Biphasic Scaffolds from Marine Collagens for Regeneration of Osteochondral Defects
Abstract
1. Introduction
2. Results
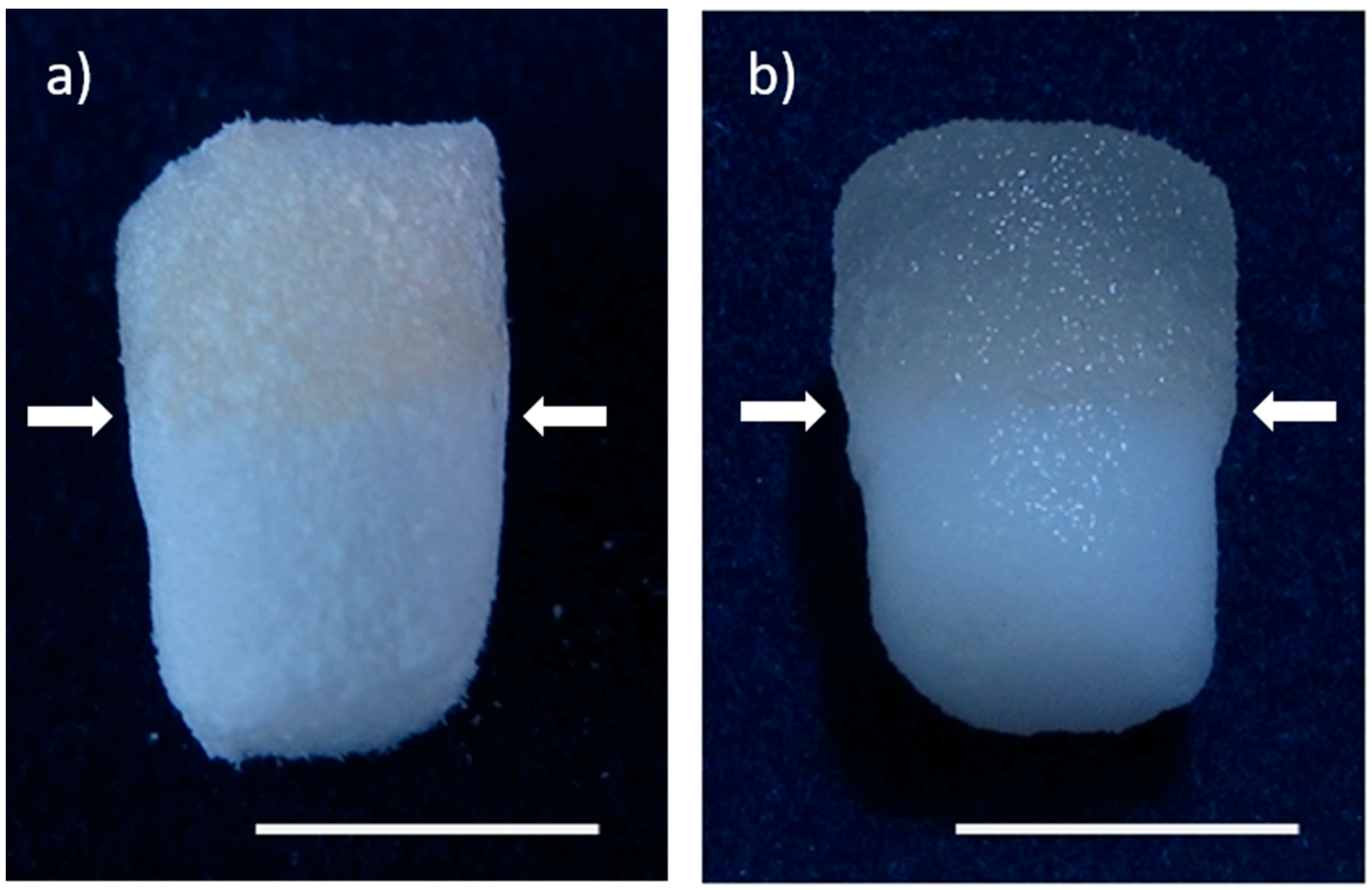
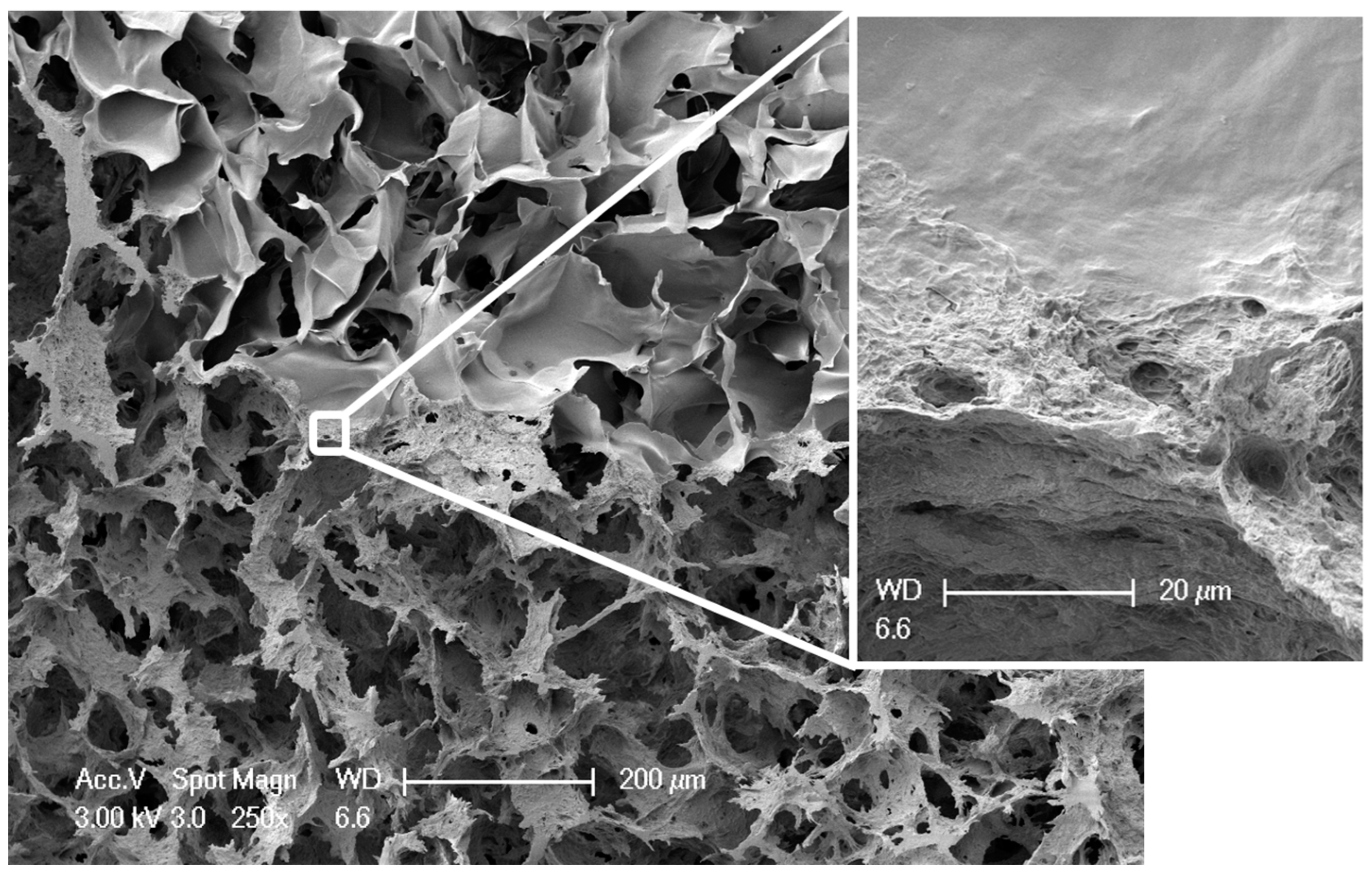
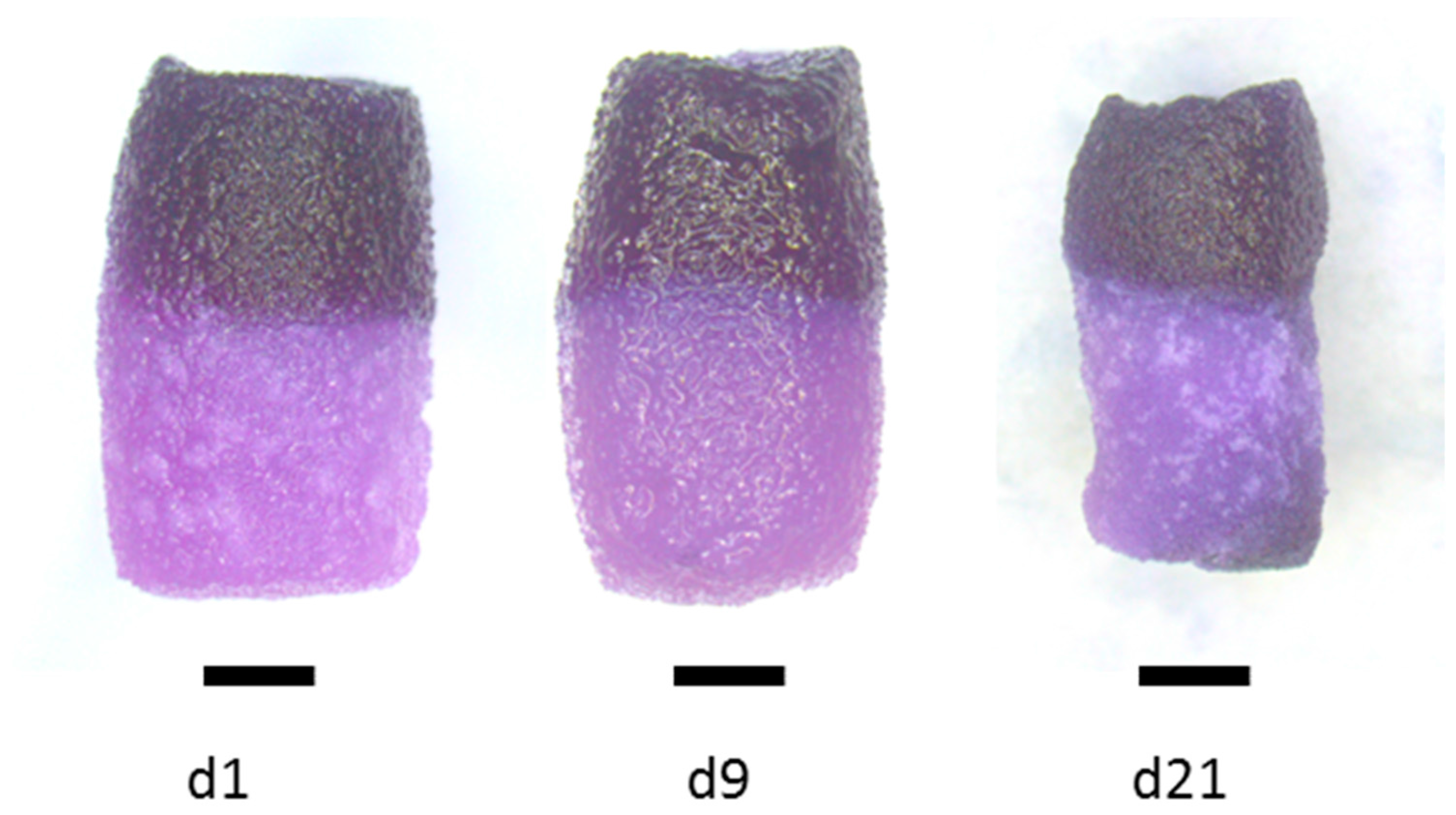
2.1. Preparation and Characterization of Biphasic Scaffolds from Marine Collagen
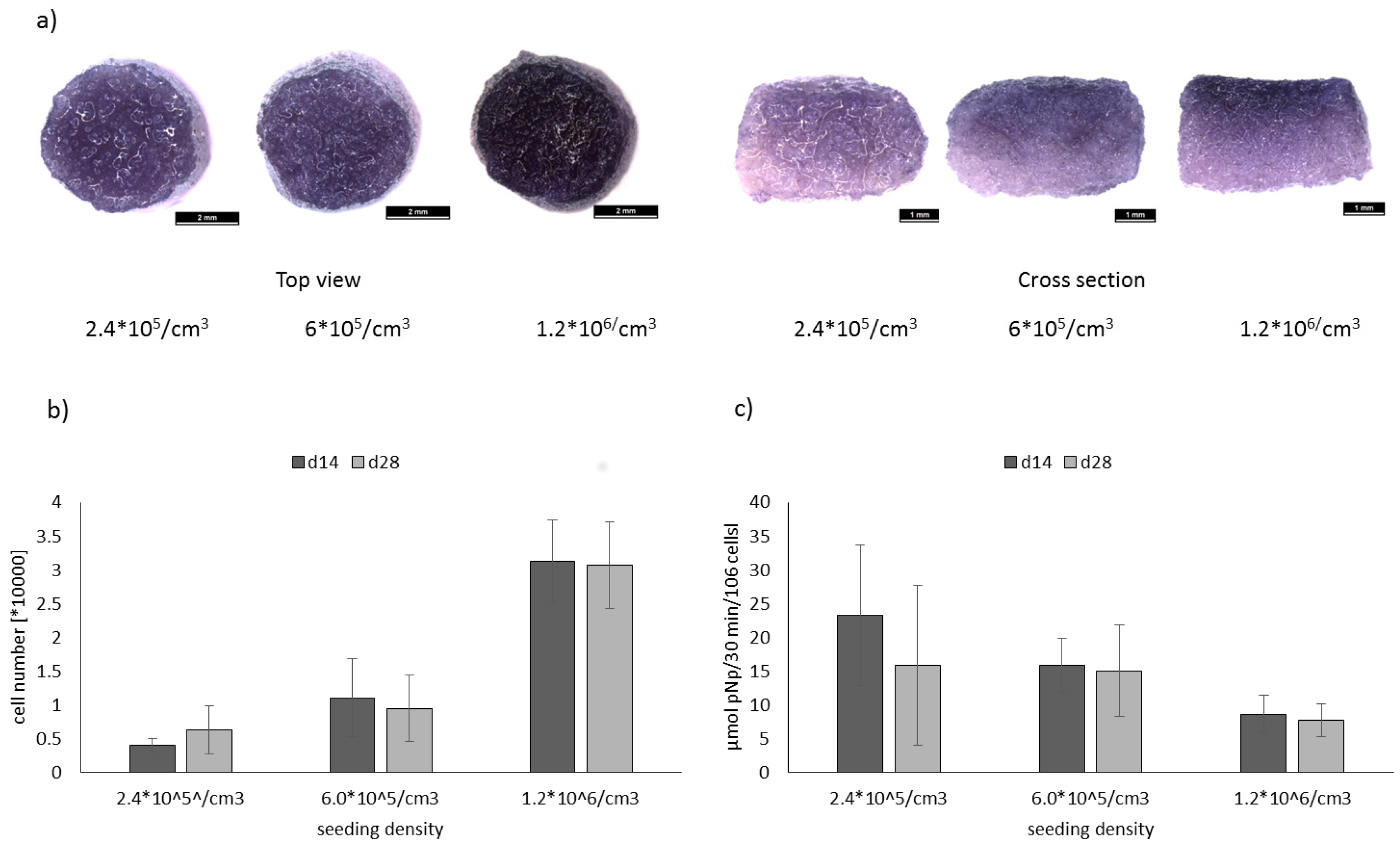
2.2. Evaluation of Optimal Seeding Density for the Osteogenic Differentiation of hMSC
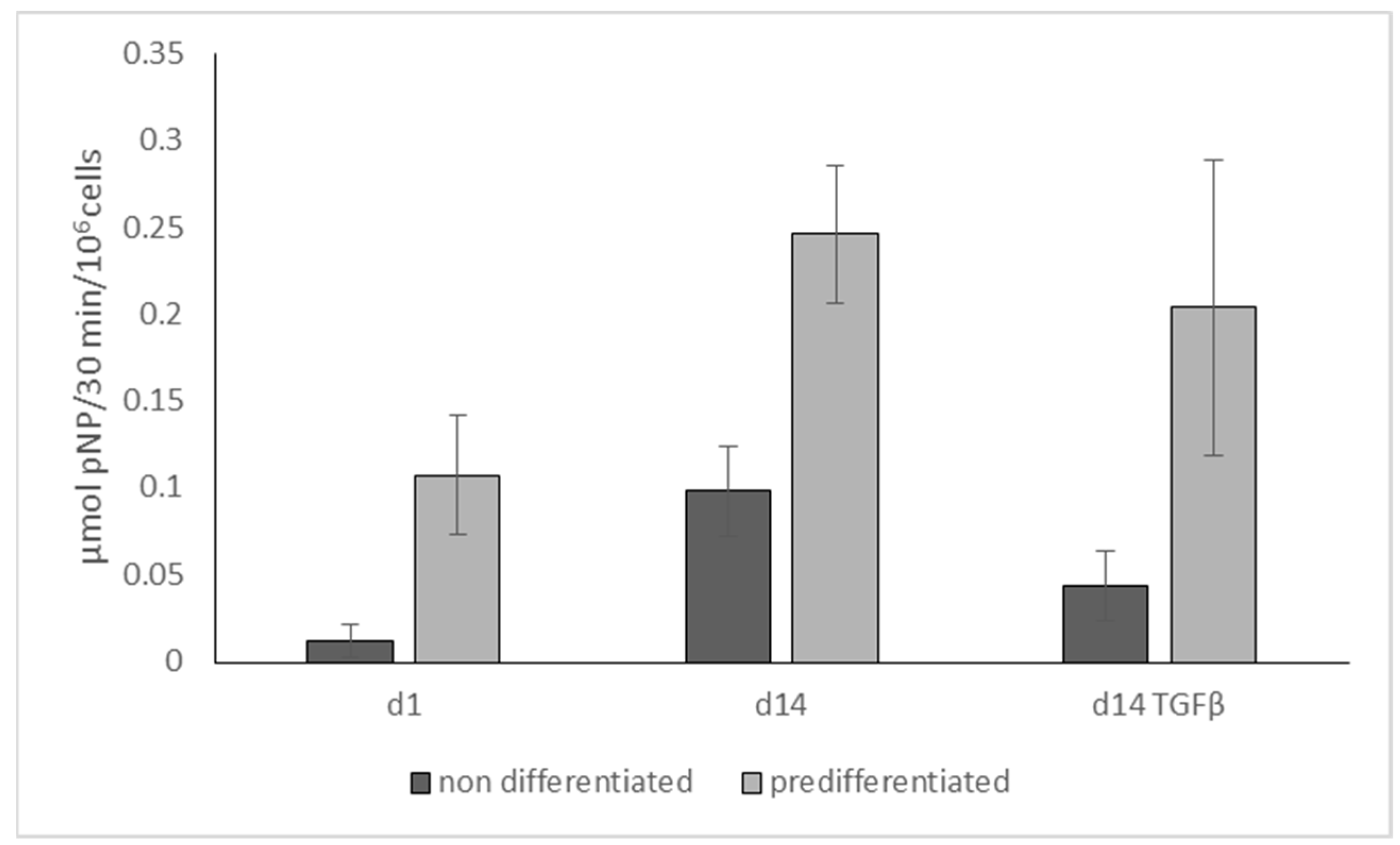
2.3. Influence of TGF-β on Osteogenic Differentiation
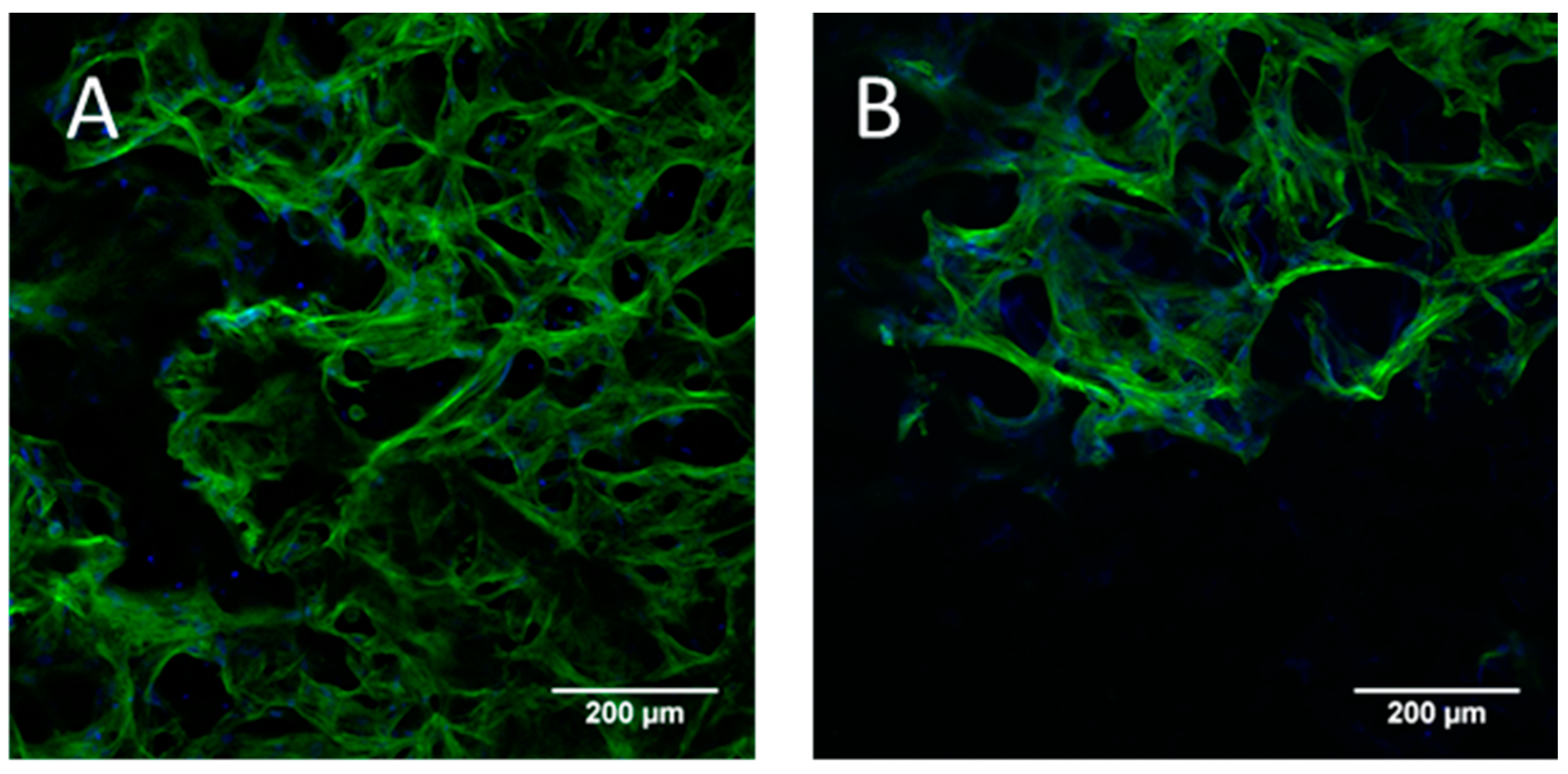
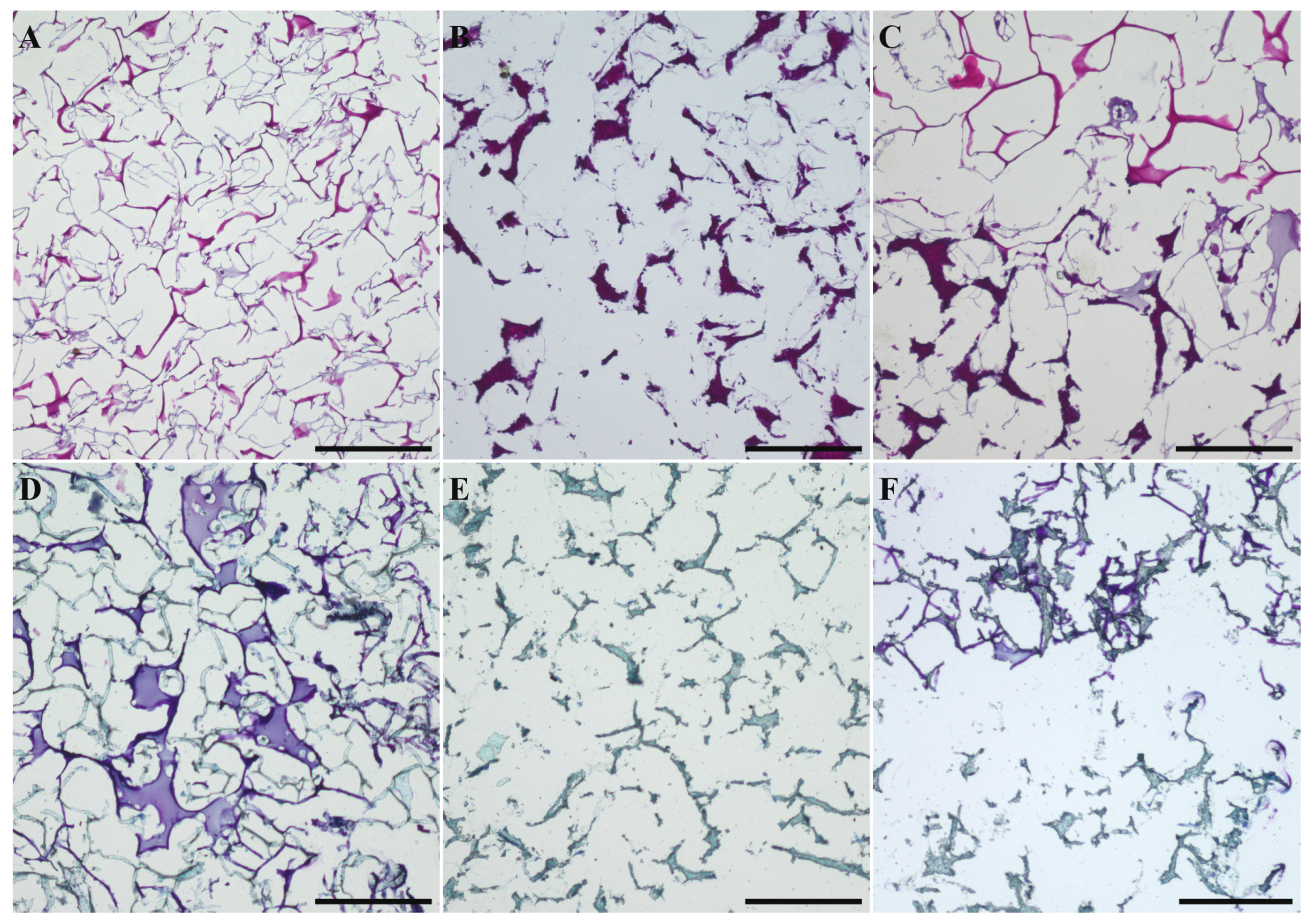
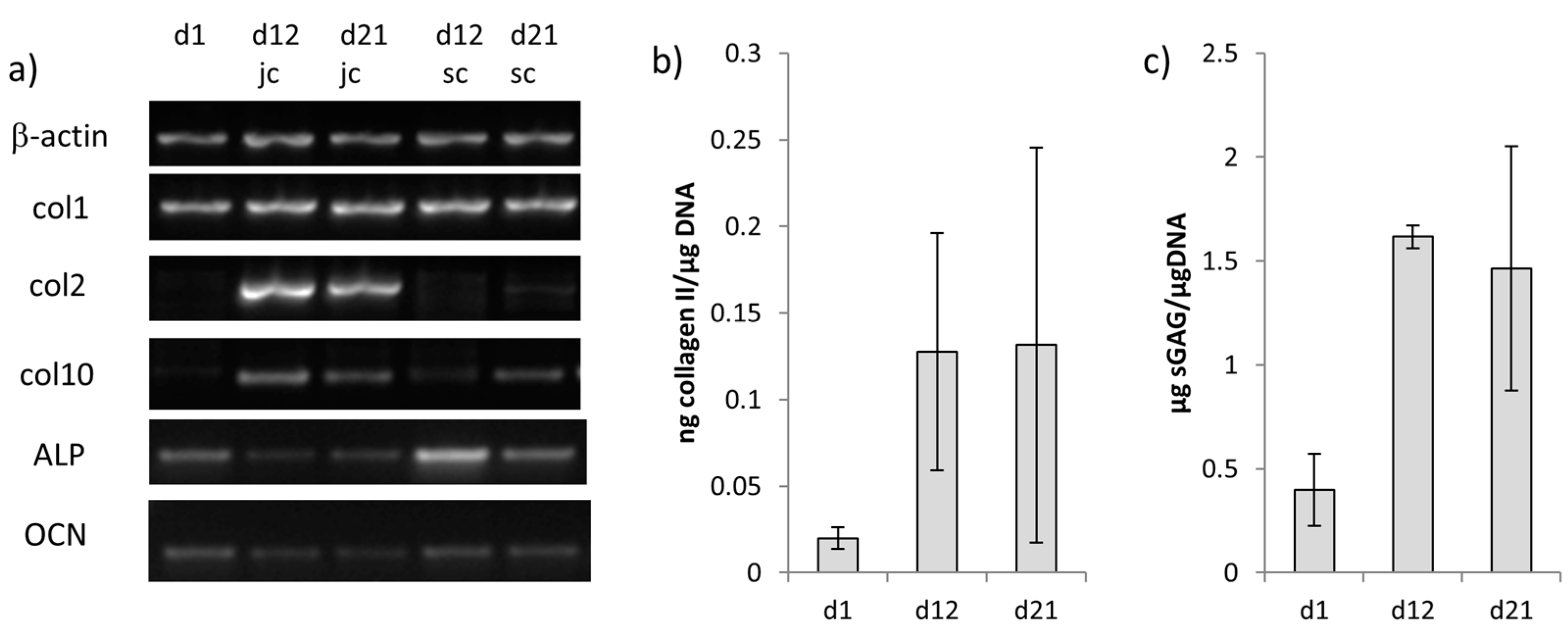
2.4. Chondrogenic and Osteogenic Differentiation of hMSC in Biphasic Marine Scaffolds
3. Discussion
4. Materials and Methods
4.1. Preparation of Biphasic Scaffolds
4.2. Cultivation of hMSC
4.3. Osteogenic Differentiation of hMSC in Mineralized Salmon Collagen Scaffolds
4.4. Cultivation of Osteochondral Constructs
4.5. MTT Staining
4.6. Fluorescence Staining and Confocal Laser Scanning Microscopy
4.7. Gene Expression Analysis
4.8. Analysis of DNA Content, ALP Activity in Monophasic Scaffolds from Mineralized Salmon Collagen sGAG Content and Collagen II Content
4.9. sGAG Content and Collagen II Content in Biphasic Scaffolds
4.10. Histological Investigations on Biphasic Scaffolds
4.11. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Silvipriya, K.; Kumar, K.; Bhat, A.; Kumar, B.; John, A.; Lakshmanan, P. Collagen: Animal Sources and Biomedical Application. J. Appl. Pharm. Sci. 2015, 5, 123–127. [Google Scholar] [CrossRef]
