Production, Characterization and Biocompatibility Evaluation of Collagen Membranes Derived from Marine Sponge Chondrosia reniformis Nardo, 1847
Abstract
1. Introduction
2. Results
2.1. Fibrillar Collagen Suspensions Extraction and Characterization
2.2. Viscosity Evaluation
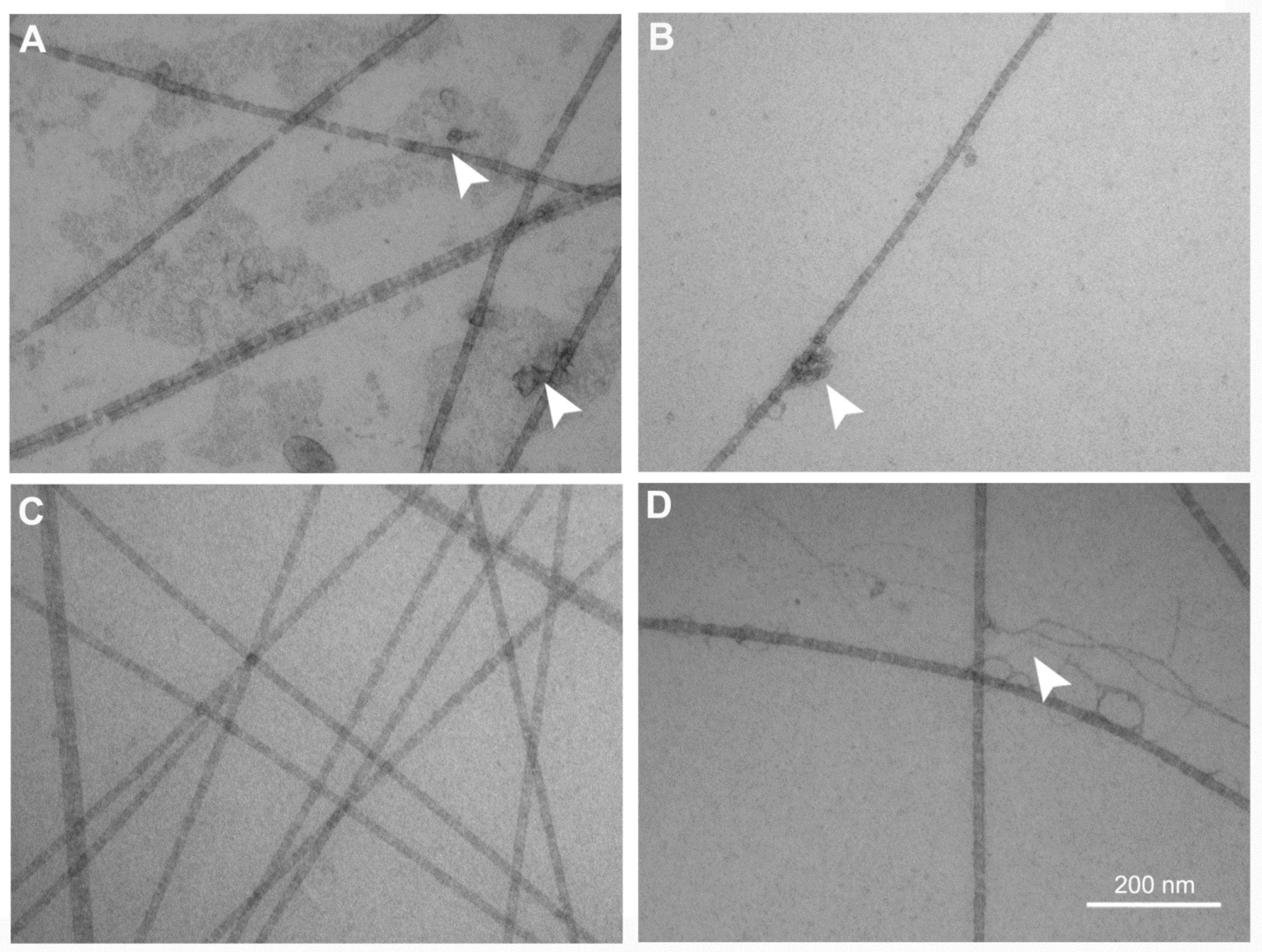
2.3. Transmission Electron Microscopy Analysis of the FSs
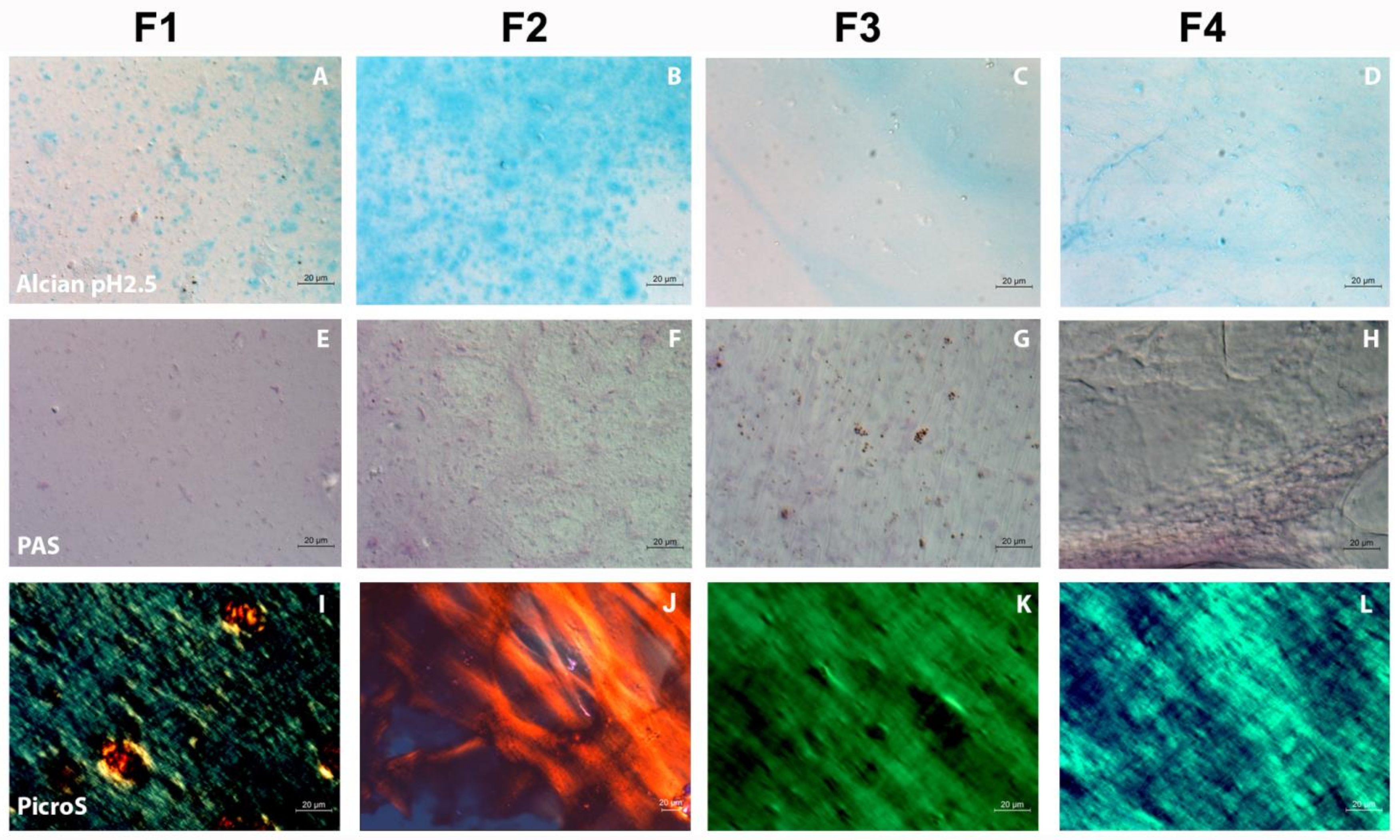
2.4. Qualitative Evaluation of the FSs by Histological Methods
2.5. Sponge Collagen Membrane Production
2.6. SCMs Characterization
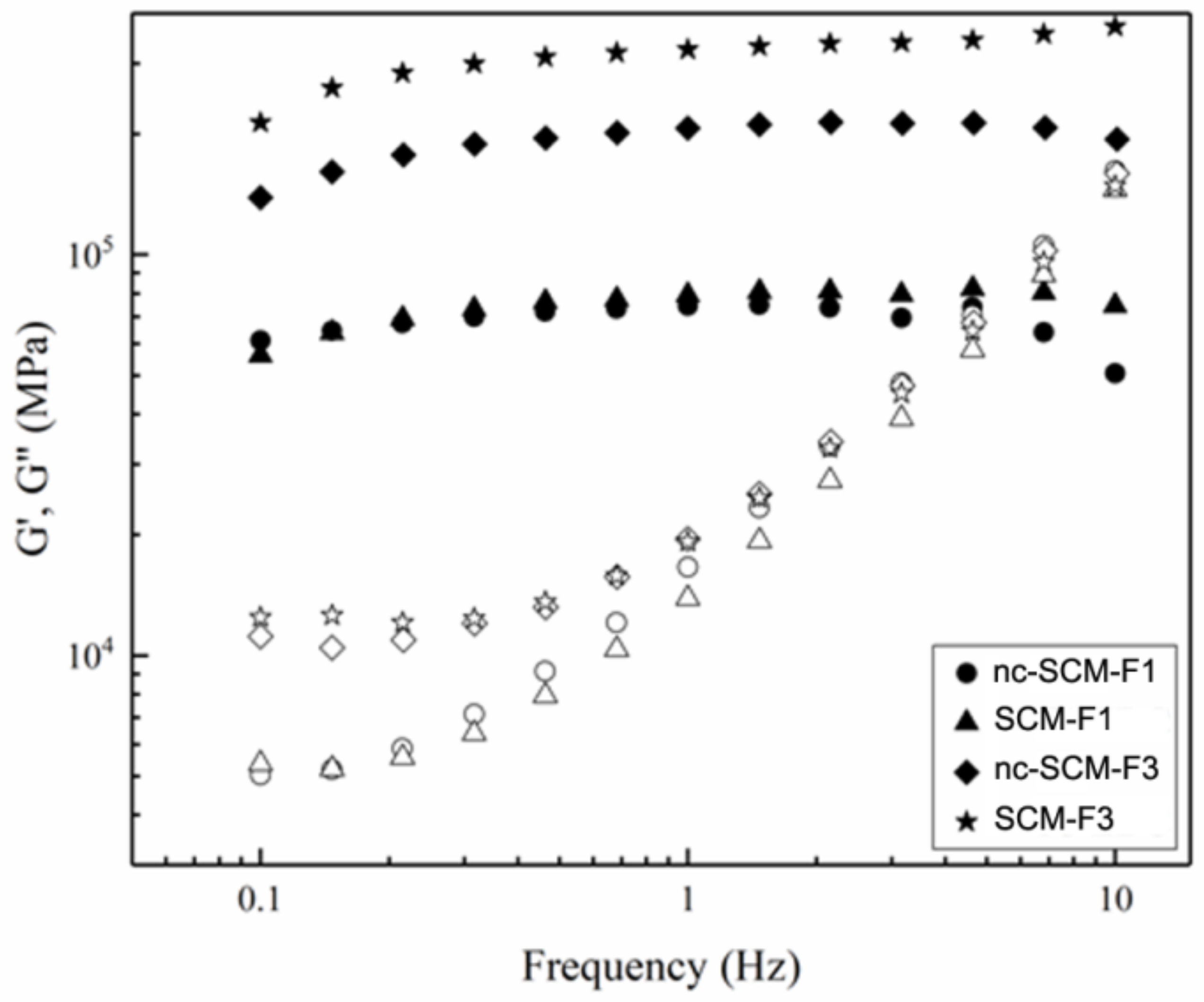
2.6.1. Mechanical Tests
2.6.2. In Vitro Resistance to Enzymatic Degradation
2.6.3. Water Binding Capacity
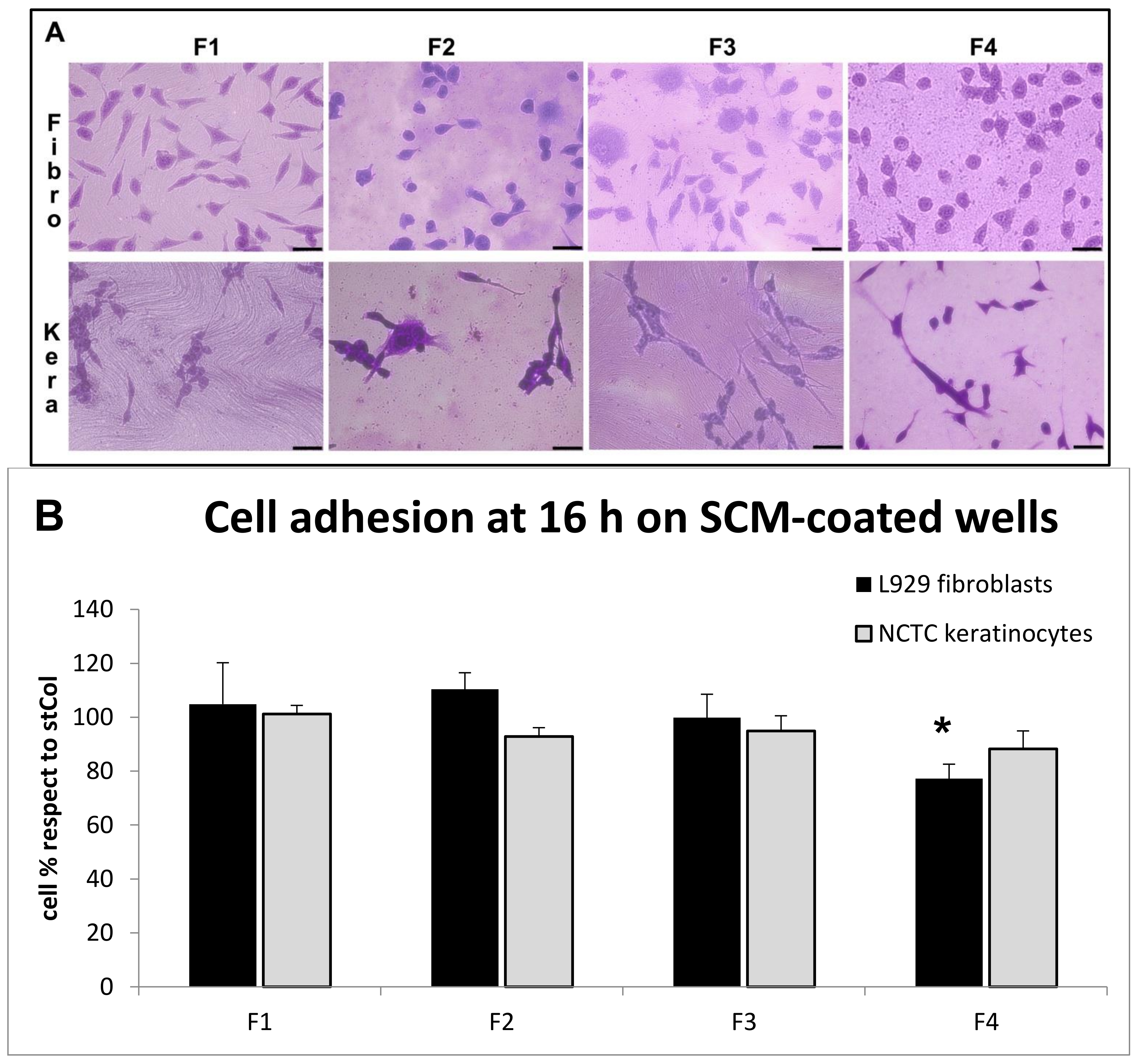
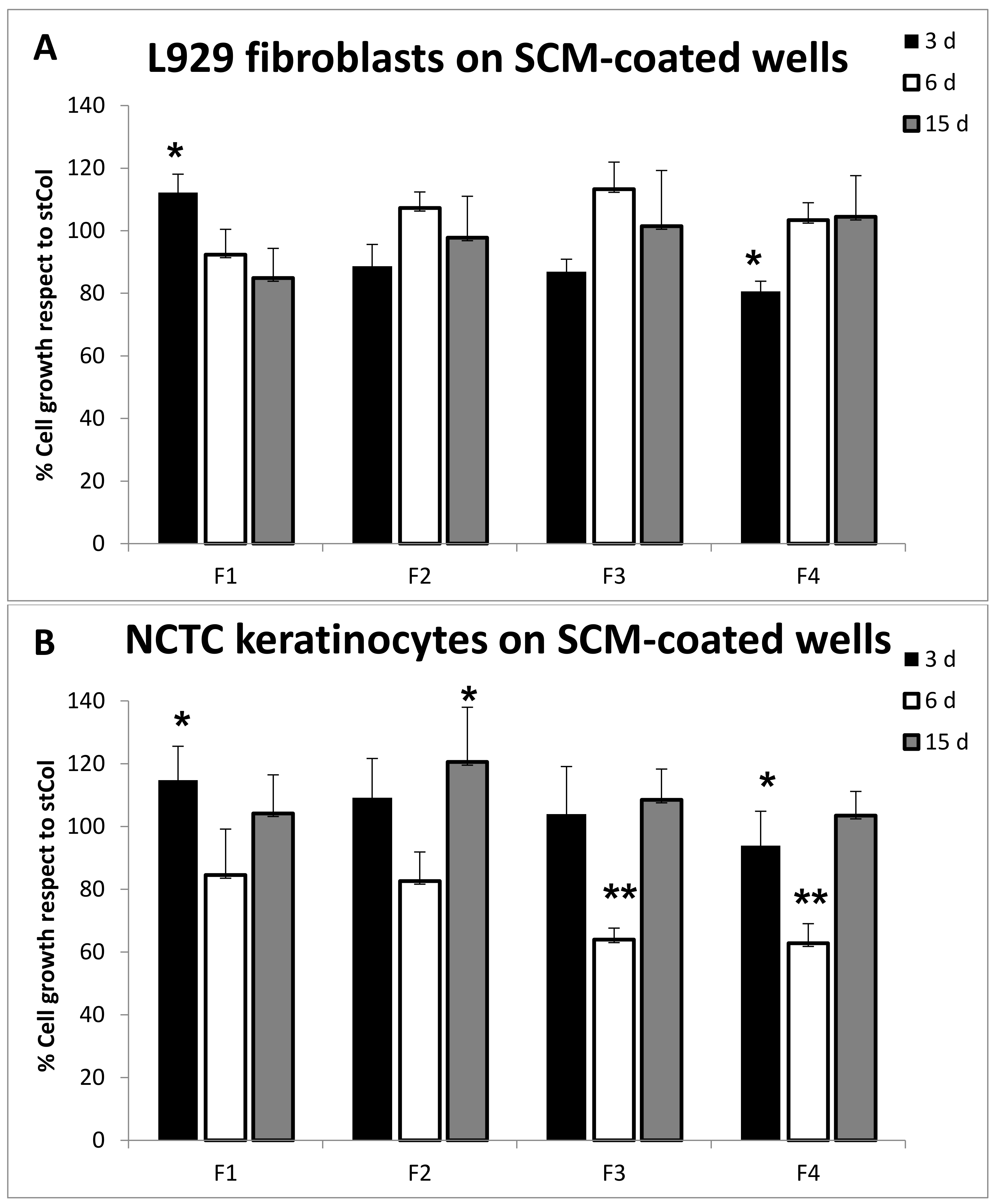
2.6.4. Biocompatibility
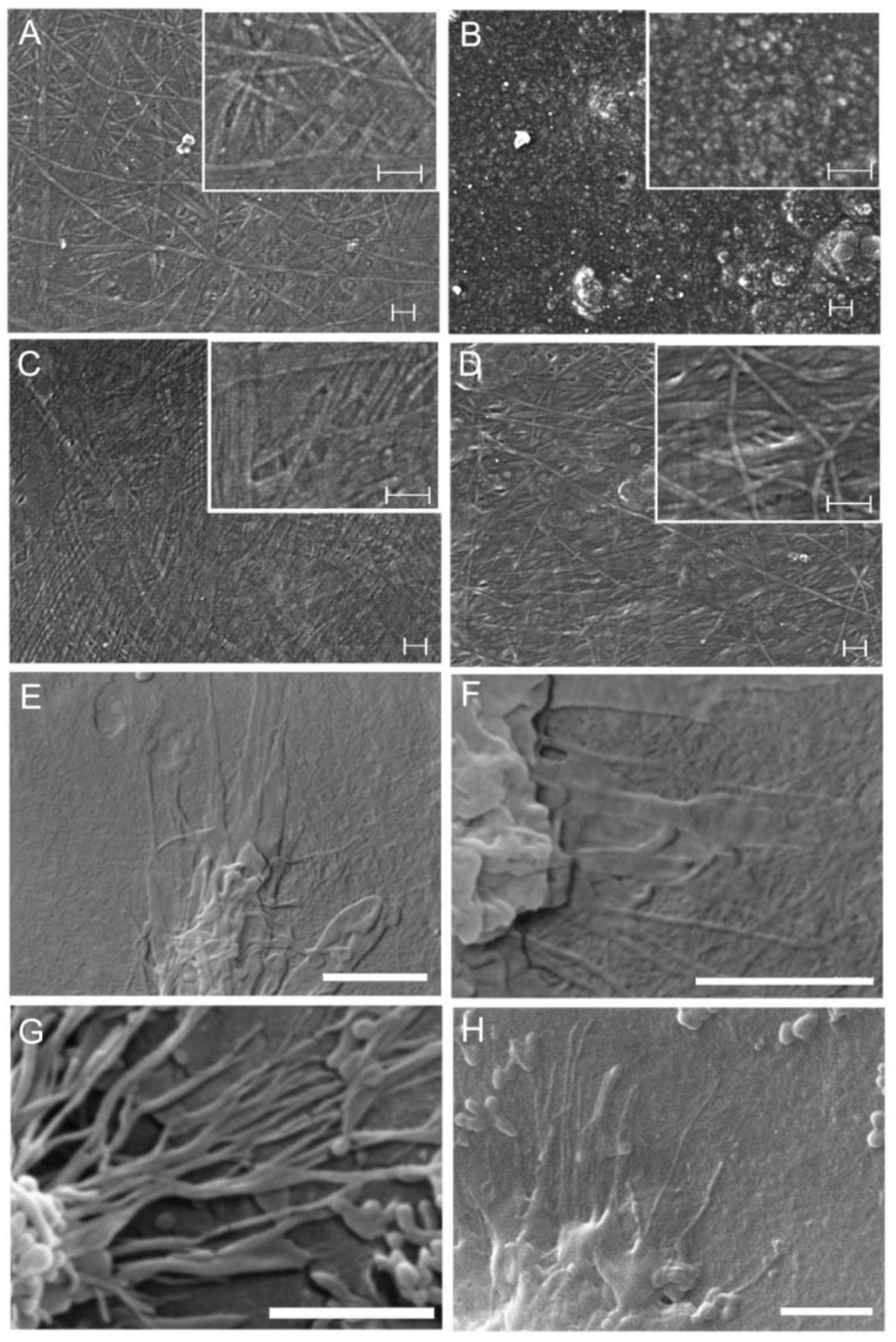
2.6.5. Environmental Scanning Electron Microscope (ESEM) Analysis
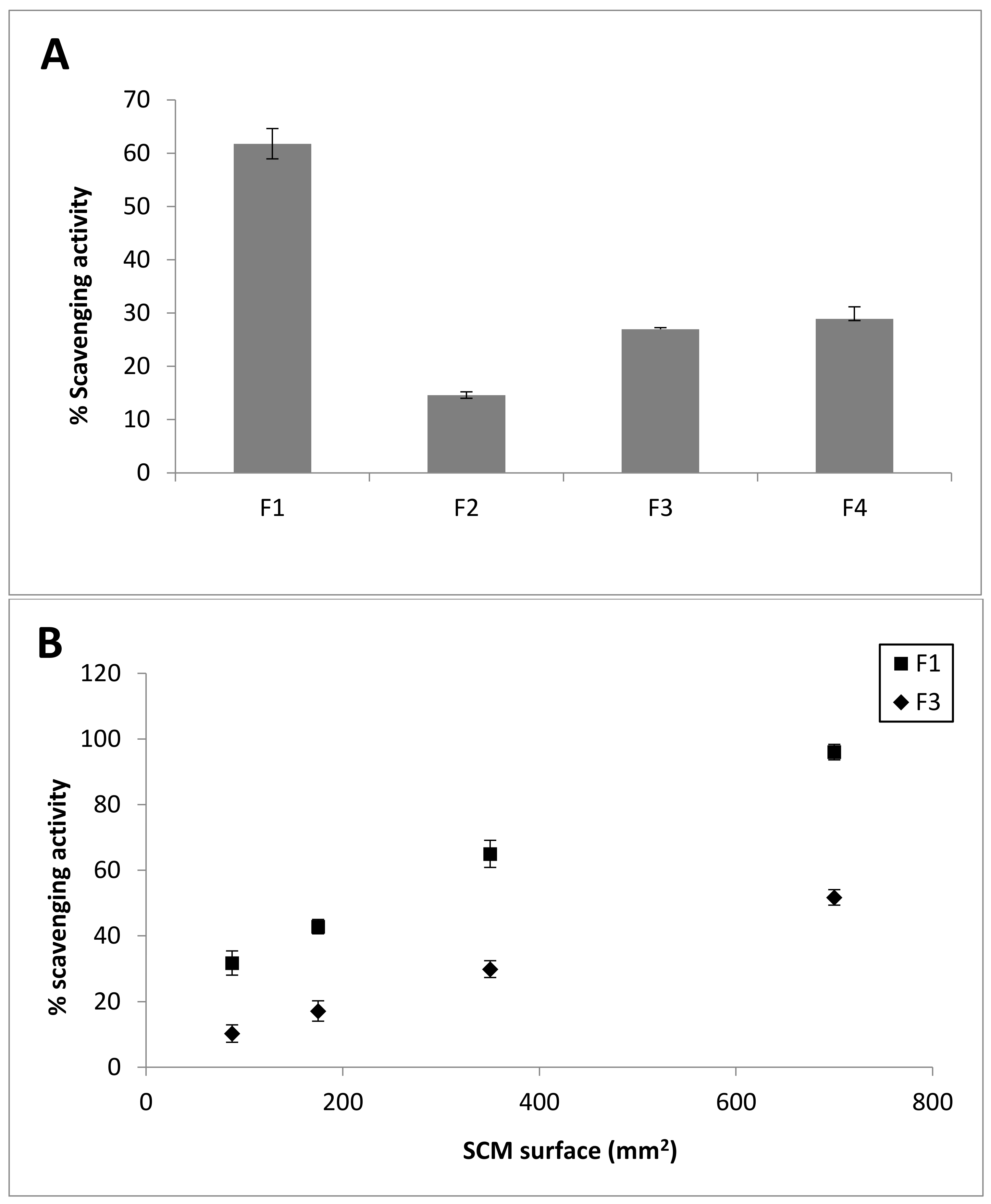
2.7. DPPH Radical Scavenging Activity
3. Discussion
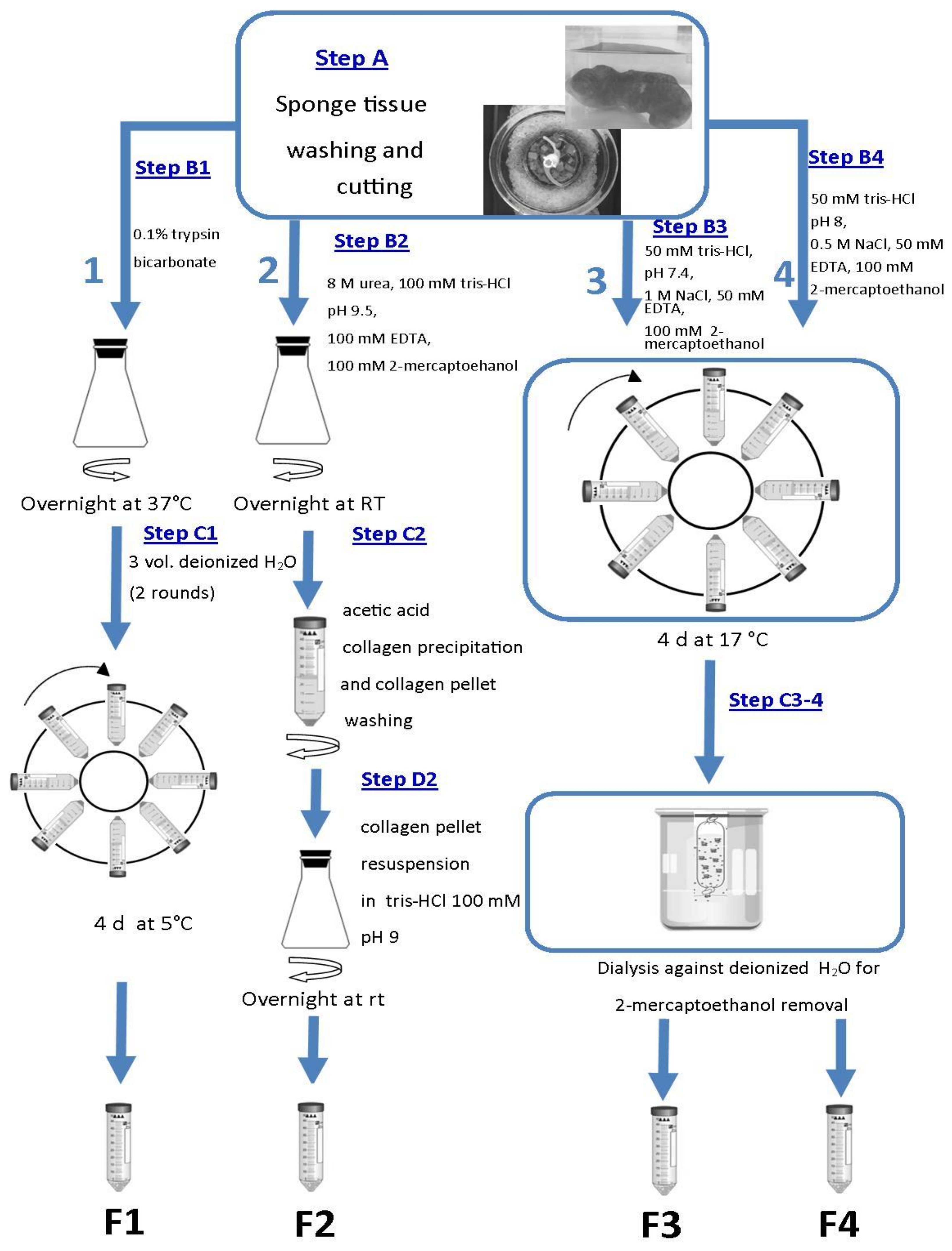
4. Materials and Methods
4.1. Sponge Sampling
4.2. Fibrillar Collagen Suspension Extracts
4.3. FS Characterization
4.3.1. BCA Total Protein Quantification
4.3.2. Collagen Quantification
4.3.3. Alcian Blue GAG Assay
4.3.4 Transmission Electron Microscopy: Negative Staining
4.3.5. FS Qualitative Evaluation by Histological Methods
4.3.6. Rheological Characterization
4.4. SCM Production
4.5. SCM Characterization
4.5.1. In Vitro Enzymatic Resistance
4.5.2. Water Binding Capacity
4.5.3. Dynamic Mechanical Tests
4.6. SCM Biocompatibility Evaluation
4.6.1. Cell Cultures
4.6.2. Cell Growth and Cell Adhesion
4.6.3. Light and ESEM Microscopy
4.7. DPPH Radical Scavenging Activity
4.8. Statistical Analyses
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ellis, D.L.; Yannas, I.V. Recent advances in tissue synthesis in vivo by use of collagen glycosaminoglycan copolymers. Biomaterials 1996, 17, 291–299. [Google Scholar] [CrossRef]
- Chen, P.; Marsilio, E.; Goldstein, R.H.; Yannas, I.V.; Spector, M. Formation of lung alveolar like structures in collagen-glycosaminoglycan scaffolds in vitro. Tissue Eng. 2005, 11, 1436–1448. [Google Scholar] [CrossRef] [PubMed]
- Buijtenhuijs, P.; Buttafoco, L.; Poot, A.A.; Daamen, W.F.; van Kuppevelt, T.H.; Dijkstra, P.; de Vos, R.A.; Ster, L.M.; Geelkerken, B.R.; Feijen, J.; et al. Tissue engineering of bloodvessels: Characterization of smooth-muscle cells for culturing on collagen-and-elastin-based scaffolds. Biotechnol. Appl. Biochem. 2004, 39, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Garcia, Y.; Hemantkumar, N.; Collighan, R.; Griffin, M.; Rodriguez-Cabello, J.C.; Pandit, A. In vitro characterization of a collagen scaffold enzymatically cross-linked with a tailored elastin-like polymer. Tissue Eng. Part A 2009, 15, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Damour, O.; Gueugniaud, P.Y.; Berthin-Maghit, M.; Rousselle, P.; Berthod, F.; Sahuc, F.; Collombel, C. A dermal substrate made of collagen-GAG-chitosan for deep burn coverage: First clinical uses. Clin. Mater. 1994, 15, 273–276. [Google Scholar] [CrossRef]
