Review on Molecular Mechanisms of Antifouling Compounds: An Update since 2012
Abstract
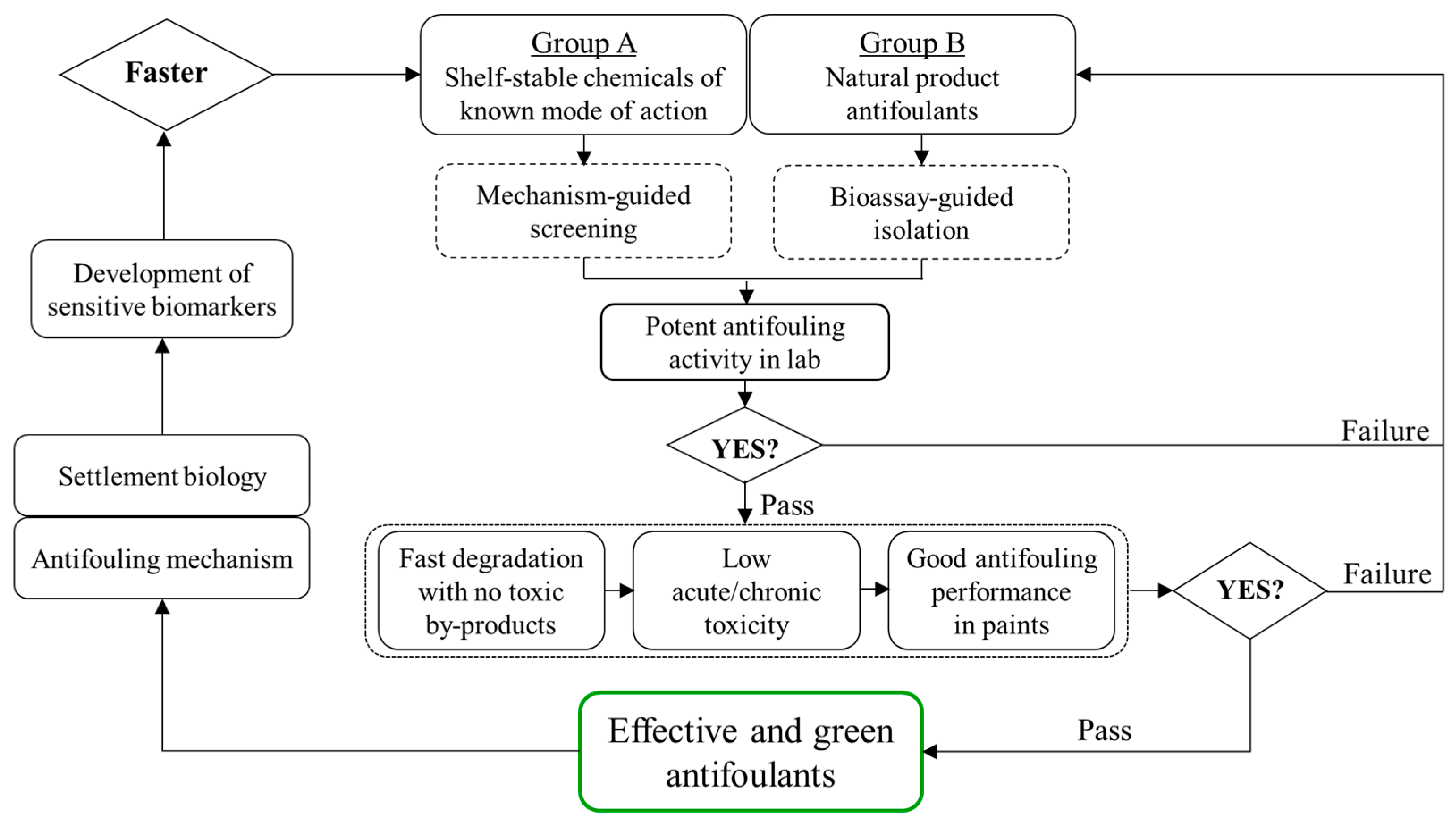
1. Introduction
2. Antifouling Compounds with Proposed Specific Targets
2.1. Inhibitors of Transmembrane Transport
2.2. Quorum Sensing Inhibitors
2.3. Neurotransmission Blockers
2.4. Adhesive Production/Release Inhibitors
2.5. Enzyme/Protein Inhibitors
3. Antifouling Compounds with Proposed General Targets
3.1. Protein Expression/Metabolic Activity Regulators
3.2. Oxidative Stress Inducers
3.3. Neurotransmission Blockers
3.4. Surface Modifiers
3.5. Biofilm Inhibitors
3.6. Adhesive Production/Release Inhibitors
3.7. Toxic Killing
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Antifouling technology-past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coat. 2004, 50, 75–104. [Google Scholar] [CrossRef]
- Callow, J.A.; Callow, M.E. Trends in the development of environmentally friendly fouling-resistant marine coatings. Nat. Commun. 2011, 2, 244. [Google Scholar] [CrossRef] [PubMed]
- Omae, I. Organotin antifouling paints and their alternatives. Appl. Organomet. Chem. 2003, 17, 81–105. [Google Scholar] [CrossRef]
- Voulvoulis, N. Antifouling paint booster biocides: Occurrence and partitioning in water and sediments. Handb. Environ. Chem. 2006, 5, 155–170. [Google Scholar]
- Qian, P.Y.; Chen, L.; Xu, Y. Mini-review: Molecular mechanisms of antifouling compounds. Biofouling 2013, 29, 381–400. [Google Scholar] [CrossRef] [PubMed]
- Fusetani, N. Biofouling and antifouling. Nat. Prod. Rep. 2004, 21, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Qian, P.Y.; Li, Z.; Xu, Y.; Li, Y.; Fusetani, N. Mini-review: Marine natural products and their synthetic analogs as antifouling compounds: 2009–2014. Biofouling 2015, 31, 101–122. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EU) No 528/2012 of the European Parliament and of the Council of 22 May 2012 Concerning the Making Available on the Market and Use of Biocidal Products; Official Journal of the European Union No. L167 of 27 June 2012; Office for Official Publications of the European Communities: Luxembourg, 2012.
- Soliman, Y.A.; Mohamed, A.S.; NaserGomaa, M. Antifouling activity of crude extracts isolated from two Red Sea puffer fishes. Egypt. J. Aquat. Res. 2014, 40, 1–7. [Google Scholar] [CrossRef]
- Yang, C.Y.; Yu, Y.N.; Sun, W.J.; Xia, C.H. Indole derivatives inhibited the formation of bacterial biofilm and modulated Ca2+ efflux in diatom. Mar. Pollut. Bull. 2014, 88, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Sun, W.; Liu, S.; Xia, C. Comparative effects of indole derivatives as antifouling agents on the growth of two marine diatom species. Chem. Ecol. 2015, 31, 299–307. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Wang, X.; Guo, Y.W.; Schröder, H.C. Potentiation of the cytotoxic activity of copper by polyphosphate on biofilm-producing bacteria: A bioinspired approach. Mar. Drugs 2012, 10, 2369–2387. [Google Scholar] [CrossRef] [PubMed]
- Bowie, D.; Parvizi, P.; Duncan, D.; Nelson, C.J.; Fyles, T.M. Chemical-genetic identification of the biochemical targets of polyalkyl guanidinium biocides. Org. Biomol. Chem. 2013, 11, 4359. [Google Scholar] [CrossRef] [PubMed]
