Phycoerythrin Peptide from Pyropia yezoensis Alleviates Endoplasmic Reticulum Stress Caused by Perfluorooctane Sulfonate-Induced Calcium Dysregulation
Abstract
:1. Introduction
2. Results
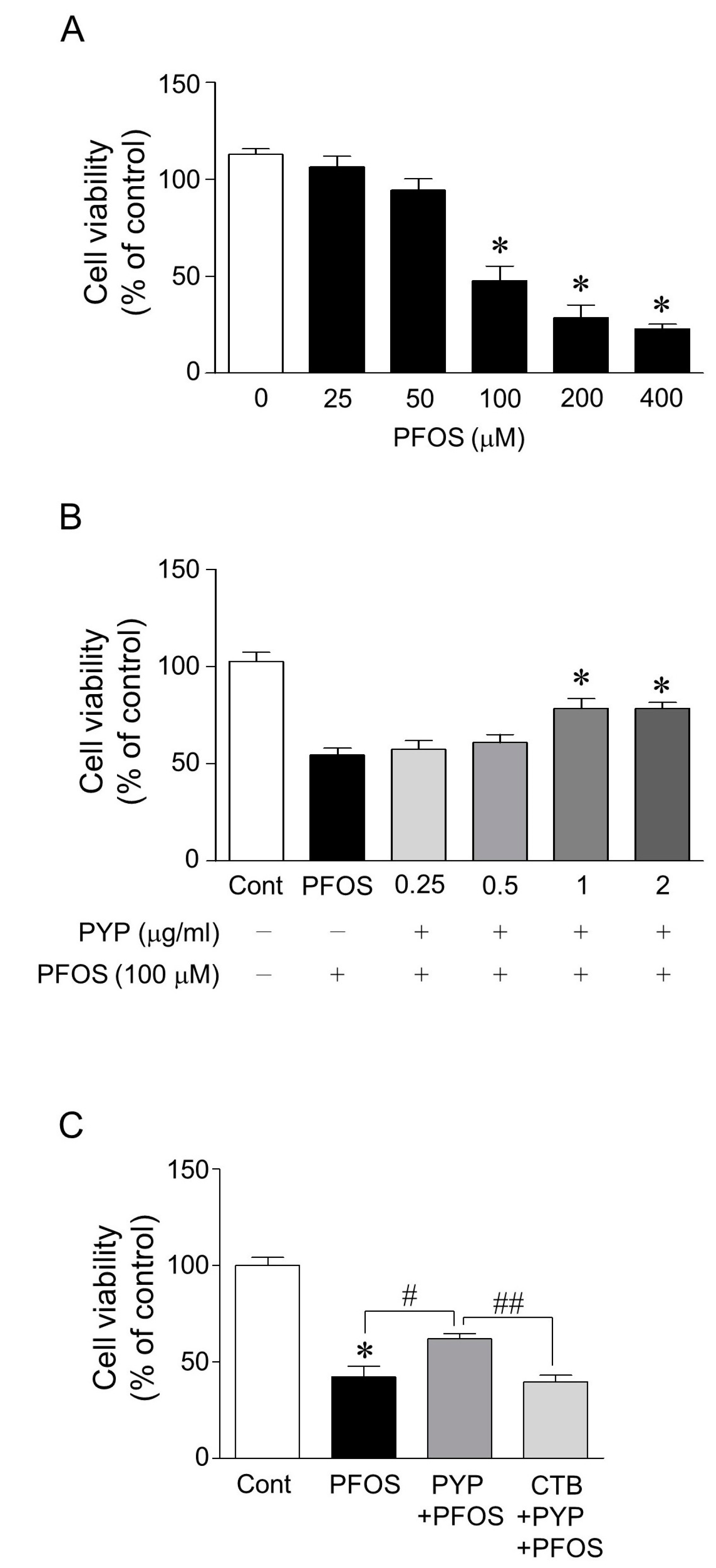
2.1. PYP Pretreatment Attenuated PFOS-Induced Decrease in Viability of Frontal Cortical Neurons and the PYP-Induced Enhancement of Cell Viability Was Downregulated by Inhibiting TrkB Receptor
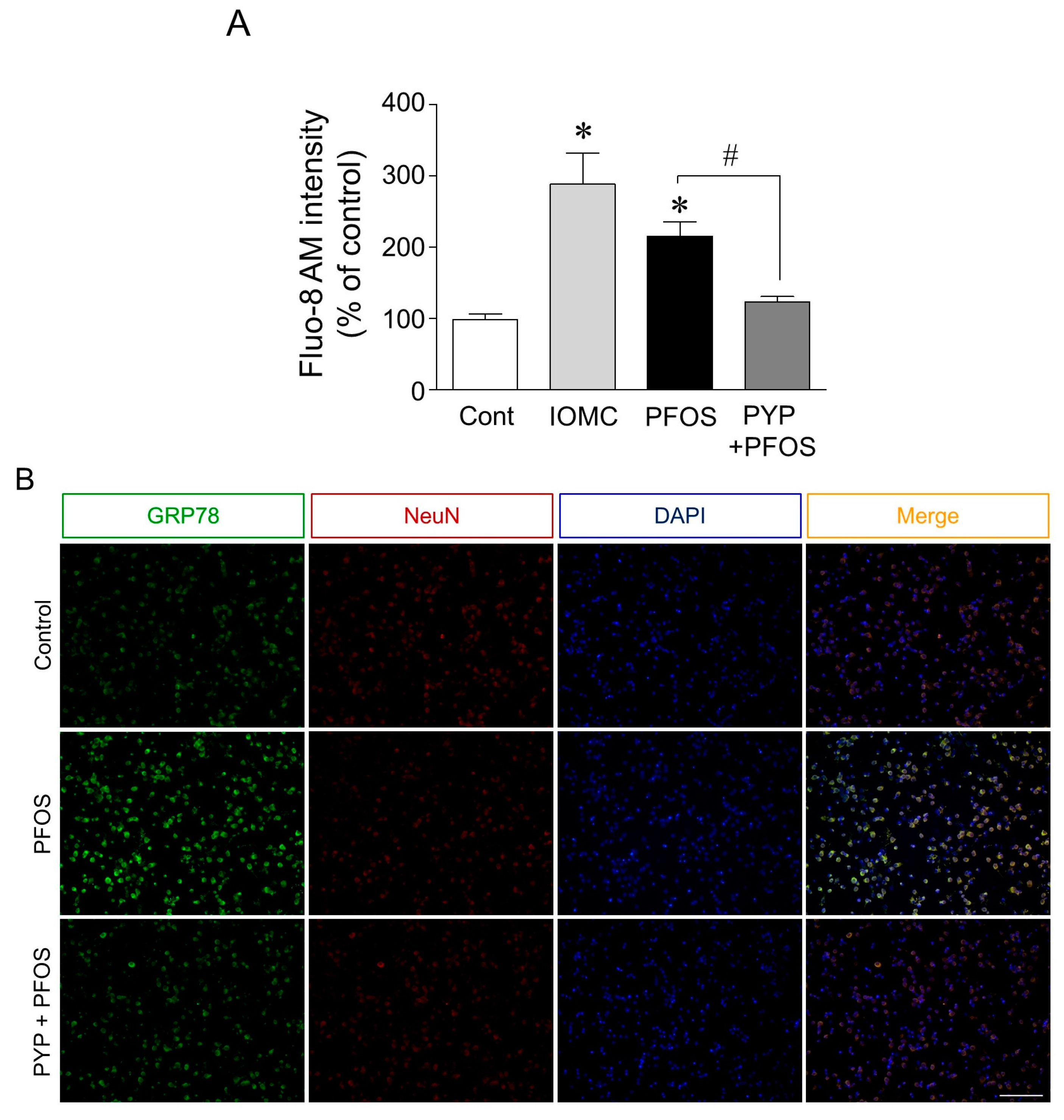
2.2. PYP Pretreatment Downregulated PFOS-Induced Increase in GRP78 Expression and Intracellular Calcium Levels in Frontal Cortical Neurons
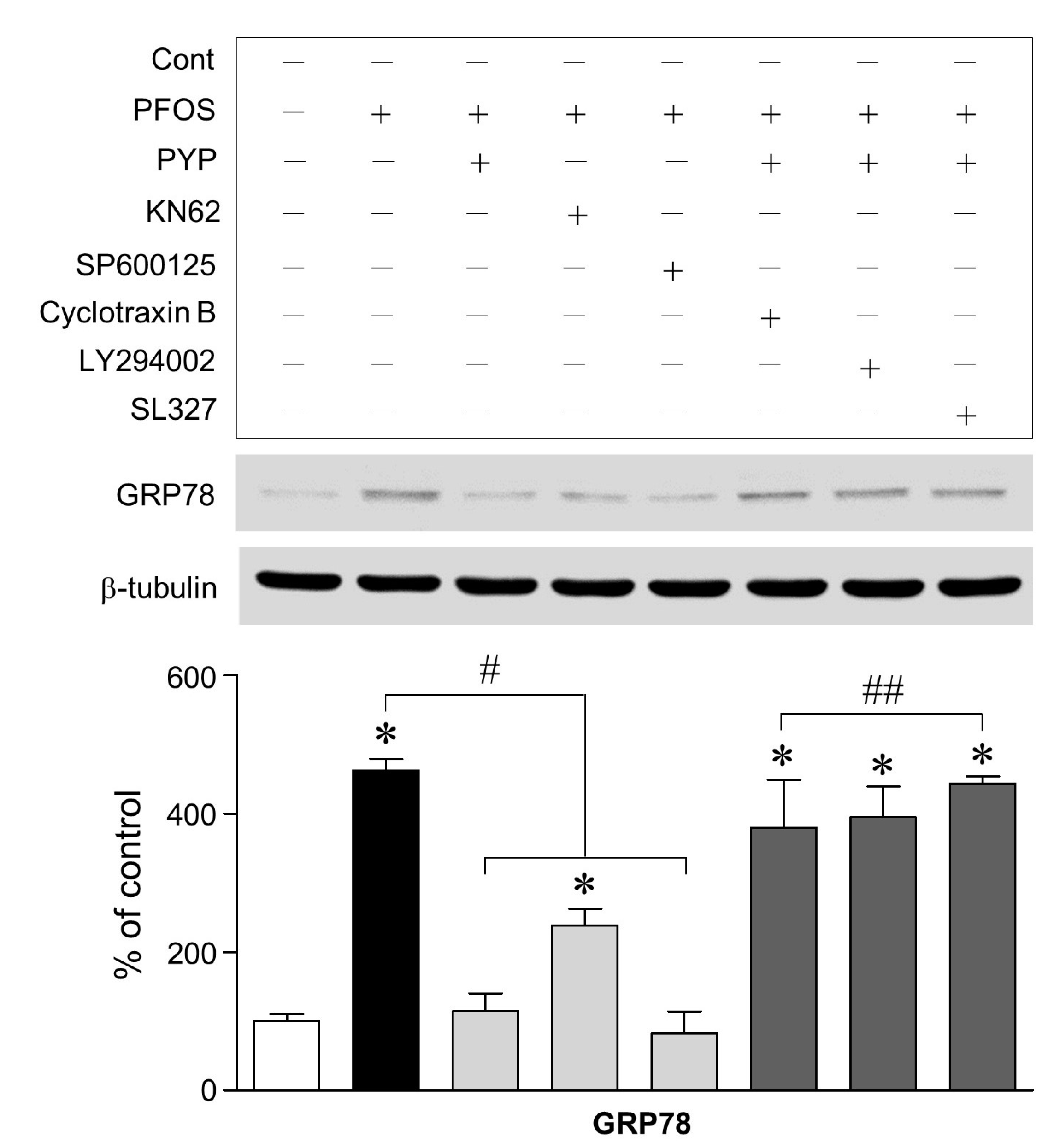
2.3. PFOS-Induced GRP78 Expression Was Mediated by Phosphorylation of JNK Linked to CaMKII and PYP-Mediated Decrease in GRP78 Expression Was Carried Out by Activating TrkB Receptor-PI3K-ERK1/2 Signaling
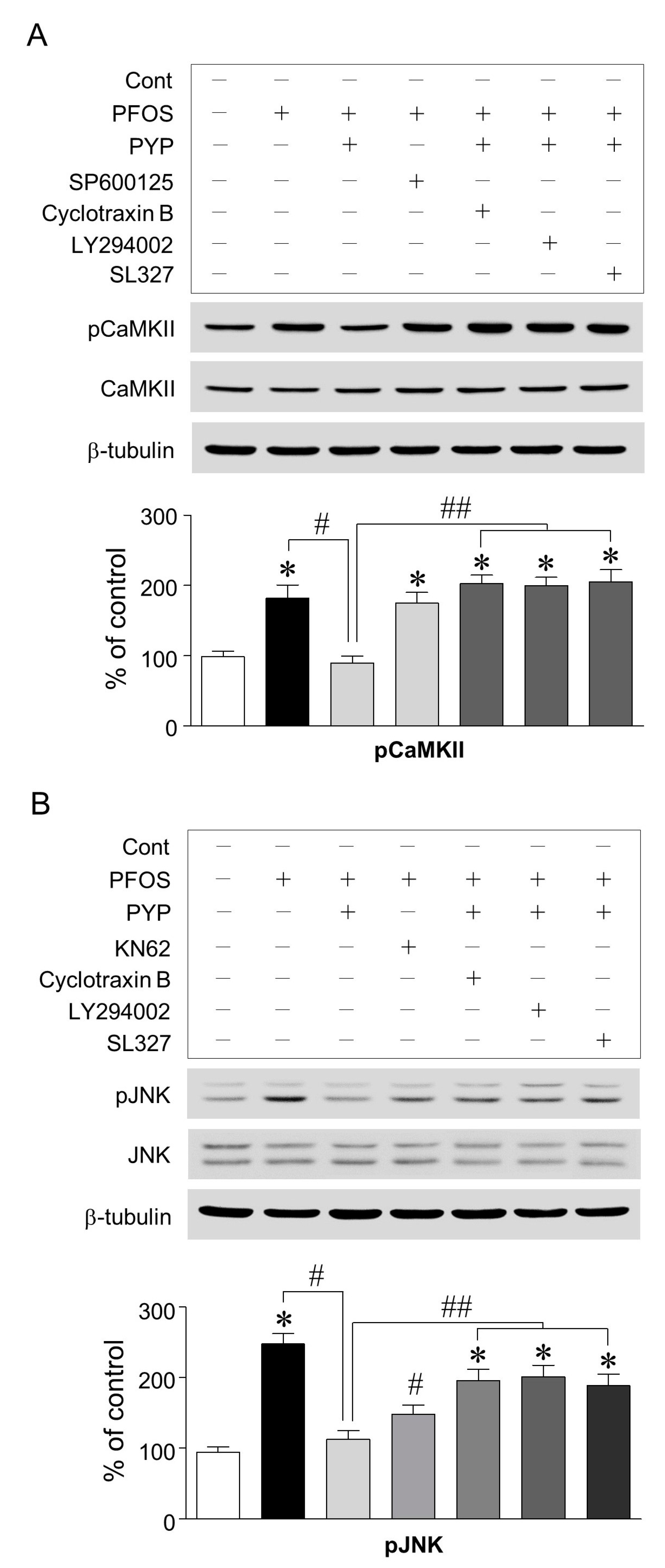
2.4. PYP Pretreatment Downregulated the Increase in PFOS-Induced CaMKII and JNK Phosphorylation and Inhibition of CaMKII Attenuated the Increase in PFOS-Induced JNK Phosphorylation in Frontal Cortical Neurons
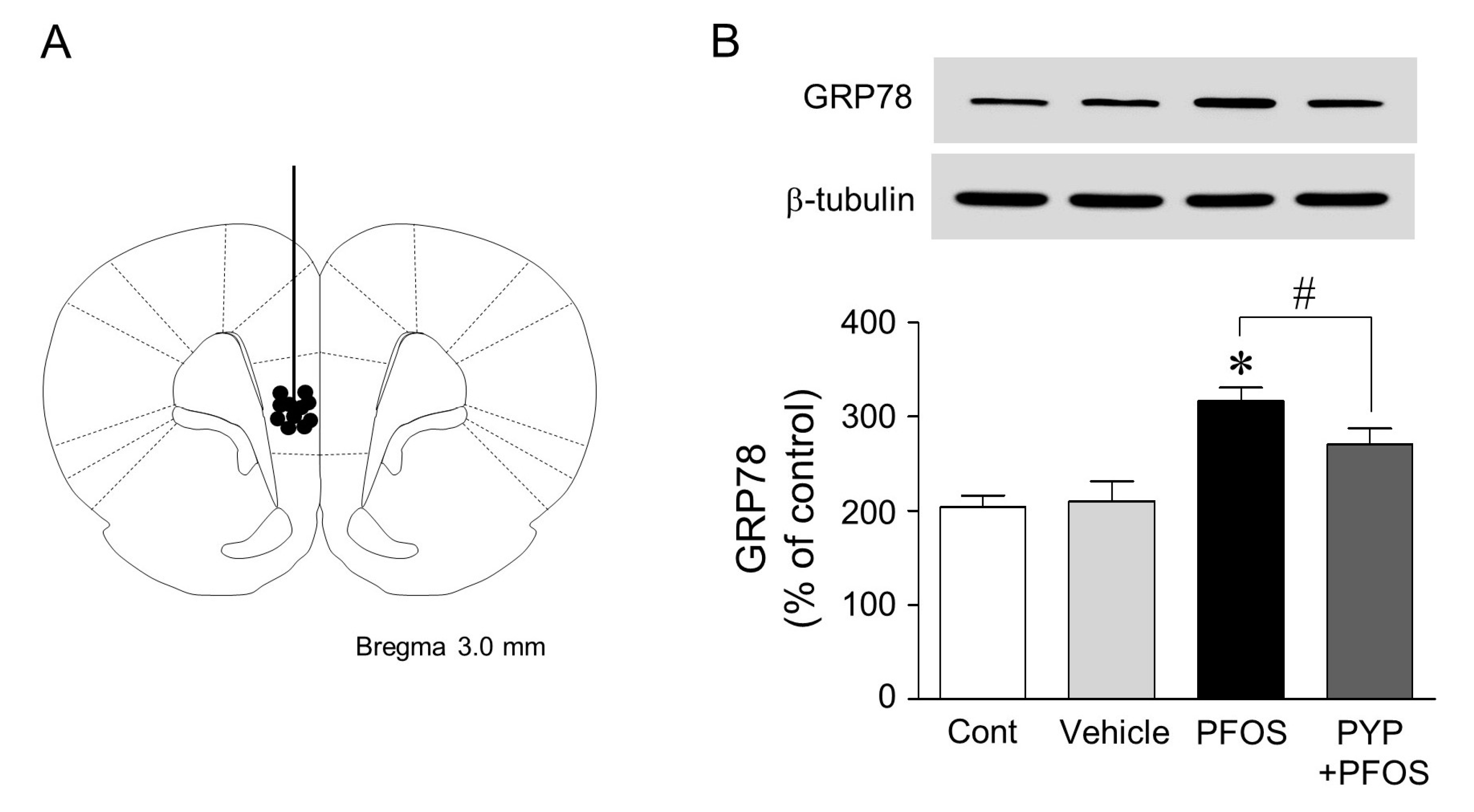
2.5. Microinjection of PYP Downregulated the Increase in PFOS-Induced ER Stress in Rat Prefrontal Cortex
3. Discussion
4. Materials and Methods
4.1. Peptide and Chemicals
4.2. Primary Cortical Neuron Culture
4.3. Cell Viability Assay
4.4. Double-Immunofluorescence Staining
4.5. Immunoblotting
4.6. Intracellular Calcium Level
4.7. Rat Surgery and Microinjection