- Shahabeddin, L.; Berthod, F.; Damour, O.; Collombel, C. Characterization of skin reconstructed on a chitosan-cross-linked collagen-glycosaminoglycan matrix. Skin Pharmacol. 1990, 3, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Raines, R.T. Review collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.; Moreira-Silva, J.; Marques, A.; Domingues, A.; Bayon, Y.; Reis, R. Marine origin collagens and its potential applications. Mar. Drugs 2014, 12, 5881–5901. [Google Scholar] [CrossRef] [PubMed]
- Pati, F.; Adhikar, B.; Dhara, S. Isolation and characterization of fish scale collagen of higher thermal stability. Biores. Technol. 2010, 101, 3737–3742. [Google Scholar] [CrossRef] [PubMed]
- Jridi, M.; Bardaa, S.; Moalla, D.; Rebaii, T.; Souissi, N.; Sahnoun, Z.; Nasri, M. Microstructure, rheological and wound healing properties of collagen-based gel from cuttlefish skin. Int. J. Biol. Macromol. 2015, 77, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Boero, F.; Bouillon, J.; Gravili, C.; Miglietta, M.P.; Parsons, T.; Piraino, S. Gelatinous plankton: Irregularities rule the world (sometimes). Mar. Ecol. Prog. Ser. 2008, 356, 299–310. [Google Scholar] [CrossRef]
- Song, E.; Kim, S.Y.; Chun, T.; Byun, H.J.; Lee, Y.M. Collagen scaffolds derived from a marine source and their biocompatibility. Biomaterials 2006, 27, 2951–2961. [Google Scholar] [CrossRef] [PubMed]
- Feuda, R.; Dohrmann, M.; Pett, W.; Philippe, H.; Rota-Stabelli, O.; Lartillot, N.; Wörheide, G.; Pisani, D. Improved modeling of compositional heterogeneity supports sponges as sister to all other animals. Curr. Biol. 2017, 27, 3864–3870. [Google Scholar] [CrossRef] [PubMed]
- Simpson, T.L. Collagen fibrils, spongin, matrix substances. In The Cell Biology of Sponges; Springer: New York, NY, USA, 1984; ISBN 978-1-46-129740-6. [Google Scholar]
- Garrone, R. Phylogenesis of Connective Tissue: Morphological Aspects and Biosynthesis of Sponge Intercellular Matrix; Karger, S., Ed.; University of Michigan: Ann Arbor, MI, USA, 1978; pp. 1–250. ISBN 978-3-80-552767-5. [Google Scholar]
- Junqua, S.; Robert, L.; Garrone, R.; Pavans de Ceccatty, M.; Vacelet, J. Biochemical and morphological studies on collagens of horny sponges. Ircinia filaments compared to spongines. Connect. Tissue Res. 1974, 2, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Exposito, J.Y.; Garrone, R. Characterization of a fibrillar collagen gene in sponges reveals the early evolutionary appearance of two collagen gene families. Proc. Natl. Acad. Sci. USA 1990, 87, 6669–6673. [Google Scholar] [CrossRef] [PubMed]
- Exposito, J.Y.; Le Guellec, D.; Lu, Q.; Garrone, R. Short chain collagens in sponges are encoded by a family of closely related genes. J. Biol. Chem. 1991, 266, 21923–21928. [Google Scholar] [PubMed]
