Formation of Silver Nanoparticles Using Fluorescence Properties of Chitosan Oligomers
Abstract
:1. Introduction
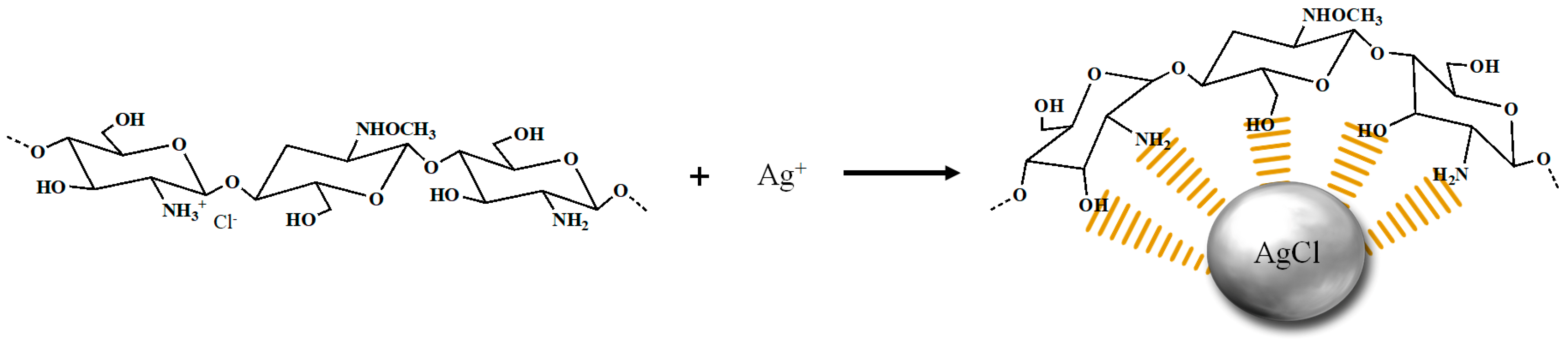
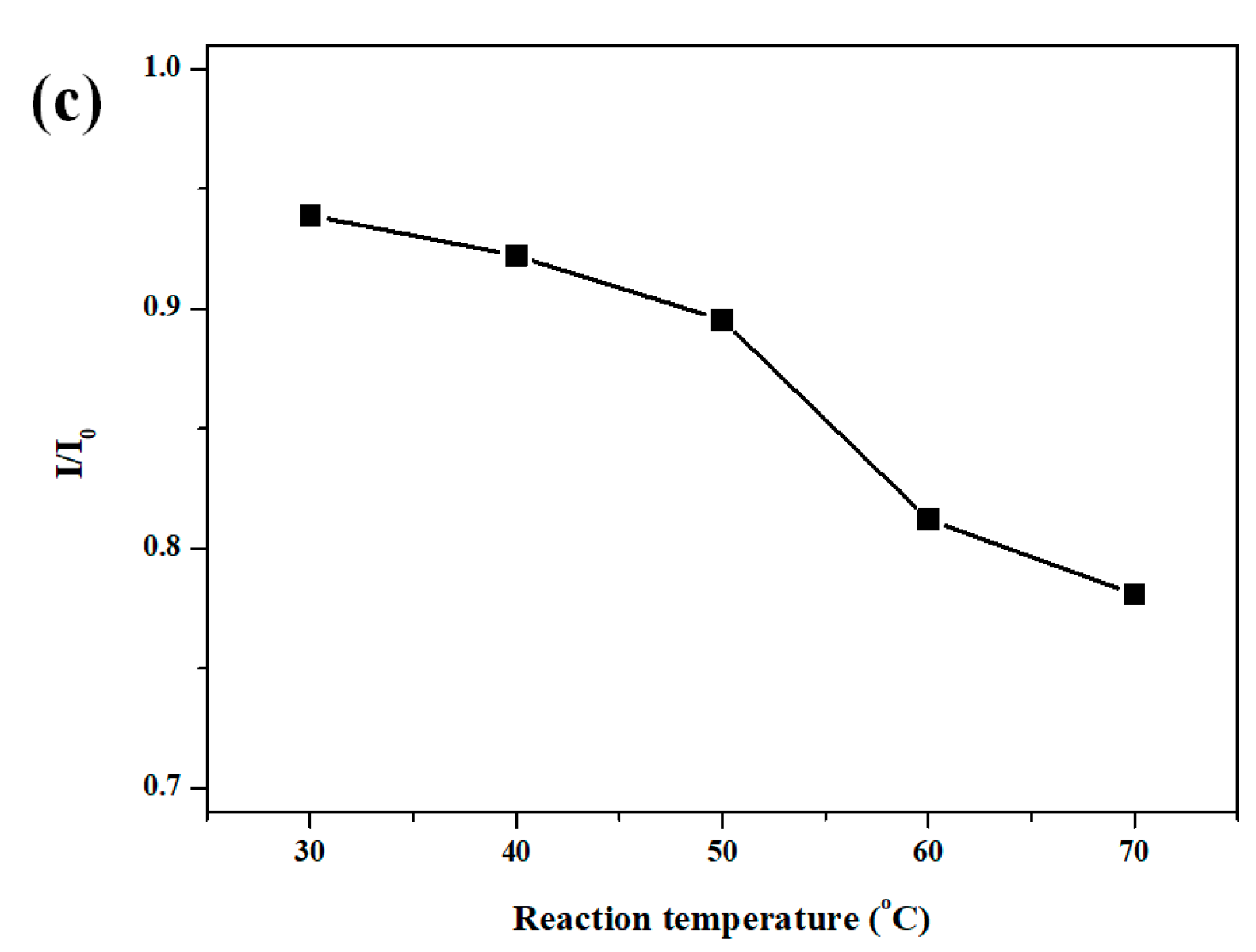
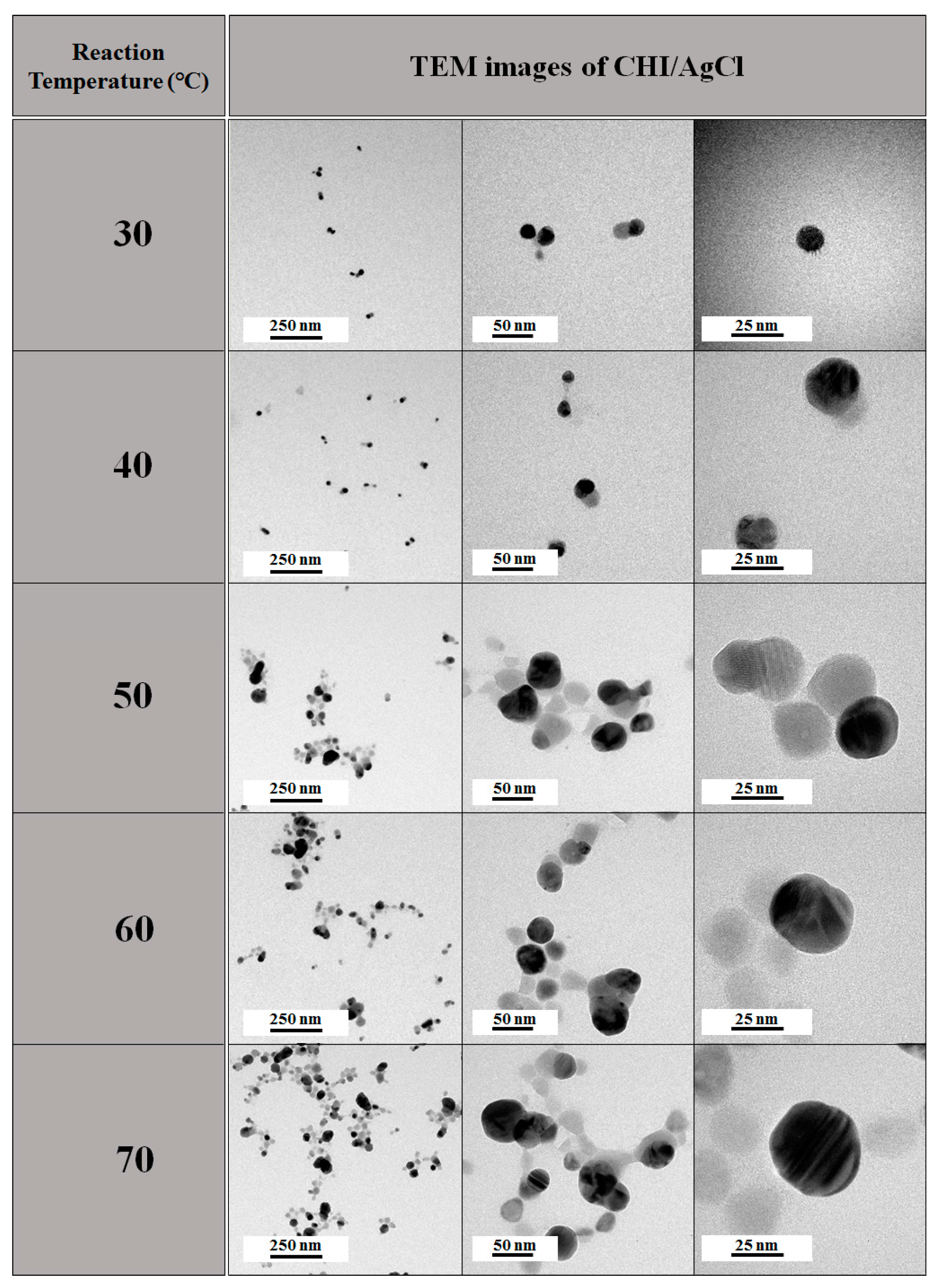
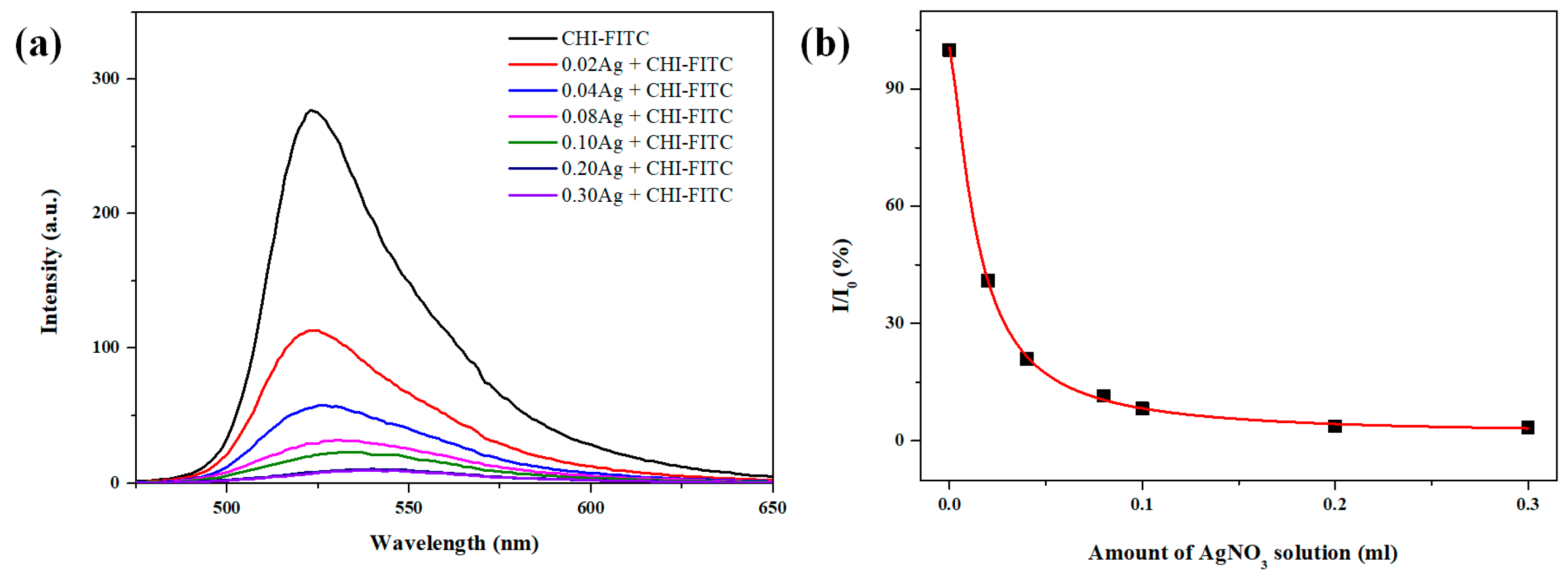
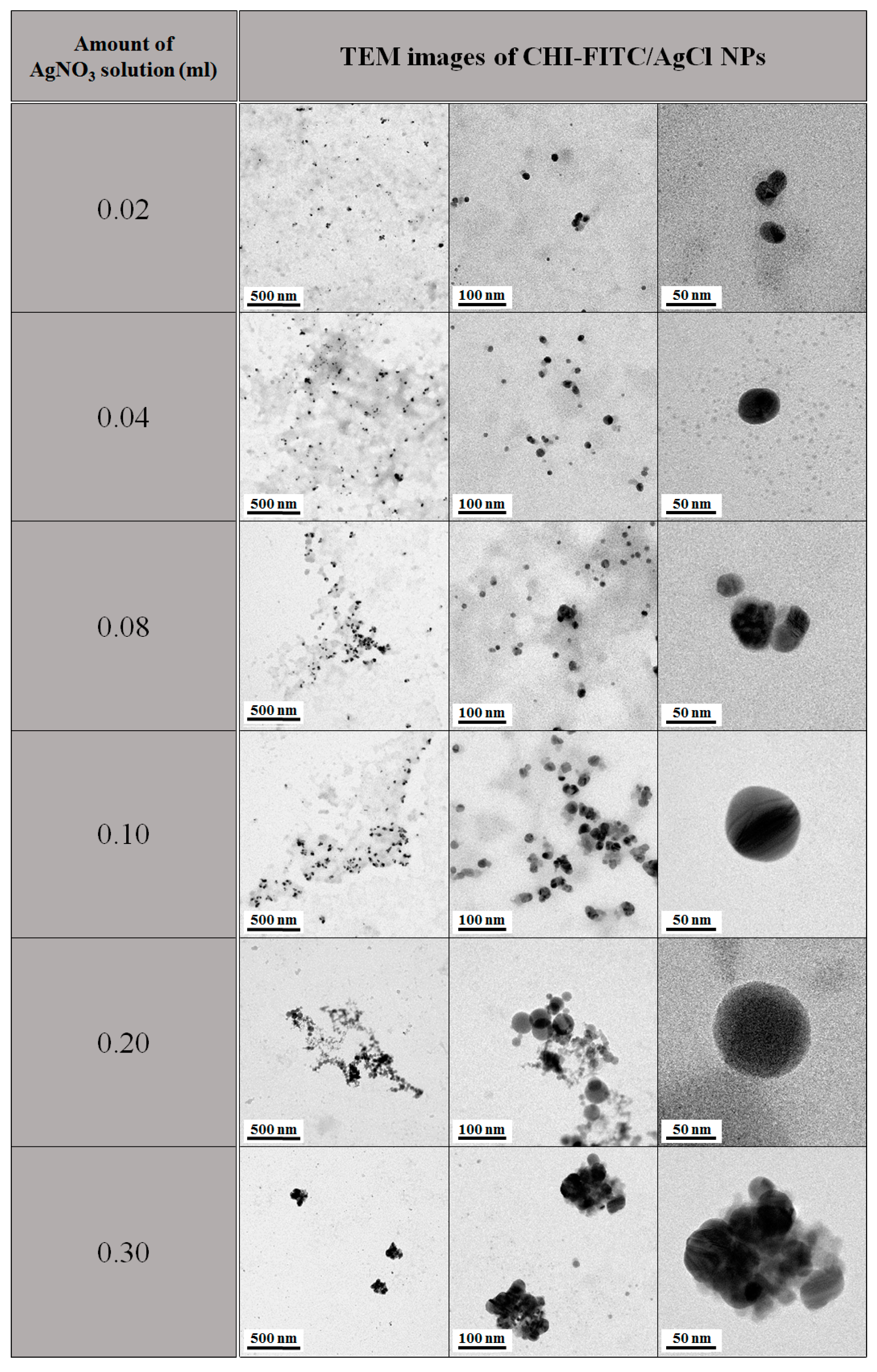
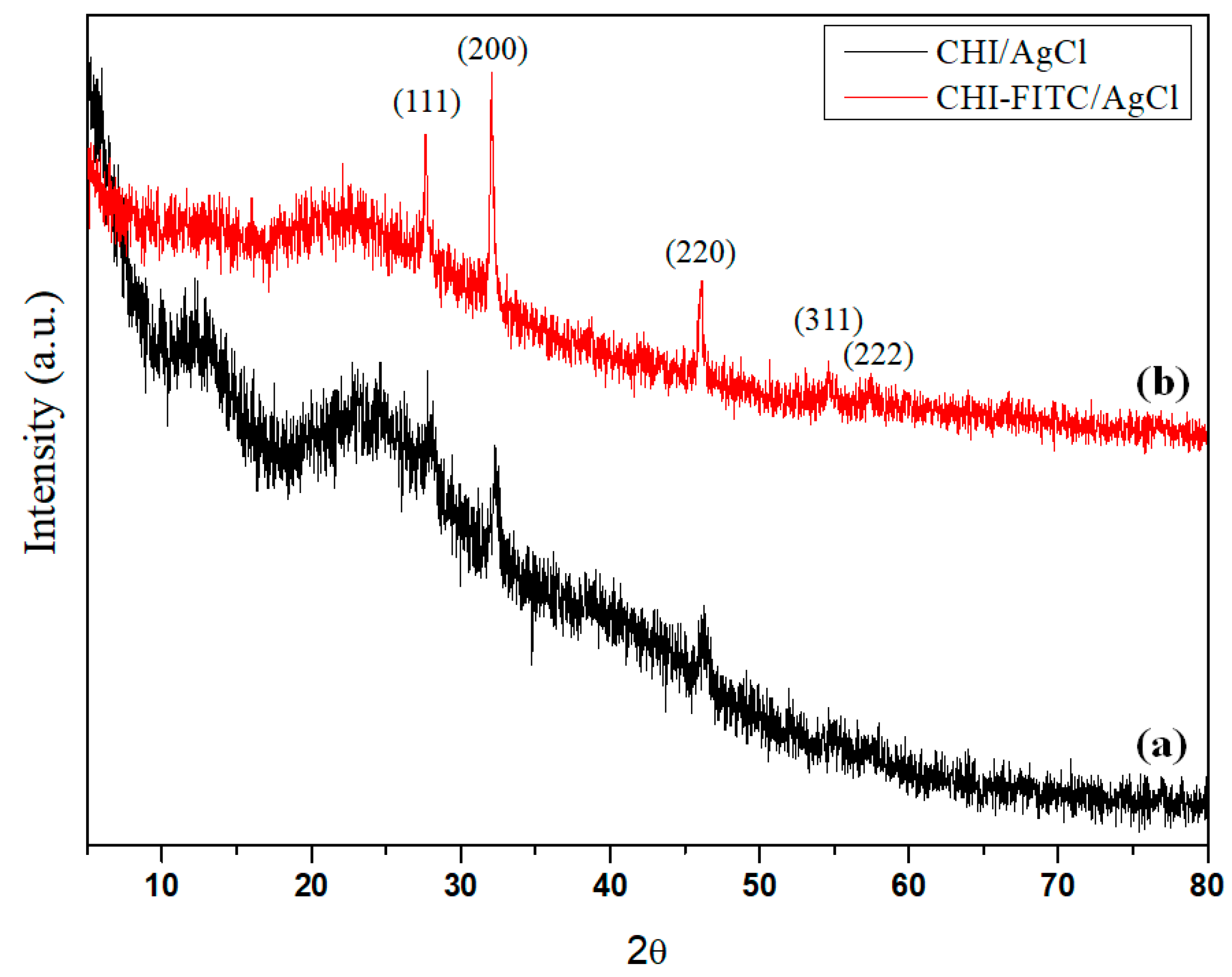
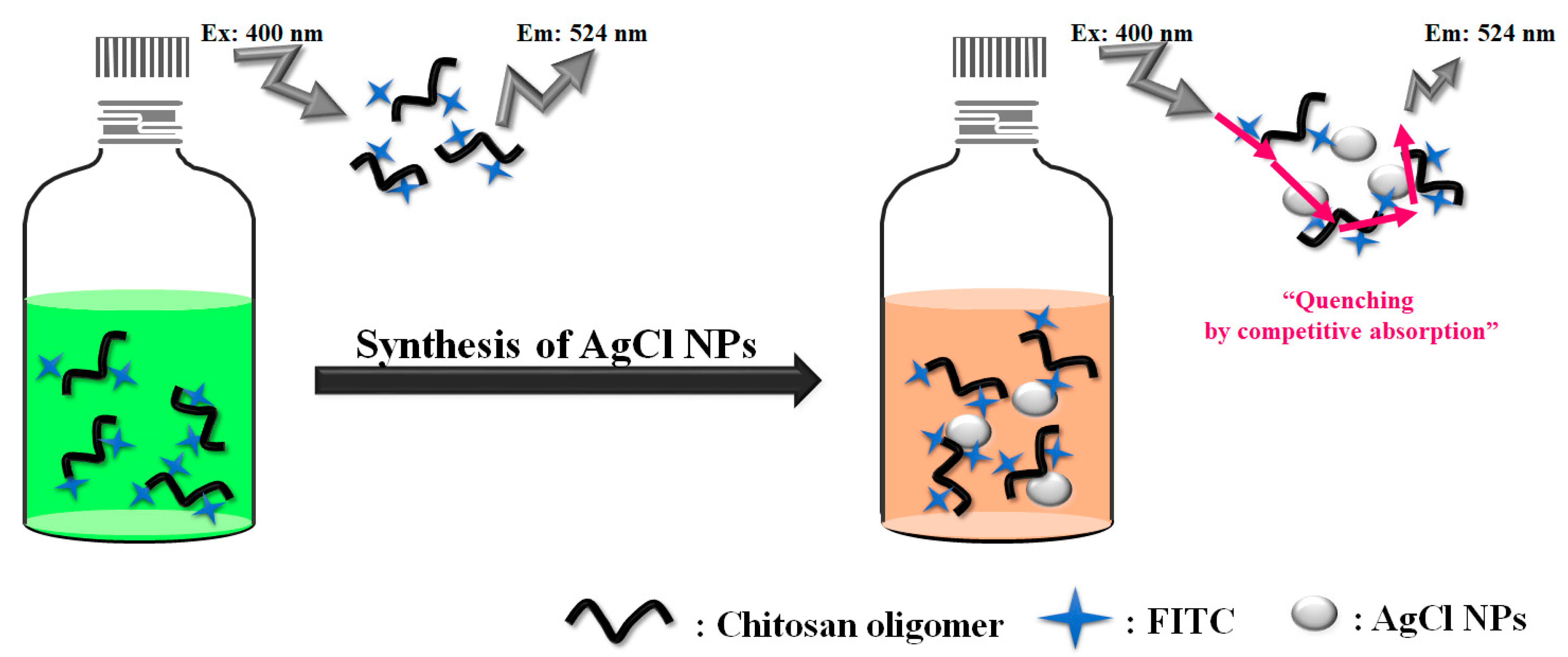
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Preparation of CHI/AgCl NPs and CHI-FITC/AgCl NPs Complexes
3.3. Characterizations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Barikani, M.; Oliaei, E.; Seddiqi, H.; Honarkar, H. Preparation and application of chitin and its derivatives: A review. Iran. Polym. J. 2014, 23, 307–326. [Google Scholar] [CrossRef]