- Quintana, J.; Brango-Vanegas, J.; Costa, G.M.; Castellanos, L.; Arévalo, C.; Duque, C. Marine organisms as source of extracts to disrupt bacterial communication: Bioguided isolation and identification of quorum sensing inhibitors from Ircinia felix. Rev. Bras. Farmacogn. 2015, 25, 199–207. [Google Scholar] [CrossRef]
- Jha, B.; Kavita, K.; Westphal, J.; Hartmann, A.; Schmitt-Kopplin, P. Quorum sensing inhibition by Asparagopsis taxiformis, a marine macro alga: Separation of the compound that interrupts bacterial communication. Mar. Drugs 2013, 11, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.; García, M.; Sánchez, M.; Stupak, M.; Mazzuca, M.; Palermo, J.A.; Blustein, G. Effect of secochiliolide acid isolated from the Patagonian shrub Nardophyllum bryoides as active component in antifouling paints. Int. Biodeter. Biodegrad. 2014, 89, 37–44. [Google Scholar] [CrossRef]
- Schwartz, N.; Dobretsov, S.; Rohde, S.; Schupp, P.J. Comparison of antifouling properties of native and invasive Sargassum (Fucales, Phaeophyceae) species. Eur. J. Phycol. 2017, 52, 116–131. [Google Scholar] [CrossRef]
- Batista, D.; Carvalho, A.; Costa, R.; Coutinho, R.; Dobretsov, S. Extracts of macroalgae from the Brazilian coast inhibit bacterial quorum sensing. Bot. Mar. 2014, 57, 441–447. [Google Scholar] [CrossRef]
- Abed, R.M.M.; Dobretsov, S.; Al-Fori, M.; Gunasekera, S.P.; Sudesh, K.; Paul, V.J. Quorum-sensing inhibitory compounds from extremophilic microorganisms isolated from a hypersaline cyanobacterial mat. J. Ind. Microbiol. Biotechnol. 2013, 40, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Kalinovskaya, N.I.; Romanenko, L.A.; Kalinovsky, A.I. Antibacterial low-molecular-weight compounds produced by the marine bacterium Rheinheimera japonica KMM 9513T. Anton. Van Leeuwenhoek 2017, 110, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Golberg, K.; Pavlov, V.; Marks, R.S.; Kushmaro, A. Coral-associated bacteria, quorum sensing disrupters, and the regulation of biofouling. Biofouling 2013, 29, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Martín-Rodríguez, A.J.; Reyes, F.; Martín, J.; Pérez-Yépez, J.; León-Barrios, M.; Couttolenc, A.; Espinoza, C.; Trigos, A.; Martín, V.S.; Norte, M.; et al. Inhibition of bacterial quorum sensing by extracts from aquatic fungi: First report from marine endophytes. Mar. Drugs 2014, 12, 5503–5526. [Google Scholar] [CrossRef] [PubMed]
- Hunt, L.R.; Smith, S.M.; Downum, K.R.; Mydlarz, L.D. Microbial regulation in gorgonian corals. Mar. Drugs 2012, 10, 1225–1243. [Google Scholar] [CrossRef] [PubMed]
- Tello, E.; Castellanos, L.; Arévalo-Ferro, C.; Duque, C. Disruption in quorum-sensing systems and bacterial biofilm inhibition by cembranoid diterpenes isolated from the octocoral Eunicea knighti. J. Nat. Prod. 2012, 75, 1637–1642. [Google Scholar] [CrossRef] [PubMed]