- Kim, M.M.; Mendis, E.; Rajapakse, N.; Lee, S.H.; Kim, S.K. Effect of spongin derived from Hymeniacidon sinapium on bone mineralization. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Green, D.; Howard, D.; Yang, X.; Kelly, M.; Oreffo, R.O. Natural marine sponge fibre skeleton: A biomimetic scaffold for human osteoprogenitor cell attachment, growth, and differentiation. Tissue Eng. 2003, 9, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Solomon, K.L.; Zhang, X.; Pavlos, N.J.; Abel, T.; Willers, C.; Dai, K.; Xu, J.; Zheng, Q.; Zheng, M. In vitro evaluation of natural marine sponge collagen as a scaffold for bone tissue engineering. Int. J. Biol. Sci. 2011, 7, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.K.; Kundu, B.; Mahato, A.; Thakur, N.L.; Joardar, S.N.; Mandal, B.B. In vitro and in vivo evaluation of the marine sponge skeleton as a bone mimicking biomaterial. Integr. Biol. 2015, 7, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Norman, M.; Bartczak, P.; Zdarta, J.; Tylus, W.; Szatkowski, T.; Stelling, A.L.; Ehrlich, H.; Jesionowski, T. Adsorption of C.I. Natural Red 4 onto spongin skeleton of marine demosponge. Materials 2014, 8, 96–116. [Google Scholar] [CrossRef] [PubMed]
- Norman, M.; Bartczak, P.; Zdarta, J.; Ehrlich, H.; Jesionowski, T. Anthocyanin dye conjugated with Hippospongia communis marine demosponge skeleton and its antiradical activity. Dyes Pigment. 2016, 134, 541–552. [Google Scholar] [CrossRef]
- Norman, M.; Zdarta, J.; Bartczak, P.; Piasecki, A.; Petrenko, I.; Ehrlich, H. Marine sponge skeleton photosensitized by copper phthalocyanine: A catalyst for Rhodamine B degradation. Open Chem. 2016, 14, 243–254. [Google Scholar] [CrossRef]
- Norman, M.; Bartczak, P.; Zdarta, J.; Tomala, W.; Żurańska, B.; Dobrowolska, A.; Piasecki, A.; Czaczyk, K.; Ehrlich, H.; Jesionowsk, T. Sodium copper chlorophyllin immobilization onto Hippospongia communis marine demosponge skeleton and its antibacterial activity. Int. J. Mol. Sci. 2016, 17, 1564. [Google Scholar] [CrossRef] [PubMed]
- Norman, M.; Żółtowska-Aksamitowska, S.; Zgoła-Grześkowiak, A.; Ehrlich, H.; Jesionowski, T. Iron(III) phthalocyanine supported on a spongin scaffold as an advanced photocatalyst in a highly efficient removal process of halophenols and bisphenol A. J. Hazard. Mater. 2018, 347, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Zdarta, J.; Norman, M.; Smułek, W.; Moszynski, D.; Kaczorek, E.; Stelling, A.L.; Ehrlich, H.; Jesionowski, T. Spongin-based scaffolds from Hippospongia communis demosponge as an effective support for lipase immobilization. Catalysts 2017, 7, 147. [Google Scholar] [CrossRef]
- Szatkowski, T.; Wysokowski, M.; Lota, G.; Pęziak, D.; Bazhenov, V.V.; Nowaczyk, G.; Walter, J.; Molodtsov, S.L.; Stöcker, H.; Himcinschi, C.; et al. Novel nanostructured hematite-spongin composite developed using extreme biomimetic approach. RSC Adv. 2015, 5, 79031–79040. [Google Scholar] [CrossRef]
