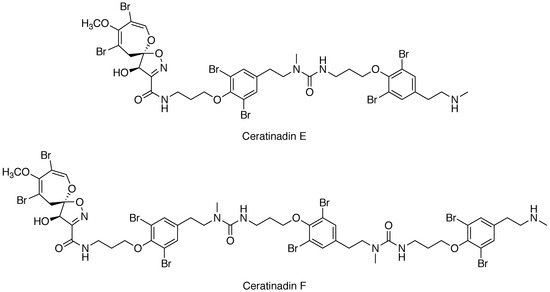
Ceratinadins E and F, New Bromotyrosine Alkaloids from an Okinawan Marine Sponge Pseudoceratina sp.
Abstract
1. Introduction
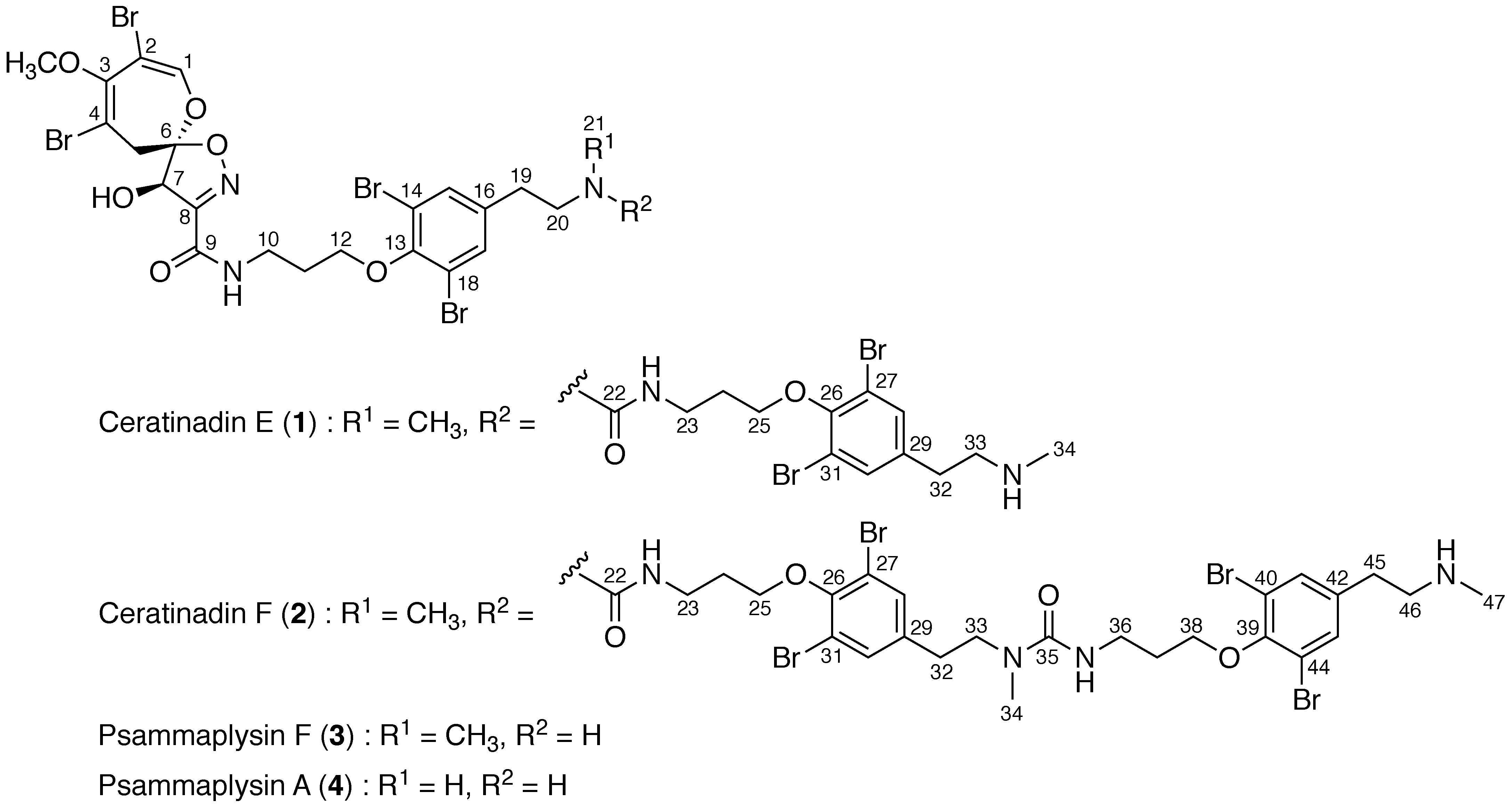
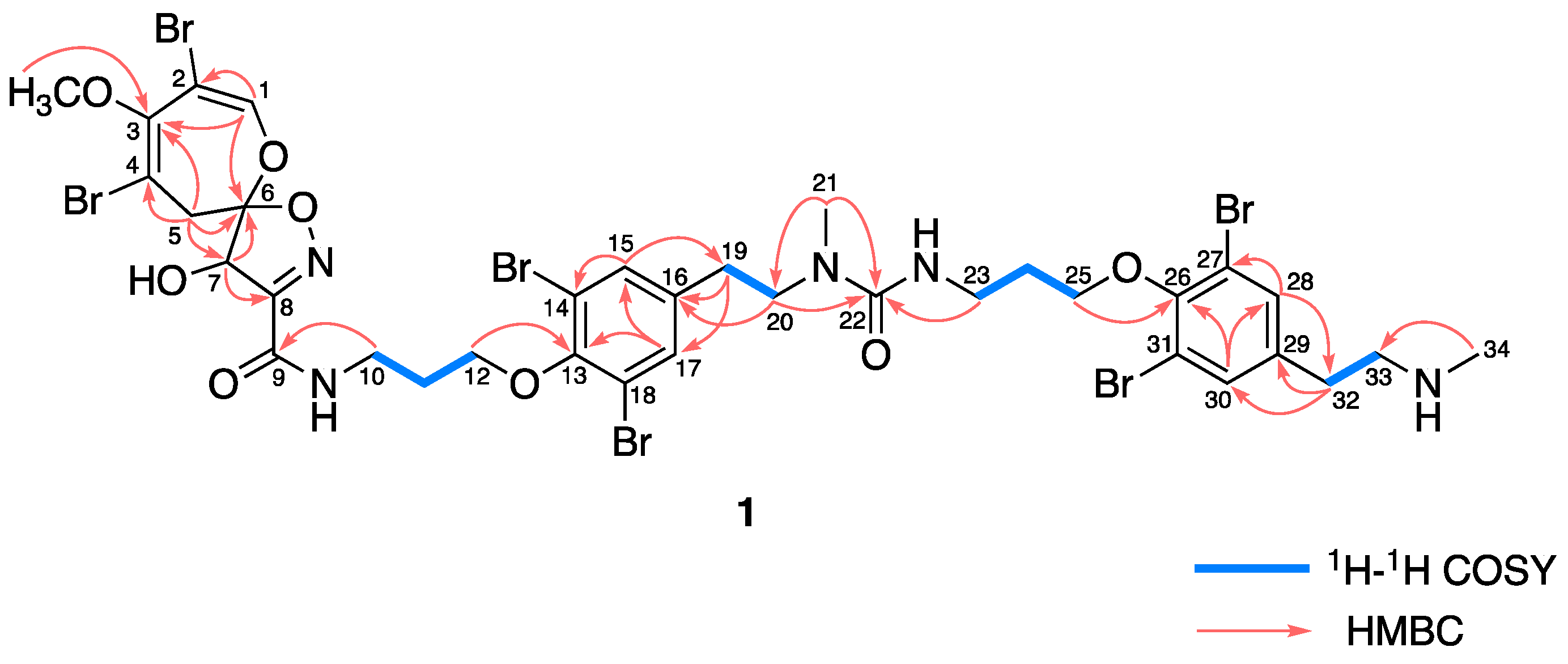
2. Results
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Extraction and Isolation
4.3. Ceratinadin E (1)
4.4. Ceratinadin F (2)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Malaria Report 2017; WHO Press: Geneva, Switzerland, 2017. [Google Scholar]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Fu, X.; Schmitz, F.J.; Kelly-Borges, M. Psammaplysin F, a new bromotyrosine derivative from a sponge, Aplysinella sp. J. Nat. Prod. 1997, 60, 614–615. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Davis, R.A.; Buchanan, M.S.; Duffy, S.; Avery, V.M.; Camp, D.; Quinn, R.J. Antimalarial Bromotyrosine derivatives from the Australian marine sponge Hyattella sp. J. Nat. Prod. 2010, 73, 985–987. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Andrews, K.T.; Birell, G.W.; Tran, T.L.; Camp, D.; Davis, R.A.; Quinn, R.J. Psammaplysin H, a new antimalarial bromotyrosine alkaloid from a marine sponge of the genus Pseudoceratina. Bioorg. Med. Chem. Lett. 2011, 21, 846–848. [Google Scholar] [CrossRef] [PubMed]
- Mudianta, I.W.; Skinner-Adams, T.; Andrews, K.T.; Davis, R.A.; Hadi, T.A.; Hayes, P.Y.; Garson, M.J. Psammaplysin derivatives from the Balinese marine sponge Aplysinella strangylata. J. Nat. Prod. 2012, 75, 2132–2143. [Google Scholar] [CrossRef] [PubMed]