- Ramshaw, J.A.M. Biomedical applications of collagens. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H. Biological Materials of Marine Origin: Invertebrates; Biologically-Inspired Systems; Springer: Dordrecht, The Netherlands; New York, NY, USA, 2010; ISBN 978-90-481-9129-1. [Google Scholar]
- Ehrlich, H. Biological Materials of Marine Origin Vertebrates; Springer: Dordrecht, The Netherlands, 2015; ISBN 978-94-007-5730-1. [Google Scholar]
- Silva, T.; Moreira-Silva, J.; Marques, A.; Domingues, A.; Bayon, Y.; Reis, R. Marine Origin Collagens and Its Potential Applications. Mar. Drugs 2014, 12, 5881–5901. [Google Scholar] [CrossRef] [PubMed]
- Subhan, F.; Ikram, M.; Shehzad, A.; Ghafoor, A. Marine Collagen: An Emerging Player in Biomedical applications. J. Food Sci. Technol. 2015, 52, 4703–4707. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, B.; Bernhardt, A.; Heinemann, S.; Stachel, I.; Meyer, M.; Gelinsky, M. Biomimetically mineralized salmon collagen scaffolds for application in bone tissue engineering. Biomacromolecules 2012, 13, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Sewing, J.; Klinger, M.; Notbohm, H. Jellyfish collagen matrices conserve the chondrogenic phenotype in two- and three-dimensional collagen matrices. J. Tissue Eng. Regen. Med. 2017, 11, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, B.; Bernhardt, A.; Lode, A.; Heinemann, S.; Sewing, J.; Klinger, M.; Notbohm, H.; Gelinsky, M. Jellyfish collagen scaffolds for cartilage tissue engineering. Acta Biomater. 2014, 10, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Gelinsky, M.; Eckert, M.; Despang, F. Biphasic, but monolithic scaffolds for the therapy of osteochondral defects. Int. J. Mater. Res. 2007, 98, 749–755. [Google Scholar] [CrossRef]
