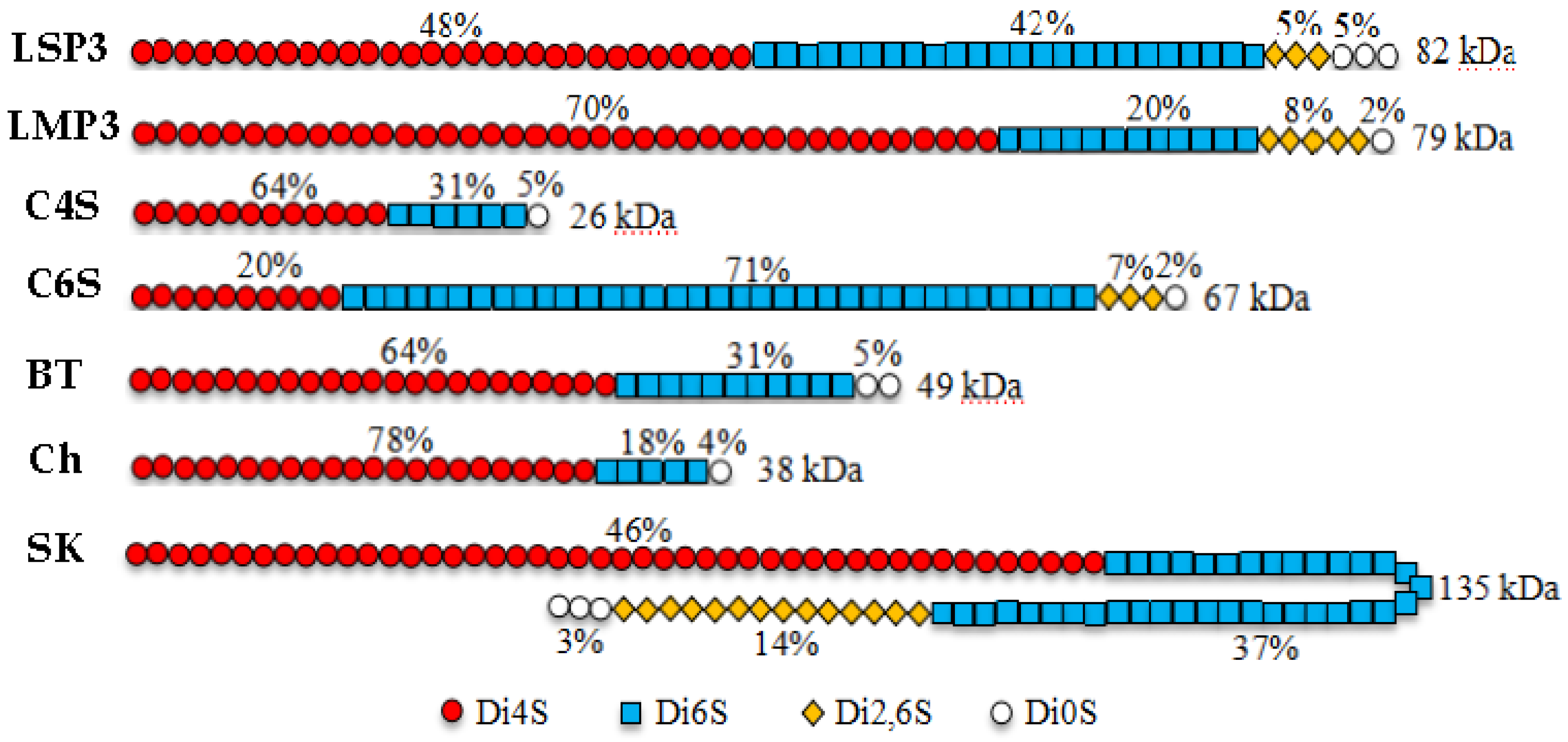
2.1. Chemical Composition
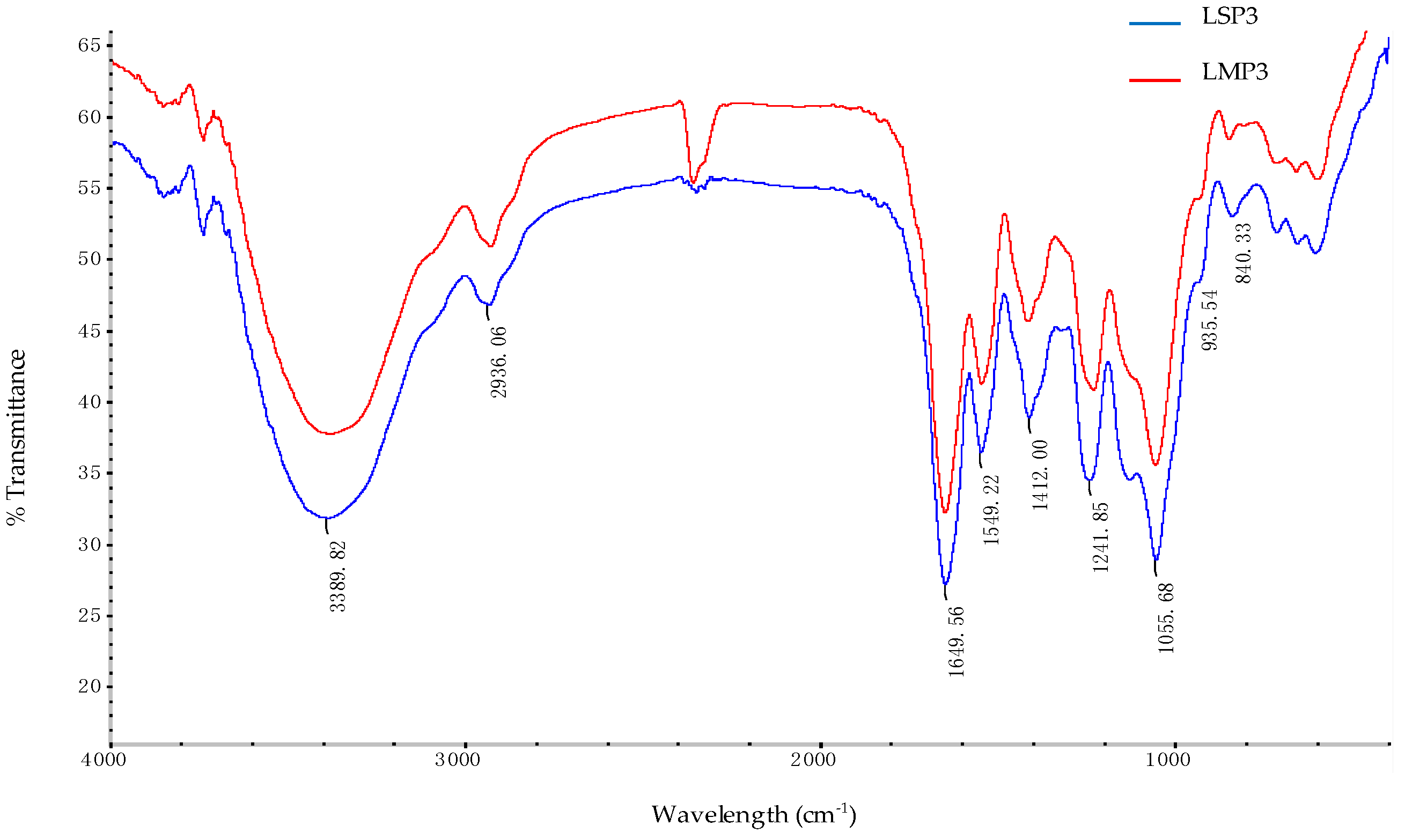
2.2. FT-IR Spectroscopy of LSP3 and LMP3
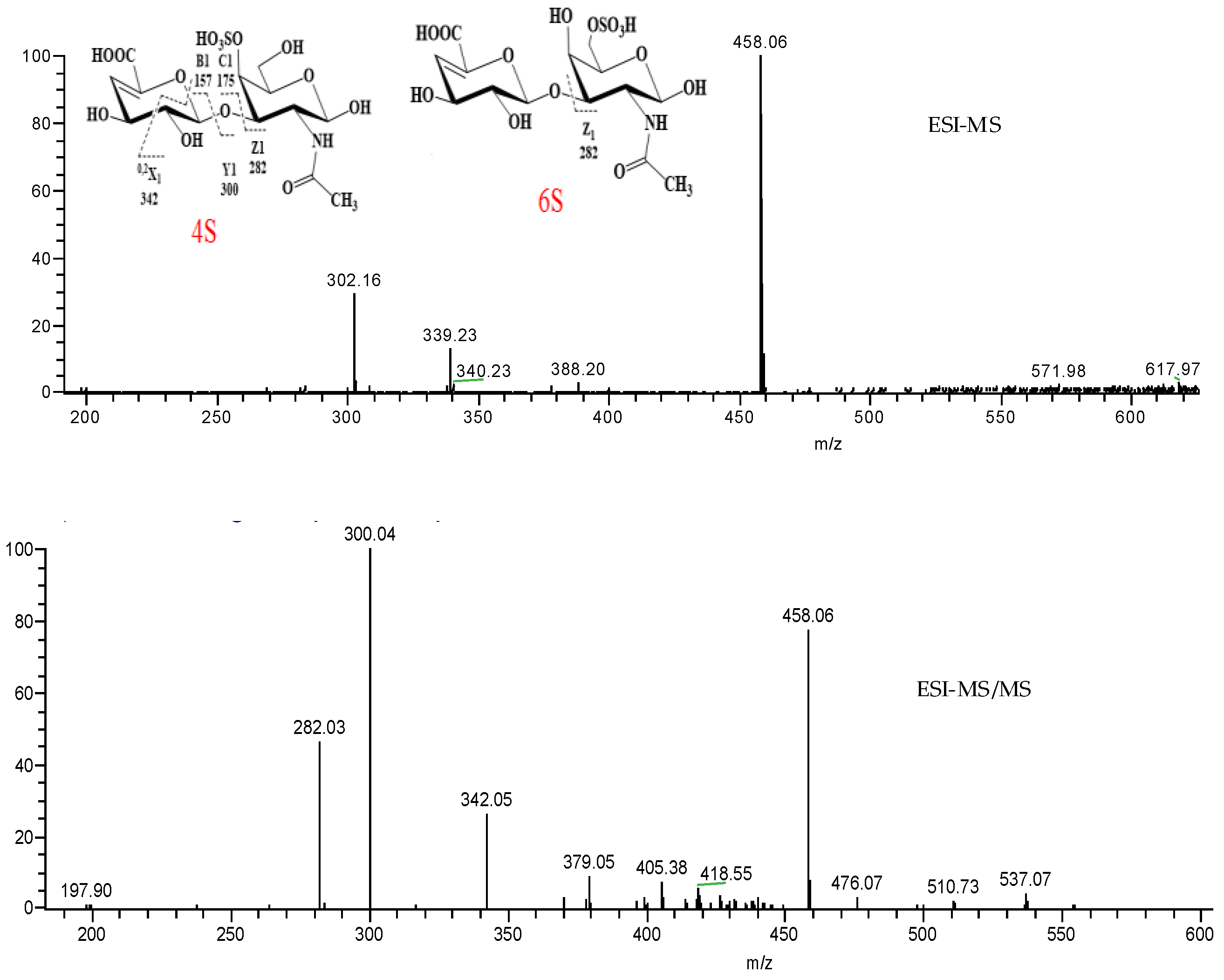
2.3. ESI-MS Analysis of LSP3 and LMP3
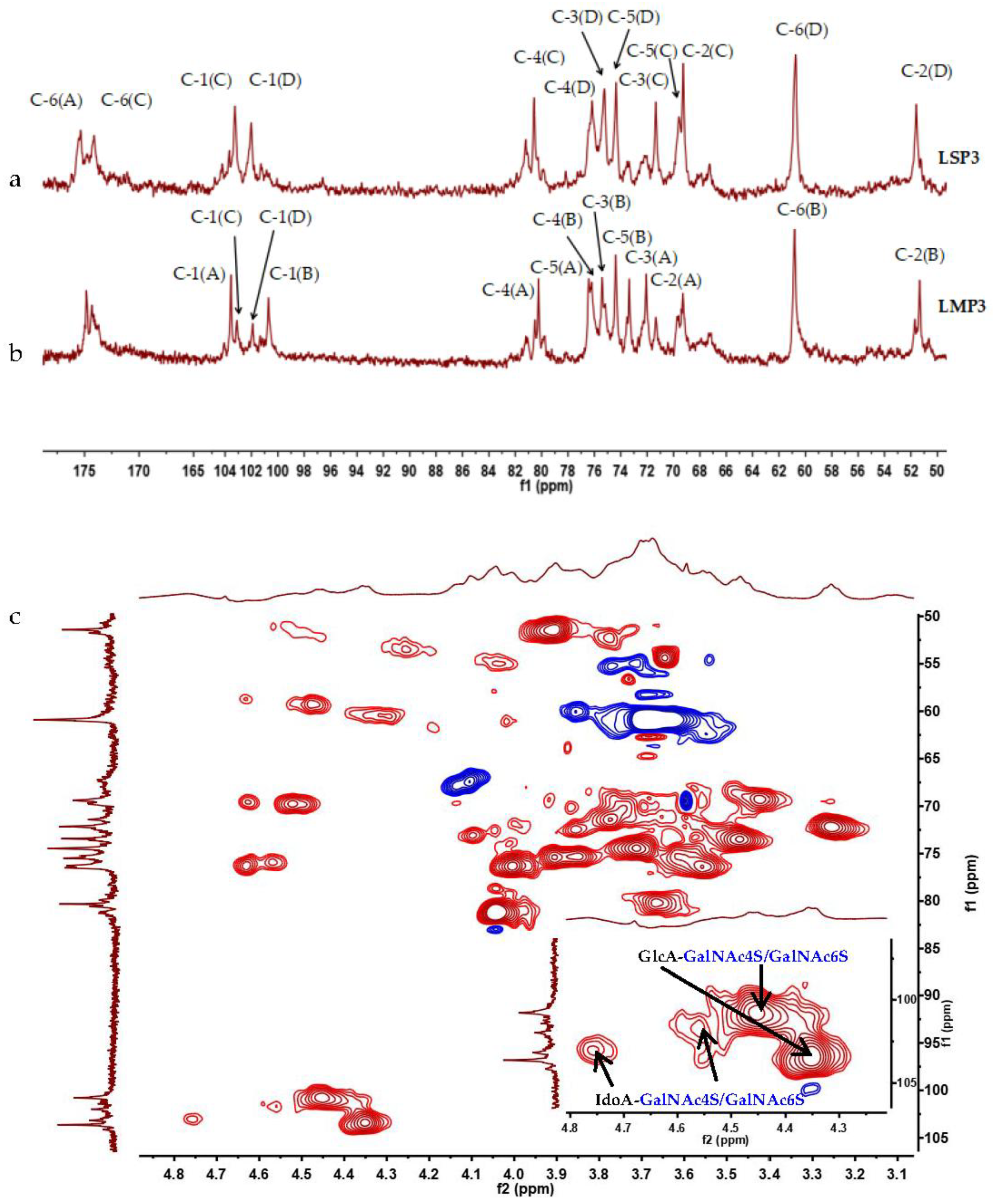
2.4. NMR Spectroscopy Analysis of LSP3 and LMP3
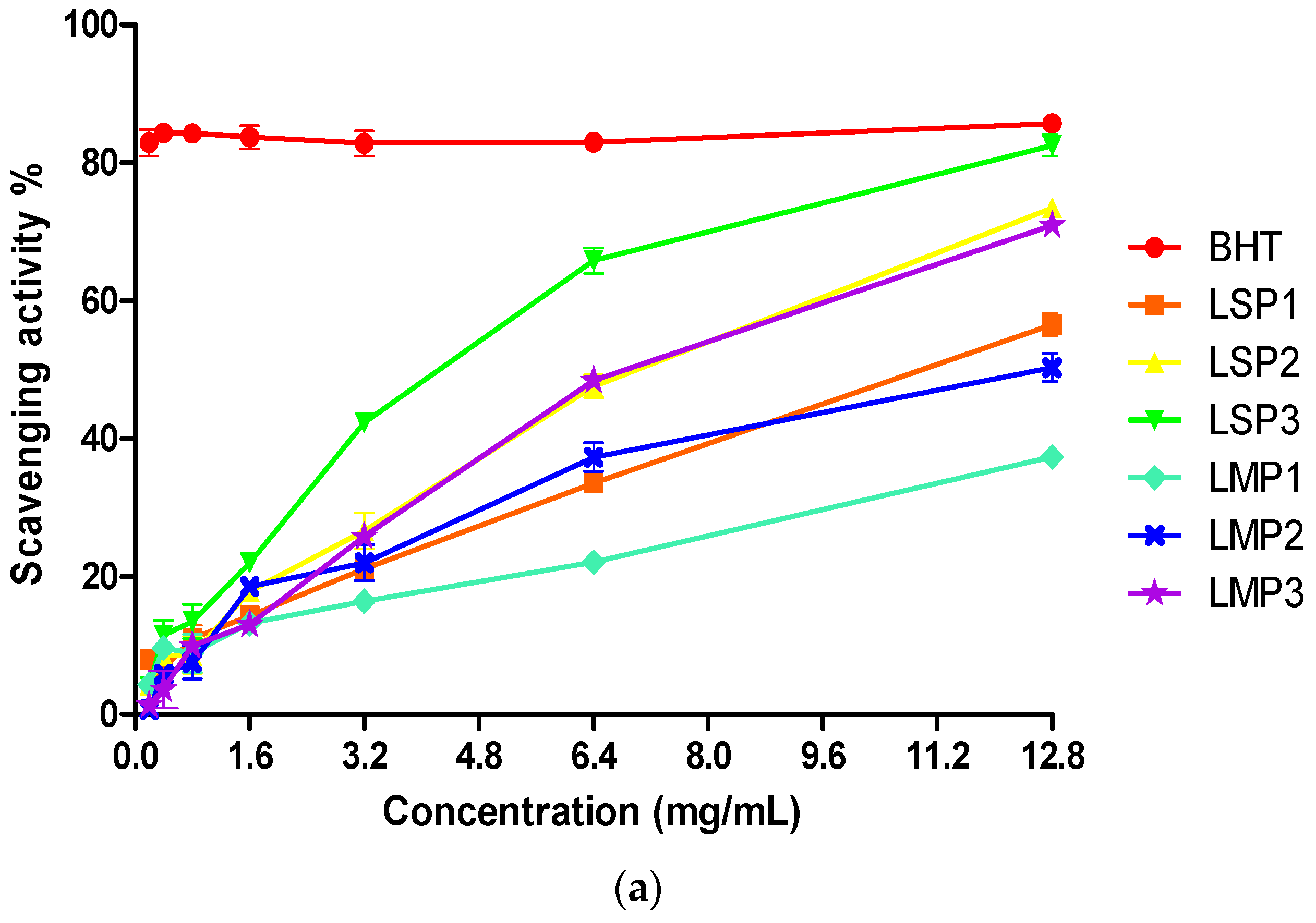
2.5. Antioxidant Activity of Fractions
3. Materials and Methods
3.1. Materials and Reagents
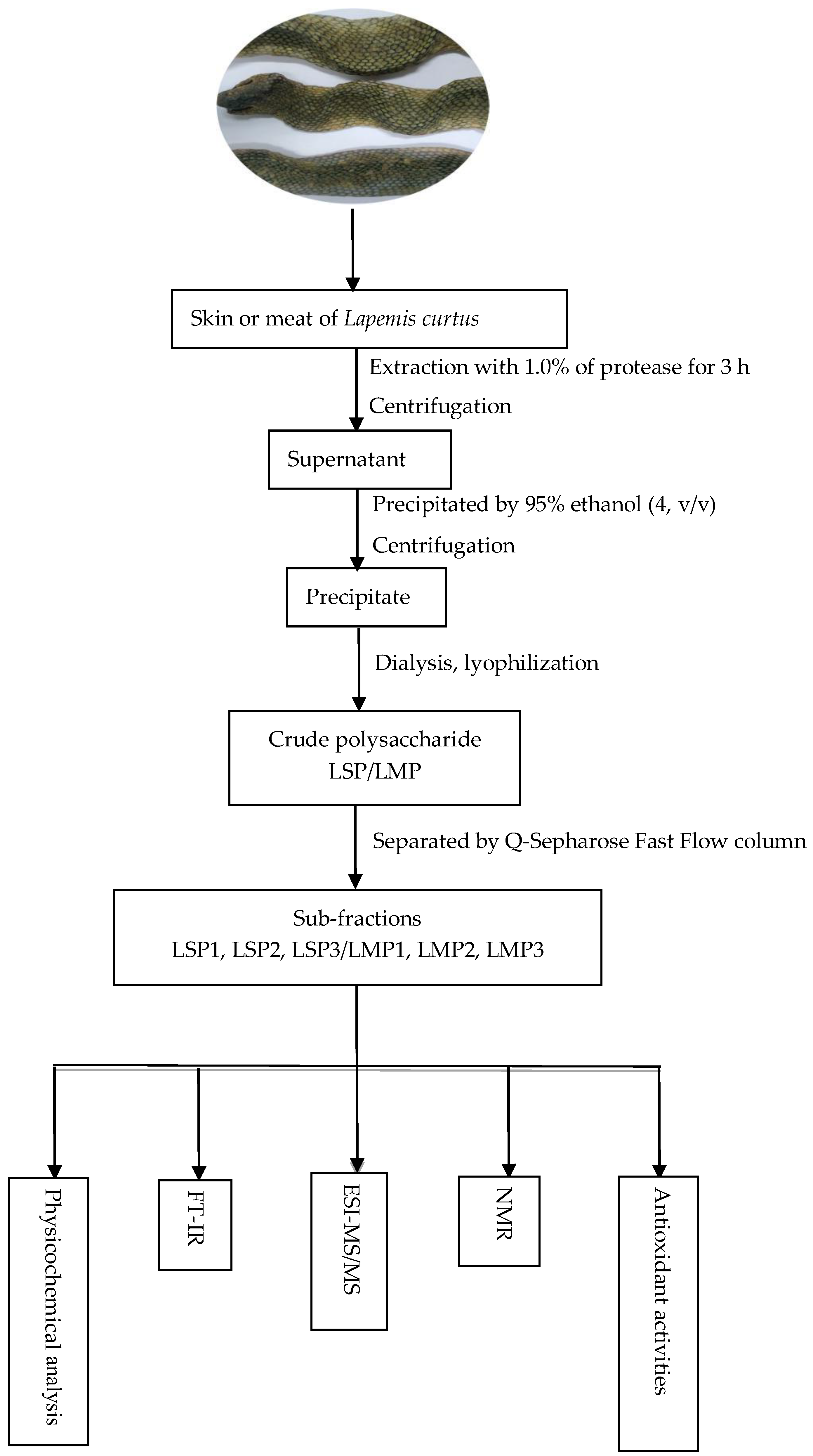
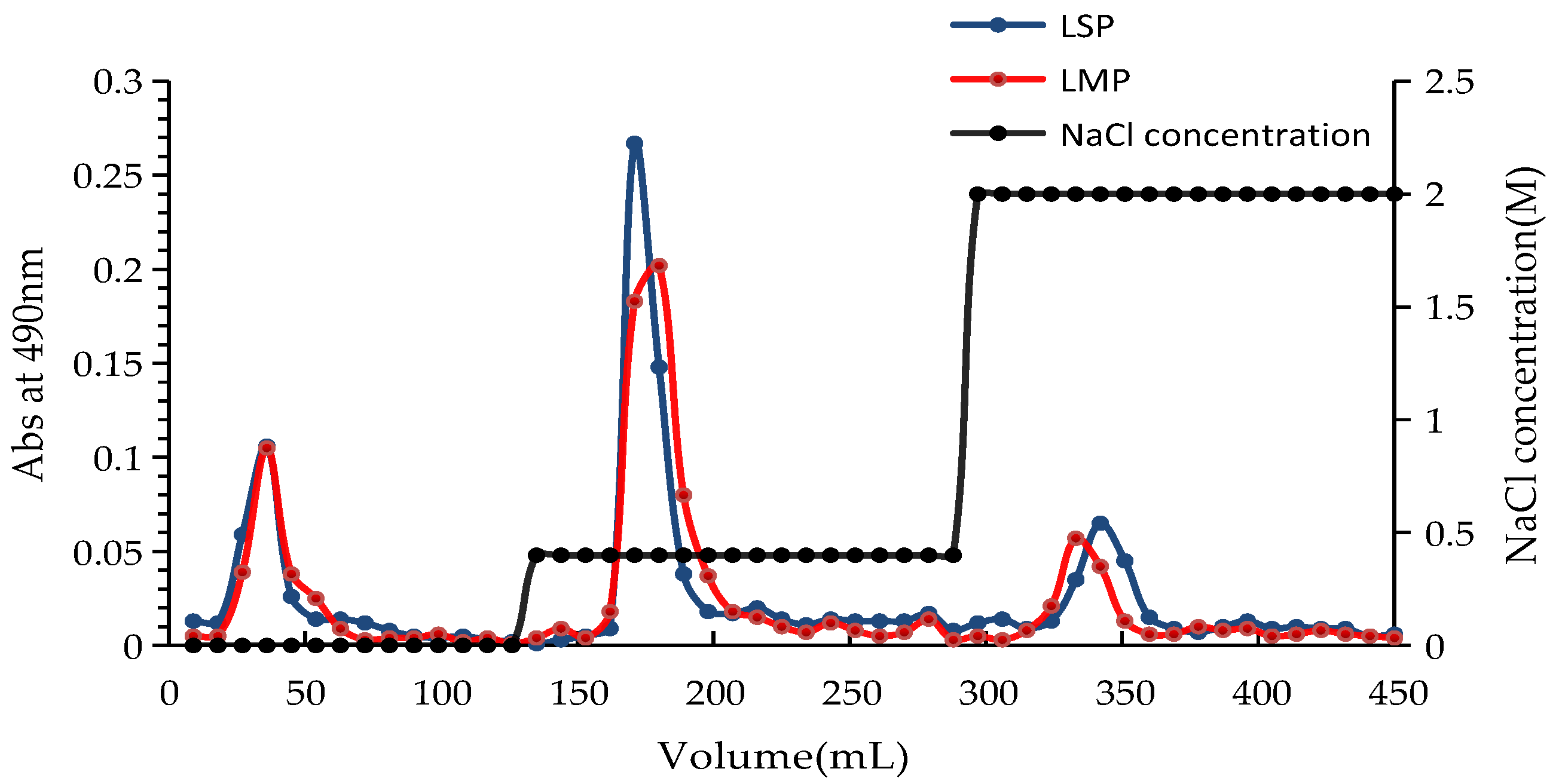
3.2. Extraction and Purification
3.3. Chemical Analysis and Molecular Weight Analysis
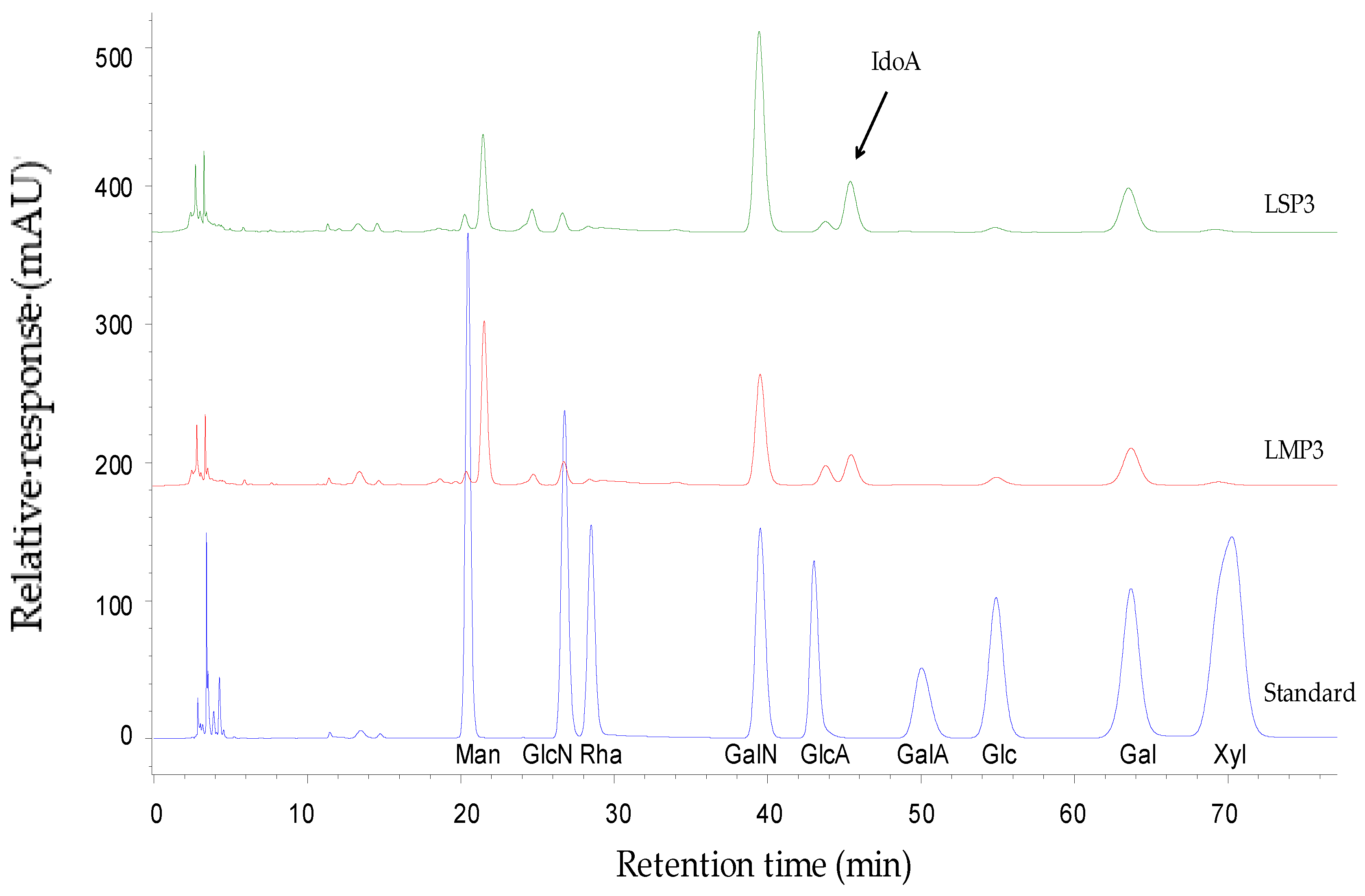
3.4. Composition Analysis
3.5. Fourier Transform Infrared (FT-IR) Spectroscopy Analysis
3.6. Electrospray Mass Spectroscopy (ESI-MS) Analysis
3.7. NMR Spectroscopy Analysis
3.8. Determination of Antioxidant Activity
3.8.1. DPPH Free Radical Scavenging Activity
3.8.2. Iron (Fe2+) Chelating Activity
3.8.3. Total Antioxidant Capacity Assay Kit with ABTS Method
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Panagos, C.G.; Thomson, D.; Moss, C.; Bavington, C.D.; Ólafsson, H.G.; Uhrín, D. Characterisation of hyaluronic acid and chondroitin/dermatan sulfate from the lumpsucker fish, C. lumpus. Carbohydr. Polym. 