Abstract
Palladium-catalyzed dehydrogenative coupling is an efficient synthetic strategy for the construction of quinoline scaffolds, a privileged structure and prevalent motif in many natural and biologically active products, in particular in marine alkaloids. Thus, quinolines and 1,2-dihydroquinolines can be selectively obtained in moderate-to-good yields via intramolecular C–H alkenylation reactions, by choosing the reaction conditions. This methodology provides a direct method for the construction of this type of quinoline through an efficient and atom economical procedure, and constitutes significant advance over the existing procedures that require preactivated reaction partners.
1. Introduction
Marine organisms are an increasingly important source of bioactive natural products, which in some cases have found application as pharmaceuticals (e.g., anticancer drugs) [1,2]. Quinoline core is a common structural motif among many marine alkaloids [3,4]. For example, the pyridoacridine family (e.g., ascididemin), a large class of marine alkaloids isolated from sessile organisms (sponges, corals, ascidians, bryozoans) [5,6], which display different types of biological activities, e.g., cytotoxicity, production of reactive oxygen species (ROS), and topoisomerase inhibition [7,8,9,10]. Marinoquinolines A–F are pyrroloquinolines isolated from gliding bacterium Ohtaekwangia kribbensis (Bacteroidetes) [11], whereas Veranamine is a benzonaphthyridine isolated from Florida sponges, namely, Verongula rigida, with significant antidepressant activity [12,13]. Besides, two quinoline alkaloid glycosides have been isolated in Puerto Rico from extracts of the cyanobacterium Lyngbya majuscule [14,15] (Figure 1). On the other hand, quinstatins, derived from dolastatin 10 (exceptionally anticancer drug contained in the sea hare Dolabella auricularia) by replacing the C-terminal dolaphenine (Doe) unit with a carefully designed quinoline, have been reported to be exceptional cancer cell growth inhibitors [16,17]. Cyanobacterial metabolites calothrixins have shown their potential as human DNA topisomerase I poisons for their cytotoxicity in cancer [18].
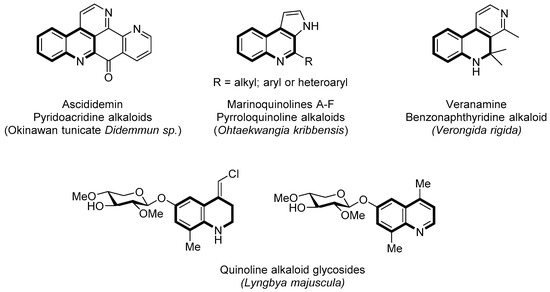
Figure 1.
Selected bioactive marine alkaloids bearing a quinoline core.
The outstanding biological activities of these marine alkaloids have attracted the attention of numerous research groups working toward the total synthesis of members of these natural products or analogues, either to get enough quantities or to establish structure-activity relationships for drug development. For example, it has been claimed that synthetic 4-alkylcarbonylmethyl or 4-alkoxycarbonylmethyl substituted quinolines show inhibitory activity against drug resistant Mycobacterium tuberculosis [19] and potent antimicrobial activity against Helicobacter pylori [20]. More recently, based on SAR studies, it has been demonstrated that the presence of a methoxyl group at C-5 position of the quinoline nucleus is structural feature common to a new class of Enhancer of Zeste Homologue 2 (EZH2) inhibitors, which could be useful for the treatment of several cancer types (lymphoma, colon, prostate, breast, and lung cancer) (Figure 2) [21].
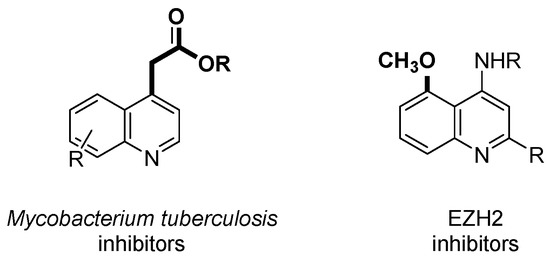
Figure 2.
Characteristic structural features of some bioactive quinoline alkaloid analogues.
Therefore, the development of new methodologies for the synthesis of quinolines and their dihydro/tetrahydro counterparts is well documented in the literature [22,23,24,25]. Among the several variants for synthesis of quinolines, the palladium-mediated cyclization processes [26,27,28,29] and, in particular, the intramolecular Mizoroki–Heck reaction [30,31,32,33] stand out as valuable synthetic protocols. In our research program on quinoline synthesis [34,35,36], we have reported [37] an effective protocol for the synthesis of 2-substituted 4-alkylidenetetrahydroquinoline derivatives, which employs a 6-exo-trig Mizoroki–Heck cyclization of N-alkenyl-substituted 2-haloanilines (Figure 3a). When non-substituted alkenes are used, the reaction can be directed towards the formation of an exocyclic or endocyclic carbon-carbon double bond, while 4-alkylidenetehtrahydroquinolines are obtained regioselectively with substituted alkenes. However, the Fujiwara-Moritani reaction offers notable advantages over traditional cross-coupling chemistry [38,39,40,41,42]. Reactions can be performed under air atmosphere even in aqueous media, and there is no need to prepare specifically functionalized cyclization precursors (i.e., o-haloanilines). This transformation, also known as oxidative Mizoroki–Heck reaction, consists in a direct coupling between two C–H centers (an aromatic C–H bond and an olefinic C–H bond), so it can be considered as either a C–H activation reaction or a C–H olefination. The reaction is catalyzed by Pd(II) and an external oxidant is required to regenerate the active catalytic species. Control of site selectivity is one of the most important challenges in this chemistry because organic molecules can contain a wide variety of C–H bonds [43]. The most common strategies for addressing this issue are the use of electronically activated substrates, directing groups [44,45,46] or ligands [47,48,49,50], which are able to coordinate to the metal center and deliver the catalyst to the targeted C–H bond. Intermolecular Fujiwara-Moritani reactions have received much attention recently, but examples of intramolecular variants are still scarce [51]. In particular, a number of reports have dealt with the construction of five membered rings via 5-exo-trig processes, and in many cases, the alkenylation of electron-rich heteroarenes is involved [52,53]. For example, intramolecular reaction of substituted N-phenylacrylamides catalyzed by Pd(II)-catalysts afforded oxindoles in moderate to good yields [54]. However, 6-endo-trig cyclizations are rare in Fujiwara-Moritani reactions and have been described only when the 5-exo process is blocked, not allowing the palladium hydride elimination. Nevertheless, we have been able to complete an unprecedented selective 6-endo-trig intramolecular C–H alkenylation of N-phenylacrylamides that led to 4-substituted quinolin-2[1H]-ones (Figure 3b) [55]. The adequate choice of the catalyst, oxidant, and experimental conditions allowed us to presumably change the steric and electronic properties around the metal center and direct the reaction to the β-position of the unsaturated moiety.
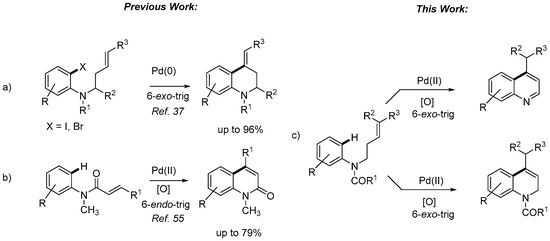
Figure 3.