4.8. Statistics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lemal, D.M. Perspective on fluorocarbon chemistry. J. Org. Chem. 2004, 69, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K.; Tao, L.; Sinclair, E.; Pastva, S.D.; Jude, D.J.; Giesy, J.P. Perfluorinated compounds in aquatic organisms at various trophic levels in a Great Lakes food chain. Arch. Environ. Contam. Toxicol. 2005, 48, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Loccisano, A.E.; Campbell, J.L.; Andersen, M.E.; Clewell, H.J. Evaluation and prediction of pharmacokinetics of PFOA and PFOS in the monkey and human using a PBPK model. Regul. Toxicol. Pharmacol. 2011, 59, 157–175. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, K.T.; Sørensen, M.; McLaughlin, J.K.; Lipworth, L.; Tjønneland, A.; Overvad, K.; Raaschou-Nielsen, O. Perfluorooctanoate and perfluorooctanesulfonate plasma levels and risk of cancer in the general Danish population. J. Natl. Cancer Inst. 2009, 101, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Bonefeld-Jorgensen, E.C.; Long, M.; Bossi, R.; Ayotte, P.; Asmund, G.; Krüger, T.; Ghisari, M.; Mulvad, G.; Kern, P.; Nzulumiki, P.; et al. Perfluorinated compounds are related to breast cancer risk in Greenlandic Inuit: A case control study. Environ. Health 2011, 10, 88. [Google Scholar] [CrossRef] [PubMed]
- Fei, C.; McLaughlin, J.K.; Lipworth, L.; Olsen, J. Maternal levels of perfluorinated chemicals and subfecundity. Hum. Reprod. 2009, 24, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Z.; Hu, D.C. Effects of perfluorooctanoate and perfluorooctane sulfonate exposure on hepatoma Hep G2 cells. Arch. Toxicol. 