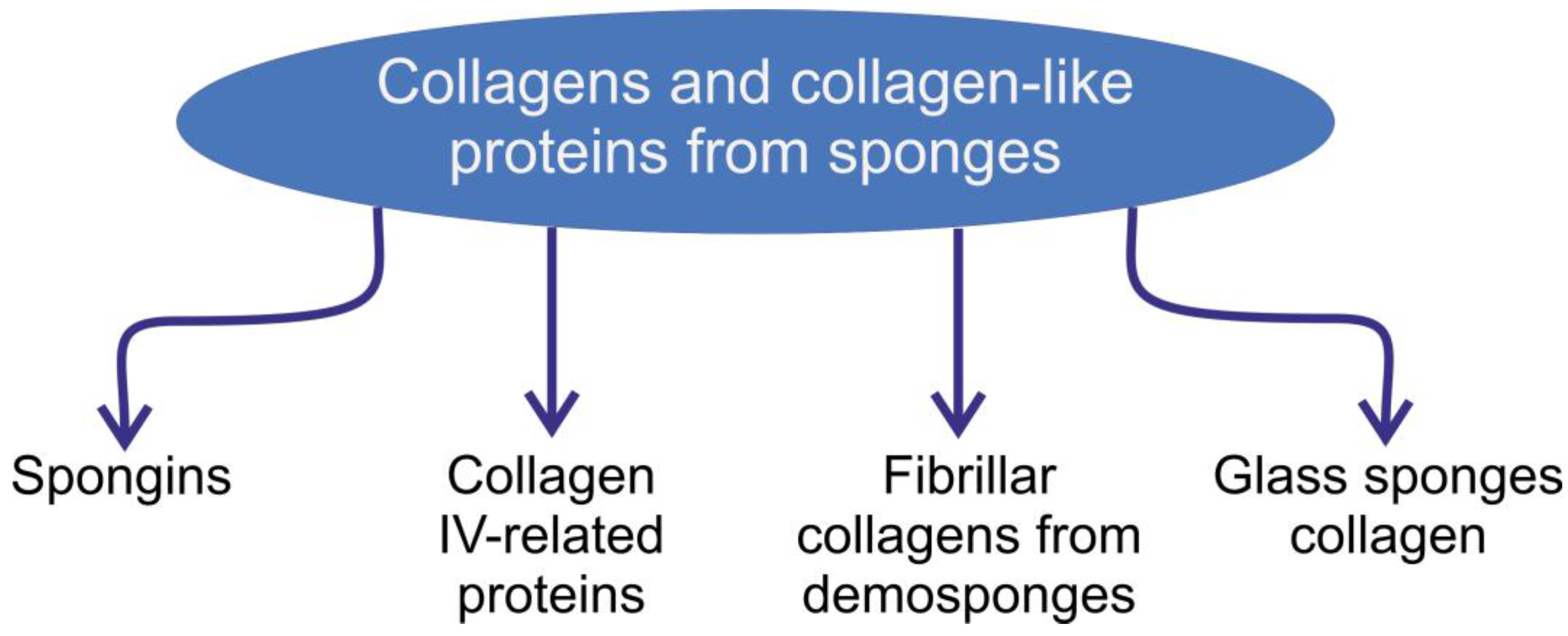
Collagens of Poriferan Origin
Abstract
1. Introduction
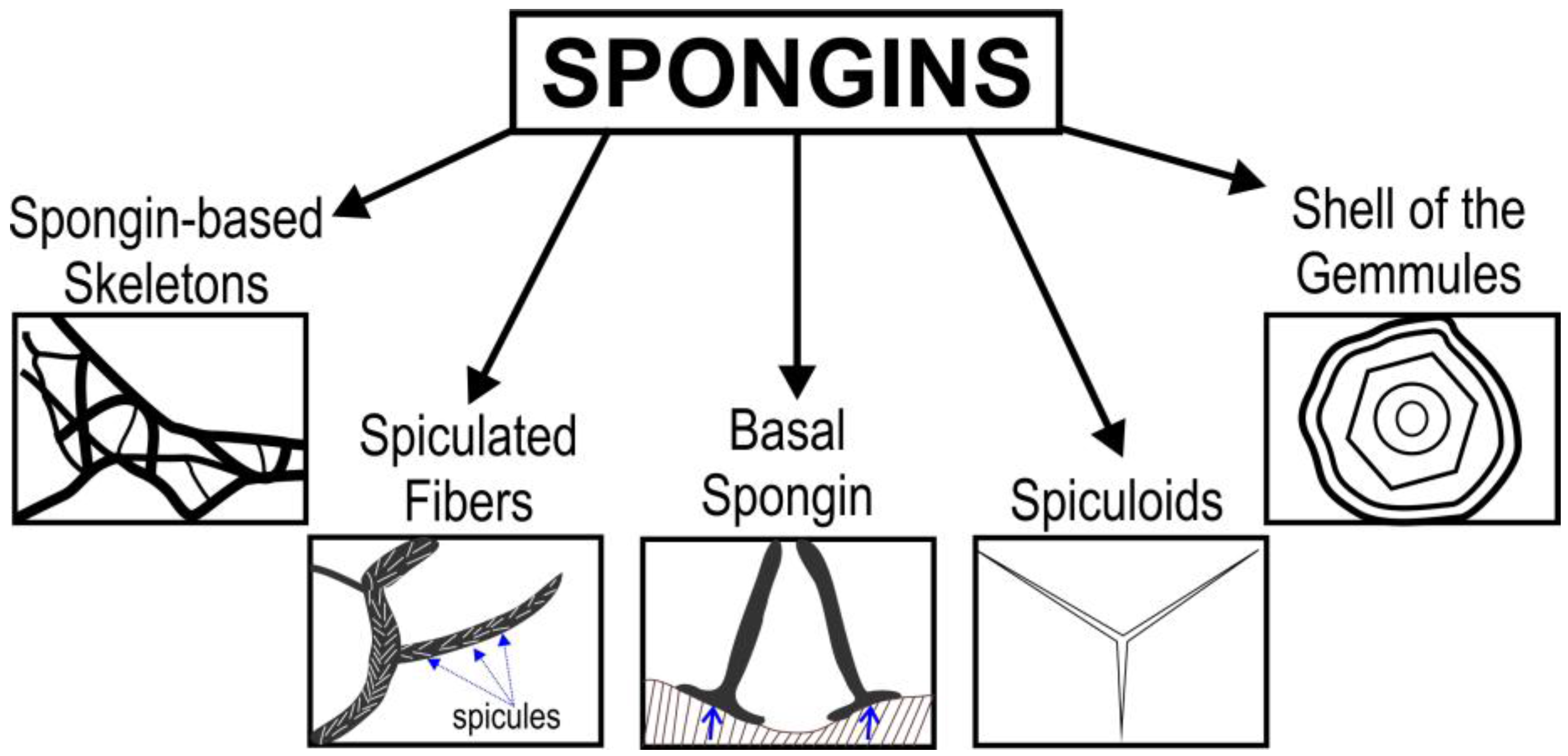
2. Spongins as Enigmatic Structural Proteins in Sponges
Trends in the Applications of Spongins
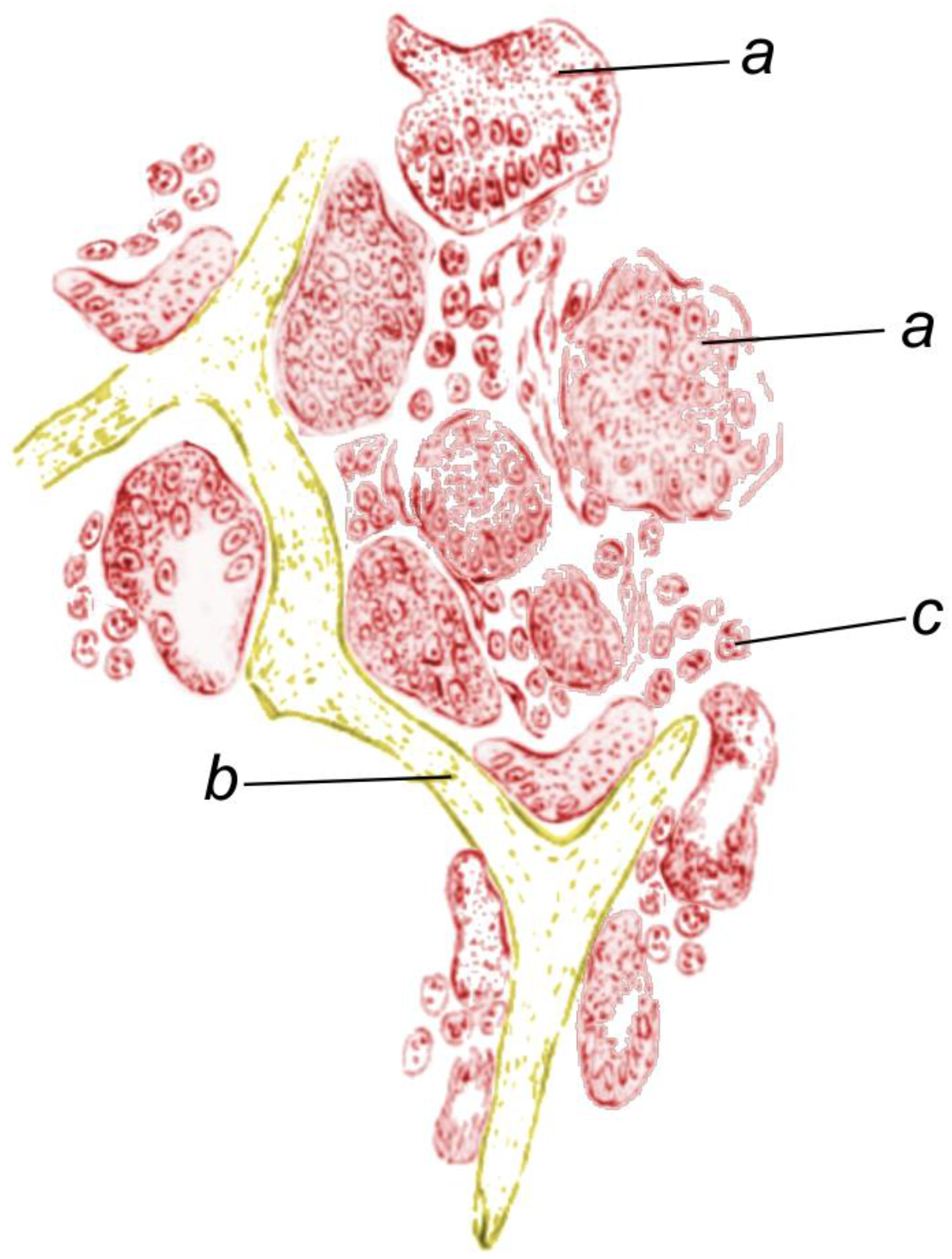
3. Collagen IV and Related Proteins in Sponges
4. Fibrillar Collagens in the Mesohyl of Demosponges
5. Chondrosia Collagens
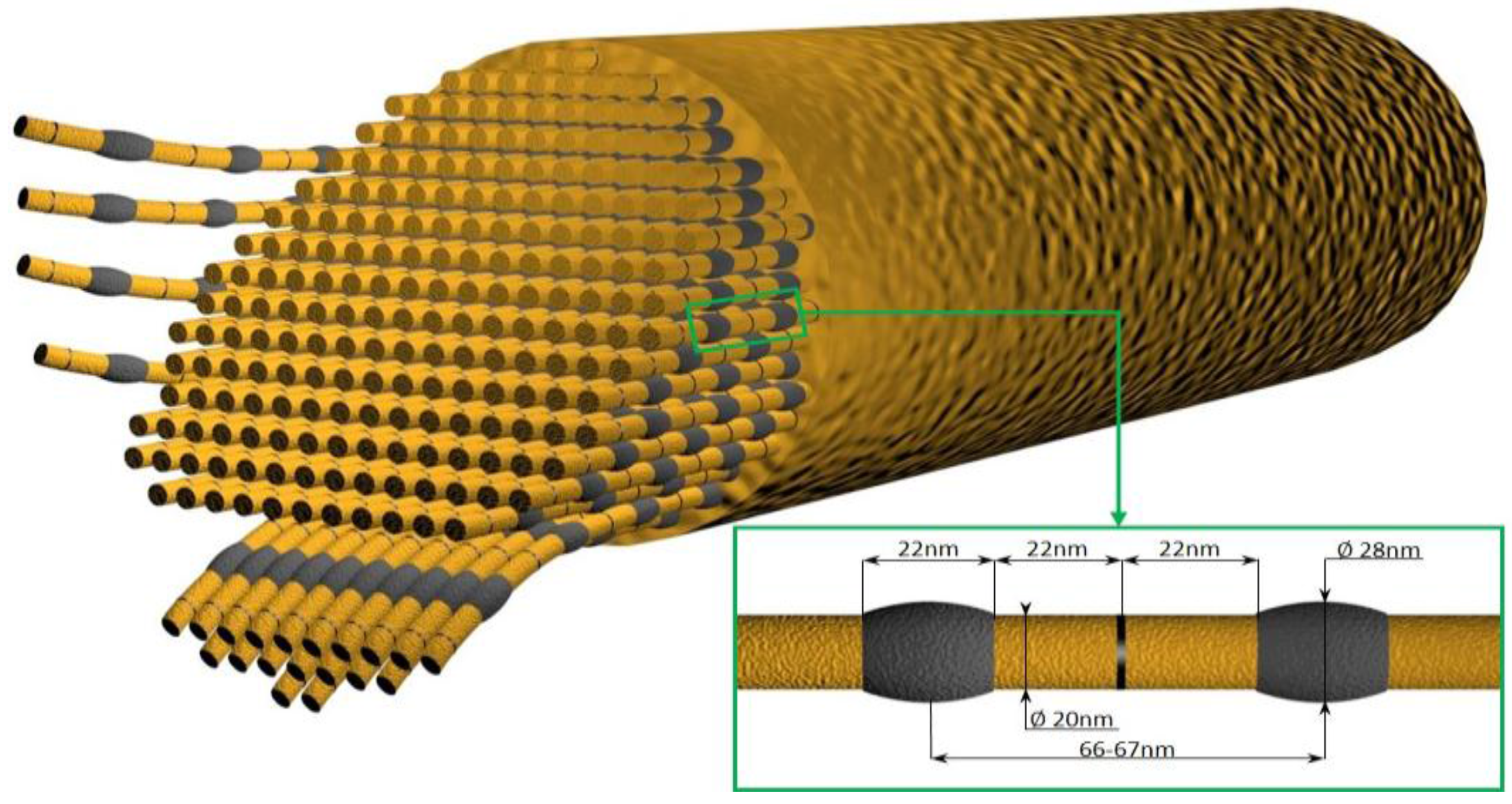
6. Glass Sponge Collagen
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Luo, Y.; Sinkeviciute, D.; He, Y.; Karsdal, M.; Henrotin, Y.; Mobasheri, A.; Önnerfjord, P.; Bay-Jensen, A. The minor collagens in articular cartilage. Protein Cell 2017, 8, 560–572. [Google Scholar] [CrossRef] [PubMed]
- Tzaphlidou, M.; Berillis, P. Structural alterations caused by lithium in skin and liver collagen using an image processing method. J. Trace Microprobe Tech. 2002, 20, 493–504. [Google Scholar] [CrossRef]
- Ehrlich, H.; Deutzmann, R.; Brunner, E.; Cappellini, E.; Koon, H.; Solazzo, C.; Yang, Y.; Ashford, D.; Thomas-Oates, J.; Lubeck, M.; et al. Mineralization of the metre-long biosilica structures of glass sponges is templated on hydroxylated collagen. Nat. Chem. 2010, 2, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Shahar, R.; Weiner, S. Open questions on the 3D structures of collagen containing vertebrate mineralized tissues: A perspective. J. Struct. Biol. 2018, 201, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Palamara, J.E.A.; Burrow, M.F. Effects of collagen crosslinkers on dentine: A literature review. Calcif. Tissue Int. 2018, 102, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, G.N.; Kartha, G. Structure of collagen. Nature 1955, 176, 593–595. [Google Scholar] [CrossRef] [PubMed]
- Rich, A.; Crick, F.H.C. The structure of collagen. Nature 1955, 175, 863–864. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [PubMed]
- Squire, J.M.; Parry, D.A. Fibrous protein structures: Hierarchy, history and heroes. Subcell. Biochem. 2017, 82, 929–958. [Google Scholar]
- Bella, J.; Hulmes, D.J. Fibrillar collagens. Subcell. Biochem. 2017, 82, 457–490. [Google Scholar] [PubMed]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed]
- Porfírio, E.; Fanaro, G.B. Collagen supplementation as a complementary therapy for the prevention and treatment of osteoporosis and osteoarthritis: A systematic review. Rev. Bras. Geriatr. Gerontol. 2016, 19, 153–164. [Google Scholar] [CrossRef]
- Pawelec, K.M.; Best, S.M.; Cameron, R.E. Collagen: A network for regenerative medicine. J. Mater. Chem. B 2016, 4, 6484–6496. [Google Scholar] [CrossRef] [PubMed]
- Conway, J.R.W.; Vennin, C.; Cazet, A.S.; Herrmann, D.; Murphy, K.J.; Warren, S.C.; Wullkopf, L.; Boulghourjian, A.; Zaratzian, A.; Da Silva, A.M.; et al. Three-dimensional organotypic matrices from alternative collagen sources as pre-clinical models for cell biology. Sci. Rep. 2017, 7, 16887. [Google Scholar] [CrossRef] [PubMed]
- Avila Rodríguez, M.I.; Rodríguez Barroso, L.G.; Sánchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2018, 17, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Hashim, P.; Mohd Ridzwan, M.S.; Bakar, J.; Hashim, M. Collagen in food and beverage industries. Int. Food Res. J. 2015, 22, 1–8. [Google Scholar]
- Raspanti, M.; Requzzoni, M.; Protasoni, M.; Basso, P. Not only tendons: The other architecture of collagen fibrils. Int. J. Biol. Macromol. 2018, 107, 1668–1674. [Google Scholar] [CrossRef] [PubMed]
- Uzel, S.G.; Buehler, M.J. Nanomechanical sequencing of collagen: Tropocollagen features heterogeneous elastic properties at the nanoscale. Integr. Biol. 2009, 1, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H. Biological Materials of Marine Origin. Vertebrates; Springer Science + Business Media: Dordecht, The Netherlands, 2015. [Google Scholar]
- Alves, A.L.P.; Marques, A.L.; Martins, E.; Silva, T.H.; Reis, R.L. Cosmetic potential of marine fish skin collagen. Cosmetics 2017, 4, 39. [Google Scholar] [CrossRef]