Palladium-catalyzed approaches to quinoline core.
In this work we describe the use of the intramolecular C–H alkenylation reaction of N-buten-3-ylanilines for the synthesis of quinolines and dihydroquinolines, through 6-exo-trig cyclization processes. Thus, starting from the same precursors, conditions will be selected to favor nitrogen deprotection and oxidation to obtain the quinolines, or to avoid this over-oxidation and obtain 1,2-dihydroquinolines after isomerization of the double bond. The extension to different substitution patterns on the alkene or on the aromatic ring will also be described.
2. Results and Discussion
The study began with the intramolecular Fujiwara-Moritani reaction of the N-substituted N-alkenylanilines 1a,b (Scheme 1). To check the viability of the reaction, we first carried out a stoichiometric reaction with 1 equiv. of Pd(OAc)2 in acetic acid at reflux. In both cases, the N-protecting group was lost in the reaction, leading to quinoline 2a in low yield (12%) through a 6-exo-trig cyclization, followed by isomerization of the double bond and aromatization. Pd(II)-catalytic conditions were subsequently investigated (Scheme 1, Table 1). Due to extensive decomposition observed at high temperature, Pd(II)-catalysis at room temperature was first studied. Pd(OAc)2 was initially used as Pd(II) source in acetic acid in the presence of p-toluenesulfonic acid, as these conditions have been found to be optimal for the cyclization of related amides [55]. Besides, among the wide range of oxidants that can be used for reoxidation of Pd(0) to Pd(II), we selected PhCO3tBu [56], Cu(OAc)2 [57], p-benzoquinone [58] and N-fluoro-2,4,6-trimethylpyridinium triflate (F+) [59] for this preliminary screening.
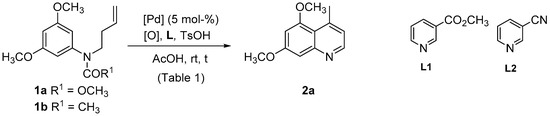
Scheme 1.
Pd(II)-catalyzed cyclization of 1a,b.

Table 1.
Optimization of cyclization conditions for 1a,b.
Thus, quinoline 2a was obtained at room temperature with generally low yields, irrespective the oxidant, although the reactions were faster (5.5 h vs. 24 h) when acetate 1b was used (Table 1, entries 1, 4, 7, 10, 13, 16). The use of ligands for palladium to increase the reactivity was also studied. In this context, pyridine ligands have been shown to enhance not only the reaction rate but also the site selectivity in Pd(II)-catalyzed reactions, and they have been used in intramolecular reactions, in combination with different oxidants [54,60,61,62]. We selected two pyridine ligands: ethyl nicotinate (L1) and 3-cyanopyridine (L2). However, the addition of these ligands for palladium had a detrimental effect in combination of all oxidants used, except for p-benzoquinone, for which a slight increase of the reactivity was observed (Table 1, entries 13 vs. 15 and 16 vs. 17). Various Pd(II) sources were also tested and Pd(dba)2 was revealed as a better catalyst than Pd(OAc)2 (Table 1, entries 19–22 vs. entries 1, 7, and 13). Finally, the best conditions implied the use of PdCl2(CH3CN)2 as Pd(II) source, and a combination of PhCO3tBu and Cu(OAc)2 (5 mol %) as oxidative system, affording 2a in moderate yields from both 1a and 1b, although the reaction was much faster with 1b (Table 1, entries 23 and 25).
With these conditions in hand, the reaction was extended for the synthesis of quinolines with different substitution at C-4 (Scheme 2, Table 2). Thus, different electron withdrawing groups were incorporated in the alkene from 1a,b through cross metathesis to obtain 1c–1j (see Supplementary Materials). However, when phenylsulfonyl derivative 1d was submitted to the previously optimized conditions, only low conversion (<10%) of the starting material was observed at rt (Table 2, entry 1), while decomposition was also observed when higher temperature was used (entry 2). The use of a more powerful oxidant, such as F+ and 70 °C were necessary to obtain 4-phenylsufonylmethylquinoline 2b in moderate yield (Table 2, entry 4). The yield could be improved using the corresponding carbamate 1c as starting material (Table 2, entry 5). Similarly, acetamide 1f showed lower reactivity than the corresponding carbamate 1e, since longer reaction time is needed to bring the reaction to completion (Table 2, entries 6 and 7), even using a higher catalyst loading. In both cases 2c was obtained in moderate yields. When F+ was substituted for PhCO3tBu as the oxidant, longer reaction times were required to obtain 2c in lower yield (Table 2, entry 8). The scope of the reaction was further studied using only substrates possessing a carbamate-protecting group 1g–1j due to their higher reactivity. Thus, the reaction proceeded efficiently for the synthesis of quinolines 2d–e that bear different ester moieties (Table 2, entries 10–12), but failed when a trisubstituted olefin was used (Table 2, entry 9).
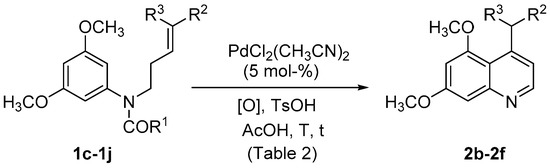
Scheme 2.
Synthesis of 4-substituted quinolines 2b–f.

Table 2.
Extension to substituted alkenes. Synthesis of quinolines 2b–f.
As has been shown, the intramolecular Fujiwara-Moritani reaction allows the synthesis of 4-substituted quinolines through a 6-exo-trig cyclization followed by deprotection and aromatization. On the other hand, to apply this protocol for the synthesis of 1,2-dihydroisoquinolines, deprotection of the nitrogen atom should be avoided, thus preventing further oxidation. This would imply the use of milder reaction conditions, avoiding the use of acetic acid as solvent [63]. After some experimentation, we found that the cyclization could be efficiently performed in dioxane at room temperature, using p-benzoquinone as an oxidant in the presence of p-toluenesulfonic acid (Scheme 3). Under these reaction conditions, both protecting groups in 1a and 1b were stable, and dihydroquinolines 3a and 3b were obtained in good yields (Table 3, entries 1 and 3). Once again, the carbamate protected aniline 1a was more reactive than 1b, leading to a good yield of 3a in shorter reaction time (7.5 h vs. 25 h). An increase of the reaction temperature to 70 °C led to a more efficient reaction, obtaining 3a in high yield (89%) in only 10 min (Table 3, entry 2). Once again, the use of ligands L1 and L2 for palladium was detrimental, completely precluding the reaction. Then, the extension to other substitution patterns on the aromatic ring was studied. Interestingly, with a more electron rich aromatic ring (1k), the reaction was less efficient, and no cyclization was observed at room temperature after 24 h (Table 3, entry 6). An increase of the temperature was required to obtain 3c in low yield (Table 3, entry 7), which could be improved increasing the catalyst loading (Table 3, entry 8), although an increase of the reaction time led to decomposition, lowering the isolated yield of 3c (Table 3, entry 9). An electron-donor group ortho to the cyclization position appears to be necessary, as the reaction did not proceed at all for the 3,4-disubstituted substrates 1l and 1m (Table 3, entries 10 and 11). However, when weakly donor methyl groups are incorporated in 3,5-positions, the cyclization took place, but in this case isomerization of the double bond led to the formation of the 1,4-dihydroquinoline 4d.
