Bacillibactin and Bacillomycin Analogues with Cytotoxicities against Human Cancer Cell Lines from Marine Bacillus sp. PKU-MA00093 and PKU-MA00092
Abstract
1. Introduction
2. Results and Discussion
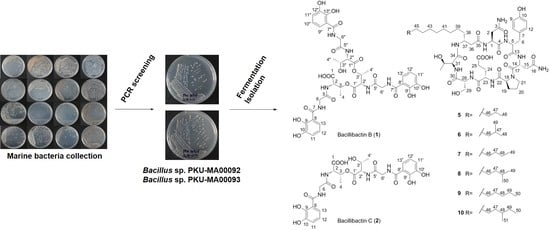
2.1. Genome Mining for Nonribosomal Peptides Discovery from Marine Bacteria Library Based on a PCR Screening Method
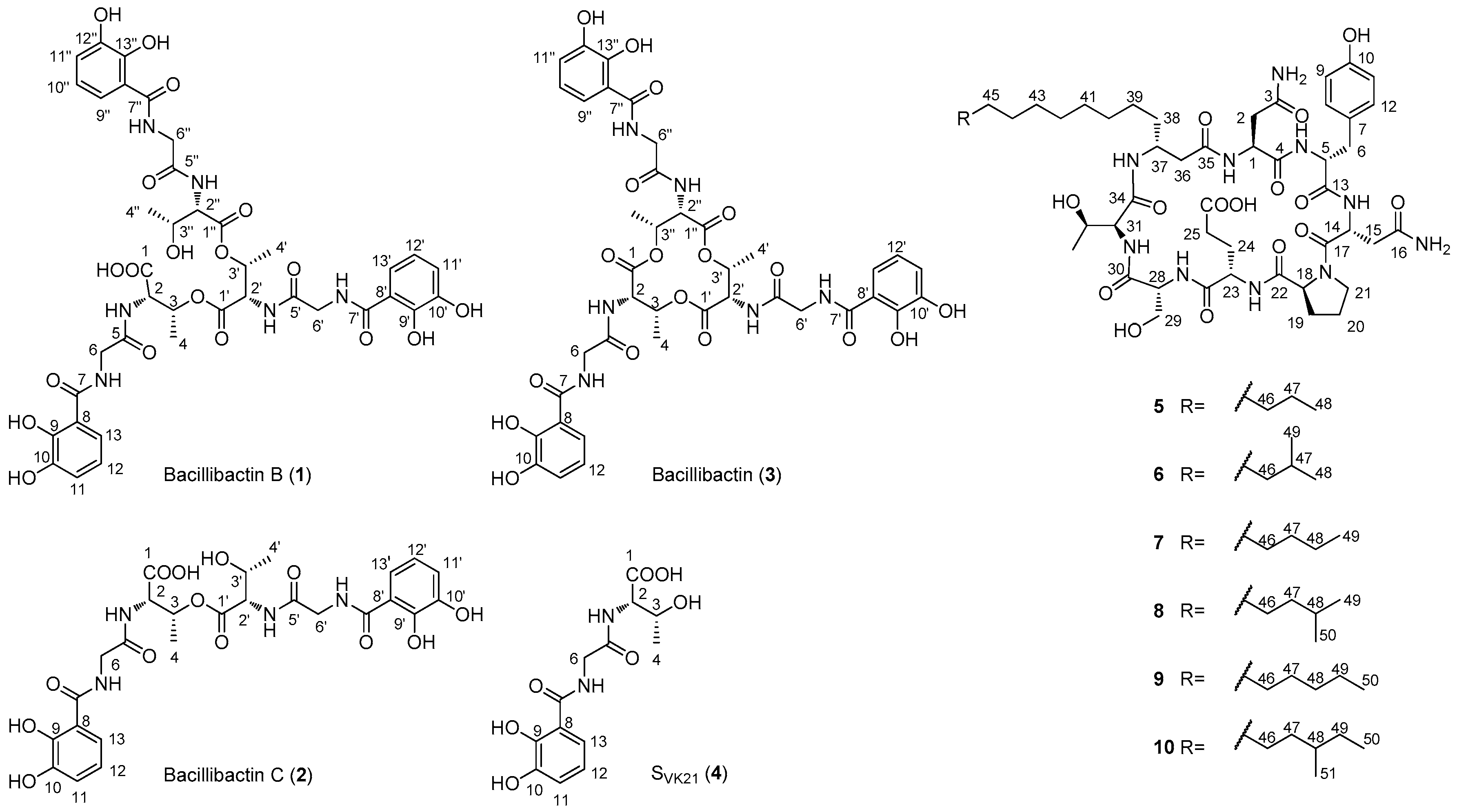
2.2. Structural Elucidation of Compounds 1–10
2.3. Cytotoxicity Assays against Human Cancer Cell Lines
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Marine Bacterial Strains Isolation
3.3. PCR Screening for Potential Producers of Nonribosomal Peptides
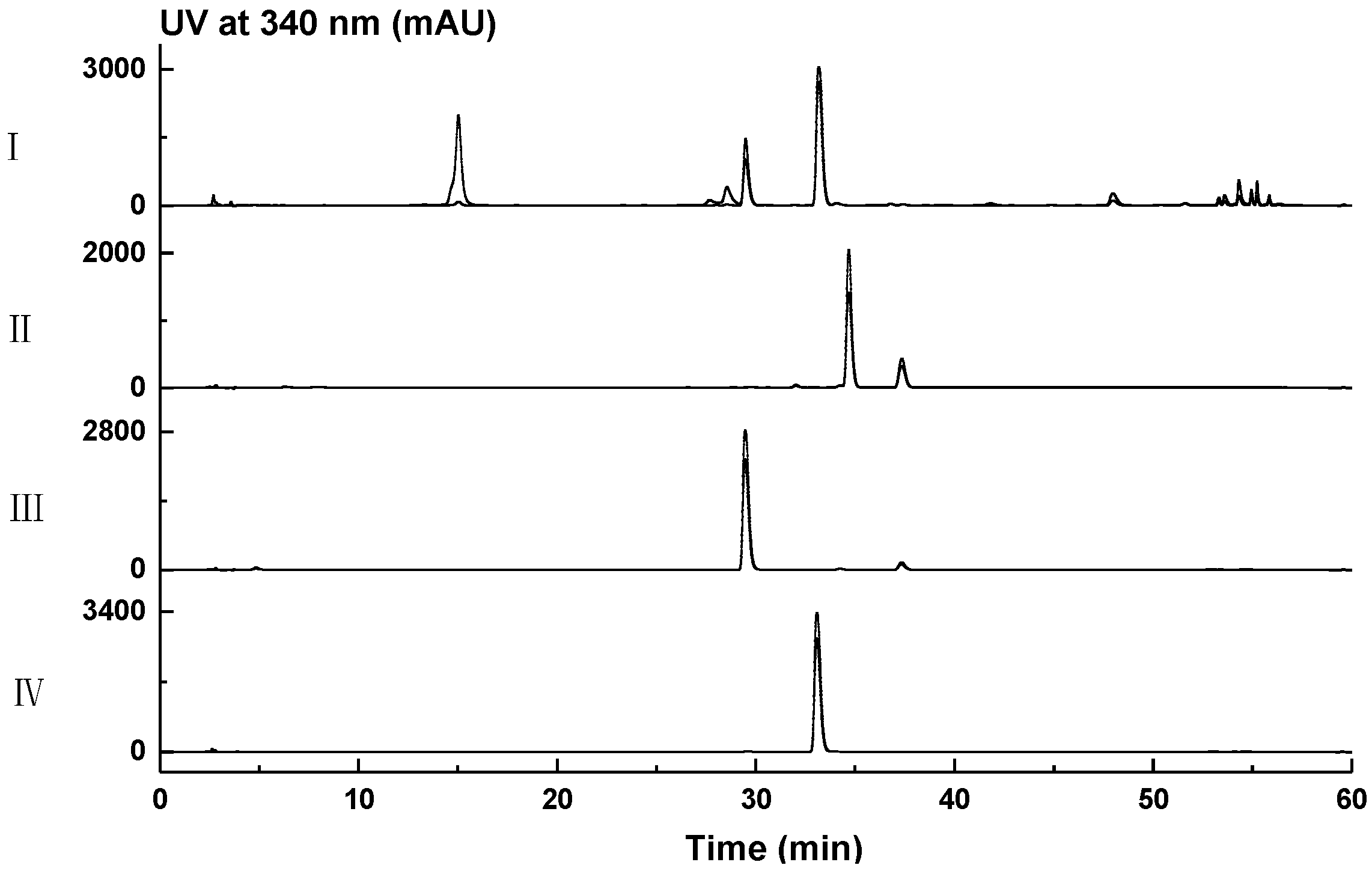
3.4. Small-Scale Fermentation, HPLC
3.5. Phylogenetic Analysis
3.6. Large-Scale Fermentation and Isolation
3.7. Marfey’s Analysis for Compounds 2 and 5
3.8. Cytotoxicity Assays for Compounds 1–10
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Johnson, B.A.; Anker, H.; Meleney, F.L. Bacitracin: A new antibiotic produced by a member of the B. subtilis group. Science 1945, 102, 376–377. [Google Scholar] [CrossRef] [PubMed]
- Danders, W.; Marahiel, M.A.; Krause, M.; Kosui, N.; Kato, T.; Izumiya, N.; Kleinkauf, H. Antibacterial action of gramicidin S and tyrocidines in relation to active transport, in vitro transcription, and spore outgrowth. Antimicrob. Agents Chemother. 1982, 22, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.W.; Hung, Y.J.; Yang, C.H.; Chen, Y.C. The antimicrobial activity of gramicidin A is associated with hydroxyl radical formation. PLoS ONE 2015, 10, e0117065. [Google Scholar] [CrossRef] [PubMed]
- Felnagle, E.A.; Jackson, E.E.; Chan, Y.A.; Podevels, A.M.; Berti, A.D.; McMahon, M.D.; Thomas, M.G. Nonribosomal peptide synthetases involved in the production of medically relevant natural products. Mol. Pharm. 2008, 5, 191–211. [Google Scholar] [CrossRef] [PubMed]
- Mootz, H.D.; Marahiel, M.A. The tyrocidine biosynthesis operon of Bacillus brevis: Complete nucleotide sequence and biochemical characterization of functional internal adenylation domains. J. Bacteriol. 1997, 179, 6843–6850. [Google Scholar] [CrossRef] [PubMed]