- Venkatesan, J.; Anil, S.; Kim, S.K.; Shim, M.S. Marine fish proteins and peptides for cosmeceuticals: A review. Mar. Drugs 2017, 15, 143. [Google Scholar] [CrossRef] [PubMed]
- Berillis, P. Marine Collagen: Extraction and applications. In Research Trends in Biochemistry, Molecular Biology and Microbiology; Saxena, M., Ed.; SM Group open access eBooks: Dover, DE, USA, 2015. [Google Scholar]
- Zhang, J.; Sun, Y.; Zhao, Y.; Wei, B.; Xu, C.; He, L.; Oliveira, C.L.P.; Wang, H. Centrifugation-induced fibrous orientation in fish-sourced collagen matrices. Soft Matter 2017, 13, 9220–9228. [Google Scholar] [CrossRef] [PubMed]
- Adams, E. Invertebrate collagens. Science 1978, 202, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.F. The nature of collagen. In Comprehensive Biochemistry, Extracellular and Supporting Structures; Florkin, M., Stotz, E.H., Eds.; Elsevier: Amsterdam, The Netherlands, 1968. [Google Scholar]
- Engel, J. Versatile collagens in invertebrates. Science 1997, 277, 1785–1786. [Google Scholar] [CrossRef] [PubMed]
- Exposito, J.Y.; Cluzel, C.; Garrone, R.; Lethias, C. Evolution of collagens. Anat. Rec. 2002, 268, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Garrone, R. Evolution of metazoan collagens. Prog. Mol. Subcell. Biol. 1999, 21, 119–139. [Google Scholar]
- Gross, J.; Sokal, Z.; Rougvie, M. Structural and chemical studies on the connective tissue of marine sponges. J. Histochem. Cytochem. 1956, 4, 227–246. [Google Scholar] [CrossRef] [PubMed]
- Tanzer, M.L. The biological diversity of collagenous proteins. Trends Biochem. Sci. 1978, 3, 15–17. [Google Scholar] [CrossRef]
- Silva, T.H.; Moreira-Silva, J.; Marques, A.L.P.; Domingues, A.; Bayon, Y.; Reis, R.L. Marine origin collagens and its potential applications. Mar. Drugs 2014, 12, 5881–5901. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.L.; Holmes, D.F. Collagenous extracellular matrix biomaterials for tissue engineering: Lessons from the common sea urchin tissue. Int. J. Mol. Sci. 2017, 18, 901. [Google Scholar] [CrossRef] [PubMed]
- Betancourt-lozano, M.; Gonzalez-farias, F.; Garcıa-gasca, A. Variation of antimicrobial activity of the sponge Aplysina fistularis (Pallas, 1766) and its relation to associated fauna. J. Exp. Mar. Biol. Ecol. 1998, 223, 1–18. [Google Scholar] [CrossRef]
- Pallela, R.; Ehrlich, H. Marine Sponges: Chemicobiological and Biomedical Applications; Springer India: New Delhi, India, 2016. [Google Scholar]
- Reitner, J.; Mehl, D. Monophyly of the Porifera. Verh. Naturwiss. Ver. Hambg. 1996, 36, 5–32. [Google Scholar]
- Nichols, S.; Wörheide, G. Sponges: New views of old animals. Integr. Comp. Biol. 2005, 45, 333–334. [Google Scholar] [CrossRef] [PubMed]
- Moldowan, J.M. C30 steranes, novel markers for marine petroleums and sedimentary rocks. Geochim. Cosmochim. Acta 1984, 48, 2767–2768. [Google Scholar] [CrossRef]
- Moldowan, J.M.; Seifert, W.K.; Gallegos, E.J. Relationship between petroleum composition and depositional environment of petroleum source rocks. Am. Assoc. Pet. Geol. Bull. 1985, 69, 1255–1268. [Google Scholar]
- Moldowan, J.M.; Fago, F.J.; Lee, C.Y.; Jacobson, S.R.; Watt, D.S.; Slougui, N.-E.; Jeganathan, A.; Young, D.C. Sedimentary 24+propylcholestanes, molecular fossils diagnostic of marine algae. Science 1990, 247, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Wainright, P.O.; Hinkle, G.; Sogin, M.L.; Stickel, S.K. Monophyletic origins of the Metazoa: An evolutionary link with fungi. Science 1993, 360, 340–342. [Google Scholar] [CrossRef]
- Wood, R. Reef-building sponges. Am. Sci. 1990, 78, 224–235. [Google Scholar]
- Van Soest, R.W.M.; Boury-Esnault, N.; Vacelet, J.; Dohrmann, M.; Erpenbeck, D.; de Voogd, N.J.; Santodomingo, N.; Vanhoorne, B.; Kelly, M.; Hooper, J.N.A. Global diversity of sponges (Porifera). PLoS ONE 2012, 7, e35105. [Google Scholar] [CrossRef] [PubMed]
- Garrone, R. Phylogenesis of Connective Tissue: Morphological Aspects and Biosynthesis of Sponge Intercellular Matrix; John Wiley & Sons: Hoboken, NJ, USA, 1978. [Google Scholar]