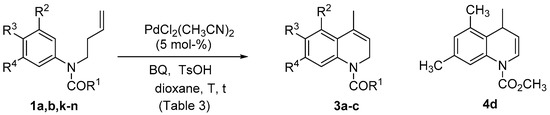
Scheme 3.
Synthesis of 4-substituted dihydroquinolines 3a–c and 4d.

Table 3.
Synthesis of 4-substituted dihydroquinolines 3a–c and 4d.
These results are in agreement with the mechanistic proposal shown in Scheme 4. Thus, the first step would be the formation if the arylpalladium(II) intermediate I. Different mechanisms have been proposed for this palladation step, which is highly dependent on the substrate [50]. Thus, concerted metalation deprotonation (CMD), oxidative addition or electrophilic palladation mechanisms have been proposed. In this case, the electronic effects of the substituents on the aromatic ring would agree with an electrophilic palladation mechanism, favored by the electron-donor effects of the substituents on the aromatic ring. The subsequent migratory insertion followed by β-hydride elimination would form quinoline II, with an exocyclic double bond that would isomerize to the endocyclic position forming 3, probably due to a higher thermodynamic stability. Pd(0) is finally oxidized to the Pd(II) active species by the oxidant.
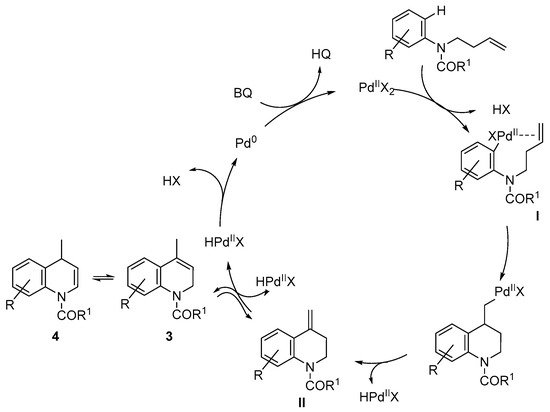
Scheme 4.
Mechanistic proposal.
In conclusion, it has been shown that both quinolines and 1,2-dihydroquinolines can be selectively obtained in moderate to good yields via palladium(II)-catalyzed C–H alkenylation reactions, choosing the reaction conditions. Thus, when the reactions are carried out in acetic acid, deprotection and further oxidation leads to the one pot formation of 4-substituted quinolines 2a–f. On the other hand, under milder reaction conditions, deprotection and over-oxidation can be avoided, leading to 1,2-dihydroquinolines 3a–c. This procedure is complementary to the related Mizoroki-Heck reaction [37] that led to the formation of 4-methylidenetetrahydroquinolines, with exocyclic double bonds, with the advantage that this procedure does not require the prior functionalization of the substrates. However, the method is so far limited to the use of electron rich aromatic rings.
3. Materials and Methods
3.1. General Experimental Methods
Melting points were determined in unsealed capillary tubes and are uncorrected. IR spectra were obtained in film over NaCl pellets, or using an ATR. NMR spectra were recorded at 20–25 °C, at 300 MHz for 1H and 75.5 MHz for 13C or at 500 MHz for 1H and 125.7 MHz for 13C in CDCl3 solutions. Assignments of individual 13C and 1H resonances are supported by DEPT experiments and 2D correlation experiments (COSY, HSQCed or HMBC). Selective NOE or NOESY experiments were performed when necessary. Mass spectra were recorded under electron impact (EI) at 70 eV or under chemical ionization (CI) at 230 eV, or using Electrospray ionization (ESI+). Exact mass was obtained using a TOF detector. TLC was carried out with 0.2 mm thick silica gel plates. Visualization was accomplished by UV light. Flash column chromatography was performed on silica gel (230–400 mesh) or on alumina (70–230 mesh). All solvents used in reactions were anhydrous and purified according to standard procedures. All air- or moisture-sensitive reactions were performed under argon; the glassware was dried (130 °C) and purged with argon. Palladium catalysts were purchased from Sigma-Aldrich Química SL (Madrid, Spain), and were used without further purification: Pd(OAc)2 98% purity, PdCl2(CH3CN)2, 99% purity.
3.2. Synthesis of 4-Substituted Quinolines 2
5,7-Dimethoxy-4-methylquinoline (2a) (Table 1, entry 23). Over a solution of methyl but-3-en-1-yl(3,5-dimethoxyphenyl)carbamate (1a) (94.3 mg, 0.36 mmol) in AcOH (1.4 mL), PhCO3tBu (0.09 mL, 0.43 mmol), Cu(OAc)2 (3.3 mg, 0.018 mmol), TsOH (64.8 mg, 0.36 mmol) and PdCl2(CH3CN)2 (4.7 mg, 0.018 mmol) were added. The mixture was stirred at room temperature for 24 h, and then the solvent was removed under vacuum. The residue was dissolved in AcOEt (5 mL) and it was washed with a 2 M aqueous solution of Na2CO3 (2 × 10 mL) and brine (2 × 10 mL). The aqueous phase was re-extracted with AcOEt (10 mL) and the combined organic extracts were dried (Na2SO4) and concentrated in vacuo. Flash column chromatography (silica gel, hexane/AcOEt 6/4) afforded 23 (40.3 mg, 56%) as an oil: IR (ATR) 1612 cm−1 (C=N); 1H NMR (CDCl3): δ 2.81 (s, 3H, CH3), 3.89 (s, 3H, OCH3), 3.92 (s, 3H, OCH3), 6.48 (d, J = 2.3 Hz, 1H, H6), 6.95 (d, J = 4.5 Hz, 1H, H3), 7.02 (d, J = 2.3 Hz, 1H, H8), 8.56 (d, J = 4.5 Hz, 1H, H2); 13C NMR (CDCl3): δ 24.3 (CH3), 55.5 (2 × OCH3), 98.6 (C6), 100.4 (C3), 116.8 (C4a), 121.4 (C8), 146.0 (C4), 150.0 (C2), 151.35 (C8a), 158.6 (C5), 160.4 (C7); MS (EI) m/z (rel intensity) 204.1 (M+ + 1, 13), 203.1 (M+, 100), 188 (28), 174.1 (12), 160.1 (11), 145 (14), 117 (11); HRMS (CI) calcd. for C12H14NO2 [MH+], 204.1025; found: 204.1025.
General Procedure for the Synthesis of 4-Substituted Quinolines 2b–f
Over a solution of the corresponding butenyl aniline 1c–j (1 mmol) in AcOH (11 mL), TsOH (1 mmol), N-fluoro-2,4,6-trimethylpyridinium triflate (F+) (1.2 mmol), Cu(OAc)2 (0.05 mmol) and PdCl2(CH3CN)2 (0.05 or 0.1 mmol) were added. The mixture was stirred at 70 °C for the specified time, and then the solvent was removed under vacuum. The residue was dissolved in AcOEt (5 mL) and it was washed with a 2 M aqueous solution of Na2SO4 (2 × 10 mL) and brine (2 × 10 mL). The aqueous phase was re-extracted with AcOEt (10 mL) and the combined organic extracts were dried (Na2SO4) and concentrated in vacuo. Flash column chromatography (silica gel, hexane/AcOEt) afforded the corresponding quinolines 2b–f (Table 2).