- Martín-Rodríguez, A.J.; Babarro, J.M.F.; Lahoz, F.; Sansón, M.; Martín, V.S.; Norte, M.; Fernández, J.J. From broad-spectrum biocides to quorum sensing disruptors and mussel repellents: Antifouling profile of alkyl triphenylphosphonium salts. PLoS ONE 2015, 10, e0123652. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, I.; Nam, J.; Hwang, D.S.; Yeon, K.M.; Kim, J. Immobilization and stabilization of acylase on carboxylated polyaniline nanofibers for highly effective antifouling application via quorum quenching. ACS Appl. Mater. Interfaces 2017. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Serra, S.; Abreu, A.C.; Saavedra, M.J.; Salgado, A.; Simões, M. Evaluation of the effects of selected phytochemicals on quorum sensing inhibition and in vitro cytotoxicity. Biofouling 2014, 30, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Liaqat, I.; Bachmann, R.T.; Edyvean, R.G.J. Type 2 quorum sensing monitoring, inhibition and biofilm formation in marine microrganisms. Curr. Microbiol. 2014, 68, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Nong, X.H.; Wang, Y.F.; Zhang, X.Y.; Zhou, M.P.; Xu, X.Y.; Qi, S.H. Territrem and butyrolactone derivatives from a marine-derived fungus Aspergillus terreus. Mar. Drugs 2014, 12, 6113–6124. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, M.; Svenson, J.; Sepcic, K.; Trembleau, L.; Engqvist, M.; Andersen, J.H.; Jaspars, M.; Stensvåg, K.; Haug, T. Isolation and synthesis of pulmonarins A and B, acetylcholinesterase inhibitors from the colonial ascidian Synoicum pulmonaria. J. Nat. Prod. 2014, 77, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Trepos, R.; Cervin, G.; Hellio, C.; Pavia, H.; Stensen, W.; Stensvåg, K.; Svendsen, J.-S.; Haug, T.; Svenson, J. Antifouling compounds from the sub-arctic ascidian Synoicum pulmonaria: Synoxazolidinones A and C, pulmonarins A and B, and synthetic analogues. J. Nat. Prod. 2014, 77, 2105–2113. [Google Scholar] [CrossRef] [PubMed]
- Grandič, M.; Zovko, A.; Frangež, R.; Turk, T.; Sepčić, K. Binding and permeabilization of lipid bilayers by natural and synthetic 3-alkylpyridinium polymers. Bioorg. Med. Chem. 2012, 20, 1659–1664. [Google Scholar] [CrossRef] [PubMed]
- Piazza, V.; Dragić, I.; Sepčić, K.; Faimali, M.; Garaventa, F.; Turk, T.; Berne, S. Antifouling activity of synthetic alkylpyridinium polymers using the barnacle model. Mar. Drugs 2014, 12, 1959–1976. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Qiu, J.; Miao, L.; Feng, K.; Zhou, X. Antifouling activities of anti-histamine compounds against the barnacle Amphibalanus (= Balanus) amphitrite. J. Exp. Mar. Biol. Ecol. 2014, 452, 47–53. [Google Scholar] [CrossRef]
- Jin, C.; Qiu, J.; Yu, S.; Miao, L.; Zhou, X. Histamine promotes the larval metamorphic competence of barnacle Amphibalanus amphitrite. Mar. Biol. Res. 2014, 10, 799–806. [Google Scholar] [CrossRef]
- Al-Aidaroos, A.M.; Satheesh, S.; Devassy, R.P. Effects of pharmacological compounds on the barnacle larval development, metabolism and settlement. Int. Biodeter. Biodegrad. 2017, 117, 190–196. [Google Scholar] [CrossRef]