- Junqua, S.; Robert, L.; Garrone, R. Biochemical and morphological studies on the collagens of horny sponges. Ircinia filaments compared to spongines. Connect. Tissue Res. 1974, 2, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Städeler, G. Untersuchungen über das Fibroin, Spongin und Chitin, nebst Bemerkungen über den tierischen Schleim. Eur. J. Organ. Chem. 1859, 111, 12–28. [Google Scholar]
- Green, D.; Howard, D.; Yang, X.; Kelly, M.; Oreffo, R.O.C. Natural marine sponge fiber skeleton: A biomimetic scaffold for human osteoprogenitor cell attachment, growth, and differentiation. Tissue Eng. 2003, 9, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Green, D.W.; Lee, J.-M.; Jung, H.-S. Marine structural biomaterials in medical biomimicry. Tissue Eng. Part B Rev. 2015, 21, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Green, D.W.; Padula, M.P.; Santos, J.; Chou, J.; Milthorpe, B.; Ben-Nissan, B. A therapeutic potential for marine skeletal proteins in bone regeneration. Mar. Drugs 2013, 11, 1203–1220. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Solomon, K.L.; Zhang, X.; Pavlos, N.J.; Abel, T.; Willers, C. In vitro evaluation of natural marine sponge collagen as a scaffold for bone tissue engineering. Int. J. Biol. Sci. 2011, 7, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Pallela, R.; Venkatesan, J.; Bhatnagar, I.; Shim, Y.; Kim, S. Application of marine collagen–based scaffolds in bone tissue engineering. In Marine Biomaterials: Isolation, Characterization and Applications; Kim, S.-K., Ed.; CRC-Taylor & Francis: Boca Raton, FL, USA, 2011; pp. 519–528. [Google Scholar]
- Nandi, S.K.; Kundu, B.; Mahato, A.; Thakur, N.L.; Joardar, S.N.; Mandal, B.B. In vitro and in vivo evaluation of the marine sponge skeleton as a bone mimicking biomaterial. Integr. Biol. 2015, 7, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Langasco, R.; Cadeddu, B.; Formato, M.; Lepedda, A.J.; Cossu, M.; Giunchedi, P.; Pronzato, R.; Rassu, G.; Manconi, R.; Gavini, R. Natural collagenic skeleton of marine sponges in pharmaceutics: Innovative biomaterial for topical drug delivery. Mater. Sci. Eng. C 2017, 70, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Tziveleka, L.A.; Ioannou, E.; Tsiourvas, D.; Berillis, P.; Foufa, E.; Roussis, V. Collagen from the marine sponges Axinella cannabina and Suberites carnosus: Isolation and morphological, biochemical, and biophysical characterization. Mar. Drugs 2017, 15, 152. [Google Scholar] [CrossRef] [PubMed]
- De Laubenfels, M.; Storr, J. The taxonomy of American commercial sponges. Bull. Mar. Sci. 1958, 8, 99–117. [Google Scholar]
- Schulze, F.E. Untersuchungen über den Bau und die Entwicklung der Spongien. Z. wiss. Zool. 1877, 28, 1–48. [Google Scholar]
- Cresswell, E. Sponges: Their Nature, History, Modes of Fishing, Varieties, Cultivation, etc.; Sir Isaac Pitman & Sons Ltd.: London, UK, 1922. [Google Scholar]
- Geoffroy, C.J. Analyse chimique de l’eponge de la moyenne espece. Hist. Acad. R. Sci. 1705, 660–661. [Google Scholar]
- Fyfe, A. Account of some experiments, made with the view of ascertaining the different substances from which iodine can be produced. Edinb. Philos. 1819, 1, 254–258. [Google Scholar]
- Croockewit, J.H. Ueber die Zusammensetzung des Badeschwammes. Eur. J. Organ. Chem. 1843, 48, 43–56. [Google Scholar] [CrossRef]
- Schlossberger, J. Ueber Fibroin und die Substanz des Badeschwamms. Arch. Pharm. 1859, 147, 62–65. [Google Scholar] [CrossRef]
- Von Kölliker, A. Der feinere Bau der Protozoen; Wilhelm Engelman: Leipzig, Germany, 1864. [Google Scholar]
- Hundeshagen, F. Über jodhaltige Spongien and Jodospongin. Angew. Chem. Int. Ed. 1895, 8, 473–476. [Google Scholar] [CrossRef]
- Harnack, E. Ueber das iodospongin, die jodhaltige eiweissartige Subsanz aus dem Badeschwamm. Z. Physiol. Chem. 1898, 25, 412–424. [Google Scholar] [CrossRef][Green Version]
- Clancey, V.J. The constitution of sponges. The common bath sponge, Hippospongia equina. Biochem. J. 1926, 20, 1186–1189. [Google Scholar] [CrossRef] [PubMed]