2014, 106, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Thelin, M.A.; Bartolini, B.; Axelsson, J.; Gustafsson, R.; Tykesson, E.; Pera, E.; Oldberg, A.; Maccarana, M.; Malmstrom, A. Biological functions of iduronic acid in chondroitin/dermatan sulfate. FEBS J. 2013, 280, 2431–2446. [Google Scholar] [CrossRef] [PubMed]
- Abdelhedi, O.; Nasri, R.; Souissi, N.; Nasri, M.; Jridi, M. Sulfated polysaccharides from common smooth hound: Extraction and assessment of anti-ACE, antioxidant and antibacterial activities. Carbohydr. Polym. 2016, 152, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar]
- Campo, G.M.; Avenoso, A.; Campo, S.; Ferlazzo, A.M.; Calatroni, A. Antioxidant Activity of Chondroitin Sulfate. Adv. Pharmacol. 2006, 53, 417–431. [Google Scholar] [CrossRef] [PubMed]
- Sereshk, Z.H.; Bakhtiari, A.R. Distribution patterns of PAHs in different tissues of annulated sea snake (Hydrophis cyanocinctus) and short sea snake (Lapemis curtus) from the Hara Protected Area on the North Coast of the Persian Gulf, Iran. Ecotox. Environ. Safe 2014, 109, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Shirai, N.; Suzuki, H.; Shimizu, R. Fatty acid composition of oil extracted from the fat sack of the Erabu sea snake Laticauda semifasciata in the Pacific Ocean and South China Sea. Fish. Sci. 2002, 68, 239–240. [Google Scholar] [CrossRef]
