Overview on the Application of Modern Methods for the Extraction of Bioactive Compounds from Marine Macroalgae
Abstract
1. Introduction
2. Novel Extraction Techniques of Bioactive Compounds from Marine Macroalgae
2.1. Supercritical Fluid Extraction (SFE)
2.2. Ultrasound-Assisted Extraction (UAE)
2.3. Subcritical Water Extraction (SWE)
2.4. Microwave-Assisted Extraction (MAE)
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Pal, A.; Kamthania, M.C.; Kumar, A. Bioactive compounds and properties of seaweeds—A review. OALibJ 2014, 1, 1–17. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant. Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Al-Enazi, N.M.; Al-Homaidan, A.A.; Ibraheem, I.B.M.; Al-Othman, M.R.; Hatamleh, A.A. Antibacterial β-amyrin isolated from Laurencia microcladia. Arab. J. Chem. 2015, 8, 32–37. [Google Scholar] [CrossRef]
- Wang, H.M.D.; Li, X.C.; Lee, D.J.; Chang, J.S. Potential biomedical applications of marine algae. Bioresour. Technol. 2017, 244, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.; Valentao, P.; Andrade, P. Bioactive compounds from macroalgae in the new millenium: implications for neurodegenerative diseases. Mar. Drugs 2014, 12, 4934–4972. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.; Sousa, S.; Silva, A.; Amorim, M.; Pereira, L.; Rocha-Santos, T.A.P.; Gomes, A.M.P.; Duarte, A.C.; Freitas, A.C. Impact of enzyme- and ultrasound-assisted extraction methods on biological properties of red, brown and green seaweeds from the central west coast of portugal. J. Agr. Food Chem. 2015, 63, 3177–3188. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Wijesekara, I.; Li, Y.; Kim, S.K. Phlorotannins as bioactive agents from brown algae. Process. Biochem. 2011, 46, 2219–2224. [Google Scholar] [CrossRef]
- Montero, L.; Sanchez-Camargo, A.P.; Garcia-Canas, V.; Tanniou, A.; Stiger-Pouvreau, V.; Russo, M.; Rastrelli, L.; Cifuentes, A.; Herrero, M.; Ibanez, E. Anti-proliferative and chemical characterization by comprehensive two-dimensional liquid chromatography coupled to mass spectrometry of phlorotannins from the brown macroalga Sargassum muticum collected on North-Atlantic coasts. J. Chromatogr. A 2016, 1428, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.V.; Kim, S.K. Potential pharmacological applications of polyphenolic derivatives from marine brown algae. Environ. Toxicol. Pharmacol. 2011, 32, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Ruperez, P.; Saura-Calixto, F. Dietary fibre and physicochemical properties of edible spanish seaweeds. Eur. Food Res. Technol. 2001, 212, 349–354. [Google Scholar] [CrossRef]
- Saravana, P.S.; Cho, Y.J.; Park, Y.B.; Woo, H.C. Structural, antioxidants and emulsifying activities of fucoidan from Saccharina japonica using pressurized liquid extraction. Carbohydr. Polym. 2016, 153, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Liu, X.; Hao, J.; Cai, C.; Fan, F.; Dun, Y.; Zhao, X.; Liu, X.; Li, C.; Yu, G. In vitro and in vivo hypoglycemic effects of brown algal fucoidans. Int. J. Biol. Macromol. 2016, 82, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, S.; Plaza, M.; Jaime, L.; Ibanez, E.; Reglero, G.; Senorans, J. Pressurized liquids as an alternative green process to extract antiviral agents from the edible seaweed Himanthalia elongata. J. Appl. Phycol. 2011, 23, 909–917. [Google Scholar] [CrossRef]