- Hirano, K.; Kubota, T.; Tsuda, M.; Watanabe, K.; Fromont, J.; Kobayashi, J. Ma’edamines A and B, cytotoxic bromotyrosine alkaloids with a unique 2(1H)pyrazinone ring from sponge Suberea sp. Tetrahedron 2000, 56, 8107–8110. [Google Scholar] [CrossRef]
- Mukai, H.; Kubota, T.; Aoyama, K.; Mikami, Y.; Fromont, J.; Kobayashi, J. Tyrokeradines A and B, new bromotyrosine alkaloids with an imidazolyl-quinolinone moiety from a Verongid sponge. Bioorg. Med. Chem. Lett. 2009, 19, 1337–1339. [Google Scholar] [CrossRef] [PubMed]
- Kon, Y.; Kubota, T.; Shibazaki, A.; Gonoi, T.; Fromont, J.; Kobayashi, J. Ceratinadins A-C, new bromotyrosine alkaloids from an Okinawan marine sponge Pseudoceratina sp. Bioorg. Med. Chem. Lett. 2010, 20, 4569–4572. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T.; Watase, S.; Mukai, H.; Fromont, J.; Kobayashi, J. Tyrokeradines C-F, new bromotyrosine alkaloids from the Verongid sponges. Chem. Pharm. Bull. 2012, 60, 1599–1601. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T.; Watase, S.; Sakai, K.; Fromont, J.; Gonoi, T.; Kobayashi, J. Tyrokeradines G and H, new bromotyrosine alkaloids from an Okinawan Verongid sponge. Bioorg. Med. Chem. Lett. 2015, 25, 5221–5223. [Google Scholar] [CrossRef] [PubMed]
- Rotem, M.; Carmely, S.; Kashman, Y. Two new antibiotics from the red sea sponge Psammaplysilla purpurea: Total 13C-NMR line assignment of psammaplysins A and B and aerothionin. Tetrahedron 1983, 39, 667–676. [Google Scholar] [CrossRef]
- Roll, D.M.; Chang, C.W.J.; Scheuer, P.J.; Gray, G.A.; Shoolery, J.M.; Matsumoto, G.K.; Duyne, G.D.V.; Clardy, J. Structure of the psammaplysins. J. Am. Chem. Soc. 1985, 107, 2916–2920. [Google Scholar] [CrossRef]