- Szatkowski, T.; Stefańska, K.S.; Wysokowski, M.; Stelling, A.L.; Joseph, Y.; Ehrlich, H.; Jesionowski, T. Immobilization of titanium(IV) oxide onto 3D spongin scaffolds of marine sponge origin according to extreme biomimetics principles for removal of C.I. Basic Blue 9. Biomimetics 2017, 2, 4. [Google Scholar] [CrossRef]
- Szatkowski, T.; Kopczyński, K.; Motylenko, M.; Borrmann, H.; Mania, B.; Graś, M.; Lota, G.; Bazhenov, V.V.; Rafaja, D.; Roth, F.; et al. Extreme Biomimetics: Carbonized 3D spongin scaffold as a novel support for nanostructured manganese oxide(IV) and its electrochemical applications. Nano Res. 2018. [Google Scholar] [CrossRef]
- Heinemann, S.; Ehrlich, H.; Knieb, C.; Hanke, T. Biomimetically inspired hybrid materials based on silicified collagen. Int. J. Mater. Res. 2007, 98, 603–608. [Google Scholar] [CrossRef]
- Heinemann, S.; Heinemann, C.; Ehrlich, H.; Meyer, M.; Baltzer, H.; Worch, H.; Hanke, T. A novel biomimetic hybrid material made of silicified collagen: Perspectives for bone replacement. Adv. Eng. Mater. 2007, 9, 1061–1068. [Google Scholar] [CrossRef]
- Ehrlich, H.; Heinemann, S.; Heinemann, C.; Simon, P.; Bazhenov, V.V.; Shapkin, N.P.; Born, R.; Zabachnick, K.R.; Hanke, T.; Worch, H. Nanostructural organisation of naturally occuring composites: Part I. Silica-Collagen-Based Biocomposites. J. Nanomater. 2008. [Google Scholar] [CrossRef]
- Ehrlich, H.; Deutzmann, R.; Brunner, E.; Cappellini, E.; Koon, H.; Solazzo, C.; Yang, Y.; Ashford, D.; Thomas-Oates, J.; Lubeck, M.; et al. Mineralization of the Meter-long Biosilica Structures of Glass Sponges is template on Hydroxylated Collagen. Nat. Chem. 2010, 2, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Mehbub, M.F.; Lei, J.; Franco, C.; Zhang, W. Marine Sponge Derived Natural Products between 2001 and 2010: Trends and Opportunities for Discovery of Bioactives. Mar. Drugs 2014, 12, 4539–4577. [Google Scholar] [CrossRef] [PubMed]
- Leal, M.C.; Puga, J.; Serôdio, J.; Gomes, N.C.; Calado, R. Trends in the discovery of new marine natural products from invertebrates over the last two decades—Where and what are we bioprospecting? PLoS ONE 2012, 7, e30580. [Google Scholar] [CrossRef] [PubMed]
- Sipkema, D.; Osinga, R.; Schatton, W.; Mendola, D.; Tramper, J.; Wijffels, R.H. Large-scale production of pharmaceuticals by marine sponges: Sea, cell, or synthesis? Biotechnol. Bioeng. 2005, 90, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, C.; Valderrama, K.; Zea, S.; Castellanos, L. Mariculture and natural production of the antitumoural (+)-discodermolide by the Caribbean marine sponge Discodermia dissoluta. Mar Biotechnol. 2013, 15, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Custodio, M.R.; Prokic, I.; Steffen, R.; Koziol, C.; Borojevic, R.; Brümmer, F.; Nickel, M.; Müller, W.E. Primmorphs generated from dissociated cells of the sponge Suberites domuncula: A model system for studies of cell proliferation and cell death. Mech. Ageing Dev. 1998, 105, 45–59. [Google Scholar] [CrossRef]
- Pozzolini, M.; Mussino, F.; Cerrano, C.; Scarfì, S.; Giovine, M. Sponge cell cultivation: Optimization of the model Petrosia ficiformis (Poiret 1789). J. Exp. Mar. Biol. Ecol. 2014, 454, 70–77. [Google Scholar] [CrossRef]