- Huheihel, M.; Ishanu, V.; Tal, J.; Arad, S. Activity of Porphyridium sp. Polysaccharide against herpes simplex in vitro and in vivo. J. Biochem. Bioph. Meth. 2002, 20, 189–200. [Google Scholar] [CrossRef]
- Ye, H.; Wang, K.; Zhou, C.; Liu, J.; Zeng, X. Purification, antitumor and antioxidant activities in vitro of polysaccharides from the brown seaweed Sargassum pallidum. Food Chem. 2008, 111, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vaquero, M.; Rajauria, G.; O’Doherty, J.; Torres, S. Polysaccharides from macroalgae: Recent advances, innovative technologies and challenges in extraction and purification. Food Res. Int. 2017, 99, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Christaki, E.; Bonos, E.; Giannenas, I.; Florou-Paneri, P. Functional properties of carotenoids originating from algae. J. Sci. Food Agric. 2012, 93, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, M.; Wanezaki, S.; Miyauchi, K.; Kurihara, H.; Kohno, H.; Kawabata, J.; Odashima, S.; Takahashi, K. Apoptosis-inducing effect of fucoxanthin on human leukemia cell line HL-60. Food Sci. Technol. Res. 1999, 5, 243. [Google Scholar] [CrossRef]
- Heffernan, N.; Smyth, T.J.; FitzGerald, R.J.; Villa-Soler, A.; Mendiola, J.; Ibanez, E.; Brunton, N.P. Comparison of extraction methods for selected carotenoids from macroalgae and the assessment of their seasonal/spatial variation. Innov. Food Sci. Emerg. Technol. 2016, 37, 221–228. [Google Scholar] [CrossRef]
- Fabrowska, J.; Ibanez, E.; Leska, B.; Herrero, M. Supercritical fluid extraction as a tool to valorize underexploited freshwater green algae. Algal Res. 2016, 19, 237–245. [Google Scholar] [CrossRef]
- Sivagnanam, S.P.; Yin, S.; Choi, J.H.; Park, Y.B.; Woo, H.C.; Chun, B.S. Biological properties of fucoxanthin in oil recovered from two brown seaweeds using supercritical CO2 extraction. Mar. Drugs 2015, 13, 3422–3442. [Google Scholar] [CrossRef] [PubMed]
- Dawczynski, C.; Schubert, R.; Jahreis, G. Amino acids, fatty acids and dietary fibre in edible seaweed products. Food Chem. 2007, 103, 891–899. [Google Scholar] [CrossRef]
- Silva, G.; Pereira, R.B.; Valentao, P.; Andrade, P.B.; Sousa, C. Distinct fatty acid profile of ten brown macroalgae. Rev. Bras. Farmacogn. Braz. J. Pharmacogn. 2013, 23, 608–613. [Google Scholar] [CrossRef]
- Andrade, P.B.; Barbosa, M.; Matos, R.P.; Lopes, G.; Vinholes, J.; Mouga, T.; Valentao, P. Valuable compounds in macroalgae extracts. Food Chem. 2013, 138, 1819–1828. [Google Scholar] [CrossRef] [PubMed]
- Duarte, K.; Justino, C.I.L.; Pereira, R.; Freitas, A.C.; Gomes, A.M.; Duarte, A.C.; Rocha-Santos, T.A.P. Green analytical methodologies for the discovery of bioactive compounds from marine sources. Trends Environ. Anal. Chem. 2014, 3, 43–52. [Google Scholar] [CrossRef]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K. Algal extracts: Technology and advances. Eng. Life Sci. 2014, 14, 1–32. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Application of novel extraction technologies for extraction of bioactives from marine algae. J. Agric. Food Chem. 2013, 61, 4667–4675. [Google Scholar] [CrossRef] [PubMed]
- Grosso, C.; Valentao, P.; Ferreres, F.; Andrade, P.B. Alternative and efficient extraction methods for marine-derived compounds. Mar. Drugs 2015, 13, 3182–3230. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.; Cifuentes, A.; Ibanez, E. Sub- and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae. A review. Food Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef]