- Jeon, J.E.; Vaquette, C.; Klein, T.J.; Hutmacher, D.W. Perspectives in Multiphasic Osteochondral Tissue Engineering: Perspectives in Multiphasic Osteochondral Tissue Engineering. Anat. Rec. 2014, 297, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, K.; Moriguchi, Y.; Murawski, C.D.; Yoshikawa, H.; Nakamura, N. Osteochondral Tissue Engineering with Biphasic Scaffold: Current Strategies and Techniques. Tissue Eng. Part B Rev. 2014, 20, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, A.-M.; Hoque, M.E.; Prasad, R.G.S.V.; Uth, N. Current strategies in multiphasic scaffold design for osteochondral tissue engineering: A review: Current Strategies in Multiphasic Scaffold Design. J. Biomed. Mater. Res. A 2015, 103, 2460–2481. [Google Scholar] [CrossRef] [PubMed]
- Gadjanski, I.; Vunjak-Novakovic, G. Challenges in engineering osteochondral tissue grafts with hierarchical structures. Expert Opin. Biol. Ther. 2015, 15, 1583–1599. [Google Scholar] [CrossRef] [PubMed]
- Mano, J.F.; Reis, R.L. Osteochondral defects: Present situation and tissue engineering approaches. J. Tissue Eng. Regen. Med. 2007, 1, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mareddy, S.; Tan, D.M.; Crawford, R.; Long, X.; Miao, X.; Xiao, Y. A minimal common osteochondrocytic differentiation medium for the osteogenic and chondrogenic differentiation of bone marrow stromal cells in the construction of osteochondral graft. Tissue Eng. Part A 2009, 15, 2481–2490. [Google Scholar] [CrossRef] [PubMed]