- Mándi, A.; Mudianta, I.W.; Kurtán, T.; Garson, M.J. Absolute configuration and conformational study of psammaplysins A and B from the Balinese marine sponge Aplysinella strongylata. J. Nat. Prod. 2015, 78, 2051–2056. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, S.; Kobayashi, H.; van Soest, R.W.M.; Fusetani, N. Novel bromotyrosine derivatives that inhibit growth of the fish pathogenic bacterium Aeromonas hydrophila, from a marine sponge Hexadella sp. J. Org. Chem. 2005, 70, 1893–1896. [Google Scholar] [CrossRef] [PubMed]
- Otoguro, K.; Kohana, A.; Manabe, C.; Ishiyama, A.; Ui, H.; Shiomi, K.; Yamada, H.; Ōmura, S. Potent antimalarial activities of polyether antibiotic, X-206. J. Antibiot. 2001, 54, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Tymiak, A.A.; Rinehert, K.L., Jr. Biosynthesis of dibromotyrosine-derived antimicrobial compounds by the marine sponge Aplysina fistularis (Verongia aurea). J. Am. Chem. Soc. 1981, 103, 6763–6765. [Google Scholar] [CrossRef]

| Ceratinadin E (1) | Ceratinadin F (2) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Position | δH a | Multi (J in Hz) | δC b | Multi | Position | δH a | Multi (J in Hz) | δC b | Multi |
| 1 | 7.18 | s | 147.6 | d | 1 | 7.17 | s | 147.6 | d |
| 2 | − | 105.1 | s | 2 | − | 105.1 | s | ||
| 3 | − | 150.7 | s | 3 | − | 150.7 | s | ||
| 3-OCH3 | 3.69 d | s | 60.1 | q | 3-OCH3 | 3.69 d | s | 60.1 | q |
| 4 | - | 105.3 | s | 4 | - | 105.3 | s | ||
| 5a | 3.40 | me | 39.1 | t | 5a | 3.41 | d (16.4) | 39.1 | t |
| 5b | 3.11 | d (15.7) | 5b | 3.10 | d (16.4) | ||||
| 6 | - | 121.7 | s | 6 | - | 121.6 | s | ||
| 7 | 5.03 | s | 81.2 | d | 7 | 5.02 | s | 81.2 | d |
| 8 | - | 159.5 | s | 8 | - | 159.5 | s | ||
| 9 | - | 161.5 | s | 9 | - | 161.5 | s | ||