5,7-Dimethoxy-4-[(phenylsulfonyl)methyl]quinoline (2b) (Table 2, entry 5). Prepared from 1c (68.7 mg, 0.17 mmol), TsOH (32.7 mg, 0.17 mmol), F+ (59.4 mg, 0.20 mmol), Cu(OAc)2 (1.6 mg, 0.008 mmol) and PdCl2(CH3CN)2 (2.2 mg, 0.008 mmol) in AcOH (1.5 mL). The mixture was stirred at 70 °C for 24 h. After work-up and purification by flash column chromatography (silica gel, hexane/AcOEt 2/8), 2d was obtained (35.3 mg, 61%) as an oil: IR (ATR) 1620 cm−1 (C=N), 1325 cm−1, 1135 cm−1 (R-SO2-R); 1H NMR (CDCl3): δ 3.71 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 5.22 (s, 2H, CH2), 6.36 (d, J = 2.4 Hz, 1H, H6), 7.01–7.05 (m, 2H, H3, H8), 7.28–7.37 (m, 2H, H3′, H5′), 7.46–7.53 (m, 3H, H2′, H4′, H6′), 8.69 (d, J = 4.5 Hz, 1H, H2); 13C NMR (CDCl3): δ 55.5 (OCH3), 55.6 (OCH3), 62.0 (CH2), 99.7 (C6), 101.1 (C8), 115.2 (C4a), 123.5 (C3), 128.6 (C2′, C3′, C5′, C6′), 133.6 (C4′), 134.1 (C4), 138.4 (C1′), 149.9 (C2), 151.7 (C8a), 156.7 (C5), 160.5 (C7); MS (ESI+) m/z (rel intensity) 345.1 (MH+ + 1, 19), 344.1 (MH+, 100); HRMS (ESI+) calcd. for C18H18NO4S [MH+], 344.0957; found: 344.0970.
Methyl 2-(5,7-dimethoxyquinolin-4-yl)acetate (2c) (Table 2, entry 6). Prepared from 1e (93.3 mg, 0.29 mmol), TsOH (54.9 mg, 0.29 mmol), F+ (0.10 g, 0.35 mmol), Cu(OAc)2 (2.6 mg, 0.014 mmol) and PdCl2(CH3CN)2 (3.7 mg, 0.014 mmol) in AcOH (3.2 mL). The mixture was stirred at 70 °C for 19 h. After work-up and purification by flash column chromatography (silica gel, hexane/AcOEt 2/8), 2c was obtained (40.8 mg, 54%) as a solid: mp (CH2Cl2) 77–80 °C; IR (ATR) 1735 cm−1(C=O); 1H NMR (CDCl3): δ 3.67 (s, 3H, COOCH3), 3.83 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 4.09 (s, 2H, CH2COOCH3), 6.49 (d, J = 2.2 Hz, 1H, H6), 6.95 (d, J = 4.4 Hz, 1H, H3), 7.02 (d, J = 2.2 Hz, 1H, H8), 8.67 (d, J = 4.4 Hz, 1H, H2); 13C NMR (CDCl3): δ 42.8 (CH2COOCH3), 51.8 (COOCH3), 55.2 (OCH3), 55.5 (OCH3), 99.0 (C6), 100.9 (C8), 116.0 (C4a), 122.2 (C3), 140.2 (C4), 150.4 (C2), 151.6 (C8a), 157.2 (C5), 160.5 (C7), 171.4 (CO); MS (EI) m/z (rel intensity) 262.1 (M+ + 1, 17), 261.2 (M+, 100), 229.1 (12), 202.1 (10), 186.1 (19), 172.1 (36); HRMS (ESI+) calcd. for C14H16NO4 [MH+], 262.1079; found: 262.1091.
2,2,2-Trifluoroethyl 2-(5,7-dimethoxyquinolin-4-yl)acetate (2d) (Table 2, entry 10). Prepared from 1h (0.15 g, 0.39 mmol), TsOH (73.4 mg, 0.39 mmol), F+ (0.13 g, 0.46 mmol), Cu(OAc)2 (3.5 mg, 0.019 mmol) and PdCl2(CH3CN)2 (5.0 mg, 0.019 mmol) in AcOH (4.3 mL). The mixture was stirred at 70 °C for 21 h. After work-up and purification by flash column chromatography (silica gel, hexane/AcOEt 2/8), 6b was obtained (58.3 mg, 46%) as a solid: mp (CH2Cl2) 95–97 °C; IR (ATR) 1745 cm−1(C=O); 1H NMR (CDCl3): δ 3.81 (s, 3H, OCH3), 3.90 (s, 3H, OCH3), 4.19 (s, 2H, CH2COOCH2), 4.48 (q, J = 8.5 Hz, 2H, COOCH2CF3), 6.50 (d, J = 2.2 Hz, 1H, H6), 6.95 (d, J = 4.4 Hz, 1H, H3), 7.06 (d, J = 2.2 Hz, 1H, H8), 8.67 (d, J = 4.4 Hz, 1H, H2); 13C NMR (CDCl3): δ 42.2 (CH2COOCH2), 55.3 (OCH3), 55.6 (OCH3), 60.5 (q, J = 36.6 Hz, COOCH2CF3), 99.3 (C6), 100.8 (C8), 115.8 (C4a), 122.3 (C3), 112.9 (q, J = 275.8 Hz, CF3), 139.2 (C4), 150.3 (C2), 151.5 (C8a), 157.0 (C5), 160.7 (C7), 169.5 (CO); MS (EI) m/z (rel intensity) 330.1 (M+ + 1, 17), 329.1 (M+, 100), 186 (21), 172.1 (27); HRMS (ESI+) calcd. for C15H15F3NO4 [MH+], 330.0953; found: 330.0956.