- Pieper, J.S.; van der Kraan, P.M.; Hafmans, T.; Kamp, J.; Buma, P.; van Susante, J.L.C.; van den Berg, W.B.; Veerkamp, J.H.; van Kuppevelt, T.H. Crosslinked type II collagen matrices: Preparation, characterization, and potential for cartilage engineering. Biomaterials 2002, 23, 3183–3192. [Google Scholar] [CrossRef]
- Nicoletti, A.; Fiorini, M.; Paolillo, J.; Dolcini, L.; Sandri, M.; Pressato, D. Effects of different crosslinking conditions on the chemical–physical properties of a novel bio-inspired composite scaffold stabilised with 1,4-butanediol diglycidyl ether (BDDGE). J. Mater. Sci. Mater. Med. 2013, 24, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Sartori, M.; Pagani, S.; Ferrari, A.; Costa, V.; Carina, V.; Figallo, E.; Maltarello, M.C.; Martini, L.; Fini, M.; Giavaresi, G. A new bi-layered scaffold for osteochondral tissue regeneration: In vitro and in vivo preclinical investigations. Mater. Sci. Eng. C 2017, 70, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Bermueller, C.; Schwarz, S.; Elsaesser, A.F.; Sewing, J.; Baur, N.; von Bomhard, A.; Scheithauer, M.; Notbohm, H.; Rotter, N. Marine collagen scaffolds for nasal cartilage repair: Prevention of nasal septal perforations in a new orthotopic rat model using tissue engineering techniques. Tissue Eng. Part A 2013, 19, 2201–2214. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.U.; Barthel, T.S.; Nishimura, K.; Solchaga, L.; Caplan, A.I.; Goldberg, V.M.; Johnstone, B. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J. Bone Jt. Surg. Am. 1998, 80, 1745–1757. [Google Scholar] [CrossRef]