- Damotharan, P.; Veeruraj, A.; Arumugam, M.; Balasubramanian, T. Biological and Biochemical Potential of Sea Snake Venom and Characterization of Phospholipase A2 and Anticoagulation Activity. Ind. J. Clin. Biochem. 2016, 31, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Garnjanagoonchorn, W.; Wongekalak, L.; Engkagul, A. Determination of chondroitin sulfate from different sources of cartilage. Chem. Eng. Process. 2007, 46, 465–471. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Bilan, M.I.; Dmitrenok, A.S.; Borodina, E.Y.; Stonik, V.A.; Nifantiev, N.E.; Usov, A.I. A highly regular fucosylated chondroitin sulfate from the sea cucumber Massinium magnum: Structure and effects on coagulation. Carbohydr. Polym. 2017, 167, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Zamfir, A.; Seidler, D.G.; Kresse, H.; Peter-Katalinić, J. Structural characterization of chondroitin/dermatan sulfate oligosaccharides from bovine aorta by capillary electrophoresis and electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2002, 16, 2015–2024. [Google Scholar] [CrossRef] [PubMed]
- Huckerby, T.N.; Lauder, R.M.; Brown, G.M.; Nieduszynski, I.A.; Anderson, K.; Boocock, J.; Sandall, P.L.; Weeks, S.D. Characterization of oligosaccharides from the chondroitin sulfates. FEBS J. 2001, 268, 1181–1189. [Google Scholar] [CrossRef]