- Hanssen, K.Ø.; Cervin, G.; Trepos, R.; Petitbois, J.; Haug, T.; Hansen, E.; Andersen, J.H.; Pavia, H.; Hellio, C.; Svenson, J. The bromotyrosine derivative ianthelline isolated from the arctic marine sponge Stryphnus fortis inhibits marine micro- and macrobiofouling. Mar. Biotechnol. 2014, 16, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Niemann, H.; Hagenow, J.; Chung, M.Y.; Hellio, C.; Weber, H.; Proksch, P. SAR of sponge-inspired hemibastadin congeners inhibiting blue mussel phenoloxidase. Mar. Drugs 2015, 13, 3061–3071. [Google Scholar] [CrossRef] [PubMed]
- Ananda Priya, K.; Satheesh, B.; Ashok Kumar, P.; Varalakshmi, G.; Sivakumar, N. Antifouling activity of prodigiosin from estuarine isolate of Serratia marcescens CMST 07. Microbiol. Res. Agro-Ecosyst. Manag. 2013, 16, 326. [Google Scholar]
- Cahill, P.; Heasman, K.; Jeffs, A.; Kuhajek, J.; Mountfort, D. Preventing ascidian fouling in aquaculture: Screening selected allelochemicals for anti-metamorphic properties in ascidian larvae. Biofouling 2012, 28, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Nong, X.H.; Zheng, Z.H.; Zhang, X.Y.; Lu, X.H.; Qi, S.H. Polyketides from a marine-derived fungus Xylariaceae sp. Mar. Drugs 2013, 11, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Villa, F.; Remelli, W.; Forlani, F.; Vitali, A.; Cappitelli, F. Altered expression level of Escherichia coli proteins in response to treatment with the antifouling agent zosteric acid sodium salt. Environ. Microbiol. 2012, 14, 1753–1761. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xia, C.; Qian, P.Y. Optimization of antifouling coatings incorporating butenolide, a potent antifouling agent via field and laboratory tests. Prog. Org. Coat. 2017, 109, 22–29. [Google Scholar] [CrossRef]
- Chen, L.; Xu, Y.; Wang, W.X.; Qian, P.Y. Degradation kinetics of a potent antifouling agent, butenolide, under various environmental conditions. Chemosphere 2015, 119, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ye, R.; Xu, Y.; Gao, Z.; Au, D.W.T.; Qian, P.Y. Comparative safety of the antifouling compound butenolide and 4,5-dichloro-2-n-octyl-4-isothiazolin-3-one (DCOIT) to the marine medaka (Oryzias melastigma). Aquat. Toxicol. 2014, 149, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, H.; Sun, J.; Wong, Y.H.; Han, Z.; Au, D.W.T.; Bajic, V.B.; Qian, P.Y. Proteomic changes in brain tissues of marine medaka (Oryzias melastigma) after chronic exposure to two antifouling compounds: Butenolide and 4,5-dichloro-2-n-octyl-4-isothiazolin-3-one (DCOIT). Aquat. Toxicol. 2014, 157, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Sun, J.; Zhang, H.; Au, D.W.T.; Lam, P.K.S.; Zhang, W.; Bajic, V.B.; Qiu, J.W.; Qian, P.Y. Hepatic proteomic responses in marine medaka (Oryzias melastigma) chronically exposed to antifouling compound butenolide [5-octylfuran-2(5H)-one] or 4,5-dichloro-2-n-octyl-4-isothiazolin-3-one (DCOIT). Environ. Sci. Technol. 2015, 49, 1851–1859. [Google Scholar] [CrossRef] [PubMed]