Dodecyl 2-(5,7-dimethoxyquinolin-4-yl)acetate (2e) (Table 2, entry 11). Prepared from 1i (0.14 g, 0.29 mmol), TsOH (54.3 mg, 0.29 mmol), F+ (99.1 mg, 0.34 mmol), Cu(OAc)2 (3.1 mg, 0.017 mmol) and PdCl2(CH3CN)2 (4.4 mg, 0.017 mmol) in AcOH (3.2 mL). The mixture was stirred at 70 °C for 21 h. After work-up and purification by flash column chromatography (silica gel, hexane/AcOEt 3/7), 2f was obtained (59.5 mg, 50%) as a solid: mp (CH2Cl2) 54–56 °C; IR (ATR) 1731 cm−1 (C=O); 1H NMR (CDCl3): δ 0.87 (t, J = 6.7 Hz, 3H, CH3), 1.10–1.35 (m, 18H, OCH2CH2(CH2)9CH3), 1.42–1.64 (m, 2H, CO2CH2CH2), 3.83 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 4.05 (t, J = 6.7 Hz, 2H, COOCH2), 4.09 (s, 2H, CH2COOCH2), 6.49 (d, J = 1.7 Hz, 1H, H6), 6.96 (d, J = 4.1 Hz, 1H, H3), 7.08 (d, J = 1.7 Hz, 1H, H8), 8.67 (br s, 1H, H2); 13C NMR (CDCl3): δ 14.1 (CH3), 22.7 (CH3CH2), 25.9 (COOCH2CH2CH2), 28.6, 29.2, 29.3, 29.5, 29.6, 29.7 (7 × CH2), 31.9 (CH3CH2CH2), 43.2 (CH2COOCH2), 55.2 (OCH3), 55.5 (OCH3), 64.9 (COOCH2), 99.0 (C6), 100.9 (C8), 116.0 (C4a), 122.2 (C3), 140.5 (C4), 150.4 (C2), 151.6 (C8a), 157.2 (C5), 160.5 (C7), 171.0 (CO); MS (EI) m/z (rel intensity) 416.3 (M+ + 1, 8), 415.3 (M+, 30), 386.3 (24), 372.2 (23), 358.2 (21), 344.2 (18), 330.2 (18), 316.2 (21), 302.1 (21), 248.1 (12), 247.1 (10), 204.1 (11), 203.1 (72), 202.1 (25), 189.1 (10), 188.1 (100), 173.1 (18), 172.1 (56), 129 (10), 57.1 (12), 55.1 (19); HRMS (ESI+) calcd. for C25H38NO4 [MH+], 416.2801; found: 416.2809.
Benzyl 2-(5,7-dimethoxyquinolin-4-yl)acetate (2f) (Table 2, entry 12). Prepared from 1j (0.11 g, 0.28 mmol), TsOH (53.9 mg, 0.28 mmol), F+ (98.3 mg, 0.34 mmol), Cu(OAc)2 (2.6 mg, 0.014 mmol) and PdCl2(CH3CN)2 (3.7 mg, 0.014 mmol) in AcOH (3.1 mL). The mixture was stirred at 70 °C for 21 h. After work-up and purification by flash column chromatography (silica gel, hexane/AcOEt 2/8), 2g was obtained (58.9 mg, 62%) as an oil: IR (ATR) 1735 cm−1 (C=O); 1H NMR (CDCl3): δ 3.54 (s, 3H, OCH3), 3.92 (s, 3H, OCH3), 4.12 (s, 2H, CH2COOCH2Ph), 5.12 (s, 2H, CH2Ph) 6.41 (d, J = 2.3 Hz, 1H, H6), 6.97 (d, J = 4.5 Hz, 1H, H3), 7.06 (d, J = 2.3 Hz, 1H, H8), 7.14-7.49 (m, 5H, Ph), 8.67 (d, J = 4.5 Hz, 1H, H2); 13C NMR (CDCl3): δ 43.1 (CH2COOCH2Ph), 54.9 (OCH3), 55.5 (OCH3), 66.3 (CH2Ph), 99.0 (C6), 100.8 (C8), 116.0 (C4a), 122.3 (C3), 128.2 (C2′, C6′), 128.5 (C3′, C4′, C5′), 136.0 (C1′), 140.2 (C4), 150.4 (C2), 151.6 (C8a), 157.1 (C5), 160.5 (C7), 170.7 (CO); MS (EI) m/z (rel intensity) 338.1 (M+ + 1, 20), 337.1 (M+, 92), 172.1 (47), 91.1 (100); HRMS (ESI+) calcd. for C20H20NO4 [MH+], 338.1392; found: 338.1418.
3.3. Synthesis of 4-Substituted Dihydroquinolines 3 and 4
General Procedure
Over a solution of the corresponding N-substituted but-3-en-1-ylaniline 1a,b,k–n (1 mmol) in dioxane (66.7 mL), TsOH (1 mmol), p-benzoquinone (1 mmol) and PdCl2(CH3CN)2 (0.05 mmol) were added. The reaction mixture was stirred for the specified time at room temperature or at 70 °C. Afterwards, water was added to quench the reaction and the mixture was extracted with CH2Cl2 (3 × 10 mL). The combined organic extracts were washed with brine (10 mL), dried (Na2SO4) and concentrated in vacuo. Flash column chromatography (silica gel, hexane/AcOEt) afforded the corresponding 1,2-dihydroquinoles 3a–c or 1,4-dihydroquinoline 4d.
Methyl 5,7-dimethoxy-4-methylquinoline-1(2H)-carboxylate (3a) (Table 3, entry 2). Prepared from carbamate 1a (106.5 mg, 0.40 mmol), TsOH (77.5 mg, 0.40 mmol), p-benzoquinone (44.1 mg, 0.40 mmol) and PdCl2(CH3CN)2 (5.3 mg, 0.020 mmol) in dioxane (31 mL). The reaction mixture was stirred for 10 min at 70 °C and after work-up and purification by flash column chromatography (silica gel, hexane/AcOEt 8/2), 3a was obtained (94.2 mg, 89%) as an oil: IR (ATR) 1706 cm−1 (C=O); 1H NMR (CDCl3): δ 2.16 (d, J = 1.3 Hz, 3H, CH3) 3.77 (s, 3H, COOCH3), 3.79 (s, 3H, OCH3), 3.81 (s, 3H, OCH3), 4.10–4.15 (m, 2H, CH2), 5.64 (td, J = 4.8, 1.3 Hz, 1H, H3), 6.29 (d, J = 2.4 Hz, 1H, H6), 6.80 (br s, 1H, H8); 13C NMR (CDCl3): δ 21.9 (CH3), 42.7 (C2), 53.0 (COOCH3), 55.4 (OCH3), 55.5 (OCH3), 96.1 (C6), 101.4 (C8), 113.6 (C4a), 119.9 (C3), 132.7 (C4), 139.4 (C8a), 154.4 (CO), 158.0 (C5), 159.0 (C7); MS (EI) m/z (rel intensity) 264.1 (M+ + 1, 13), 263.1 (M+, 79), 249.1 (14), 248.1 (100), 205.1 (11), 204.1 (84), 203.1 (50), 189.1 (29), 188.1 (24), 174.1 (13), 160.1 (16), 146.1 (11), 145.1 (11), 130.1 (10), 117.1 (10); HRMS (ESI+) calcd. for C14H18NO4 [MH+], 264.1236; found: 264.1259.