- Abderhalden, E.; Strauss, E. Die Spaltprodukte des Spongins mit Sauren. Hoppe-Seyler’s Z. Physiol. Chem. 1906, 48, 49–53. [Google Scholar] [CrossRef]
- Strauss, E. Studien ueber die Albuminoide mit Besonderer Berucksichtigung des Spongins und der Keratine; Carl Witer’s Universitatsbuchhandlung: Munchen, Germany, 1904. [Google Scholar]
- Jenkins, C.L. Insights on the conformational stability of collagen. Nat. Prod. Rep. 2002, 19, 49–59. [Google Scholar] [PubMed]
- Block, R.J.; Bolling, D. The amino acid composition of keratins. J. Biol. Chem. 1939, 127, 685–693. [Google Scholar]
- Ackermann, D.; Burchard, C. Zur Kenntnis der Spongine. Hoppe-Seyler’s Z. Physiol. Chem. 1941, 271, 183–189. [Google Scholar] [CrossRef]
- Ackermann, D.; Müller, I. Über das Vorkommen von Dibromotyrosin neben Dijodtyrosin im Spongin. Hoppe-Seyler’s Z. Physiol. Chem. 1941, 269, 146–157. [Google Scholar] [CrossRef]
- Low, E.M. Halogenated amino acids of the bath sponge. J. Mar. Res. 1951, 10, 239–245. [Google Scholar]
- Katzman, R.L.; Halford, M.H.; Reinhold, V.N.; Jeanloz, R.W. Invertebrate connective tissue. IX. Isolation and structure determination of glucosylgalactosylhydroxylysine from sponge and sea anemone collagen. Biochemistry 1972, 11, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Gaino, E.; Pronzato, R. Ultrastructural evidence of bacterial damage to Spongia officinalis fibres (Porifera, Demospongiae). Dis. Aquat. Organ. 1989, 6, 67–74. [Google Scholar] [CrossRef]
- Ehrlich, H.; Maldonado, M.; Spindler, K.; Eckert, C.; Hanke, T.; Born, R.; Simon, P.; Heinemann, S.; Worch, H. First evidence of chitin as a component of the skeletal fibers of marine sponges. Part I. Verongida (Demospongia: Porifera). J. Exp. Zool. Part B 2007, 356, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Ilan, M.; Maldonado, M.; Muricy, G.; Bavestrello, G.; Kljajic, Z.; Carballo, J.L.; Schiaparelli, S.; Ereskovsky, A.; Schupp, P.; et al. Three-dimensional chitin-based scaffolds from Verongida sponges (Demospongiae: Porifera). Part I. Isolation and identification of chitin. Int. J. Bol. Macromol. 2010, 47, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Wysokowski, M.; Bazhenov, V.V.; Tsurkan, M.V.; Galli, R.; Stelling, A.L.; Stöcker, H.; Kaiser, S.; Niederschlag, E.; Gärtner, G.; Behm, T.; et al. Isolation and identification of chitin in three-dimensional skeleton of Aplysina fistularis marine sponge. Int. J. Biol. Macromol. 2013, 62, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Kaluzhnaya, O.V.; Tsurkan, M.V.; Ereskovsky, A.; Tabachnick, K.R.; Ilan, M.; Stelling, A.; Galli, R.; Petrova, O.V.; Nekipelov, S.V.; et al. First report on chitinous holdfast in sponges (Porifera). Proc. R. Soc. Lond. B 2013, 280, 20130339. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Kaluzhnaya, O.V.; Brunner, E.; Tsurkan, M.V.; Ereskovsky, A.; Ilan, M.; Tabachnick, K.R.; Bazhenov, V.V.; Paasch, S.; Kammer, M.; et al. Identification and first insights into the structure and biosynthesis of chitin from the freshwater sponge Spongilla lacustris. J. Struct. Biol. 2013, 183, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Wysokowski, M.; Petrenko, I.; Stelling, A.; Stawski, D.; Jesionowski, T.; Ehrlich, H. Poriferan chitin as a versatile template for extreme biomimetics. Polymers 2015, 7, 235–265. [Google Scholar] [CrossRef]
- Shavandi, A.; Silva, T.H.; Bekhit, A.A.; Bekhit, A.E. Keratin: Dissolution, extraction and biomedical application. Biomater. Sci. 2017, 5, 1699–1735. [Google Scholar] [CrossRef] [PubMed]
- Cerrano, C.; Calcinai, B.; Gioia, C.; Camillo, D.; Valisano, L.; Bavestrello, G. How and why do sponges incorporate foreign material? Strategies in porifera. In Porifera Research: Biodiversity, Innovation and Sustainability; Custódio, M.R., Lôbo-Hajdu, G., Hajdu, E., Muricy, G., Eds.; Série Livros 28; Museu Nacional: Rio de Janeiro, Brazil, 2007; pp. 239–246. [Google Scholar]