- Linne, U.; Stein, D.B.; Mootz, H.D.; Marahiel, M.A. Systematic and quantitative analysis of protein-protein recognition between nonribosomal peptide synthetases investigated in the tyrocidine biosynthetic template. Biochemistry 2003, 42, 5114–5124. [Google Scholar] [CrossRef] [PubMed]
- Krätzschmar, J.; Krause, M.; Marahiel, M.A. Gramicidin S biosynthesis operon containing the structural genes grsA and grsB has an open reading frame encoding a protein homologous to fatty acid thioesterases. J. Bacteriol. 1989, 171, 5422–5429. [Google Scholar] [CrossRef] [PubMed]
- Conti, E.; Stachelhaus, T.; Marahiel, M.A.; Brick, P. Structural basis for the activation of phenylalanine in the non-ribosomal biosynthesis of gramicidin S. EMBO J. 1997, 16, 4174–4183. [Google Scholar] [CrossRef] [PubMed]
- May, J.J.; Wendrich, T.M.; Marahiel, M.A. The dhb operon of Bacillus subtilis encodes the biosynthetic template for the catecholic siderophore 2,3-dihydroxybenzoate-glycine-threonine trimeric ester bacillibactin. J. Biol. Chem. 2001, 276, 7209–7217. [Google Scholar] [CrossRef] [PubMed]
- Segond, D.; Abi Khalil, E.; Buisson, C.; Daou, N.; Kallassy, M.; Lereclus, D.; Arosio, P.; Bou-Abdallah, F.; Nielsen Le Roux, C. Iron acquisition in Bacillus cereus: The roles of IlsA and bacillibactin in exogenous ferritin iron mobilization. PLoS Pathog. 2014, 10, e1003935. [Google Scholar] [CrossRef] [PubMed]
- Inès, M.; Dhouha, G. Lipopeptide surfactants: Production, recovery and pore forming capacity. Peptides 2015, 71, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Roongsawang, N.; Washio, K.; Morikawa, M. Diversity of nonribosomal peptide synthetases ivolved in the biosynthesis of lipopeptide biosurfactants. Int. J. Mol. Sci. 2011, 12, 141–172. [Google Scholar] [CrossRef] [PubMed]
- Mondol, M.A.M.; Shin, H.J.; Islam, M.T. Diversity of secondary metabolites from marine Bacillus species: Chemistry and biological activity. Mar. Drugs 2013, 11, 2846–2872. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, Z.; Miao, X.; Zhang, F. The screening of antimicrobial bacteria with diverse novel nonribosomal peptide synthetase (NRPS) genes from South China Sea sponges. Mar. Biotechnol. 2009, 11, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Toledo, G.; Green, W.; Gonzalez, R.A.; Christoffersen, L.; Podar, M.; Chang, H.W.; Hemscheidt, T.; Trapido-Rosenthal, H.G.; Short, J.M.; Bidigare, R.R.; et al. High throughput cultivation for isolation of novel marine microorganisms. Oceanography 2006, 19, 120–125. [Google Scholar] [CrossRef]