- Mishra, V.K.; Temelli, F.; Ooraikul, B. Extraction and purification of ω-3 fatty acids with an emphasis on supercritical fluid extraction-A review. Food Res. Int. 1993, 26, 217–226. [Google Scholar] [CrossRef]
- Duarte, K.; Justino, C.I.L.; Gomes, A.M.; Rocha-Santos, T.A.P.; Duarte, A.C. Green Analytical methodologies for preparation of extracts and analysis of bioactive compounds. Compr. Anal. Chem. 2014, 65, 59–78. [Google Scholar] [CrossRef]
- del Pilar Sanchez Camargo, A.; Ibanez, E.; Cifuentes, A.; Herrero, M. Bioactives obtained from plants, seaweeds, microalgae and food by-products using pressurized liquid extraction and supercritical fluid extraction. Compr. Anal. Chem. 2017, 76, 1–24. [Google Scholar] [CrossRef]
- Silva, R.P.F.F.; Rocha-Santos, T.A.P.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef]
- Tanniou, A.; Esteban Serrano, L.; Vandanjon, L.; Ibanez, E.; Mendiola, J.A.; Cerantola, S.; Kervarec, N.; La Barre, S.; Marchal, L.; Stiger-Pouvreau, V. Green improved processes to extract bioactive phenolic compounds from brown macroalgae using Sargassum muticum as model. Talanta 2013, 104, 44–52. [Google Scholar] [CrossRef]
- Ospina, M.; Casto-Vargas, H.I.; Parada-Alfonso, F. Antioxidant capacity of Colombian seaweeds: 1. Extracts obtained from Gracilaria mammillaris by means of supercritical fluid extraction. J. Supercrit. Fluids 2017, 128, 314–322. [Google Scholar] [CrossRef]
- Roh, M.K.; Uddin, M.S.; Chun, B.S. Extraction of Fucoxanthin and Polyphenol from Undaria pinnatifida Using Supercritical Carbon Dioxide with Co-Solvent. Biotechnol. Bioproc. Eng. 2008, 13, 724–729. [Google Scholar] [CrossRef]
- Saravana, P.S.; Tilahun, A.; Cho, Y.J.; Choi, J.H.; Park, Y.B.; Woo, H.C.; Chin, B.S. Influence of co-solvents on fucoxanthin and phlorotannin recovery from brown seaweed using supercritical CO2. J. Supercrit. Fluids 2017, 120, 295–303. [Google Scholar] [CrossRef]
- Crampon, C.; Boutin, O.; Badens, E. Supercritical Carbon Dioxide Extraction of Molecules of Interest from Microalgae and Seaweeds. Ind. Eng. Chem. Res. 2011, 50, 8941–8953. [Google Scholar] [CrossRef]
- Cheung, P.C.K. Temperature and pressure effects on supercritical carbon dioxide extraction of n-3 fatty acids from red seaweed. Food Chem. 1999, 65, 399–403. [Google Scholar] [CrossRef]
- Quitain, A.T.; Kai, T.; Sasaki, M.; Goto, M. Supercritical carbon dioxide extraction of fucoxanthin from Undaria pinnatifida. J. Agric. Food Chem. 2013, 61, 5792–5797. [Google Scholar] [CrossRef] [PubMed]
- Hattab, M.; Culioli, G.; Piovetti, L.; Chitour, S.E.; Valls, R. Comparison of various extraction methods for identification and determination of volatile metabolites from brown alga Dictyopteris membranacea. J. Chromatogr. A 2007, 1143, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Klejdus, B.; Lojkova, L.; Plaza, M.; Šnoblova, M.; Šterbova, D. Hyphenated technique for the extraction and determination of isoflavones in algae: Ultrasound-assisted supercritical fluid extraction followed by fast chromatography with tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 7956–7965. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Gorka, B.; Wieczorek, P.P.; Roj, E.; Lipok, J.; Leska, B.; Messyasz, B.; Wilk, R.; Schroeder, G.; Dobrzynska-Inger, A.; et al. Supercritical fluid extraction of algae enhances levels of biologically active compounds promoting plant growth. Eur. J. Phycol. 2016, 51, 1–10. [Google Scholar] [CrossRef]