- Castritsi-Catharios, J.; Zaoutsos, S.P.; Berillis, P.; Zouganelis, G.D.; Ekonomou, G.; Kefalas, E.; Pantelis, J. Kalymnos, the island which made history in sponge fishery. Data on physical parameters, elemental composition and DNA barcode preliminary results of the most common bath sponge species in Aegean Sea. Reg. Stud. Mar. Sci. 2017, 13, 71–79. [Google Scholar] [CrossRef]
- Castritsi-Catharios, J.; Zaoutsos, S.P.; Ekonomou, G.; Berillis, P. Physical parameters and chemical composition of four sponge species. Preliminary results. In Proceedings of the HydroMedit 2016, Messolonghi, Greece, 10–12 November 2016; pp. 156–159. [Google Scholar]
- Castritsi-Catharios, J.; Magli, M.; Vacelet, J. Evaluation of the quality of two commercial sponges by tensile strength measurement. J. Mar. Biol. Assoc. UK 2007, 87, 1765–1771. [Google Scholar] [CrossRef]
- Dandy, A. On the occurrence of gelatinous spicules, and their mode of origin, in a new genus of siliceous sponges. Proc. R. Soc. Lond. B 1916, 89, 315–322. [Google Scholar] [CrossRef]
- Szatkowski, T.; Jesionowski, T. Hydrothermal synthesis of spongin-based materials. In Extreme Biomimetics; Ehrlich, H., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 251–274. [Google Scholar]
- Von Raimann, E. Über den Nutzen des Bade- oder Waschschwammes bey heftigen Blutun-gen. Med. Jahrbücher 1939, 27, 306–307. [Google Scholar]
- White, C. An account of the successful use of the sponge, in the stoppage of an Haemorrhage, occasioned by amputation below the knee; and of the remarkable effects of that application in preventing the absorption of matter. In Cases in Surgery with Remarks: Part 1; Johnston: London, England, 1770; pp. 151–158. [Google Scholar]
- Zschiesche, P. Die Anwendung des Pressschwammes in der Gynaekologie und Seine Gefahren; Universitaet Greifswald: Greifswald, Germany, 1873. [Google Scholar]
- Haussmann, D. Kann die Erweiterung des Verengten Muttermundes durch Pressschwamm die Empfangniss erleichtern? Gesellschaft für Geburtshilfe und Gynäkologie Berlin: Berlin, Germany, 1878; pp. 311–327. [Google Scholar]
- Petrus, C. Naauwkeurige Afbelding en Beschryving van eene Geheel en al Verloorene, Maar door Kunst Herstelde Neus en Verhemelte; J.C.SEPP Boekverkooper: Amsterdam, The Netherlands, 1771. [Google Scholar]
- Hamilton, D.J. On sponge-grafting. Edinb. Med. J. 1881, 27, 385–413. [Google Scholar]
- Kim, M.M.; Mendis, E.; Rajapakse, N.; Lee, S.H.; Kim, S.K. Effect of spongin derived from Hymeniacidon sinapium on bone mineralization. J. Biomed. Mater. Res. B 2009, 90, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Norman, M.; Bartczak, P.; Zdarta, J.; Ehrlich, H. Anthocyanin dye conjugated with Hippospongia communis marine demosponge skeleton and its antiradical activity. Dyes Pigments 2016, 134, 541–552. [Google Scholar] [CrossRef]
- Norman, M.; Bartczak, P.; Zdarta, J.; Tomala, W.; Żurańska, B.; Dobrowolska, A.; Piasecki, A.; Czaczyk, K.; Ehrlich, H.; Jesionowski, T. Sodium copper chlorophyllin immobilization onto Hippospongia communis marine demosponge skeleton and its antibacterial activity. Int. J. Mol. Sci. 2016, 17, 1564. [Google Scholar] [CrossRef] [PubMed]
- Zdarta, J.; Norman, M.; Smułek, W.; Moszyński, D.; Kaczorek, E.; Stelling, A.L.; Ehrlich, H.; Jesionowski, T. Spongin-based scaffolds from Hippospongia communis demosponge as an effective support for lipase immobilization. Catalysts 2017, 7, 147. [Google Scholar] [CrossRef]
- Szatkowski, T.; Siwińska-Stefańska, K.; Wysokowski, M.; Stelling, A.; Joseph, Y.; Ehrlich, H.; Jesionowski, T. Immobilization of titanium(IV) oxide onto 3D spongin scaffolds of marine sponge origin according to extreme biomimetics principles for removal of C.I. Basic Blue 9. Biomimetics 2017, 2, 4. [Google Scholar] [CrossRef]
- Szatkowski, T.; Wysokowski, M.; Lota, G.; Pęziak, D.; Bazhenov, V.V.; Nowaczyk, G.; Walter, J.; Molodtsov, S.L.; Stöcker, H.; Himcinschi, C.; et al. Novel nanostructured hematite–spongin composite developed using an extreme biomimetic approach. RSC Adv. 2015, 5, 79031–79040. [Google Scholar] [CrossRef]