- Chandramouli, K.H.; Dash, S.; Zhang, Y.; Ravasi, T.; Qian, P.Y. Proteomic and metabolomic profiles of marine Vibrio sp. 010 in response to an antifoulant challenge. Biofouling 2013, 29, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, S.R.; Santhiyagu, P.; Singamuthu, M.; Ahila, N.K.; Jayaraman, R.; Ethiraj, K. Synthesis and characterization of silver and gold nanoparticles using aqueous extract of seaweed, Turbinaria conoides, and their antimicrofouling activity. Sci. World J. 2014. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, M.; Sivanandham, V.; Hans-Uwe, D.; Murugaiah, S.G.; Seeni, P.; Gopalan, S.; Rathinam, A.J. Antifouling assessments on biogenic nanoparticles: A field study from polluted offshore platform. Mar. Pollut. Bull. 2015, 101, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, G.; Sun, J.; Xu, Y.; Han, Z.; Liu, L.; Shao, C.; Liu, Q.; Wang, C.; Qian, P.Y. Cochliomycin A inhibits the larval settlement of Amphibalanus amphitrite by activating the NO/cGMP pathway. Biofouling 2016, 32, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Su, P.; Gu, Q.; Li, W.D.; Guo, J.L.; Qiao, W.; Feng, D.Q.; Tang, S.A. Antifouling activity against bryozoan and barnacle by cembrane diterpenes from the soft coral Sinularia flexibilis. Int. Biodeter. Biodegrad. 2017, 120, 97–103. [Google Scholar] [CrossRef]
- Lai, D.; Liu, D.; Deng, Z.; van Ofwegen, L.; Proksch, P.; Lin, W. Antifouling eunicellin-type diterpenoids from the gorgonian Astrogorgia sp. J. Nat. Prod. 2012, 75, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.; García, M.; Stupak, M.; Blustein, G. Synthesis and characterization of ferric sorbate and aluminum sorbate as antifouling pigments for marine paints. Ind. Eng. Chem. Res. 2014, 53, 3570–3577. [Google Scholar] [CrossRef]
- Fujiwara, S.; Akima, C.; Nogata, Y.; Yoshimura, E.; Chiba, K.; Kitano, Y. Bio-organic and anti-barnacle studies of fluorescence-labeled probe compounds against cyprids of barnacles. J. Exp. Mar. Biol. Ecol. 2013, 445, 88–92. [Google Scholar] [CrossRef]
- Salta, M.; Wharton, J.A.; Dennington, S.P.; Stoodley, P.; Stokes, K.R. Anti-biofilm performance of three natural products against initial bacterial attachment. Int. J. Mol. Sci. 2013, 14, 21757–21780. [Google Scholar] [CrossRef] [PubMed]
- Faÿ, F.; Carteau, D.; Linossier, I.; Delbury, M.; Vallée-Réhel, K. Joint-action of antifouling substances in copper-free paints. Colloid Surf. B 2013, 102, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Carteau, D.; Vallée-Réhel, K.; Linossier, I.; Quiniou, F.; Davy, R.; Compère, C.; Delbury, M.; Faÿ, F. Development of environmentally friendly antifouling paints using biodegradable polymer and lower toxic substances. Prog. Org. Coat. 2014, 77, 485–493. [Google Scholar] [CrossRef]
- Mostafaei, A.; Nasirpouri, F. Preparation and characterization of a novel conducting nanocomposite blended with epoxy coating for antifouling and antibacterial applications. J. Coat. Technol. Res. 2013, 10, 679–694. [Google Scholar] [CrossRef]
- Al-Naamani, L.; Dobretsov, S.; Dutta, J.; Burgess, J.G. Chitosan-zinc oxide nanocomposite coatings for the prevention of marine biofouling. Chemosphere 2017, 168, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Abiraman, T.; Balasubramanian, S. Synthesis and characterization of large-scale (<2 nm) chitosan-decorated copper nanoparticles and their application in antifouling coating. Ind. Eng. Chem. Res. 2017, 56, 1498–1508. [Google Scholar]