- Punin Crespo, M.O.; Lage Yusty, M.A. Comparison of supercritical fluid extraction and Soxhlet extraction for the determination of aliphatic hydrocarbons in seaweed samples. Ecotoxicol. Environ. Saf. 2006, 64, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Florez-Fernandez, N.; Gonzalez Munoz, M.J. Ultrasound-assisted extraction of bioactive carbohydrates. In Water extraction of Bioactive Compounds; Dominguez Gonzalez, H., Gonzalez Munoz, M.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 317–331. ISBN 978-0-12-809380-1. [Google Scholar]
- Oh, S.H.; Ahn, J.; Kang, D.H.; Lee, H.Y. The effect of ultrasonificated extracts of spirulina maxima on the anticancer activity. Mar. Biotechnol. 2011, 13, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.T.; Vuong, Q.V.; Schreider, M.J.; Bowyer, M.C.; Van Altena, I.A.; Scarlett, C.J. Optimisation of ultrasound-assisted extraction conditions for phenolic content and antioxidant activities of the alga Hormosira banksii using response surface methodology. J. Appl. Phycol. 2017, 29, 3161–3173. [Google Scholar] [CrossRef]
- Lee, S.H.; Kang, M.C.; Moon, S.H.; Jeon, B.T.; Jeon, Y.J. Potential use of ultrasound in antioxidant extraction from Ecklonia cava. Algae 2013, 28, 371–378. [Google Scholar] [CrossRef]
- Topuz, O.K.; Gokoglu, N.; Yerlikaya, P.; Ucak, I.; Gumus, B. Optimization of Antioxidant Activity and Phenolic Compound Extraction Conditions from Red Seaweed (Laurencia obtuse). J. Aquat. Food Prod. Technol. 2016, 25, 414–422. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; Smyth, T.J.; O’Donnell, C.P. Optimization of ultrasound assisted extraction of bioactive components from brown seaweed Ascophyllum nodosum using response surface methodology. Ultrason. Sonochem. 2015, 23, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Kadam, S.U.; O’Donnell, C.P.; Rai, D.K.; Hossain, M.B.; Burgess, C.M.; Walsh, D.; Tiwari, B.K. Laminarin from irish brown seaweeds ascophyllum nodosum and laminaria hyperborea: ultrasound assisted extraction, characterization and bioactivity. Mar. Drugs 2015, 13, 4270–4280. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Tavanandi, H.A.; Mantri, V.A.; Raghavarao, K.S.M.S. Ultrasound assisted methods for enhanced extraction of Phycobiliproteins from marine macro-algae, Gelidium pusillum (Rhodophyta). Ultrason. Sonochem. 2017, 38, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Guo, X.Y.; Zhang, D.N.; Wu, Y.; Wu, T.; Chen, Z.G. Ultrasound-assisted extraction and purification of taurine from the red algae Porphyra yezoensis. Ultrason. Sonochem. 2015, 24, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.; Ibanez, E. Pressurized hot water extraction and processing. In Enhancing Extraction Processes in the Food Industry; Lebovka, N., Vorobiev, E., Chemat, F., Eds.; Taylor & Francis Group, LLC: Abingdon, UK, 2011; pp. 223–254. ISBN 9781138199330. [Google Scholar]
- Zakaria, S.M.; Kamal, S.M.M. Subcritical Water Extraction of Bioactive Compounds from Plants and Algae: Applications in Pharmaceutical and Food Ingredients. Food Eng. Rev. 2016, 8, 23–34. [Google Scholar] [CrossRef]