- Ehrlich, H. Extreme Biomimetics; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Chioran, A.; Duncan, S.; Catalano, A.; Brown, T.J.; Ringuette, M.J. Collagen IV trafficking: The inside-out and beyond story. Dev. Biol. 2017, 431, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Fidler, A.L.; Darris, C.E.; Chetyrkin, S.V.; Pedchenko, V.K.; Boudko, S.P.; Brown, K.L.; Gray Jerome, W.; Hudson, J.K.; Rokas, A.; Hudson, B.G. Collagen IV and basement membrane at the evolutionary dawn of metazoan tissues. eLife 2017, 6, e24176. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Pascual, F.; Slatter, D.A. Collagen cross-linking: Insights on the evolution of metazoan extracellular matrix. Sci. Rep. 2016, 6, 37374. [Google Scholar] [CrossRef] [PubMed]
- Boute, N.; Exposito, J.Y.; Boury-Esnault, N.; Vacelet, J.; Nor, N.; Miyazaki, K.; Yoshizato, K.; Garrone, R. Type IV collagen in sponges, the missing link in basement membrane ubiquity. Biol. Cell 1996, 88, 37–44. [Google Scholar] [CrossRef]
- Leys, S.P.; Riesgo, A. Epithelia, an evolutionary novelty of metazoans. J. Exp. Zool. Part B 2011, 314, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Riesgo, A.; Taboada, S.; Sánchez-Vila, L.; Solà, J.; Bertran, A.; Avila, C. Some like it fat: Comparative ultrastructure of the embryo in two demosponges of the genus Mycale (order Poecilosclerida) from Antarctica and the Caribbean. PLoS ONE 2015, 10, e0118805. [Google Scholar] [CrossRef] [PubMed]
- Exposito, J.Y.; Le Guellec, D.; Lu, Q.; Garrone, R. Short chain collagens in sponges are encoded by a family of closely related genes. J. Biol. Chem. 1991, 266, 21923–21928. [Google Scholar] [PubMed]
- Aouacheria, A.; Geourjon, C.; Aghajari, N.; Navratil, V.; Deléage, G.; Lethias, C.; Exposito, J.Y. Insights into early extracellular matrix evolution: Spongin short chain collagen-related proteins are homologous to basement membrane type IV collagens and form a novel family widely distributed in invertebrates. Mol. Biol. Evol. 2006, 23, 2288–2302. [Google Scholar] [CrossRef] [PubMed]
- Rocha Moreira Da Silva, J.C.; Soares Diogo Carlos, G.; De Sousa E Silva Barros Prata, M.; Quinteiros Lopes Henriques Da Silva, T.J.; Pinto Marques, A.M.; Gonçalves Dos Reis, R.L.; Teixeira Cerqueira, M.; Vieira Pereira Ferreira, M.S. Marine-Sponge Type IV Collagen Membranes Its Production and Biomedical Applications Thereof. Patent WO2,015,186,118, 10 December 2015. [Google Scholar]
- Cavalier-Smith, T. Origin of animal multicellularity: Precursors, causes, consequences—The choanoflagellate/sponge transition, neurogenesis and the Cambrian explosion. Philos. Trans. R. Soc. Lond. B 2017, 372, 20150476. [Google Scholar] [CrossRef] [PubMed]
- Simpson, T.L. Collagen Fibrils, Spongin, Matrix Substances. In The Cell Biology of Sponges; Springer: New York, NY, USA, 1984. [Google Scholar]
- Garrone, R.; Pottu, J. Collagen biosynthesis in sponges—Elaboration of spongin by spongocytes. J. Submicrosc. Cytol. 1973, 5, 199–218. [Google Scholar]
- Bairati, A.; Garrone, R. Biology of Invertebrate and Lower Vertebrate Collagens; Plenum: New York, NY, USA, 1985. [Google Scholar]
- Imhoff, J.M.; Garrone, R. Solubilization and characterization of Chondrosia reniformis sponge collagen. Connect. Tissue Res. 1983, 11, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Eckert, C.; Schröder, H.C.; Brandt, D.; Perovic-Ottstadt, S.; Muller, W.E.G. Histochemical and electron microscopic analysis of spiculogenesis in the demosponge Suberites domuncula. Zootaxa 2006, 54, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H. Biological Materials of Marine Origin. Invertebrates; Springer Science + Business Media B.V.: Dordrecht, The Netherlands, 2010. [Google Scholar]
- Krasko, A.; Lorenz, B.; Batel, R.; Schröder, H.C.; Müller, I.M.; Müller, W.E.G. Expression of silicatein and collagen genes in the marine sponge Suberites domuncula is controlled by silicate and myotrophin. Eur. J. Biochem. 2000, 267, 4878–4887. [Google Scholar] [CrossRef] [PubMed]