1-[5,7-Dimethoxy-4-methylquinolin-1(2H)-yl]ethanone (3b) (Table 3, entry 3). Prepared from acetamide 1b (0.12 g, 0.46 mmol), TsOH (88.1 mg, 0.46 mmol), p-benzoquinone (50.1 mg, 0.46 mmol) and PdCl2(CH3CN)2 (6.0 mg, 0.023 mmol) in dioxane (31 mL). The reaction mixture was stirred for 25 h at rt, and after work-up and purification by flash column chromatography (silica gel, hexane/AcOEt 6/4), 3b was obtained (71.2 mg, 62%) as a mixture of rotamers in a 6:4 ratio and as an oil: IR (ATR) 1699 (C=O); 1H NMR (CDCl3): δ 2.19 (s, 3H, CH3, both rotamers), 2.23 (s, 3H, COCH3, both rotamers), 3.83 (s, 6H, 2 × OCH3, both rotamers), 4.21 (br s, 2H, CH2, both rotamers), 5.71 (br s, 6.36, major rotamer: 2H, H6, H8; minor rotamer: 1H, H6), 6.75 (br s, 1H, H8, minor rotamer); 13C NMR (CDCl3): δ 21.9 (CH3, both rotamers), 22.7 (COCH3, both rotamers), 40.9 (C2, both rotamers), 55.4 (OCH3, both rotamers), 55.5 (OCH3, both rotamers), 96.5 (C6, both rotamers), 102.3 (C8, major rotamer), 114.1 (C4a, both rotamers), 116.1 (C8, minor rotamer), 122.2 (C3, both rotamers), 132.2 (C4, both rotamers), 139.9 (C8a, minor rotamer), 149.84 (C8a, major rotamer), 157.8 (C5, both rotamers), 158.8 (C7, both rotamers), 169.7 (CO, both rotamers); MS (EI) m/z (rel intensity) 248.1 (M+ + 1, 3), 247.1 (M+, 18), 205.1 (15), 204.1 (100), 203.1 (10), 190.1 (28), 189.1 (19); HRMS (ESI+) calcd. for C14H18NO3 [MH+], 248.1287, found: 248.1294.
Methyl 5,6,7-trimethoxy-4-methylquinoline-1(2H)-carboxylate (3c) (Table 3, entry 8). Prepared from carbamate 1k (0.107 g, 0.36 mmol), TsOH (70 mg, 0.36 mmol), p-benzoquinone (40 mg, 0.36 mmol) and PdCl2(CN)2 (9.5 mg, 0.036 mmol) in dioxane (31 mL). The reaction mixture was stirred for 2 h at 70 °C, and after work-up and purification by flash column chromatography (silica gel, hexane/AcOEt 6/4) affording 3c as an oil (42 mg, 40%): IR (ATR) 1685 cm−1 (C=O); 1H NMR (CDCl3): δ 2.18 (s, 3H, CH3), 3.77 (s, 3H, CO2CH3), 3.82 (s, 3H, OCH3), 3.84 (s, 3H, OCH3), 3.86 (s, 3H, OCH3), 4.11 (brs, 2H, NCH2), 5.69 (brs, 1H, H3), 6.98 (br s, 1H, H8); 13C NMR (CDCl3): δ 21.3 (CH3), 42.6 (C2), 53.0 (CO2CH3), 56.0 (OCH3), 60.8 (OCH3), 61.1 (OCH3), 104.3 (C8), 117.8 (C4a), 121.2 (C3), 132.2 (C4), 133.5 (C8a), 139.8 (C6), 150.8 (C5), 151.9 (C7), 154.5 (CO); MS (EI) m/z (rel intensity): 293 (M+, 69), 278 (100), 262 (8), 234 (40), 218 (24), 207 (27), 204 (21), 190 (17), 176 (13); HRMS (ESI+) calcd. for C15H20NO5 [M + H]+, 294.1341; found: 294.1361.
Methyl 4,5,7-trimethylquinoline-1(4H)-carboxylate (4d) (Table 3, entry 12). Prepared from carbamate 1n (110.0 mg, 0.47 mmol), TsOH (91.1 mg, 0.47 mmol), p-benzoquinone (52.0 mg, 0.47 mmol) and PdCl2(CN)2 (12.4 mg, 0.047 mmol) in dioxane (31 mL). The reaction mixture was stirred for 24 h at 70 °C, and after work-up and purification by flash column chromatography (silica gel, hexane/AcOEt 9:1) affording the product 4d as an oil (34.4 mg, 32%): IR (ATR) 1730 cm−1 (C=O); 1H NMR (CDCl3): δ 1.16 (d, J = 6.9 Hz, 3H, CHCH3), 2.29 (s, 3H, CH3), 2.31 (s, 3H, CH3), 3.44–3.52 (m, 1H, H4), 3.87 (s, 3H, OCH3), 5.48 (t, J = 6.9 Hz, 1H, H3), 6.84 (s, 1H, H6), 6.95 (d, J = 6.9 Hz, 1H, H2), 7.62 (s, 1H, H8); 13C NMR (CDCl3): δ 18.7 (CHCH3), 21.2 (CH3), 29.0 (C4), 53.1 (OCH3), 116.3 (C3), 120.3 (C8), 125.9 (C6), 127.8 (C4a), 129.4 (C2), 134.6 (C8a), 135.3 (C7), 136.1 (C5), 153.3 (CO); MS (EI) m/z (rel intensity): 231 (M+, 65), 215 (100), 199 (8), 171 (84), 156 (21); HRMS (ESI+) calcd. for C14H18NO2 [M + H]+, 232.1338; found: 232.1344.
Supplementary Materials
The following are available online at www.mdpi.com/1660-3397/15/9/276/s1: Preparation procedures for the substrates 1a–n. Copies of 1H and 13C NMR spectra of compounds 1–4.
Acknowledgments
Ministerio de Economía y Competitividad (CTQ2013-41229-P, CTQ2016-74881-P), Gobierno Vasco (IT1045-16) and Universidad del País Vasco/Euskal Herriko Unibertsitatea UPV/EHU are gratefully acknowledged for their financial support. V.O.-d.-E., A.C.-M. wish to thank Gobierno Vasco for grants. Technical and human support provided by Servicios Generales de Investigación SGIker (UPV/EHU, MINECO, GV/EJ, ERDF and ESF) is also acknowledged.