| 10 | 3.65 c | td (6.6, 1.2) | 38.8 | t | 10 | 3.65 c | td (6.6, 2.7) | 38.8 | t |
| 11 | 2.15 c | dt (6.6, 6.6) | 31.3 | t | 11 | 2.15 c | dt (6.6, 6.6) | 31.3 | t |
| 12 | 4.08 c | me | 72.9 | t | 12 | 4.08 c | me | 72.9 | t |
| 13 | - | 153.5 | s | 13 | - | 153.5 | s | ||
| 14, 18 | - | 119.7 | s | 14, 18 | - | 119.8 | s | ||
| 15, 17 | 7.49 | s | 135.2 | d | 15, 17 | 7.49 | s | 135.2 | d |
| 16 | - | 140.8 | s | 16 | - | 140.8 | s | ||
| 19 | 2.81 c | t (7.1) | 34.6 | t | 19 | 2.81 c | me | 34.8 | t |
| 20 | 3.52 c | t (7.1) | 51.7 | t | 20 | 3.51 c | me | 51.7 | t |
| 21 | 2.88 d | s | 35.8 | q | 21 | 2.88 d | s | 35.8 | q |
| 22 | - | 161.2 | s | 22 | - | 161.2 | s | ||
| 23 | 3.45 c | me | 40.1 | t | 23 | 3.45 c | me | 40.2 | t |
| 24 | 2.06 c | dt (6.4, 6.4) | 32.4 | t | 24 | 2.05 c | me | 32.5 | t |
| 25 | 4.09 c | me | 73.7 | t | 25 | 4.08 c | me | 73.7 | t |
| 26 | - | 154.4 | s | 26 | - | 153.6 | s | ||
| 27, 31 | - | 120.3 | s | 27, 31 | - | 119.8 | s | ||
| 28, 30 | 7.59 | s | 135.2 | d | 28, 30 | 7.49 | s | 135.2 | d |
| 29 | - | 138.0 | s | 29 | - | 140.7 | s | ||
| 32 | 2.98 | br t (7.6) | 33.0 | t | 32 | 2.81 | me | 34.8 | t |
| 33 | 3.27 | br t (7.6) | 51.9 | t | 33 | 3.52 | me | 51.7 | t |
| 34 | 2.70 d | s | 34.7 | q | 34 | 2.88 d | s | 35.8 | q |
| 35 | - | 161.2 | s | ||||||
| 36 | 3.44 c | me | 40.2 | t | |||||
| 37 | 2.05 c | me | 32.4 | t | |||||
| 38 | 4.05 c | me | 73.6 | t | |||||
| 39 | - | 154.5 | s | ||||||
| 40, 44 | - | 120.3 | s | ||||||
| 41, 43 | 7.56 | s | 135.2 | d | |||||
| 42 | - | 138.0 | s | ||||||
| 45 | 2.93 | br t (7.8) | 33.0 | t | |||||
| 46 | 3.18 | me | 51.9 | t | |||||