- Pustlauk, W.; Paul, B.; Gelinsky, M.; Bernhardt, A. Jellyfish collagen and alginate: Combined marine materials for superior chondrogenesis of hMSC. Mater. Sci. Eng. C 2016, 64, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Lode, A.; Bernhardt, A.; Gelinsky, M. Cultivation of human bone marrow stromal cells on three-dimensional scaffolds of mineralized collagen: Influence of seeding density on colonization, proliferation and osteogenic differentiation. J. Tissue Eng. Regen. Med. 2008, 2, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Vater, C.; Kasten, P.; Stiehler, M. Culture media for the differentiation of mesenchymal stromal cells. Acta Biomater. 2011, 7, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Pustlauk, W.; Paul, B.; Brueggemeier, S.; Gelinsky, M.; Bernhardt, A. Modulation of chondrogenic differentiation of human mesenchymal stem cells in jellyfish collagen scaffolds by cell density and culture medium: Chondrogenic differentiation of hMSCs in jellyfish collagen scaffolds. J. Tissue Eng. Regen. Med. 2017, 11, 1710–1722. [Google Scholar] [CrossRef] [PubMed]
- Binder, B.Y.K.; Sagun, J.E.; Leach, J.K. Reduced Serum and Hypoxic Culture Conditions Enhance the Osteogenic Potential of Human Mesenchymal Stem Cells. Stem Cell Rev. Rep. 2015, 11, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Mackay, A.M.; Beck, S.C.; Murphy, J.M.; Barry, F.P.; Chichester, C.O.; Pittenger, M.F. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998, 4, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Glueck, M.; Gardner, O.; Czekanska, E.; Alini, M.; Stoddart, M.J.; Salzmann, G.M.; Schmal, H. Induction of Osteogenic Differentiation in Human Mesenchymal Stem Cells by Crosstalk with Osteoblasts. BioRes. Open Access 2015, 4, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Mohan, N.; Berkland, C.J.; Detamore, M.S. Microsphere-Based Scaffolds Carrying Opposing Gradients of Chondroitin Sulfate and Tricalcium Phosphate. Front. Bioeng. Biotechnol. 2015, 3, 96. [Google Scholar] [CrossRef] [PubMed]
- Caliari, S.R.; Harley, B.A.C. Collagen-GAG Scaffold Biophysical Properties Bias MSC Lineage Choice in the Presence of Mixed Soluble Signals. Tissue Eng. Part A 2014, 20, 2463–2472. [Google Scholar] [CrossRef] [PubMed]
- Buxton, A.N.; Bahney, C.S.; Yoo, J.U.; Johnstone, B. Temporal Exposure to Chondrogenic Factors Modulates Human Mesenchymal Stem Cell Chondrogenesis in Hydrogels. Tissue Eng. Part A 2011, 17, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Fensky, F.; Reichert, J.C.; Traube, A.; Rackwitz, L.; Siebenlist, S.; Nöth, U. Chondrogenic predifferentiation of human mesenchymal stem cells in collagen type I hydrogels. Biomed. Tech. 2014, 59, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Erickson, I.E.; Choudhury, M.; Pleshko, N.; Mauck, R.L. Transient exposure to TGF-β3 improves the functional chondrogenesis of MSC-laden hyaluronic acid hydrogels. J. Mech. Behav. Biomed. Mater. 