2009, 83, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Jones, P.D.; Upham, B.L.; Trosko, J.E.; Lau, C.; Giesy, J.P. Inhibition of gap junctional intercellular communication by perfluorinated compounds in rat liver and dolphin kidney epithelial cell lines in vitro and Sprague-Dawley rats in vivo. Toxicol. Sci. 2002, 68, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, S.; Colomina, M.T.; Vicens, P.; Franco-Pons, N.; Domingo, J.L. Concurrent exposure to perfluorooctane sulfonate and restraint stress during pregnancy in mice: Effects on postnatal development and behavior of the offspring. Toxicol. Sci. 2007, 98, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, W.; Song, J.; Yu, H.; Jin, Y.; Oami, K.; Sato, I.; Saito, N.; Tsuda, S. A comparative study on oxidative damage and distributions of perfluorooctane sulfonate (PFOS) in mice at different postnatal developmental stages. J. Toxicol. Sci. 2009, 34, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Borg, D.; Bogdanska, J.; Sundström, M.; Nobel, S.; Håkansson, H.; Bergman, Å.; DePierre, J.W.; Halldin, K.; Bergström, U. Tissue distribution of 35S-labelled perfluorooctane sulfonate (PFOS) in C57Bl/6 mice following late gestational exposure. Reprod. Toxicol. 2010, 30, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Johansson, N.; Fredriksson, A.; Eriksson, P. Neonatal exposure to perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) causes neurobehavioural defects in adult mice. Neurotoxicology 2008, 29, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Mariussen, E. Neurotoxic effects of perfluoroalkylated compounds: Mechanisms of action and environmental relevance. Arch. Toxicol. 