- Pavao, M.S.; Aiello, K.R.; Werneck, C.C.; Silva, L.C.F.; Valente, A.P.; Mulloy, B.; Colwell, N.S.; Tollefsen, D.M.; Mourão, P.A. Highly sulfated dermatan sulfates from ascidians structure versus anticoagulant activity of these glycosaminoglycans. J. Biol. Chem. 1998, 273, 27848–27857. [Google Scholar] [CrossRef] [PubMed]
- Valcarcel, J.; Novoa-Carballal, R.; Pérez-Martín, R.I.; Reis, R.L.; Vázquez, J.A. Glycosaminoglycans from marine sources as therapeutic agents. Biotechnol Adv. 2017, 35, 711–725. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, A.L.; Aguiar, J.A.; da Silva, F.S.C.; Michelacci, Y.M. Do chondroitin sulfates with different structures have different activities on chondrocytes and macrophages? Int. J. Biol. Macromol. 2017, 103, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Azam, M.S.; Kim, E.J.; Yang, H.S.; Kim, J.K. High antioxidant and DNA protection activities of N -acetylglucosamine (GlcNAc) and chitobiose produced by exolytic chitinase from Bacillus cereus, EW5. Springerplus 2014, 3, 354–365. Available online: http://www.springerplus.com/content/3/1/354 (accessed on 11 July 2014). [CrossRef] [PubMed]
- Bitter, T.; Muir, H.M. A modified uronic acid carbazole reaction. Anal. Biochem. 1962, 4, 330–334. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Xue, Y.T.; Ren, L.; Li, S.; Wang, L.L.; He, X.X.; Zhao, X.; Yu, G.L.; Guan, H.S.; Li, C.X. Study on quality control of sulfated polysaccharide drug, propylene glycol alginate sodium sulfate (PSS). Carbohydr. Polym. 