2012, 11, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Lee, W.Y.W.; Feng, Q.; Xu, L.; Wang, B.; Man, G.C.W.; Chen, Y.; Jiang, X.; Bian, L.; Cui, L.; et al. Synergistic effects on mesenchymal stem cell-based cartilage regeneration by chondrogenic preconditioning and mechanical stimulation. Stem Cell Res. Ther. 2017, 8, 221. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Toben, D.; Lienau, J.; Schell, H.; Bail, H.J.; Matziolis, G.; Duda, G.N.; Kaspar, K. Locally applied osteogenic predifferentiated progenitor cells are more effective than undifferentiated mesenchymal stem cells in the treatment of delayed bone healing. Tissue Eng. Part A 2009, 15, 2947–2954. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Han, N.; Zhang, P.; Jiang, B. Local transplantation of osteogenic pre-differentiated autologous adipose-derived mesenchymal stem cells may accelerate non-union fracture healing with limited pro-metastatic potency. Int. J. Clin. Exp. Med. 2015, 8, 1406–1410. [Google Scholar] [PubMed]
- Jaiswal, N.; Haynesworth, S.E.; Caplan, A.I.; Bruder, S.P. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J. Cell. Biochem. 1997, 64, 295–312. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, Y.-J.; Im, G.-I. Is continuous treatment with transforming growth factor-beta necessary to induce chondrogenic differentiation in mesenchymal stem cells? Cells Tissues Organs 2009, 190, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]

| Osteogenic medium | FCS | - | Low glucose | AAP | Dex | β-GP | |
| Chondrogenic medium | - | ITS | High glucose | AAP | Dex | - | TGF-β |