- Garrone, R.; Huc, A.; Junqua, S. Fine structure and physicochemical studies on the collagen of the marine sponge Chondrosia reniformis Nardo. J. Ultrastruct. Res. 1975, 52, 261–275. [Google Scholar] [CrossRef]
- Pozzolini, M.; Scarfi, S.; Mussino, F.; Ferrando, S.; Gallus, L.; Giovine, M. Molecular cloning, characterization, and expression analysis of a prolyl 4-hydroxylase from the marine sponge Chondrosia reniformis. Mar. Biotechnol. 2015, 17, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Ledger, P.W. Types of collagen fibres in the calcareous sponges Sycon and Leucandra. Tissue Cell 1974, 6, 385–389. [Google Scholar] [CrossRef]
- Pozzolini, M.; Bruzzone, F.; Berilli, V.; Mussino, F.; Cerrano, C.; Benatti, U.; Giovine, M. Molecular characterization of a nonfibrillar collagen from the marine sponge Chondrosia reniformis Nardo 1847 and positive effects of soluble silicates on its expression. Mar. Biotechnol. 2012, 14, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, S.; Ehrlich, H.; Douglas, T.; Heinemann, C.; Worch, H.; Schatton, W.; Hanke, T. Ultrastructural studies on the collagen of the marine sponge Chondrosia reniformis Nardo. Biomacromolecules 2007, 8, 3452–3457. [Google Scholar] [CrossRef] [PubMed]
- Palmer, I.; Clarke, S.A.; Nelson, J.; Schatton, W.; Dunne, N.J.; Buchanan, F. Identification of a suitable sterilisation method for collagen derived from a marine Demosponge. Int. J. Nano Biomater. 2012, 4, 148–163. [Google Scholar] [CrossRef]
- Bavestrello, G.; Cerrano, C.; Cattaneo-Vietti, R.; Sara, M.; Calabria, F.; Cortesogno, L. Selective incorporation of foreign material in Chondrosia reniformis (Porifera, Demospongiae). Ital. J. Zool. 1996, 63, 215–220. [Google Scholar] [CrossRef]
- Bavestrello, G.; Benatti, U.; Calcinai, B.; Cattaneo-Vietti, R.; Cerrano, C.; Favre, A.; Giovine, M.; Lanza, S.; Pronzato, R.; Sara, M. Body polarity and mineral selectivity in the demosponge Chondrosia reniformis. Biol. Bull. 1998, 195, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Fassini, D. Coordination Phenomena in the Marine Demosponge Chondrosia reniformis; Università Degli Studi Di Milano: Milan, Italy, 2013. [Google Scholar]
- Fassini, D.; Parma, L.; Lembo, F.; Candia Carnevali, M.; Wilkie, I.; Bonasoro, F. The reaction of the sponge Chondrosia reniformis to mechanical stimulation is mediated by the outer epithelium and the release of stiffening factor(s). Zoology 2014, 117, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Pozzolini, M.; Ferrando, S.; Gallus, L.; Gambardella, C.; Ghignone, S.; Giovine, M. Aquaporin in Chondrosia reniformis Nardo, 1847 and its possible role in the interaction between cells and engulfed siliceous particles. Biol. Bull. 2016, 230, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Nicklas, M.; Schatton, W.; Heinemann, S.; Hanke, T.; Kreuter, J. Preparation and characterization of marine sponge collagen nanoparticles and employment for the transdermal delivery of 17β-estradiol-hemihydrate. Drug Dev. Ind. Pharm. 2009, 35, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, S.; Heinemann, C.; Ehrlich, H.; Meyer, M.; Baltzer, H.; Worch, H.; Hanke, T. A novel biomimetic hybrid material made of silicified collagen: Perspectives for bone replacement. Adv. Eng. Mater. 2007, 9, 1061–1068. [Google Scholar] [CrossRef]
- Heinemann, S.; Ehrlich, H.; Knieb, C.; Hanke, T. Biomimetically inspired hybrid materials based on silicified collagen. Int. J. Mater. Res. 2007, 98, 603–608. [Google Scholar] [CrossRef]
- Ehrlich, H. Chitin and collagen as universal and alternative templates in biomineralization. Int. Geol. Rev. 2010, 52, 661–699. [Google Scholar] [CrossRef]
- Tabachnick, K.; Janussen, D.; Menschenina, L. Cold biosilicification in Metazoan: Psychrophilic glass sponges. In Extreme Biomimetics; Ehrlich, H., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 53–80. [Google Scholar]