- Margassery, L.M.; Kennedy, J.; O′Gara, F.; Dobson, A.D.; Morrissey, J.P. Diversity and antibacterial activity of bacteria isolated from the coastal marine sponges Amphilectus fucorum and Eurypon major. Lett. Appl. Microbiol. 2012, 55, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Tareq, F.S.; Lee, M.A.; Lee, H.S.; Lee, Y.J.; Lee, J.S.; Hasan, C.M.; Islam, M.T.; Shin, H.J. Non-cytotoxic antifungal agents: Isolation and structures of gageopeptides A-D from a Bacillus strain 109GGC020. J. Agric. Food Chem. 2014, 62, 5565–5572. [Google Scholar] [CrossRef] [PubMed]
- Barsby, T.; Kelly, M.T.; Gagné, S.M.; Andersen, R.J. Bogorol A produced in culture by a marine Bacillus sp. reveals a novel template for cationic peptide antibiotics. Org. Lett. 2001, 3, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Pettit, G.R.; Knight, J.C.; Herald, D.L.; Pettit, R.K.; Hogan, F.; Mukku, V.J.R.V.; Hamblin, J.S.; Dodson, M.J., II; Chapuis, J.C. Isolation and structure elucidation of bacillistatins 1 and 2 from a marine Bacillus silvestris. J. Nat. Prod. 2009, 72, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Lautru, S.; Deeth, R.J.; Bailey, L.M.; Challis, G.L. Discovery of a new peptide natural product by Streptomyces coelicolor genome mining. Nat. Chem. Biol. 2005, 1, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Gross, H.; Stockwell, V.O.; Henkels, M.D.; Nowak-Thompson, B.; Loper, J.E.; Gerwick, W.H. The genomisotopic approach: A systematic method to isolate products of orphan biosynthetic gene clusters. Chem. Biol. 2007, 14, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, M.A.; Wang, W.; Roux, C.M.; Beasley, F.C.; Heinrichs, D.E.; Dunman, P.M.; Magarvey, N.A. Staphylococcus aureus nonribosomal peptide secondary metabolites regulate virulence. Science 2010, 329, 294–296. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Ma, M.; Rateb, M.E.; Shaaban, K.A.; Yu, Z.; Huang, S.X.; Zhao, L.X.; Zhu, X.; Yan, Y.; Peterson, R.M.; et al. Biosynthetic potential-based strain prioritization for natural product discovery: A showcase for diterpenoid-producing actinomycetes. J. Nat. Prod. 2014, 77, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Ayuso-Sacido, A.; Genilloud, O. New PCR primers for the screening of NRPS and PKS-I systems in actinomycetes: Detection and distribution of these biosynthetic gene sequences in major taxonomic groups. Microb. Ecol. 2005, 49, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Sun, W.; Chen, M.; Dai, S.; Zhang, L.; Liu, Y.; Lee, K.J.; Li, X. Diversity of culturable actinobacteria isolated from marine sponge Haliclona sp. Antonie Van Leeuwenhoek 2007, 92, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Abdelmohsen, U.R.; Yang, C.; Horn, H.; Hajjar, D.; Ravasi, T.; Hentschel, U. Actinomycetes from Red Sea sponges: Sources for chemical and phylogenetic diversity. Mar. Drugs 2014, 12, 2771–2789. [Google Scholar] [CrossRef] [PubMed]
- Gontang, E.A.; Gaudêncio, S.P.; Fenical, W.; Jensen, P.R. Sequence-based analysis of secondary-metabolite biosynthesis in marine actinobacteria. Appl. Environ. Microbiol. 2010, 76, 2487–2499. [Google Scholar] [CrossRef] [PubMed]
- Tambadou, F.; Lanneluc, I.; Sablé, S.; Klein, G.L.; Doghri, I.; Sopéna, V.; Didelot, S.; Barthélémy, C.; Thiéry, V.; Chevrot, R. Novel nonribosomal peptide synthetase (NRPS) genes sequenced from intertidal mudflat bacteria. FEMS Microbiol. Lett. 2014, 357, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Oleinikova, G.K.; Kuznetsova, T.A.; Huth, F.; Laatsch, H.; Isakov, V.V.; Shevchenko, L.S.; Elyakov, G.B. Cyclic lipopetides with fungicidal activity from the sea isolate of the bacterium Bacillus subtilis. Russ. Chem. Bull. 2001, 50, 2231–2235. [Google Scholar] [CrossRef]
- Budzikiewicz, H.; Bössenkamp, A.; Taraz, K.; Pandey, A.; Meyer, J.M. Corynebactin, a cyclic catecholate siderophore from Corynebacterium glutamicum ATCC 14067 (Brevibacterium sp. DSM 20411). Z. Naturforschung 1997, 52, 551–554. [Google Scholar] [CrossRef]
- Li, J.; Liu, S.; Jiang, Z.; Sun, C. Catechol amide iron chelators produced by a mangrove-derived Bacillus subtilis. Tetrahedron 2017, 73, 5245–5252. [Google Scholar] [CrossRef]