2016, 144, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yu, G.; Guan, H.; Yue, N.; Zhang, Z.; Li, H. Preparation of low-molecular-weight polyguluronate sulfate and its anticoagulant and anti-inflammatory activities. Carbohydr. Polym. 2007, 69, 272–279. [Google Scholar] [CrossRef]
- Pu, J.; Zhao, X.; Wang, Q.; Wang, Y.; Zhou, H. Development and validation of a HPLC method for determination of degree of polymerization of xylo-oligosaccharides. Food. Chem. 2016, 213, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hao, J.; Shan, X.; Zhang, X.; Zhao, X.; Li, Q.; Wang, X.; Cai, C.; Li, G.; Yu, G. Antithrombotic activities of fucosylated chondroitin sulfates and their depolymerized fragments from two sea cucumbers. Carbohydr. Polym. 2016, 152, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Chen, X.; Zhang, X.; Liu, L.; Cong, D.; Zhao, X.; Yu, G. Acidolysis-based component mapping of glycosaminoglycans by reversed-phase high-performance liquid chromatography with off-line electrospray ionization–tandem mass spectrometry: Evidence and tags to distinguish different glycosaminoglycans. Anal. Biochem. 2014, 465, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Masuko, S.; Takieddin, M.; Xu, H.; Liu, R.; Jing, J.; Mousa, S.A.; Linhardt, R.J.; Liu, J. Chemoenzymatic synthesis of homogeneous ultralow molecular weight heparins. Science 2011, 334, 498–501. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Decker, E.A.; Welch, B. Role of ferritin as a lipid oxidation catalyst in muscle food. J. Agric. Food Chem. 1990, 38, 674–677. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Wan, Y.; Xu, J.Y.; Wu, G.H.; Li, L.; Yao, X.H. Ultrasound extraction of polysaccharides from mulberry leaves and their effect on enhancing antioxidant activity. Carbohydr. Polym. 2016, 137, 473–479. [Google Scholar] [CrossRef] [PubMed]

| Composition | Polysaccharides from Skin | Polysaccharides from Meat | ||||
|---|---|---|---|---|---|---|
| LSP1 | LSP2 | LSP3 | LMP1 | LMP2 | LMP3 | |
| Uronic acid (%) | 0.5 | 1.8 | 25.3 | 1.1 | 1.9 | 15.2 |
| Total proteins (%) | 48.3 | 46.0 | 19.2 | 42.4 | 63.8 | 12.3 |
| Sulfated groups (%) | 0.7 | 0.4 | 11.2 | 0.5 | 0.4 | 10.1 |
| Molecular weight (kDa) | 3.7 | 3.1 | 82.0 | 2.1 | 2.7 | 79.0 |
| Monosaccharide (molar ratio) | ||||||
| Mannose | 5.6 | 4.7 | - | 4.8 | 2.5 | - |
| N-acetyl Glucosamine | 12.6 | 17.2 | 1.8 | 18.1 | 13.8 | 2.0 |
| Rhamnose | - | - | - | - | - | - |
| Glucuronic acid | - | 1.1 | 16.2 | - | - | 12.3 |
| Galacturonic acid | - | - | - | - | - | - |
| N-acetyl Galactosamine | 1.1 | 1.6 | 30.1 | 2.3 | 1.5 | 15.9 |
| Glucose | 9.4 | 3.3 | 1.2 | 15.4 | 3.1 | 2.0 |