Author Contributions
N.S. and E.L. conceived and designed the experiments; A.C.-M., V.O.-d.-E. and M.M.-N. performed the experiments and analyzed the data; N.S. and E.L. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References and Note
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug development from marine natural products. Nat. Rev. Drug Discov. 2009, 8, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Andersen, R.J. Sponging off nature for new drug leads. Biochem. Pharmacol. 2017, 139, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Hu, W.-P.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2007, 24, 31–86. [Google Scholar] [CrossRef] [PubMed]
- Michael, J.P. Quinoline, quinazoline and acridone alkaloids. Nat. Prod. Rep. 2008, 25, 166–187. [Google Scholar] [CrossRef] [PubMed]
- Molinski, T.F. Marine pyridoacridine alkaloids: Structure, synthesis, and biological chemistry. Chem. Rev. 1993, 93, 1825–1838. [Google Scholar] [CrossRef]
- Skyler, D.; Heathcock, C.H. The pyridoacridine family tree: A useful scheme for designing synthesis and predicting undiscovered natural products. J. Nat. Prod. 2002, 65, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Delfourne, E.; Bastide, J. Marine pyridoacridine alkaloids and synthetic analogues as antitumor agents. Med. Res. Rev. 2003, 23, 234–252. [Google Scholar] [CrossRef] [PubMed]
- Imperatore, C.; Aiello, A.; D’Aniello, F.; Senese, M.; Menna, M.L. Alkaloids from marine invertebrates as important leads for anticancer drugs discovery and development. Molecules 2014, 19, 20391–20423. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Alfonso, E.; Avendaño, C.; Menéndez, J.C. Efficient synthesis of the pyrido[2,3,4-kl]acridin-4-one system common to several cytotoxic marine alkaloids. Tetrahedron Lett. 2003, 44, 6003–6005. [Google Scholar] [CrossRef]
- Melzer, B.; Plodek, A.; Bracher, F. Total synthesis of the marine pyridoacridine alkaloid Demethyldeoxyamphimedine. J. Org. Chem. 2014, 79, 7239–7242. [Google Scholar] [CrossRef] [PubMed]
- Okanya, P.W.; Mohr, K.I.; Gerth, K.; Jansen, R.; Müller, R. Marinoquinolines A-F, pyrroloquinolines from Ohtaekwangia kribbensis (Bacteroidetes). J. Nat. Prod. 2011, 74, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Hamann, M.T.; Kochanowska, A.J.; El-Alfy, A.; Matsumoto, R.R.; Boujos, A. Method Using Marine Sponge-Derived Compounds Having Antidepressant, Anxiolytic and Other Neurological Activity, and Compositions of Matter. U.S. Patent 20,090,093,513 A1, 9 April 2009. [Google Scholar]
- Liang, D.; Wang, Y.; Wang, Y.; Di, D. A simple synthesis of the debrominated analogue of veranamine. J. Chem. Res. 2015, 39, 105–107. [Google Scholar] [CrossRef]
- Orjala, J.; Gerwick, W.H. Two quinoline alkaloids from the Caribbean cyanobacterium Lyngbya majuscula. Phytochemistry 1997, 45, 1087–1090. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2005, 22, 15–61. [Google Scholar] [CrossRef] [PubMed]
- Pettit, G.R.; Hogan, F.; Toms, S. Antineoplastic agents. 592. Highly effective cancer cell growth inhibitory structural modifications of Dolastatin 10. J. Nat. Prod. 2011, 74, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Pettit, G.R.; Melody, N.; Chapuis, J.-C. Antineoplastic agents. 603. Quinstatins: Exceptional cancer cell growth inhibitors. J. Nat. Prod. 2017, 80, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Nijampatnam, B.; Dutta, S.; Velu, S.E. Cyanobacterial metabolite calothrixins: Recent advances in synthesis and biological evaluation. Mar. Drugs 2016, 14, 17. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Singh, P.P.; Jain, M.; Sachdeva, S.; Misra, V.; Kaul, C.L.; Kaur, S.; Vaitilingam, B.; Nayyar, A.; Bhaskar, P.P. Ring-Substituted Quinoline Analogs as Anti-Tuberculosis Agents. Indian Patent 2002DE00628, 11 March 2005. [Google Scholar]
- Khan, M.A.; Miller, K.; Rainsford, K.D.; Zhou, Y. Synthesis and antimicrobial activity of novel substituted ethyl 2-(quinolin-4-yl)-propanoates. Molecules 2013, 18, 3227–3240. [Google Scholar] [CrossRef] [PubMed]
- Xiang, P.; Jie, H.; Zhou, Y.; Yang, B.; Wang, H.-J.; Hu, J.; Hu, J.; Yang, S.-Y.; Zhao, Y.-L. 5-Methoxyquinoline derivatives as a new class of EZH2 inhibitors. Molecules 2015, 20, 7620–7636. [Google Scholar] [CrossRef] [PubMed]
- Kouznetsov, V.; Vargas Mendez, L.Y.; Melendez Gomez, C.M. Recent progress in the synthesis of quinolines. Curr. Org. Chem. 2005, 9, 141–161. [Google Scholar] [CrossRef]
- Barluenga, J.; Rodríguez, F.; Fañanás, F.J. Recent advances in the synthesis of indole and quinoline derivatives through cascade reactions. Chem. Asian J. 2009, 4, 1036–1048. [Google Scholar] [CrossRef] [PubMed]
- Alajarín, R.; Burgos, C. Six-membered heterocycles: Quinoline and isoquinoline. In Modern Heterocyclic Chemistry; Álvarez-Builla, J., Vaquero, J.J., Barluenga, J., Eds.; Wiley-VCH: Weinheim, Germany, 2011; Volume 3, pp. 1527–1629. [Google Scholar]
- Ramann, G.A.; Cowen, B.J. Recent advances in metal-free quinoline synthesis. Molecules 2016, 21, 986. [Google Scholar] [CrossRef] [PubMed]
- De Meijere, A.; Diederich, F. (Eds.) Metal Catalyzed Cross-Coupling Reactions, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Wu, X.-F.; Anbarasan, P.; Neumann, H.; Beller, M. From noble metal to Nobel Prize: Palladium-catalyzed coupling reactions as key methods in organic synthesis. Angew. Chem. Int. Ed. 2010, 49, 9047–9050. [Google Scholar] [CrossRef] [PubMed]
- Tymoshenko, D.; Jeges, G.; Gregg, B.T. Synthesis of heterocycles by palladium-catalyzed intramolecular heteroarylation. In Progress in Heterocyclic Chemistry; Gribble, G.W., Joule, J.A., Eds.; Elsevier: Oxford, UK, 2011; Volume 23, pp. 27–74. [Google Scholar]
- Majumdar, K.C.; Samanta, S.; Sinha, B. Recent developments in palladium-catalyzed formation of five- and six-membered fused heterocycles. Synthesis 2012, 44, 817–847. [Google Scholar] [CrossRef]
- Zeni, G.; Larock, R.C. Synthesis of heterocycles via palladium-catalyzed oxidative addition. Chem. Rev. 2006, 106, 4644–4680. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Gribble, G.W. (Eds.) Palladium in Heterocyclic Chemistry: A Guide for the Synthetic Chemist, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Muller, T.; Bräse, S. Formation of heterocycles. In The Mizoroki-Heck Reaction; Oestreich, M., Ed.; Wiley: Chichester, UK, 2009; pp. 215–258. [Google Scholar]
- Beletskaya, I.P.; Cheprakov, A.V. Modern Heck reactions. In RSC Catalysis Series: New Trends in Cross-Coupling: Theory and Applications; Colacot, T., Ed.; Royal Society of Chemistry: London, UK, 2015; Volume 21, pp. 355–478. [Google Scholar]
- Martínez-Estíbalez, U.; Sotomayor, N.; Lete, E. Pd-catalyzed arylation/ring-closing metathesis approach to azabicycles. Tetrahedron Lett. 2007, 48, 2919–2922. [Google Scholar] [CrossRef]