- Marfey, P. Determination of D-amino acids. II. Use of a bifunctional reagent, 1,5-difluoro-2,4-dinitroenzene. Carlsberg Res. Commun. 1984, 49, 591–596. [Google Scholar] [CrossRef]
- Temirov, Y.V.; Esikova, T.Z.; Kashparov, I.A.; Balashova, T.A.; Vinokurov, L.M.; Alakhov, Y.B. A catecholic siderophore produced by the thermoresistant Bacillus licheniformis VK21 Strain. Russ. J. Bioorg. Chem. 2003, 29, 542–549. [Google Scholar] [CrossRef]
- Dertz, E.A.; Xu, J.; Stintzi, A.; Raymond, K.N. Bacillibactin-mediated iron transport in Bacillus subtilis. J. Am. Chem. Soc. 2006, 128, 22–23. [Google Scholar] [CrossRef] [PubMed]
- May, J.J.; Kessler, N.; Marahiel, M.A.; Stubbs, M.T. Crystal structure of DhbE, an archetype for aryl acid activating domains of modular nonribosomal peptide synthetases. Proc. Natl. Acad. Sci. USA 2002, 99, 12120–12125. [Google Scholar] [CrossRef] [PubMed]
- Raza, W.; Wu, H.; Shah, M.A.A.; Shen, Q. A catechol type siderophore, bacillibactin: Biosynthesis, regulation and transport in Bacillus subtilis. J. Basic Microbiol. 2008, 48, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Oleinikova, G.K.; Dmitrenok, A.S.; Voinov, V.G.; Chaikina, E.L.; Shevchenko, L.S.; Kuznetsova, T.A. Bacillomycin D from the Marine Isolate of Bacillus subtilis KMM 1922. Chem. Nat. Compd. 2005, 41, 461–464. [Google Scholar] [CrossRef]
- Tanaka, K.; Ishihara, A.; Nakajima, H. Isolation of anteiso-C17, iso-C17, iso-C16, and iso-C15 bacillomycin D from Bacillus amyloliquefaciens SD-32 and their antifungal activities against plant pathogens. J. Agric. Food Chem. 2014, 62, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Birgit, K.; Andrea, O.; Birgit, H.; Peter, B.; Jewgenij, M.; Winfried, E. Peptides, Their Production and Use and Producing Microorganism. Patent PCT/ DE1964/1213 A1, 16 April 1998. [Google Scholar]
- Kim, J.D.; Jeon, B.J.; Han, J.W.; Park, M.Y.; Kang, S.A.; Kim, B.S. Evaluation of the endophytic nature of Bacillus amyloliquefaciens strain GYL4 and its efficacy in the control of anthracnose. Pest Manag. Sci. 2016, 72, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Li, B.; Zhang, N.; Waseem, R.; Shen, Q.; Huang, Q. Production of bacillomycin- and macrolactin-type antibiotics by Bacillus amyloliquefaciens NJN-6 for suppressing soilborne plant pathogens. J. Agric. Food Chem. 2012, 60, 2976–2981. [Google Scholar] [CrossRef] [PubMed]