- Fayad, S.; Nehme, R.; Tannoury, M.; Lesellier, E.; Pichon, C.; Morin, P. Macroalga Padina pavonica water extracts obtained by pressurized liquid extraction and microwave-assisted extraction inhibit hyaluronidase activity as shown by capillary electophoresis. J. Chromatogr. A 2017, 1497, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Saravana, P.S.; Chun, B.S. Seaweed polysaccharide isolation using subcritical water hydrolysis. In Seaweed Polysaccharides: Isolation, Biological and Biomedical Applications; Venkatesan, J., Anil, S., Kim, S.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 47–73. ISBN 978-0-12-809816-5. [Google Scholar]
- Li, Z.; Wang, B.; Zhang, Q.; Qu, Y.; Xu, H.; Li, G. Preparation and antioxidant property of extract and semipurified fractions of Caulerpa racemosa. J. Appl. Phycol. 2012, 24, 1527–1536. [Google Scholar] [CrossRef]
- Lopez, A.; Rico, M.; Rivero, A.; Suarez de Tangil, M. The effects of solvents on the phenolic contents and antioxidant activity of Stypocaulon scoparium algae extracts. Food Chem. 2011, 125, 1104–1109. [Google Scholar] [CrossRef]
- Wang, T.; Jonsdottir, R.; Olafsdottir, G. Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem. 2009, 116, 240–248. [Google Scholar] [CrossRef]
- Wijeskara, I.; Yoon, N.Y.; Kim, S.K. Phlorotannins from Ecklonia cava (Phaeophyceae): Biological activities and potential health benefits. Biofactors 2010, 36, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, N.; Smyth, T.J.; FitzGerald, R.J.; Villa-Soler, A.; Brunton, N. Antioxidant activity and phenolic content of pressurized liquid and solid-liquid extracts from four Irish origin macroalgae. Int. J. Food Sci. Technol. 2014, 49, 1765–1772. [Google Scholar] [CrossRef]
- Castro-Puyana, M.; Herrero, M.; Mendiola, J.A.; Ibanez, E. Subcritical water extraction of bioactive components from algae. In Functional Ingredients from Algae for Foods and Nutraceuticals; Dominguez, H., Ed.; Woodhead Publishing: Sawston, UK, 2013; pp. 534–560. ISBN 978-0-85709-512-1. [Google Scholar]
- Tierney, M.S.; Smyth, T.J.; Hayes, M.; Soler-Vila, A.; Croft, A.K.; Brunton, N. Influence of pressurized liquid extraction and solid-liquid extraction methods on the phenolic content and antioxidant activities of Irish macroalgae. Int. J. Food Sci. Technol. 2013, 48, 860–869. [Google Scholar] [CrossRef]
- Vo Dinh, T.; Saravana, P.S.; Woo, H.C.; Chun, B.S. Ionic liquid-assisted subcritical water enhances the extraction of phenolics from brown seaweed and its antioxidant activity. Sep. Purif. Technol. 2018, 196, 287–299. [Google Scholar] [CrossRef]
- Plaza, M.; Amigo-Benavent, M.; Castillo, M.D.; Ibanez, E.; Herrero, M. Neoformation of antioxidants in glycation model systems treated under subcritical water extraction conditions. Food Res. Int. 2010, 43, 1123–1129. [Google Scholar] [CrossRef]
- Plaza, M.; Amigo-Benavent, M.; Castillo, M.D.; Ibanez, E.; Herrero, M. Facts about the formation of new antioxidants in natural samples after subcritical water extraction. Food Res. Int. 2010, 43, 2341–2348. [Google Scholar] [CrossRef]
- Saravana, P.S.; Choi, J.H.; Park, Y.B.; Woo, H.C.; Chun, B.S. Evaluation of the chemical composition of brown seaweed (Saccharina japonica) hydrolysate by pressurized hot water extraction. Algal Res. 2016, 13, 246–254. [Google Scholar] [CrossRef]
- Rodriguez Seoane, P.; Florez-Fernandez, N.; Conde Pineiro, E.; Dominguez Gonzales, H. Chapter 6—Microwave-Assisted Water Extraction. In Water Extraction of Bioactive Compounds; Dominguez Gonzalez, H., Gonzalez Munoz, M.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 163–198. ISBN 978-0-12-809380-1. [Google Scholar]