- Wilkie, I.C.; Parma, L.; Bonasoro, F.; Bavestrello, G.; Cerrano, C.; Carnevali, M.D. Mechanical adaptability of a sponge extracellular matrix: Evidence for cellular control of mesohyl stiffness in Chondrosia reniformis Nardo. J. Exp. Biol. 2006, 209, 4436–4443. [Google Scholar] [CrossRef] [PubMed]
- Fassini, D.; Parma, L.; Lembo, F.; Candia Carnevali, M.D.; Wilkie, I.C.; Bonasoro, F. The reaction of the sponge Chondrosia reniformis to mechanical stimulation is mediated by the outer epithelium and the release of stiffening factor(s). Zoology 2014, 117, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Garrone, R.; Huc, A.; Junqua, S. Fine structure and physicochemical studies on the collagen of the marine sponge Chondrosia reniformis Nardo. J. Ultrastruct. Res. 1975, 52, 261–275. [Google Scholar] [CrossRef]
- Imhoff, J.M.; Garrone, R. Solubilization and Characterization of Chondrosia reniformis Sponge Collagen. Connect. Tissue Res. 1983, 11, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Pozzolini, M.; Bruzzone, F.; Berilli, V.; Mussino, F.; Cerrano, C.; Benatti, U.; Giovine, M. Molecular characterization of a nonfibrillar collagen from the marine sponge Chondrosia reniformis Nardo 1847 and positive effects of soluble silicates on its expression. Mar. Biotechnol. 2012, 14, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Pozzolini, M.; Scarfì, S.; Mussino, F.; Ghignone, S.; Vezzulli, L.; Giovine, M. Molecular characterization and expression analysis of the first Porifera tumor necrosis factor superfamily member and of its putative receptor in the marine sponge C. reniformis. Dev. Comp. Immunol. 2016, 57, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Pozzolini, M.; Scarfì, S.; Mussino, F.; Ferrando, S.; Gallus, L.; Giovine, M. Molecular cloning, characterization, and expression analysis of a Prolyl 4-Hydroxylase from the marine sponge Chondrosia reniformis. Mar. Biotechnol. 2015, 17, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, S.; Ehrlich, H.; Douglas, T.; Heinemann, C.; Worch, H.; Schatton, W.; Hanke, T. Ultrastructural studies on the collagen of the marine sponge Chondrosia reniformis Nardo. Biomacromolecules 2007, 8, 3452–3457. [Google Scholar] [CrossRef] [PubMed]
- Swatschek, D.; Schatton, W.; Kellermann, J.; Muller, W.; Kreuter, J. Marine sponge collagen: Isolation, characterization and effects on the skin parameters surface-pH, moisture and sebum. Eur. J. Pharm. Biopharm. 2002, 53, 107–113. [Google Scholar] [CrossRef]
- Nicklas, M.; Schatton, W.; Heinemann, S.; Hanke, T.; Kreuter, J. Enteric coating derived from marine sponge collagen. Drug Dev. Ind. Pharm. 2009, 35, 1384–1388. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, J.; Muller, W.; Swatschek, D.; Schatton, W.; Schatton, M. Method for Isolating Sponge Collagen and Producing Nanoparticulate Collagen, and the Use Thereof. U.S. Patent 20030032601 A1, 3 March 2000. [Google Scholar]