| 47 | 2.70 d | s | 34.7 | q | |||||
| Antimalarial Activity a | Cytotoxicity a | Selectivity Index | |||
|---|---|---|---|---|---|
| K1 b | FCR3 c | MRC-5d | MRC-5/K1 | MRC-5/FCR3 | |
| Psammaplysin F (3) | 3.77 | 2.45 | 12.65 | 3.4 | 5.2 |
| Ceratinadin E (1) | 1.03 | 0.77 | 15.99 | 15.5 | 20.8 |
| Ceratinadin F (2) | >12.5 | -e | >50 | >4 | -e |
| Chloroquine f | 0.34 | 0.035 | >25.80 | >75.9 | >737.1 |
| Artemisinin f | 0.010 | 0.0088 | >14.12 | >1412 | >1604.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurimoto, S.-i.; Ohno, T.; Hokari, R.; Ishiyama, A.; Iwatsuki, M.; Ōmura, S.; Kobayashi, J.; Kubota, T. Ceratinadins E and F, New Bromotyrosine Alkaloids from an Okinawan Marine Sponge Pseudoceratina sp. Mar. Drugs 2018, 16, 463. https://doi.org/10.3390/md16120463
Kurimoto S-i, Ohno T, Hokari R, Ishiyama A, Iwatsuki M, Ōmura S, Kobayashi J, Kubota T. Ceratinadins E and F, New Bromotyrosine Alkaloids from an Okinawan Marine Sponge Pseudoceratina sp. Marine Drugs. 2018; 16(12):463. https://doi.org/10.3390/md16120463
Chicago/Turabian StyleKurimoto, Shin-ichiro, Taito Ohno, Rei Hokari, Aki Ishiyama, Masato Iwatsuki, Satoshi Ōmura, Jun’ichi Kobayashi, and Takaaki Kubota. 2018. "Ceratinadins E and F, New Bromotyrosine Alkaloids from an Okinawan Marine Sponge Pseudoceratina sp." Marine Drugs 16, no. 12: 463. https://doi.org/10.3390/md16120463
APA StyleKurimoto, S.-i., Ohno, T., Hokari, R., Ishiyama, A., Iwatsuki, M., Ōmura, S., Kobayashi, J., & Kubota, T. (2018). Ceratinadins E and F, New Bromotyrosine Alkaloids from an Okinawan Marine Sponge Pseudoceratina sp. Marine Drugs, 16(12), 463. https://doi.org/10.3390/md16120463