- Perez, L.; Conde, E.; Dominguez, H. Microwave hydrodiffusion and gravity processing of Sargassum muticum. Process. Biochem. 2014, 49, 981–988. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Allen, J.D.; Kanitkar, A.; Boldor, D. Oil extraction from Scenedesmus obliquus using a continuous microwave system—Design, optimization and quality characterization. Bioresour. Technol. 2011, 102, 3396–3403. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, M.; Yuen, A.K.I.; Zhang, R.; Wright, J.T.; Taylor, R.B.; Maschmeyer, T.; de Nys, R. A comparative assessment of microwave assisted (MAE) and conventional solid-liquid (SLE) techniques for the extraction of phloroglucinol from brown seaweed. Algal Res. 2017, 23, 28–36. [Google Scholar] [CrossRef]
- Ren, B.; Chen, C.; Li, C.; Fu, X.; You, L.; Liu, R.H. Optimization of microwave-assisted extraction of Sargassum thunbergii polysaccharides and its antioxidant and hypoglycemic activities. Carbohydr. Polym. 2017, 173, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.Y.; Wang, B.; Yu, C.G.; Xu, Y.F. Optimization of Microwave-assisted Extraction of Polyphenols from Enteromorpha prolifera by Orthogonal Test. Chin. Herb. Med. 2010, 2, 321–325. [Google Scholar]
- Zhang, R.; Yuen, A.K.I.; Magnusson, M.; Wright, J.T.; de Nys, R.; Masters, A.F.; Maschmeyer, T. A comparative assessment of activity and structure of phlorotannins from the brown seaweed Carpophyllum flexuosum. Algal Res. 2018, 29, 130–141. [Google Scholar] [CrossRef]
- Rodriguez-Jasso, R.M.; Mussatto, S.I.; Pastrana, L.; Aguilar, C.N.; Teixeira, J.A. Microwave-assisted extraction of sulfated polysaccharides (fucoidan) from brown seaweed. Carbohydr. Polym. 2011, 86, 1137–1144. [Google Scholar] [CrossRef]
- Quitain, A.T.; Kai, T.; Sasaki, M.; Goto, M. Microwave-Hydrothermal Extraction and Degradation of Fucoidan from Supercritical Carbon Dioxide Deoiled Undaria pinnatifida. Ind. Eng. Chem. Res. 52, 7940–7946. [CrossRef]
- You, S.; Yang, C.; Lee, H.; Lee, B.Y. Molecular characteristics of partially hydrolyzed fucoidans from sporophyll of Undaria Pinnatifida and their in vitro anticancer activity. Food Chem. 2010, 119, 554–559. [Google Scholar] [CrossRef]
- Yuan, Y.; Macquarrie, D. Microwave assisted extraction of sulfated polysaccharides (fucoidan) from Ascophyllum nodosum and its antioxidant activity. Carbohydr. Polym. 2015, 129, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Xu, X.; Jing, C.; Zou, P.; Zhang, C.; Li, Y. Microwave assisted hydrothermal extraction of polysaccharides from Ulva prolifera: Functional properties and bioactivities. Carbohydr. Polym. 2018, 181, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Tsubaki, S.; Oono, K.; Hiraoka, M.; Onda, A.; Mitani, T. Microwave-assisted hydrothermal extraction of sulfated polysaccharides from Ulva spp. and Monostroma latissimum. Food Chem. 2016, 210, 311–316. [Google Scholar] [CrossRef] [PubMed]
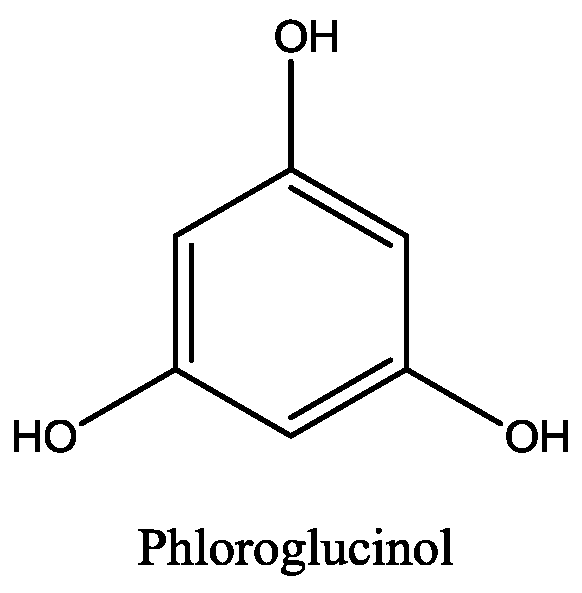
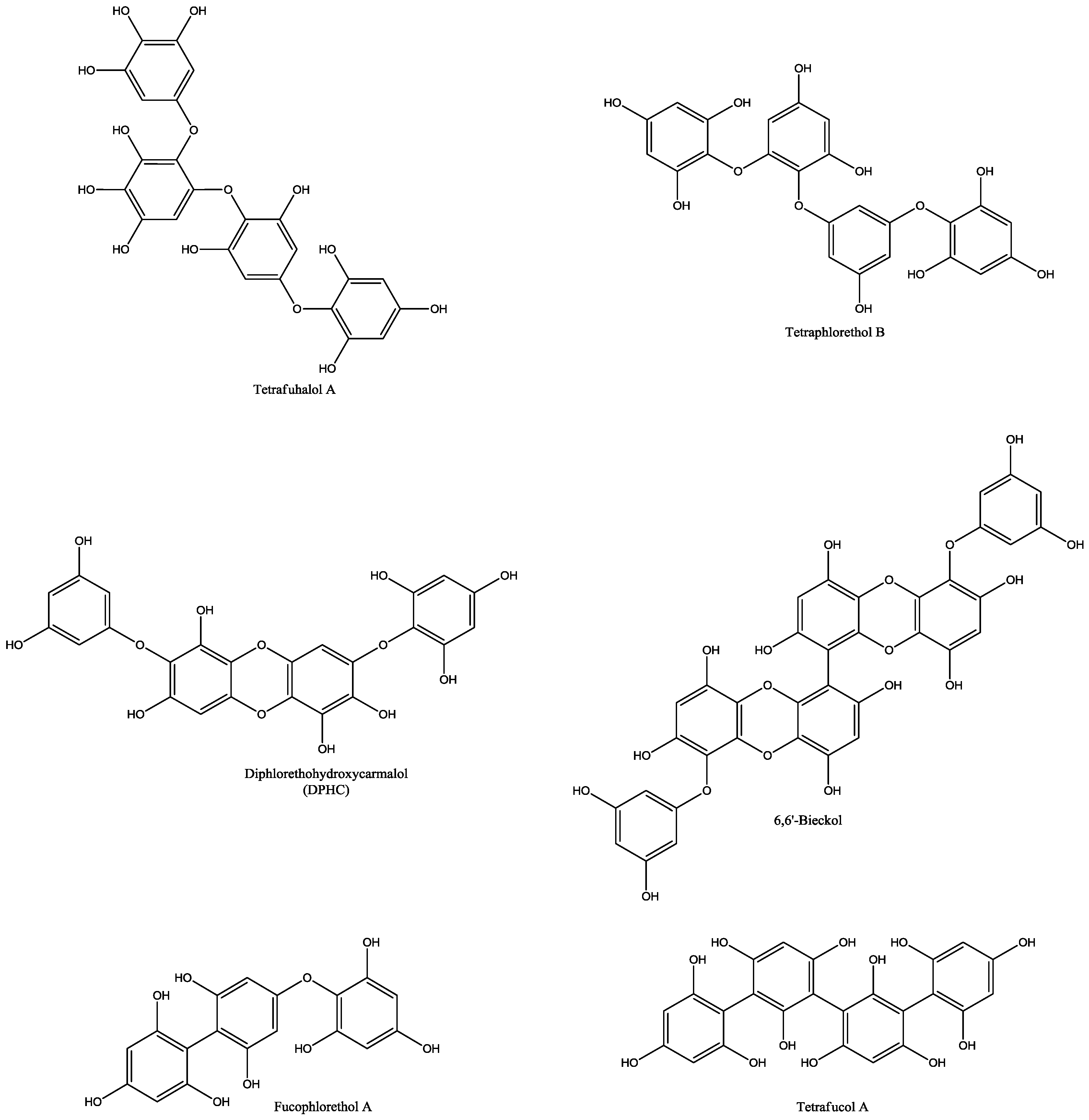
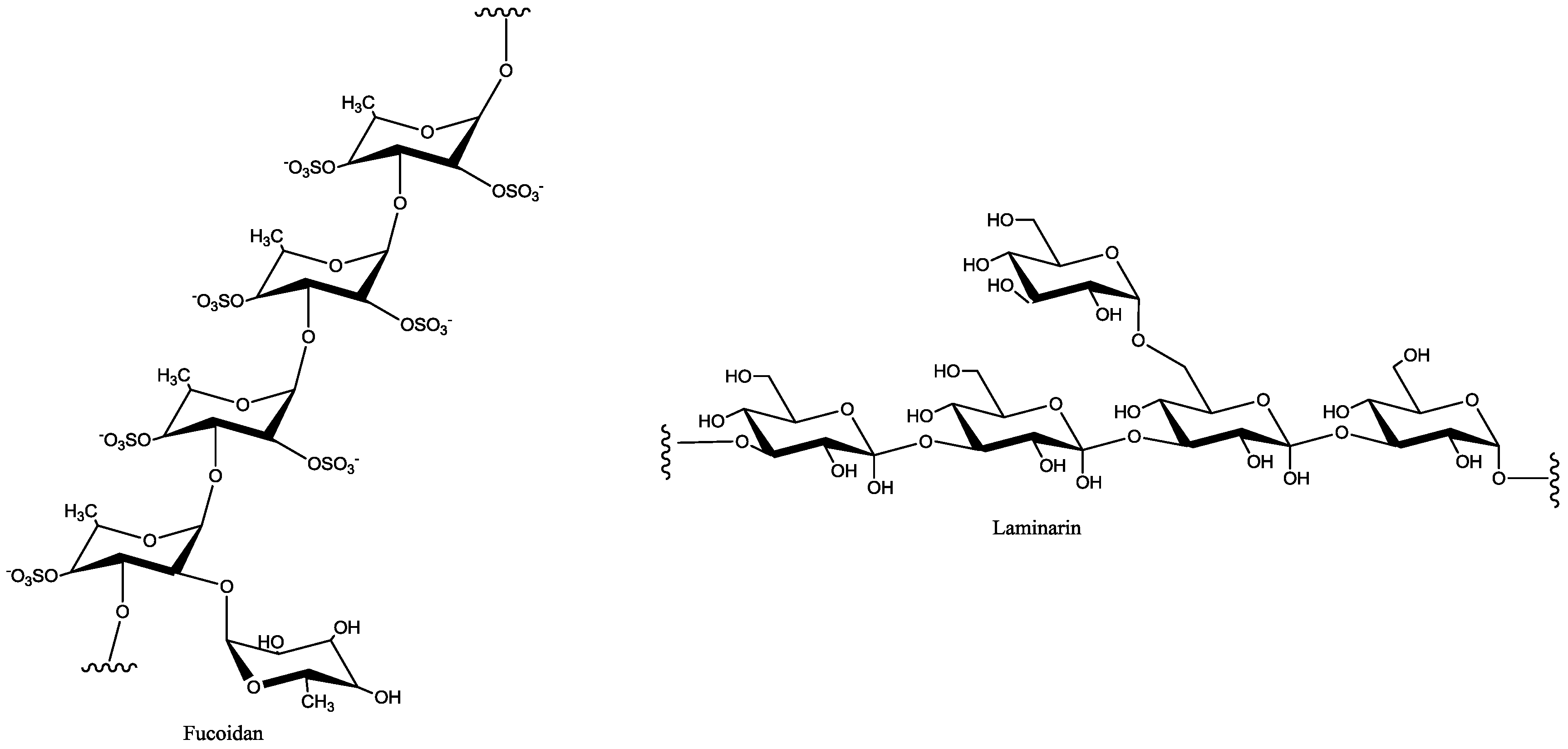
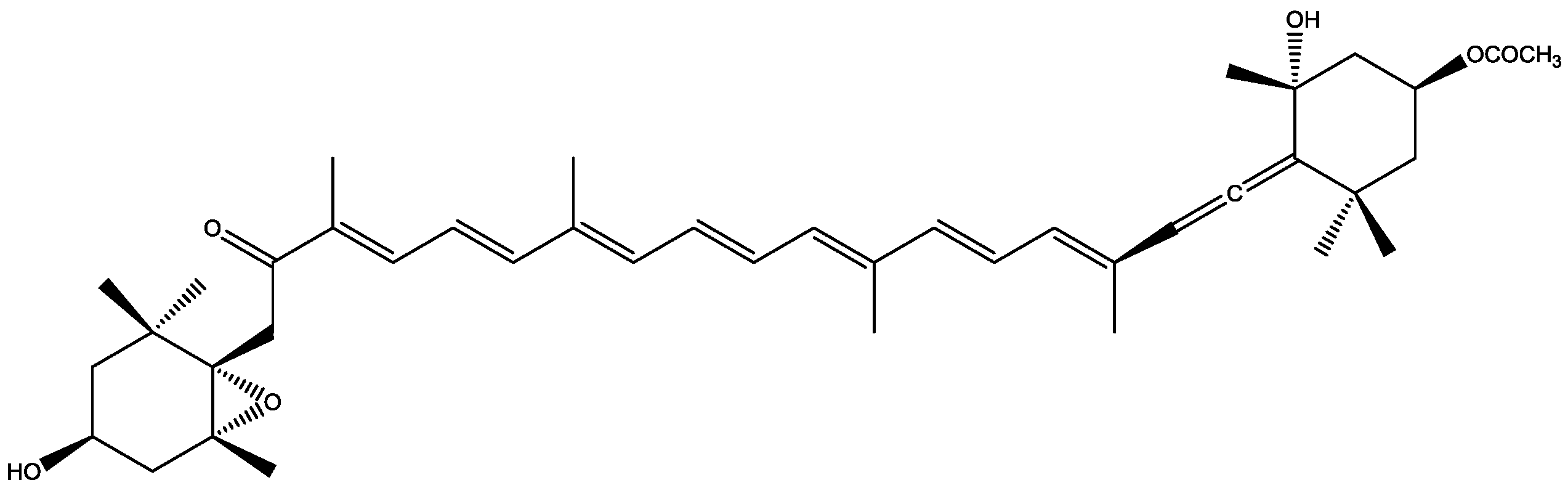
| Macroalgae Species | Extraction Parameters | Bioactive Compounds | Bioactivity | Ref. | |||
|---|---|---|---|---|---|---|---|
| Pressure [MPa] | Temp. [°C] | Time [min] | Co-Solvent [%] | ||||
| Hypnea charoides | 24.1–37.9 | 40–50 | 120 | - | Fatty acids (ω-3) | - | [41] |
| Cladophora glomerata, Ulva flexuosa, Chara fragilis | 10–30 | 40–60 | 120 | EtOH [0–15] | Carotenoids, phenols | Antioxidant | [21] |