- Gross, J.; Sokal, Z.; Rougvie, M. Structural and chemical studies on the connective tissue of marine sponges. J. Histochem. Cytochem. 1956, 4, 227–246. [Google Scholar] [CrossRef] [PubMed]
- Diehl-Seifert, B.; Kurelec, B.; Zahn, R.K.; Dorn, A.; Jericevic, B.; Uhlenbruck, G.; Müller, W.E. Attachment of sponge cells to collagen substrata: Effect of a collagen assembly factor. J. Cell Sci. 1985, 79, 271–285. [Google Scholar] [PubMed]
- Di Benedetto, C.; Barbaglio, A.; Martinello, T.; Alongi, V.; Fassini, D.; Cullorà, E.; Patruno, M.; Bonasoro, F.; Barbosa, M.A.; Carnevali, M.D.; et al. Production, characterization and biocompatibility of marine collagen matrices from an alternative and sustainable source: The sea urchin Paracentrotus lividus. Mar. Drugs 2014, 12, 4912–4933. [Google Scholar] [CrossRef] [PubMed]
- Mezger, T.G. The Rheology Handbook: For Users of Rotational and Oscillatory Rheometers, 2nd ed.; Vincentz Network: Hannover, Germany, 2006; ISBN 978-3-86-630842-8. [Google Scholar]
- Nam, K.A.; You, S.G.; Kim, S.M. Molecular and physical characteristics of squid (Todarodes pacificus) skin collagens and biological properties of their enzymatic hydrolysates. J. Food Sci. 2008, 73, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Pozzolini, M.; Valisano, L.; Cerrano, C.; Menta, M.; Schiaparelli, S.; Bavestrello, G.; Benatti, U.; Giovine, M. Influence of rocky substrata on three-dimensional sponge cells model development. In Vitro Cell. Dev. Biol. Anim. 2010, 46, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Mauch, C.; Hatamochi, A.; Scharffetter, K.; Krieg, T. Regulation of collagen synthesis in fibroblasts within a three-dimensional collagen gel. Exp. Cell Res. 1988, 178, 1508–1515. [Google Scholar] [CrossRef]
- Nusgens, B.; Merrill, C.; Lapiere, C.; Bell, E. Collagen biosynthesis by cells in a tissue equivalent matrix in vitro. Coll. Relat. Res. 1984, 4, 351–361. [Google Scholar] [CrossRef]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-based biomaterials for tissue engineering applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef]
- Badylak, S.F. The extracellular matrix as a biologic scaffold material. Biomaterials 2007, 28, 3587–3593. [Google Scholar] [CrossRef] [PubMed]
- Wilkie, I.C. Mutable collagenous tissue: Overview and biotechnological perspective. Prog. Mol. Subcell. Biol. 2005, 39, 221–250. [Google Scholar] [PubMed]
- Bondioli, E.; Fini, M.; Veronesi, F.; Giavaresi, G.; Tschon, M.; Cenacchi, G.; Cerasoli, S.; Giardino, R.; Melandri, D. Development and evaluation of a decellularized membrane from human dermis. J. Tissue Eng. Regen. Med. 2014, 8, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Eyre, D.R.; Slayter, H.S. Type VI collagen of the intervertebral disc. Biochemical and electron-microscopic characterization of the native protein. Biochem. J. 1987, 248, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Pozzolini, M.; Scarfì, S.; Gallus, L.; Ferrando, S.; Cerrano, C.; Giovine, M. Silica-induced fibrosis: An ancient response from the early metazoans. J. Exp. Biol. 2017, 220, 4007–4015. [Google Scholar] [CrossRef] [PubMed]