- Ehrlich, H.; Ereskovskii, V.; Drozdov, L.; Krylova, D.D.; Hanke, T.; Meissner, H.; Heinemann, S.; Worch, H. A modern approach to demineralization of spicules in glass sponges (Porifera: Hexactinellida) for the purpose of extraction and examination of the protein matrix. Russ. J. Mar. Biol. 2006, 32, 186–193. [Google Scholar] [CrossRef]
- Ehrlich, H.; Worch, H. Sponges as natural composites. In Porifera Research Biodiversity, Innovation and Sustainability; Custodio, M.R., Lobo-Hajdu, G., Hajdu, E., Muricy, G., Eds.; Série Livros 28; Rio de Janerio Museu National: Rio de Janeiro, Brazil, 2007; pp. 303–312. [Google Scholar]
- Ehrlich, H.; Heinemann, S.; Heinemann, C.; Simon, P.; Bazhenov, V.V.; Shapkin, N.P.; Born, R.; Tabachnick, K.R.; Hanke, T.; Worch, H. Nanostructural organization of naturally occurring composites—Part I: Silica-collagen-based biocomposites. J. Nanomater. 2008, 2008, 623838. [Google Scholar] [CrossRef]
- Niu, L.; Jiao, K.; Qi, Y.; Yiu, C.K.Y.; Ryou, H.; Arola, D.D.; Chen, J.; Breschi, L.; Pashley, D.H.; Tay, F.R. Infiltration of silica inside fibrillar collagen. Angew. Chem. Int. Ed. 2011, 50, 11688–11691. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.J.; Yang, H.Y.; Niu, L.N.; Mao, J.; Huang, C.; Pashley, D.H.; Tay, F.R. Translation of a solution-based biomineralization concept into a carrier-based delivery system via the use of expanded-pore mesoporous silica. Acta Biomater. 2016, 31, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Luo, X.; Niu, L.; Yang, H.; Yiu, C.K.; Wang, T.; Zhou, L.; Mao, J.; Huang, C.; Pashley, D.H.; Tay, F.R. Biomimetic intrafibrillar mineralization of type I collagen with intermediate precursors-loaded mesoporous carriers. Sci. Rep. 2015, 5, 11199. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-L.; Jiao, K.; Niu, L.N.; Jiao, Y.; Song, Q.; Shen, L.J.; Tay, F.R.; Chen, J.H. Intrafibrillar silicified collagen scaffold modulates monocyte to promote cell homing, angiogenesis and bone regeneration. Biomaterials 2017, 113, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Aimé, C.; Mosser, G.; Pembouong, G.; Bouteiller, L.; Coradin, T. Controlling the nano–bio interface to build collagen–silica self-assembled networks. Nanoscale 2012, 4, 7127–7134. [Google Scholar] [CrossRef] [PubMed]
- Kahn, J.L.; Eren, N.M.; Campanella, O.; Voytik-Harbin, S.L.; Rickus, J.L. Collagen-fibril matrix properties modulate the kinetics of silica polycondensation to template and direct biomineralization. J. Mater. Res. 2016, 31, 311–320. [Google Scholar] [CrossRef]
- Rickus, J.L.; Harbin, S.L.; Kahn, J.L. Cell-Collagen-Silica Composites and Methods of Making and Using the Same. Patent WO2,016,172,365, 27 October 2016. [Google Scholar]




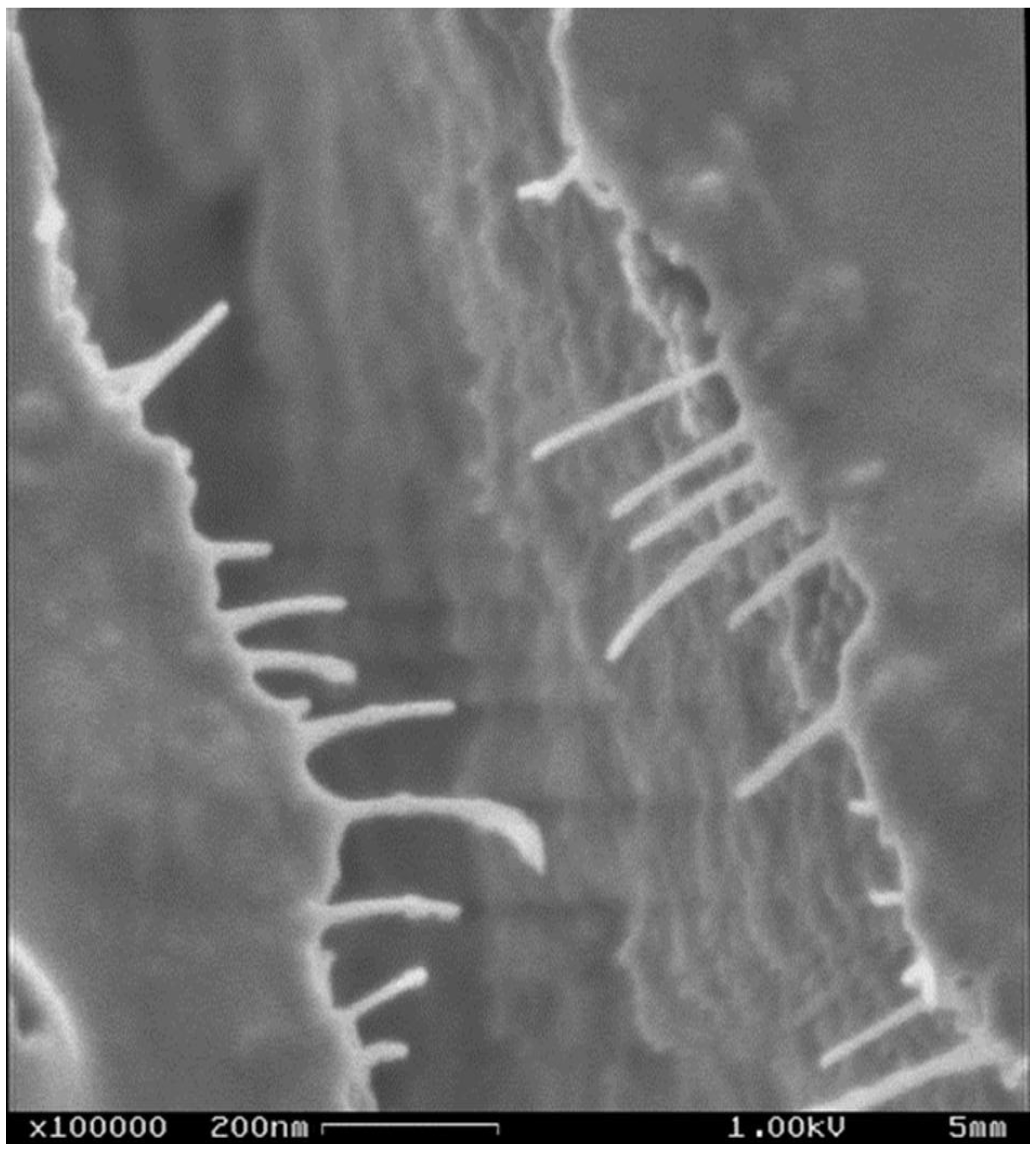
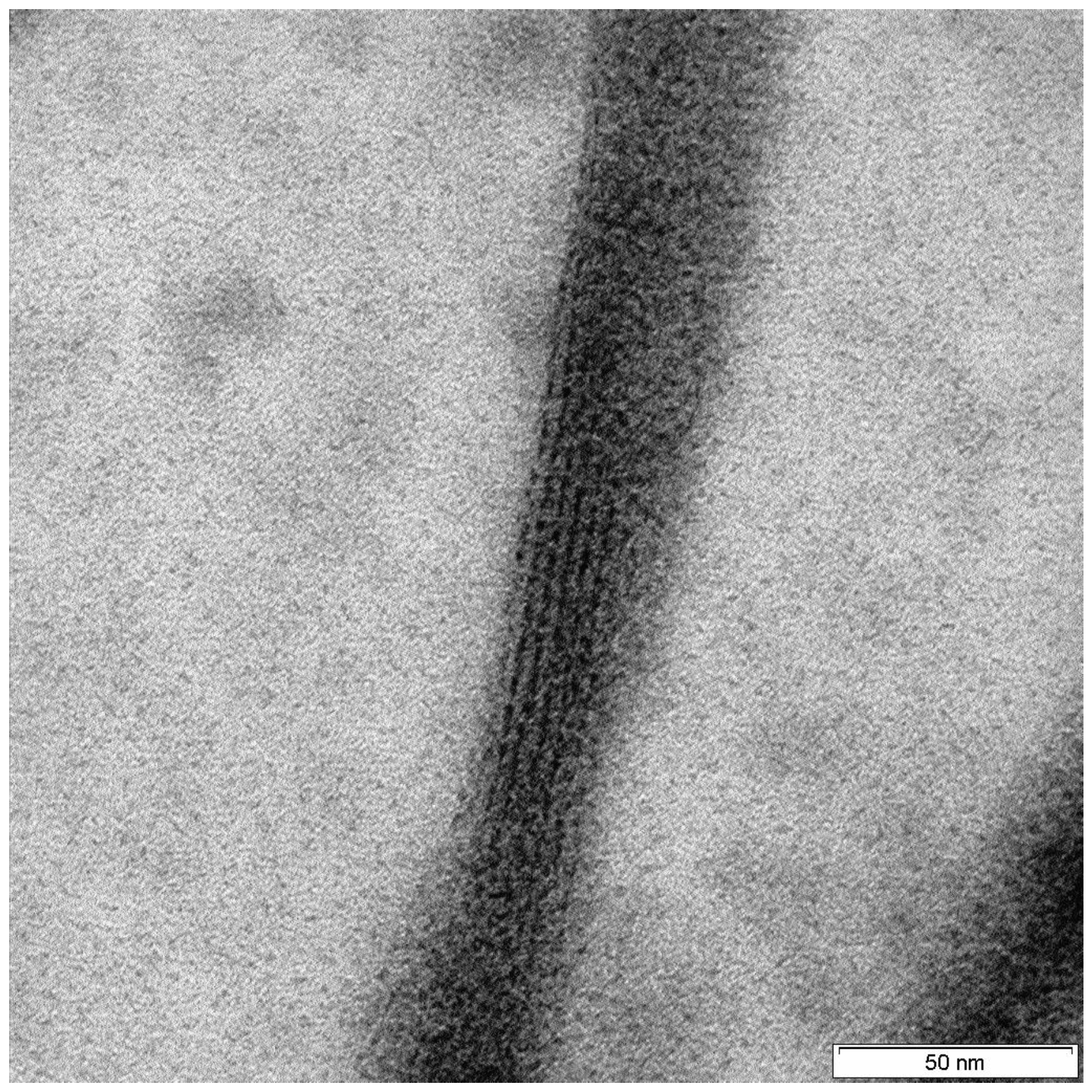
| Constituent | Content (%) |
|---|---|
| Nitrogen | 13.0–14.8 |
| Sulfur | 0.7 |
| Iodine | 0.84–1.46 |
| Histidine | 0–0.2 |
| Lysine | 3–3.6 |
| Arginine | 4.3–5.9 |
| Cystine | 2.8 |
| Tyrosine | 0–0.8 |
| Tryptophan | 0 |
| Phenylalanine | 3.3 |
| Glycine | 13.9–14.4 |
| Diiodotyrosine | 4.7 |
| Molecular ratio of lysine to arginine | 4:6 |
| C | H | N | I | Br | S | Cl | Ashes |
|---|---|---|---|---|---|---|---|
| 47.00 | 6.28 | 16.06 | 1.41 | 2.93 | 0.87 | 0 | 1.16 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ehrlich, H.; Wysokowski, M.; Żółtowska-Aksamitowska, S.; Petrenko, I.; Jesionowski, T. Collagens of Poriferan Origin. Mar. Drugs 2018, 16, 79. https://doi.org/10.3390/md16030079
Ehrlich H, Wysokowski M, Żółtowska-Aksamitowska S, Petrenko I, Jesionowski T. Collagens of Poriferan Origin. Marine Drugs. 2018; 16(3):79. https://doi.org/10.3390/md16030079
Chicago/Turabian StyleEhrlich, Hermann, Marcin Wysokowski, Sonia Żółtowska-Aksamitowska, Iaroslav Petrenko, and Teofil Jesionowski. 2018. "Collagens of Poriferan Origin" Marine Drugs 16, no. 3: 79. https://doi.org/10.3390/md16030079
APA StyleEhrlich, H., Wysokowski, M., Żółtowska-Aksamitowska, S., Petrenko, I., & Jesionowski, T. (2018). Collagens of Poriferan Origin. Marine Drugs, 16(3), 79. https://doi.org/10.3390/md16030079