| Dictyopteris membranacea | 9.1 and 10.4 | 40 | 30 | - | Volatile compounds | - | [43] |
| Fucus serratus, Laminaria digitata | 15, 22.5 and 30 | 30, 40 and 50 | 60 and 105 | - | Carotenoids | - | [20] |
| Sargassum muticum, Sargassum vulgare, Hypnea spinella, Porphyra sp., Undaria pinnatifida, Chondrus crispus, Halopytis incurvus | 10–40 | 35–75 | 10–60 | - | Isoflavones | - | [44] |
| Cladophora glomerata, Ulva flexuosa subsp. pilifera, Ulva clathrata, Polysiphoniucoides | 50 | 40 | 300, 360, 810 | - | Polyphenols, cytokinins, auxins, microelements and macroelements | Plant growth stimulation | [45] |
| Gracilaria mammillaris | 10,20 and 30 | 40, 50 and 60 | 240 | EtOH [2,5,8] | Polyphenols, carotenes | Antioxidant | [37] |
| Undaria pinnatifida | 22.9 | 45 | 50 | - | Hydrocarbons | - | [46] |
| Undaria pinnatifida | 20–40 | 25–60 | 180 | - | Fucoxanthin | - | [42] |
| Undaria pinnatifida | 8–30 | 30–60 | 50 | EtOH [3] | Fucoxanthin, polyphenols | - | [38] |
| Saccharina japonica (Laminaria japonica) | 20–30 | 45–55 | 240 | Sunflower oil, soybean oil, canola oil, EtOH and water [0.50–2.00] | Carotenoids, fucoxanthin, phlorotannins | Antioxidant | [39] |
| Saccharina japonica, Sargassum horneri | 25 | 45 | 120 | EtOH | Fatty acids, fucoxanthin, polyphenols | Anti-oxidant, anti-microbial and antihyper-tensive | [22] |
| Macroalgae Species | Ultrasound Operating Conditions | Bioactive Compounds | Bioactivity | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| Ultrasound Equipment; Frequency [kHz]; Power [W] | Sample Mass [g] | Solvent Volume [mL] | Temp. [°C] | Time [min] | ||||
| Hormosira banksii | Ultrasonic bath; 50; 150–250 | 1 | 50 (70% ethanol) | 30, 40 and 50 | 20, 40 and 60 | Polyphenols | Antioxidant | [49] |
| Ascophyllum nodosum | Ultrasound probe; 20; 750 | 4 | 40 (distilled water and 0.03 M HCl) | - | 10 | Polyphenols, fucose and uronic acid | - | [52] |
| Ascophyllum nodosum, Laminaria hyperborea | Ultrasound probe; 20; 750 | 10 | 200 (distilled water and 0.03 M HCl) | - | 15 | Polyphenols, laminarin | Antioxidant | [53] |
| Ecklonia cava | Ultrasonic bath; 40; 200 | 1 | 100 (water; 50% methanol; 100% methanol) | 30 | 360 and 720 | Polyphenols | Antioxidant | [50] |
| Gelidium pusillum | Ultrasonic bath; - 41.97 | 10 | 100 (phosphate buffer 0.1 M) | 30, 35 and 40 | 2, 4, 6, 8 and 10 | Phycobili-proteins | - | [54] |
| Sargassum muticum, Osmundea pinnatifida, Codium tomentosum | Ultrasonic bath; 50/60; 400 | 2 | 50 (deionized water) | 50 | 60 | Total phenolics, antioxidants, prebiotic compounds | Antioxidant, prebiotic, α-glucosidase inhibition | [7] |
| Laurencia obtuse | Ultrasonic bath; 40; 250 | 1 | 10–30 (95% ethanol) | 30–50 | 30–60 | Phenolic compounds, antioxidants | Antioxidant | [51] |
| Porphyra yezoensis | Ultrasonic bath; 20; 100, 200 and 300 | 10 | 200 (water) | 20, 40 and 60 | 15, 30 and 45 | Taurine | - | [55] |
| Macroalgae Species | Ultrasound Operating Conditions | Bioactive Compounds | Bioactivity | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| Sample Mass [g] | Water Volume [mL] | Pressure [MPa] | Temp. [°C] | Time [min] | ||||
| Sargassum muticum | 2 | - | 10.3 (1500 psi) | 50, 125 and 200 | 20 | Polyphenols, phlorotannins | Antioxidant | [34] |
| Padina pavonica | 0.65 | - | 15 | 60 | 10 (2 cycles) | - | Anti-hyaluronidase | [58] |
| Fucus serratus, Laminaria digitata, Gracilaria gracilis, Codium fragile | 2.5 | - | 10.3 (1500 psi) | 120 | 25 | Polyphenols | Antioxidant | [64] |