- Jemil, N.; Manresa, A.; Rabanal, F.; Ben Ayed, H.; Hmidet, N.; Nasri, M. Structural characterization and identification of cyclic lipopeptides produced by Bacillus methylotrophicus DCS1 strain. J. Chromatogr. B 2017, 1060, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Quan, C.; Jin, L.; Wang, L.; Guo, X.; Fan, S. Sequence characterization and computational analysis of the non-ribosomal peptide synthetases controlling biosynthesis of lipopeptides, fengycins and bacillomycin D, from Bacillus amyloliquefaciens Q-426. Biotechnol. Lett. 2013, 35, 2155–2163. [Google Scholar] [CrossRef] [PubMed]
- Tabbene, O.; Azaiez, S.; Grazia, A.D.; Karkouch, I.; Slimene, I.B.; Elkahoui, S.; Alfeddy, M.N.; Casciaro, B.; Luca, V.; Limam, F.; et al. Bacillomycin D and its combination with amphotericin B: Promising antifungal compounds with powerful antibiofilm activity and wound-healing potency. J. Appl. Microbiol. 2016, 120, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Moyne, A.L.; Shelby, R.; Cleveland, T.E.; Tuzun, S. Bacillomycin D: An iturin with antifungal activity against Aspergillus flavus. J. Appl. Microbiol. 2001, 90, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Hajare, S.N.; Subramanian, M.; Gautam, S.; Sharma, A. Induction of apoptosis in human cancer cells by a Bacillus lipopeptide bacillomycin D. Biochimie 2013, 95, 1722–1731. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.; Ruan, J.; Huang, Y. Diversity and biosynthetic potential of culturable actinomycetes associated with marine sponges in the China Seas. Int. J. Mol. Sci. 2012, 13, 5917–5932. [Google Scholar] [CrossRef] [PubMed]
- Kieser, T.; Bibb, M.J.; Buttner, M.J.; Chater, K.F.; Hopwood, D.A. Practical Streptomyces Genetics; The John Innes Foundation: Norwich, UK, 2000. [Google Scholar]
- Heuer, H.; Krsek, M.; Baker, P.; Smalla, K.; Wellington, E.M.H. Analysis of actinomycete communities by specific amplification of genes encoding 16S rRNA and gel-electrophoretic separation in denaturing gradients. Appl. Environ. Microbiol. 1997, 63, 3233–3241. [Google Scholar] [PubMed]