| Marker | Bp | Primer (Forward/Reverse) | Buffer/±Enhancer | Tannealing | Amplification Cycles |
|---|---|---|---|---|---|
| β-Act | 234 | 5′-GGACTTCGAGCAAGAGATGG-3′ 5′-AGCACTGTGTTGGCGTACAG-3′ | buffer S/− | 55 °C | 30x |
| Col 1 | 331 | 5′-GGATGAGGAGACTGGCAAC-3′ 5′-GAAGAAGAAATGGCAAAGAGAAAG-3′ | buffer S/− | 55 °C | 25x |
| Col 2 | 388 | 5′-GAACATCACCTACCACTGCAAG-3′ 5′-GCAGAGTCCTAGAGTGACTGAG-3′ | buffer Y/+ | 60 °C | 35x |
| Col 10 | 196 | 5′-GCCCACTACCCAACACCAAGAC-3′ 5′-CCTGGCAACCCTGGCTCTC-3′ | buffer S/− | 50 °C | 30x |
| ALP | 162 | 5′-ACCATTCCCACGTCTTCACATTTG-3′ 5′-ATTCTCTCGTTCACCGCCCAC-3′ | buffer S/− | 55 °C | 30x |
| OCN | 177 | 5′-CAA AGG TGC AGC CTT TGT GTC-3′ 5′-TCA CAG TCC GGA TTG AGC TCA-3′ | buffer S/− | 55 °C | 35x |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernhardt, A.; Paul, B.; Gelinsky, M. Biphasic Scaffolds from Marine Collagens for Regeneration of Osteochondral Defects. Mar. Drugs 2018, 16, 91. https://doi.org/10.3390/md16030091
Bernhardt A, Paul B, Gelinsky M. Biphasic Scaffolds from Marine Collagens for Regeneration of Osteochondral Defects. Marine Drugs. 2018; 16(3):91. https://doi.org/10.3390/md16030091
Chicago/Turabian StyleBernhardt, Anne, Birgit Paul, and Michael Gelinsky. 2018. "Biphasic Scaffolds from Marine Collagens for Regeneration of Osteochondral Defects" Marine Drugs 16, no. 3: 91. https://doi.org/10.3390/md16030091
APA StyleBernhardt, A., Paul, B., & Gelinsky, M. (2018). Biphasic Scaffolds from Marine Collagens for Regeneration of Osteochondral Defects. Marine Drugs, 16(3), 91. https://doi.org/10.3390/md16030091