- Haugh, M.G.; Murphy, C.M.; McKiernan, R.C.; Altenbuchner, C.; O’Brien, F.J. Crosslinking and mechanical properties significantly influence cell attachment, proliferation, and migration within collagen glycosaminoglycan scaffolds. Tissue Eng. Part A 2011, 17, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Duan, L.; Tian, Z.; Liu, W.; Li, G.; Huang, X. Rheological behavior of acylated pepsin-solubilized collagen solutions: Effects of concentration. Korea-Aust. Rheol. J. 2015, 27, 287–295. [Google Scholar] [CrossRef]
- Goo, H.C.; Hwangb, Y.-S.; Choib, Y.R.; Choc, H.N.; Suha, S. Development of collagenase-resistant collagen and its interaction with adult human dermal fibroblasts. Biomaterials 2003, 24, 5099–5113. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Zhou, Z.; Lai, H.; Xu, P.; Liao, L.; Wei, J. Biodegradable polymer membranes applied in guided bone/tissue regeneration: A review. Polymers 2016, 8, 115. [Google Scholar] [CrossRef]
- Collier, T.O.; Jenney, C.R.; DeFife, K.M.; Anderson, J.M. Protein adsorption on chemically modified surfaces. Biomed. Sci. Instrum. 1997, 33, 178–183. [Google Scholar] [PubMed]
- Pieper, J.S.; Oosterhof, A.; Dijkstra, P.J.; Veerkamp, J.H.; van Kuppevelt, T.H. Preparation and characterization of porous crosslinked collagenous matrices containing bioavailable chondroitin sulphate. Biomaterials 1999, 20, 847–858. [Google Scholar] [CrossRef]
- Liu, Y.; Ji, H.; Dong, J.; Zhang, S.; Lee, K.J.; Matthew, S. Antioxidant alkaloid from the South China Sea marine sponge Iotrochota sp. Z. Naturforsch. C 2008, 63, 636–638. [Google Scholar] [CrossRef] [PubMed]
- Alonso, E.; Alvariño, R.; Leirós, M.; Tabudravu, J.N.; Feussner, K.; Dam, M.A.; Rateb, M.E.; Jaspars, M.; Botana, L.M. Evaluation of the antioxidant activity of the marine pyrroloiminoquinone makaluvamines. Mar. Drugs 2016, 14, 197. [Google Scholar] [CrossRef] [PubMed]
- Pallela, R.; Ehrlich, H.; Bhatnagar, I. Biomedical applications of marine sponge collagens, marine sponges: Chemicobiological and biomedical applications. In Marine Sponges: Chemicobiological and Biomedical Application; Pallela, R., Ehrlich, H., Eds.; Springer: New Delhi, India, 2016; pp. 373–381. [Google Scholar]
- Fassini, D.; Duarte, A.R.C.; Reis, R.L.; Silva, T.H. Bioinspiring Chondrosia reniformis (Nardo, 1847) collagen-based hydrogel: A new extraction method to obtain a sticky and self-healing collagenous material. Mar. Drugs 2017, 15, 380. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.K.; Enwemeka, C.S. A simplified method for the analysis of hydroxyproline in biological tissues. Clin. Biochem. 1996, 29, 225–239. [Google Scholar] [CrossRef]