| Cystoseira abies-marina, Porphyra spp., Sargassum vulgare, Sargassum muticum, Undaria pinnatifida, Halopitys incurvus | 1 | - | 10.3 (1500 psi) | 120 and 200 | 20 | Polyphenols, neo-antioxidants, amino acids | Antioxidant and anti-microbial | [69] |
| Himanthalia elongata | 1 | - | 10.3 (1500 psi) | 100 | 20 | Poly-saccharides | Antiviral | [14] |
| Saccharina japonica | 9.65 | 160 | 10 | 150 | 5 | Fucoidan | Antioxidant | [12] |
| Saccharina japonica | 6 | 150 | 1.3–52 | 180–420 | 5 | Total organic carbon, minerals, amino acids, mono-saccharides | - | [70] |
| Ascophyllum nodosum, Pelvetia canaliculata, Fucus spiralis, Ulva intestinalis | 2.5 | - | 10.3 (1500 psi) | 120 | - | Polyphenols | Antioxidant | [66] |
| Saccharina japonica | 5 | 160 | 5 | 100–250 | 5 | Polyphenols | Antioxidant | [67] |
| Macroalgae Species | Ultrasound Operating Conditions | Bioactive Compounds | Bioactivity | Ref. | |||
|---|---|---|---|---|---|---|---|
| Power [w]; Frequency [MHz] | Solvent | Temp. [°C] | Time [min] | ||||
| Padina pavonica | 1000; 2450 | petroleum ether, ethanol, ethyl acetate and H2O | 40, 60, 80, 100 and 120 | 2 and 5 | - | Anti-hyaluronidase | [58] |
| Caulerpa racemosa | 100–600; - | 20–100% ethanol | 20–70 | 5–60 | Polyphenols | Antioxidant | [60] |
| Enteromorpha prolifera | 300–700; - | 10–60% ethanol | - | 5–40 (1–4 cycles) | Polyphenols | - | [76] |
| Carpophyllum flexuosum | - | H2O, acetone, ethanol, propan-1-ol, ethyl acetate | 135, 160 and 185 | 1, 3, 5, 10, 15 and 20 | Phloroglucinol | - | [74] |
| Undaria pinnatifida | 600; - | H2O | 110–120 | 5–120 | Fucoidan | - | [79] |
| Sargassum thunbergii | 200–800; - | H2O | 10–90 | 10–50 | Poly-saccharides | Antioxidant and hypoglycemic | [75] |
| Fucus vesiculosus | - | H2O | 122, 152 and 172 | 1, 16 and 31 | Poly-saccharides (fucoidan) | - | [78] |
| Ulva meridionalis, Ulva ohnoi, Monostroma latissimum | 1000; 2450 | H2O | 100–180 | 10 | Poly-saccharides (ulvan and rhamnan sulfate) | - | [83] |
| Ascophyllum nodosum | - | 0.1 M HCl | 90, 120 and 150 | 5, 15 and 30 | Fucoidan | Antioxidant | [81] |
| Ulva prolifera | 500; 2450 | 0.1 M HCl | 90, 120 and 150 | 15 | Poly-saccharides | Antioxidant, anti-hyperlipidemic | [82] |
| Carpophyllum flexuosum, Carpophyllum plumosum, Ecklonia radiata | - | H2O | 160 | 3 | Phlorotannins | Antioxidant | [77] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cikoš, A.-M.; Jokić, S.; Šubarić, D.; Jerković, I. Overview on the Application of Modern Methods for the Extraction of Bioactive Compounds from Marine Macroalgae. Mar. Drugs 2018, 16, 348. https://doi.org/10.3390/md16100348
Cikoš A-M, Jokić S, Šubarić D, Jerković I. Overview on the Application of Modern Methods for the Extraction of Bioactive Compounds from Marine Macroalgae. Marine Drugs. 2018; 16(10):348. https://doi.org/10.3390/md16100348
Chicago/Turabian StyleCikoš, Ana-Marija, Stela Jokić, Drago Šubarić, and Igor Jerković. 2018. "Overview on the Application of Modern Methods for the Extraction of Bioactive Compounds from Marine Macroalgae" Marine Drugs 16, no. 10: 348. https://doi.org/10.3390/md16100348
APA StyleCikoš, A.-M., Jokić, S., Šubarić, D., & Jerković, I. (2018). Overview on the Application of Modern Methods for the Extraction of Bioactive Compounds from Marine Macroalgae. Marine Drugs, 16(10), 348. https://doi.org/10.3390/md16100348