| Galactose | 22.9 | 14.6 | 10.8 | 19.9 | 8.8 | 8.8 |
| Xylose | - | - | 1.3 | - | - | 1.5 |
| Arabinose | - | - | - | 1.1 | 1.6 | - |
| Fucose | 1 | 1 | 1 | 1 | 1 | 1 |
| Disaccharide (%) | ||||||
| ΔDi0S | 5.0 | 1.6 | ||||
| ΔDi6S | 42.2 | 20.0 | ||||
| ΔDi4S | 47.9 | 70.5 | ||||
| ΔDi2,6S | 4.9 | 8.0 | ||||
| Signal/ppm | H1 | H2 | H3 | H4 | H5 | H6 | Residue | |
|---|---|---|---|---|---|---|---|---|
| (C1) | (C2) | (C3) | (C4) | (C5) | (C6) | |||
| A | →4)-β-GlcA-(1→ | 4.35 | 3.27 | 3.47 | 3.66 | 3.57 | - | →4)-β-GlcA-(1→3)-β-GalNAc4S-(1→ |
| (103.35) | (72.31) | (73.46) | (80.18) | (76.42) | (174.27) | |||
| B | →3)-β-GalNAc4S-(1→ | 4.44 | 3.91 | 3.90 | 4.63 | 3.71 | 3.69 | |
| (101.08) | (51.43) | (75.38) | (76.28) | (74.45) | (60.83) | |||
| C | →4)-β-IdoA-(1→ | 4.77 | 3.42 | 3.78 | 3.97 | 4.60 | - | →4)-β-IdoA-(1→3)-β-GalNAc4S-(1→ |
| (103.13) | (69.33) | (71.46) | (80.53) | (69.72) | (173.62) | |||
| D | →3)-β-GalNAc4S-(1→ | 4.56 | 3.93 | 3.84 | 4.55 | 3.71 | 3.69 | |
| (101.68) | (51.76) | (75.25) | (76.09) | (74.45) | (60.83) | |||
| A′ | →4)-β-GlcA-(1→ | 4.40 | 3.16 | 3.50 | 3.59 | 3.56 | - | →4)-β-GlcA-(1→3)-β-GalNAc6S-(1→ |
| (104.00) | (72.29) | (73.41) | (81.20) | (76.39) | (174.27) | |||
| B′ | →3)-β-GalNAc6S-(1→ | 4.42 | 3.91 | 3.74 | 4.00 | 3.86 | 4.10 | |
| (101.27) | (51.41) | (74.50) | (67.71) | (72.46) | (67.51) | |||