- Frazier, S.B.; Roodhouse, K.A.; Hourcade, D.E.; Zhang, L. The quantification of glycosaminoglycans: A comparison of HPLC, Carbazole, and Alcian Blue methods. Open Glycosci. 2008, 1, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, L.C.; Bignolas, G.; Brentani, R.R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem. J. 1979, 11, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Dong, S.; Hao, Y.; Chu, T.; Li, C.; Zhang, Z.; Zhou, Y. Demineralized bone matrix gelatin as scaffold for tissue engineering. Afr. J. Microbiol. Res. 2010, 4, 865–870. [Google Scholar] [CrossRef]
- Pozzolini, M.; Scarfi, S.; Benatti, U.; Giovine, M. Interference in MTT cell viability assay in activated macrophage cell line. Anal. Biochem. 2003, 313, 338–341. [Google Scholar] [CrossRef]



| Sample # | Extraction Method | mg FS/g Dry Tissue | mg Collagen/g Dry Tissue | mg GAG/g Dry Tissue |
| F1 | 0.1% tryp, 2 rounds in H2O 1 | 338.27 ± 25.23 | 192.13 ± 22.15 | 12.85 ± 5.10 |
| F2 | 8 M urea, tris 0.1 M, pH 9, βMe, EDTA 2 | 422.83 ± 68.15 | 24.16 ± 1.30 | 20.44 ± 8.79 |
| F3 | NaCl 1 M, tris 50 mM pH 7.4, EDTA, βMe 3 | 998.35 ± 16.12 | 355.68 ± 56.01 | 26.89 ± 7.52 |
| F4 | NaCl 0.5 M, tris 0.1 M pH 8, EDTA, βMe 4 | 837.21 ± 75.87 | 139.02 ± 44.63 | 19.78 ± 3.67 |
| Sample # | % Soluble Proteins/FS | % Collagen/Proteins | % GAG/FS | % Collagen/FS | RC/GAG |
|---|---|---|---|---|---|
| F1 | 95.00 | 57.34 | 3.80 | 56.81 | 14.95 |
| F2 | 34.00 | 16.76 | 4.83 | 5.73 | 1.18 |
| F3 | 64.80 | 48.08 | 2.68 | 35.65 | 13.23 |
| F4 | 27.39 | 60.62 | 2.36 | 16.63 | 6.99 |
| Sample # | η0 (mPa s) | η∞ (mPa s) |
|---|---|---|
| F1 | 495 | 3.84 |
| F2 | 911 | 3.93 |
| F3 | 2412 | 3.90 |
| F4 | 336 | 1.70 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozzolini, M.; Scarfì, S.; Gallus, L.; Castellano, M.; Vicini, S.; Cortese, K.; Gagliani, M.C.; Bertolino, M.; Costa, G.; Giovine, M. Production, Characterization and Biocompatibility Evaluation of Collagen Membranes Derived from Marine Sponge Chondrosia reniformis Nardo, 1847. Mar. Drugs 2018, 16, 111. https://doi.org/10.3390/md16040111
Pozzolini M, Scarfì S, Gallus L, Castellano M, Vicini S, Cortese K, Gagliani MC, Bertolino M, Costa G, Giovine M. Production, Characterization and Biocompatibility Evaluation of Collagen Membranes Derived from Marine Sponge Chondrosia reniformis Nardo, 1847. Marine Drugs. 2018; 16(4):111. https://doi.org/10.3390/md16040111
Chicago/Turabian StylePozzolini, Marina, Sonia Scarfì, Lorenzo Gallus, Maila Castellano, Silvia Vicini, Katia Cortese, Maria Cristina Gagliani, Marco Bertolino, Gabriele Costa, and Marco Giovine. 2018. "Production, Characterization and Biocompatibility Evaluation of Collagen Membranes Derived from Marine Sponge Chondrosia reniformis Nardo, 1847" Marine Drugs 16, no. 4: 111. https://doi.org/10.3390/md16040111
APA StylePozzolini, M., Scarfì, S., Gallus, L., Castellano, M., Vicini, S., Cortese, K., Gagliani, M. C., Bertolino, M., Costa, G., & Giovine, M. (2018). Production, Characterization and Biocompatibility Evaluation of Collagen Membranes Derived from Marine Sponge Chondrosia reniformis Nardo, 1847. Marine Drugs, 16(4), 111. https://doi.org/10.3390/md16040111