- Daletos, G.; Kalscheuer, R.; Koliwer-Brandl, H.; Hartmann, R.; de Voogd, N.J.; Wray, V.; Lin, W.; Proksch, P. Callyaerins from the marine sponge Callyspongia aerizusa: Cyclic peptides with antitubercular activity. J. Nat. Prod. 2015, 78, 1910–1925. [Google Scholar] [CrossRef] [PubMed]

| Position | δH (J in Hz) | δC, Type | Position | δH (J in Hz) | δC, Type | Position | δH (J in Hz) | δC, Type |
|---|---|---|---|---|---|---|---|---|
| 1 | 170.9, C | 1′ | 168.0, C | 1′′ | 169.3 b, C | |||
| 2 | 4.56, br d (8.0) | 55.1, CH | 2′ | 4.79, br d (8.0) | 54.8, CH | 2′′ | 4.38, br d (7.9) | 57.4, CH |
| 3 | 5.33, br s | 70.7, CH | 3′ | 5.38, br s | 70.7, CH | 3′′ | 4.19, br s | 66.3, CH |
| 4 | 1.09, d (6.0) | 16.4 a, CH3 | 4′ | 1.16, d (6.1) | 16.3 a, CH3 | 4′′ | 1.06, d (5.9) | 20.2, CH3 |
| 5 | 168.9 b, C | 5′ | 169.1 b, C | 5′′ | 169.2 b, C | |||
| 6 | 4.09, m | 41.8 c, CH2 | 6′ | 4.09, m | 41.9 c, CH2 | 6′′ | 4.03, m | 42.2 c, CH2 |
| 7 | 169.49 b, C | 7′ | 169.52 b, C | 7′′ | 169.7 b, C | |||
| 8 | 115.33 d, C | 8′ | 115.2 d, C | 8′′ | 115.26 d, C | |||
| 9 | 149.05 e, C | 9′ | 149.14 e, C | 9′′ | 149.2 e, C | |||
| 10 | 146.1, C | 10′ | 146.1, C | 10′′ | 146.1, C | |||
| 11 | 6.93, m | 118.8, CH | 11′ | 6.93, m | 118.8, CH | 11′′ | 6.93, m | 118.8, CH |
| 12 | 6.69, m | 118.1, CH | 12′ | 6.69, m | 118.1, CH | 12′′ | 6.69, m | 118.1, CH |
| 13 | 7.31, m | 117.7 f, CH | 13′ | 7.31, m | 117.6 f, CH | 13′′ | 7.31, m | 117.6 f, CH |
| 2-NH | 8.33, br s | 2′-NH | 8.47, br s | 2′′-NH | 8.14, br s | |||
| 6-NH | 9.04, d (5.1) | 6′-NH | 9.04, d (5.1) | 6′′-NH | 9.13, m | |||
| 9-OH | 12.31, br s | 9′-OH | 12.31, br s | 9′′-OH | 12.31, br s | |||
| 10-OH | 9.23, br s | 10′-OH | 9.23, br s | 10′′-OH | 9.23, br s |
| Position | δH (J in Hz) | δC, Type | Position | δH (J in Hz) | δC, Type |
|---|---|---|---|---|---|
| 1 | 170.7, C | 1′ | 169.4 a, C | ||
| 2 | 4.61, dd (2.5, 8.8) | 55.0, CH | 2′ | 4.39, dd (2.5, 8.5) | 57.4, CH |
| 3 | 5.33, m | 70.8, CH | 3′ | 4.21, m | 66.4, CH |
| 4 | 1.15, d (6.4) | 16.5, CH3 | 4′ | 1.05, d (6.2) | 20.1, CH3 |