2012, 86, 1349–1367. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, W.; Zhang, Q.; Zhao, H.; Quan, X. Effects of developmental perfluorooctane sulfonate exposure on spatial learning and memory ability of rats and mechanism associated with synaptic plasticity. Food Chem. Toxicol. 2015, 76, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, B.; Zhao, W.D.; Liu, Y.J.; Shang, D.S.; Fang, W.G.; Chen, Y.H. Perfluorooctane sulfonate triggers tight junction “opening” in brain endothelial cells via phosphatidylinositol 3-kinase. Biochem. Biophys. Res. Commun. 2011, 410, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.C.; Zhang, L.; Li, Y.Y.; Wang, Y.J.; Xia, W.; Lin, Y.; Wei, J.; Xu, S.Q. Inflammation-like glial response in rat brain induced by prenatal PFOS exposure. Neurotoxicology 2011, 32, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Darling, N.J.; Cook, S.J. The role of MAPK signalling pathways in the response to endoplasmic reticulum stress. Biochim. Biophys. Acta 2014, 1843, 2150–2163. [Google Scholar] [CrossRef] [PubMed]
- Zeeshan, H.M.; Lee, G.H.; Kim, H.R.; Chae, H.J. Endoplasmic reticulum stress and associated ROS. Int. J. Mol. Sci. 2016, 17, 327. [Google Scholar] [CrossRef] [PubMed]
- Gardner, B.M.; Pincus, D.; Gotthardt, K.; Gallagher, C.M.; Walter, P. Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb. Perspect. Biol. 2013, 5, a013169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhao, H.; Liu, W.; Zhang, Z.; Qin, H.; Luo, F.; Leng, S. Developmental perfluorooctane sulfonate exposure results in tau hyperphosphorylation and β-amyloid aggregation in adults rats: Incidence for link to Alzheimer’s disease. Toxicology 2016, 347, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wang, J.; Wang, S.; Xu, X. Porphyra species: A mini-review of its pharmacological and nutritional properties. J. Med. Food 2016, 19, 111–119. [Google Scholar] [CrossRef] [PubMed]
- MacArtain, P.; Gill, C.I.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional value of edible seaweeds. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Kim, E.Y.; Mikami, K.; Nam, T.J. Chemoprotective effects of a recombinant protein from Pyropia yezoensis and synthetic peptide against acetaminophen-induced Chang liver cell death. Int. J. Mol. Med. 2015, 36, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Choi, Y.H.; Choi, C.G.; Nam, T.J. Effects of the cyclophilin-type peptidylprolyl cis-trans isomerase from Pyropia yezoensis against hydrogen peroxide-induced oxidative stress in HepG2 cells. Mol. Med. Rep. 2017, 15, 4132–4138. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Kim, I.H.; Choi, Y.H.; Nam, T.J. A peptide from Porphyra yezoensis stimulates the proliferation of IEC-6 cells by activating the insulin-like growth factor I receptor signaling pathway. Int. J. Mol. Med. 2015, 35, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Kim, Y.M.; Kim, I.H.; Choi, Y.H.; Nam, T.J. Pyropia yezoensis peptide PYP1-5 protects against dexamethasone-induced muscle atrophy through the downregulation of atrogin1/MAFbx and MuRF1 in mouse C2C12 myotubes. Mol. Med. Rep. 2017, 15, 3507–3514. [Google Scholar] [CrossRef] [PubMed]