- Honarkar, H.; Barikani, M. Applications of biopolymers I: Chitosan. Monatshefte Chem. 2009, 140, 1403–1420. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Ahmed, S.; Ikram, S. Chitosan based scaffolds and their application in wound healing. Achiev. Life Sci. 2016, 10, 27–37. [Google Scholar] [CrossRef]
- Agrawal, P.; Strijkers, G.J.; Nicolay, K. Chitosan-based systems for molecular imaging. Adv. Drug Deliv. Rev. 2010, 62, 42–58. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.N.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Pan, X.; Ren, W.; Gu, L.; Wang, G.; Liu, Y. Photoluminescence from chitosan for bio-imaging. Aust. J. Chem. 2014, 67, 1422–1426. [Google Scholar] [CrossRef]
- Li, P.; Poon, Y.F.; Li, W.; Zhu, H.; Yeap, S.H.; Cao, Y.; Qi, X.; Zhou, C.; Lamrani, M.; Beuerman, R.W.; et al. A polycationic antimicrobial and biocompatible hydrogel with microbe membrane suctioning ability. Nat. Mater. 2010, 10, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Rabea, E.I.; Badawy, E.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.O.; Lee, T.S.; Park, W.H. Green synthesis and antimicrobial activity of silver chloride nanoparticles stabilized with chitosan oligomer. J. Mater. Sci.-Mater. Med. 2014, 25, 2629–2638. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Z.; Shen, W.; Gurunathan, S. Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef] [PubMed]
- Husein, M.M.; Rodil, E.; Vera, J.H. A novel method for the preparation of silver chloride nanoparticles starting from their solid powder using microemulsions. J. Colloid Interface Sci. 2005, 288, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, S.; Yu, H.; Yu, J. In situ anion-exchange