- Martínez-Estíbalez, U.; Sotomayor, N.; Lete, E. Intramolecular carbolithiation reactions for the synthesis of 2,4-disubstituted tetrahydroquinolines: Evaluation of TMEDA and (−)-sparteine as ligands in the stereoselectivity. Org. Lett. 2009, 11, 1237–1240. [Google Scholar] [CrossRef] [PubMed]
- García-Calvo, O.; Martínez-Estíbalez, U.; Lete, E.; Sotomayor, N. Synthesis of tetrahydroquinolines through intramolecular carbolithiation reactions. Heterocycles 2014, 88, 425–440. [Google Scholar] [CrossRef]
- Martínez-Estíbalez, U.; García-Calvo, O.; Ortiz-de-Elguea, V.; Sotomayor, N.; Lete, E. Intramolecular Mizoroki–Heck Reaction in the regioselective synthesis of 4-alkylidene-tetrahydroquinolines. Eur. J. Org. Chem. 2013, 2013, 3013–3022. [Google Scholar] [CrossRef]
- Ferreira, E.M.; Zhang, H.; Stoltz, B.M. Oxidative Heck-type reactions (Fujiwara-Moritani reactions). In The Mizoroki-Heck Reaction; Oestreich, M., Ed.; Wiley: Chichester, UK, 2009; pp. 345–382. [Google Scholar]
- Zhou, L.; Lu, W. Towards ideal synthesis. alkenylation of aryl C-H bonds by a Fujiwara-Moritani reaction. Chem. Eur. J. 2014, 20, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, T.; Fujiwara, Y. Dehydrogenative Heck-type reactions: The Fujiwara-Moritani reaction. In RSC Green Chemistry Series: From C-H to C-C Bonds: Cross-Dehydrogenative-Coupling; Li, C.-J., Ed.; Royal Society of Chemistry: London, UK, 2015; Volume 26, pp. 33–54. [Google Scholar]
- Topczewski, J.J.; Sanford, M.S. Carbon-hydrogen (C-H) bond activation at PdIV: A frontier in C-H functionalization catalysis. Chem. Sci. 2015, 6, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Gensch, T.; Hopkinson, M.N.; Glorius, F.; Wencel-Delord, J. Mild metal-catalyzed C-H activation: Examples and concepts. Chem. Soc. Rev. 2016, 45, 2900–2936. [Google Scholar] [CrossRef] [PubMed]
- Neufeldt, S.R.; Sanford, M.S. Controlling site selectivity in palladium-catalyzed C-H bond functionalization. Acc. Chem. Res. 2012, 45, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Rouquet, G.; Chatani, N. Catalytic functionalization of C(sp2)-H and C(sp3)-H bonds by using bidentate directing groups. Angew. Chem. Int. Ed. 2013, 52, 11726–11743. [Google Scholar] [CrossRef] [PubMed]
- Pichette Drapeau, M.; Gooßen, L.J. Carboxylic acids as directing groups for C-H bond functionalization. Chem. Eur. J. 2016, 22, 18654–18677. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Gandeepan, P.; Li, J.; Ackermann, L. Recent advances in positional-selective alkenylations: Removable guidance for twofold C-H activation. Org. Chem. Front. 2017, 4, 1435–1467. [Google Scholar] [CrossRef]
- Lyons, T.W.; Sanford, M.S. Palladium-catalyzed ligand-directed C-H functionalization reactions. Chem. Rev. 2010, 110, 1147–1169. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-H.; Engle, K.M.; Shi, B.-F.; Yu, J.-Q. Ligand-enabled reactivity and selectivity in a synthetically versatile aryl C-H olefination. Science 2010, 327, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Engle, K.M.; Yu, J.-Q. Developing Ligands for palladium(II)-catalyzed C–H functionalization: Intimate dialogue between ligand and substrate. J. Org. Chem. 2013, 78, 8927–8955. [Google Scholar] [CrossRef] [PubMed]
- Engle, K.M. The mechanism of palladium(II)-mediated C-H cleavage with mono-N-protected amino acid (MPAA) ligands: Origins of rate acceleration. Pure Appl. Chem. 2016, 88, 119–138. [Google Scholar] [CrossRef]
- Suna, E.; Shubin, K. Intramolecular coupling via C(sp2)-H activation. In Science of Synthesis. Cross Coupling and Heck-Type Reactions 3: Metal-Catalyzed Heck-Type Reactions and C-H Couplings via C-H Activation; Larhed, M., Ed.; Thieme: Stuttgart, Germany, 2013; Volume 3, pp. 643–724. [Google Scholar]
- Beck, E.M.; Gaunt, M.J. Pd-catalyzed C-H bond functionalization on the indole and pyrrole nucleus. Top. Curr. Chem. 2010, 292, 85–121. [Google Scholar] [CrossRef] [PubMed]
- Broggini, G.; Beccalli, E.M.; Fasana, A.; Gazzola, S. Palladium-catalyzed dual C-H or N-H functionalization of unfunctionalized indole derivatives with alkenes and arenes. Beilstein J. Org. Chem. 2012, 8, 1730–1746. [Google Scholar] [CrossRef] [PubMed]
- Schiffner, J.A.; Oestreich, M. All-carbon-substituted quaternary carbon atoms in oxindoles by an aerobic palladium(II)-catalyzed ring closure onto tri- and tetrasubstituted double bonds. Eur. J. Org. Chem. 2011, 2011, 1148–1154. [Google Scholar] [CrossRef]
- Ortiz-de-Elguea, V.; Sotomayor, N.; Lete, E. Two consecutive Palladium(II)-promoted C-H alkenylation reactions for the synthesis of 3-alkenylquinolones. Adv. Synth. Catal. 2015, 357, 463–473. [Google Scholar] [CrossRef]
- Grimster, N.P.; Gauntlett, C.; Godfrey, C.R.A.; Gaunt, M.J. Palladium-catalyzed intermolecular alkenylation of indoles by solvent-controlled regioselective C-H functionalization. Angew. Chem. Int. Ed. 2005, 44, 3125–3129. [Google Scholar] [CrossRef] [PubMed]
- García-Rubia, A.; Urones, B.; Gómez-Arrayás, R.; Carretero, J.C. Pd(II)-catalysed C-H functionalisation of indoles and pyrroles assisted by the removable N-(2-pyridyl)sulfonyl group: C2-alkenylation and dehydrogenative homocoupling. Chem. Eur. J. 2010, 16, 9676–9685. [Google Scholar] [CrossRef] [PubMed]
- Abbiati, G.; Beccalli, E.M.; Broggini, G.; Zoni, C. Regioselectivity on the palladium-catalyzed intramolecular cyclization of indole derivatives. J. Org. Chem. 2003, 68, 7625–7628. [Google Scholar] [CrossRef] [PubMed]
- García-Rubia, A.; Urones, B.; Gómez-Arrayás, R.; Carretero, J.C. PdII-catalyzed C-H olefination of N-(2-pyridyl)sulfonyl anilines and arylalkylamines. Angew. Chem. Int. Ed. 2011, 50, 10927–10931. [Google Scholar] [CrossRef] [PubMed]
- Kandukuri, S.R.; Schiffner, J.A.; Oestreich, M. Aerobic palladium(II)-catalyzed 5-endo-trig cyclization: An entry into the diastereoselective C-2 alkenylation of indoles with tri- and tetrasubstituted double bonds. Angew. Chem. Int. Ed. 2012, 51, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ferreira, E.M.; Stoltz, B.M. Direct oxidative Heck cyclizations: Intramolecular Fujiwara–Moritani arylations for the synthesis of functionalized benzofurans and dihydrobenzofurans. Angew. Chem. Int. Ed. 2004, 43, 6144–6148. [Google Scholar] [CrossRef] [PubMed]
- Kubota, A.; Emmert, M.H.; Sanford, M.S. Pyridine ligands as promoters in PdII/0-catalyzed C-H olefination reactions. Org. Lett. 2012, 14, 1760–1763. [Google Scholar] [CrossRef] [PubMed]
- Alternatively, to avoid the deprotection of the nitrogen, the corresponding N-(but-3-en-1-yl)-3,5-dimethoxy-N-methylaniline was prepared and submitted to cyclization conditions. However, only decomposition was observed under all conditions tested, using different palladium sources, oxidants and solvents, even in the absence of TsOH or AcOH.
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).