- Austin, M.E.; Kasturi, B.S.; Barber, M.; Kannan, K.; MohanKumar, P.S.; MohanKumar, S.M. Neuroendocrine effects of perfluorooctane sulfonate in rats. Environ. Health Perspect. 2003, 111, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Kim, E.Y.; Choi, Y.H.; Nam, T.J. Negative regulation of ERK1/2 by PI3K is required for the protective effects of Pyropia yezoensis peptide against perfluorooctane sulfonate-induced endoplasmic reticulum stress. Mol. Med. Rep. 2017, 15, 2583–2587. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jin, Y.; Liu, W.; Wang, F.; Hao, S. Possible mechanism of perfluorooctane sulfonate and perfluorooctanoate on the release of calcium ion from calcium stores in primary cultures of rat hippocampal neurons. Toxicol. In Vitro 2011, 25, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Kuan, C.Y.; Whitmarsh, A.J.; Yang, D.D.; Liao, G.; Schloemer, A.J.; Dong, C.; Bao, J.; Banasiak, K.J.; Haddad, G.G.; Flavell, R.A.; et al. A critical role of neural-specific JNK3 for ischemic apoptosis. Proc. Natl. Acad. Sci. USA 2003, 100, 15184–15189. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.D.; Kuan, C.Y.; Whitmarsh, A.J.; Rinócn, M.; Zheng, T.S.; Davis, R.J.; Rakic, P.; Flavell, R.A. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature 1997, 389, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Brecht, S.; Kirchhof, R.; Chromik, A.; Willesen, M.; Nicolaus, T.; Raivich, G.; Wessig, J.; Waetzig, V.; Goetz, M.; Claussen, M.; et al. Specific pathophysiological functions of JNK isoforms in the brain. Eur. J. Neurosci. 