| C′ | →4)-β-IdoA-(1→ | 4.73 | 3.50 | 3.74 | ND | ND | - | →4)-β-IdoA-(1→3)-β-GalNAc6S-(1→ |
| (102.98) | (69.00) | (70.85) | ND | ND | (173.62) | |||
| D′ | →3)-β-GalNAc6S-(1→ | 4.50 | 3.93 | 3.75 | 4.03 | ND | 4.13 | |
| (101.68) | (51.76) | (80.21) | (68.13) | ND | (67.90) | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, M.; Han, W.; Zhao, X.; Wang, Q.; Gao, Y.; Deng, S. Glycosaminoglycans from a Sea Snake (Lapemis curtus): Extraction, Structural Characterization and Antioxidant Activity. Mar. Drugs 2018, 16, 170. https://doi.org/10.3390/md16050170
Bai M, Han W, Zhao X, Wang Q, Gao Y, Deng S. Glycosaminoglycans from a Sea Snake (Lapemis curtus): Extraction, Structural Characterization and Antioxidant Activity. Marine Drugs. 2018; 16(5):170. https://doi.org/10.3390/md16050170
Chicago/Turabian StyleBai, Mingyue, Wenwei Han, Xia Zhao, Qingchi Wang, Yanyun Gao, and Shiming Deng. 2018. "Glycosaminoglycans from a Sea Snake (Lapemis curtus): Extraction, Structural Characterization and Antioxidant Activity" Marine Drugs 16, no. 5: 170. https://doi.org/10.3390/md16050170
APA StyleBai, M., Han, W., Zhao, X., Wang, Q., Gao, Y., & Deng, S. (2018). Glycosaminoglycans from a Sea Snake (Lapemis curtus): Extraction, Structural Characterization and Antioxidant Activity. Marine Drugs, 16(5), 170. https://doi.org/10.3390/md16050170