synthesis and photocatalytic activity of Ag8W4O16/AgCl-nanoparticle core–shell nanorods. J. Mol. Catal. A-Chem. 2011, 344, 52–59. [Google Scholar] [CrossRef]
- Zhou, Z.; Long, M.; Cai, W. Synthesis and photocatalytic performance of the efficient visible light photocatalyst Ag–AgCl/BiVO4. J. Mol. Catal. A-Chem. 2012, 353, 22–28. [Google Scholar] [CrossRef]
- Dong, L.; Liang, D.; Gong, R. In situ photoactivated AgCl/Ag nanocomposites with enhanced visible light photocatalytic and antibacterial activity. Eur. J. Inorg. Chem. 2012, 2012, 3200–3208. [Google Scholar] [CrossRef]
- Li, L.; Zhu, Y. High chemical reactivity of silver nanoparticles toward hydrochloric acid. J. Colloid Interface Sci. 2006, 303, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Shin, K.; Jang, J. Plasmonic photocatalytic system using silver chloride/silver nanostructures under visible light. J. Colloid Interface Sci. 2010, 341, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Tuncer, M.; Seker, E. Single step sol-gel made silver chloride on titania xerogels to inhibit E. coli bacteria growth: Effect of preparation and chloride ion on bactericidal activity. J. Sol-Gel Sci. Technol. 2011, 59, 304–310. [Google Scholar] [CrossRef]
- Lee, H.M.; Kim, M.H.; Yoon, Y.I.; Park, W.H. Fluorescent property of chitosan oligomer and its application as a metal ion sensor. Mar. Drugs 2017, 15, 105. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, V.; Priyadarshini, S.; Priyadharsshini, N.M.; Pandianb, K.; Velusamy, P. Biogenic synthesis of antibacterial silver chloride nanoparticles using leaf extracts of Cissus quadrangularis Linn. Mater. Lett. 2013, 91, 224–227. [Google Scholar] [CrossRef]