2005, 21, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Morishima, Y.; Gotoh, Y.; Zieg, J.; Barrett, T.; Takano, H.; Flavell, R.; Davis, R.J.; Shirasaki, Y.; Greenberg, M.E. β-Amyloid induces neuronal apoptosis via a mechanism that involves the c-Jun N-terminal kinase pathway and the induction of Fas ligand. J. Neurosci. 2001, 21, 7551–7560. [Google Scholar] [PubMed]
- Hunot, S.; Vila, M.; Teismann, P.; Davis, R.J.; Hirsch, E.C.; Przedborski, S.; Rakic, P.; Flavell, R.A. JNK-mediated induction of cyclooxygenase 2 is required for neurodegeneration in a mouse model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2004, 101, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Arnsten, A.F.T. Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 2009, 10, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Botvinick, M.; An, J. Goal-directed decision making in prefrontal cortex: A computational framework. Adv. Neural Inf. Process. Syst. 2009, 21, 169–176. [Google Scholar] [PubMed]
- Koenigs, M.; Grafman, J. The functional neuroanatomy of depression: Distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav. Brain Res. 2009, 201, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.; Liu, J.; Li, C.; Wang, J. Critical role of endoplasmic reticulum stress in cognitive impairment induced by microcystin-LR. Int. J. Mol. Sci. 2015, 16, 28077–28086. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C.; Saxena, S. ER stress and the unfolded protein response in neurodegeneration. Nat. Rev. Neurol. 2017, 13, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Tsuyama, Y.; Sanoh, S.; Ohta, S.; Kotake, Y. Perfluorooctane sulfonate induces neuronal vulnerability by decreasing GluR2 expression. Arch. Toxicol. 2017, 91, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Wessel, D.; Flügge, U.I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 1984, 138, 141–143. [Google Scholar] [CrossRef]