| 5 | 168.9 a, C | 5′ | 169.0 a, C | ||
| 6 | 4.06, m | 41.9 b, CH2 | 6′ | 4.06, m | 42.1 b, CH2 |
| 7 | 169.5 a, C | 7′ | 169.6 a, C | ||
| 8 | 115.3, C | 8′ | 115.3, C | ||
| 9 | 149.2, C | 9′ | 149.2, C | ||
| 10 | 146.1, C | 10′ | 146.1, C | ||
| 11 | 6.93, d (7.6) | 118.8, CH | 11′ | 6.93, d (7.6) | 118.8, CH |
| 12 | 6.70, t (7.6) | 118.1, CH | 12′ | 6.70, t (7.6) | 118.1, CH |
| 13 | 7.32, d (7.6) | 117.7, CH | 13′ | 7.32, d (7.6) | 117.7, CH |
| 2-NH | 8.36, d (8.8) | 2′-NH | 8.06, d (8.5) | ||
| 6-NH | 9.06, br s | 6′-NH | 9.06, br s | ||
| 9-OH | 12.36, br s | 9′-OH | 12.36, br s | ||
| 10-OH | 9.27, br s | 10′-OH | 9.27, br s |
| Cytotoxicity (IC50 Values in µM) | |||||
|---|---|---|---|---|---|
| Cell Lines | 7 | 8 | 9 | 10 | Taxol |
| HepG2 | 8.2 ± 0.2 | 5.1 ± 0.2 | 4.9 ± 0.2 | >10 | 0.075 ± 0.004 |
| MCF7 | 4.2 ± 0.1 | 2.9 ± 0.1 | 3.3 ± 0.1 | 7.2 ± 0.2 | 0.043 ± 0.003 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, M.; Liu, F.; Yang, X.; Jin, J.; Dong, X.; Zeng, K.-W.; Liu, D.; Zhang, Y.; Ma, M.; Yang, D. Bacillibactin and Bacillomycin Analogues with Cytotoxicities against Human Cancer Cell Lines from Marine Bacillus sp. PKU-MA00093 and PKU-MA00092. Mar. Drugs 2018, 16, 22. https://doi.org/10.3390/md16010022
Zhou M, Liu F, Yang X, Jin J, Dong X, Zeng K-W, Liu D, Zhang Y, Ma M, Yang D. Bacillibactin and Bacillomycin Analogues with Cytotoxicities against Human Cancer Cell Lines from Marine Bacillus sp. PKU-MA00093 and PKU-MA00092. Marine Drugs. 2018; 16(1):22. https://doi.org/10.3390/md16010022
Chicago/Turabian StyleZhou, Mengjie, Fawang Liu, Xiaoyan Yang, Jing Jin, Xin Dong, Ke-Wu Zeng, Dong Liu, Yingtao Zhang, Ming Ma, and Donghui Yang. 2018. "Bacillibactin and Bacillomycin Analogues with Cytotoxicities against Human Cancer Cell Lines from Marine Bacillus sp. PKU-MA00093 and PKU-MA00092" Marine Drugs 16, no. 1: 22. https://doi.org/10.3390/md16010022
APA StyleZhou, M., Liu, F., Yang, X., Jin, J., Dong, X., Zeng, K.-W., Liu, D., Zhang, Y., Ma, M., & Yang, D. (2018). Bacillibactin and Bacillomycin Analogues with Cytotoxicities against Human Cancer Cell Lines from Marine Bacillus sp. PKU-MA00093 and PKU-MA00092. Marine Drugs, 16(1), 22. https://doi.org/10.3390/md16010022