- Cheon, J.Y.; Kang, Y.O.; Park, W.H. Formation of Ag nanoparticles in PVA solution and catalytic activity of their electrospun PVA nanofibers. Fibers Polym. 2015, 16, 840–849. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: Berlin, Germany, 2007; pp. 277–287. ISBN 978-0387312781. [Google Scholar]
- Kim, T.H.; Choi, M.S.; Kwak, C.K.; Lee, J.H.; Lee, T.S. Fluorescent Conjugated Polymers as Integrated Sensor Materials. Polym. Sci. Technol. 2007, 18, 319–325. [Google Scholar]
- Green, N.J.; Pimblott, S.M.; Tachiya, M. Generalizations of the Stern–Volmer relation. J. Phys. Chem. 1993, 97, 196–202. [Google Scholar] [CrossRef]
- Htun, T. A negative deviation from Stern–Volmer equation in fluorescence quenching. J. Fluoresc. 2004, 14, 217–222. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheon, J.Y.; Lee, H.M.; Park, W.H. Formation of Silver Nanoparticles Using Fluorescence Properties of Chitosan Oligomers. Mar. Drugs 2018, 16, 11. https://doi.org/10.3390/md16010011
Cheon JY, Lee HM, Park WH. Formation of Silver Nanoparticles Using Fluorescence Properties of Chitosan Oligomers. Marine Drugs. 2018; 16(1):11. https://doi.org/10.3390/md16010011
Chicago/Turabian StyleCheon, Ja Young, Hun Min Lee, and Won Ho Park. 2018. "Formation of Silver Nanoparticles Using Fluorescence Properties of Chitosan Oligomers" Marine Drugs 16, no. 1: 11. https://doi.org/10.3390/md16010011
APA StyleCheon, J. Y., Lee, H. M., & Park, W. H. (2018). Formation of Silver Nanoparticles Using Fluorescence Properties of Chitosan Oligomers. Marine Drugs, 16(1), 11. https://doi.org/10.3390/md16010011