- Shevchenko, A.; Wilm, M.; Vorm, O.; Mann, M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 1996, 68, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.Y.; Wu, Y.M.; Ji, Z.; Gao, X.Y.; Pan, S.Y. A modified technique for culturing primary fetal rat cortical neurons. J. Biomed. Biotechnol. 2012, 2012, 803930. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.H.; Kim, E.-Y.; Nam, T.-J. Phycoerythrin Peptide from Pyropia yezoensis Alleviates Endoplasmic Reticulum Stress Caused by Perfluorooctane Sulfonate-Induced Calcium Dysregulation. Mar. Drugs 2018, 16, 44. https://doi.org/10.3390/md16020044
Oh JH, Kim E-Y, Nam T-J. Phycoerythrin Peptide from Pyropia yezoensis Alleviates Endoplasmic Reticulum Stress Caused by Perfluorooctane Sulfonate-Induced Calcium Dysregulation. Marine Drugs. 2018; 16(2):44. https://doi.org/10.3390/md16020044
Chicago/Turabian StyleOh, Jeong Hwan, Eun-Young Kim, and Taek-Jeong Nam. 2018. "Phycoerythrin Peptide from Pyropia yezoensis Alleviates Endoplasmic Reticulum Stress Caused by Perfluorooctane Sulfonate-Induced Calcium Dysregulation" Marine Drugs 16, no. 2: 44. https://doi.org/10.3390/md16020044
APA StyleOh, J. H., Kim, E.-Y., & Nam, T.-J. (2018). Phycoerythrin Peptide from Pyropia yezoensis Alleviates Endoplasmic Reticulum Stress Caused by Perfluorooctane Sulfonate-Induced Calcium Dysregulation. Marine Drugs, 16(2), 44. https://doi.org/10.3390/md16020044