- Castro, K.A.D.F.; Moura, N.M.M.; Fernandes, A.; Faustino, M.A.F.; Simões, M.M.Q.; Cavaleiro, J.A.S.; Nakagaki, S.; Almeida, A.; Cunha, Â. Control of Listeria innocua biofilms by biocompatible photodynamic antifouling chitosan based materials. Dyes Pigm. 2017, 137, 265–276. [Google Scholar] [CrossRef]
- Venkatnarayanan, S.; Murthy, P.S.; Kirubagaran, R.; Venugopalan, V.P. Chlorine dioxide as an alternative antifouling biocide for cooling water systems: Toxicity to larval barnacle Amphibalanus reticulatus (Utinomi). Mar. Pollut. Bull. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.Y.; Bangoura, I.; Cho, J.Y.; Joo, J.; Choi, Y.S.; Hwang, D.S.; Hong, Y.K. Antifouling effects of the periostracum on algal spore settlement in the mussel Mytilus edulis. Fish. Aquat. Sci. 2016, 19, 7. [Google Scholar] [CrossRef]
- Cahill, P.L.; Heasman, K.; Jeffs, A.; Kuhajek, J. Laboratory assessment of the antifouling potential of a soluble-matrix paint laced with the natural compound polygodial. Biofouling 2013, 29, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Moodie, L.W.K.; Trepos, R.; Cervin, G.; Larsen, L.; Larsen, D.S.; Pavia, H.; Hellio, C.; Cahill, P.; Svenson, J. Probing the structure–activity relationship of the natural antifouling agent polygodial against both micro- and macrofoulers by semisynthetic modification. J. Nat. Prod. 2017, 80, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.E.; Pandit, S.; Jeon, J.G. Identification of linoleic acid, a main component of the n-hexane fraction from Dryopteris crassirhizoma, as an anti-Streptococcus mutans biofilm agent. Biofouling 2014, 30, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Trepos, R.; Cervin, G.; Pile, C.; Pavia, H.; Hellio, C.; Svenson, J. Evaluation of cationic micropeptides derived from the innate immune system as inhibitors of marine biofouling. Biofouling 2015, 31, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Y.P.; Yao, K.; Wilbon, P.A.; Zhang, W.; Ren, L.; Zhou, J.; Nagarkatti, M.; Wang, C.; Chu, F.; He, X.; Decho, A.W.; Tang, C. Robust antimicrobial compounds and polymers derived from natural resin acids. Chem. Commun. 2012, 48, 916–918. [Google Scholar] [CrossRef] [PubMed]
- Protasov, A.; Bardeau, J.-F.; Morozovskaya, I.; Boretska, M.; Cherniavska, T.; Petrus, L.; Tarasyuk, O.; Metelytsia, L.; Kopernyk, I.; Kalashnikova, L.; Dzhuzha, O.; Rogalsky, S. New promising antifouling agent based on polymeric biocide polyhexamethylene guanidine molybdate. Environ. Toxicol. Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.; García, M.; Blustein, G. Evaluation of low copper content antifouling paints containing natural phenolic compounds as bioactive additives. Mar. Environ. Res. 2015, 109, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Mesarič, T.; Sepčič, K.; Piazza, V.; Gambardella, C.; Garaventa, F.; Drobne, D.; Faimali, M. Effects of nano carbon black and single-layer graphene oxide on settlement, survival and swimming behaviour of Amphibalanus amphitrite larvae. Chem. Ecol. 2013, 29, 643–652. [Google Scholar] [CrossRef]
- Viju, N.; Satheesh, S.; Vincent, S.G.P. Antibiofilm activity of coconut (Cocos nucifera Linn.) husk fibre extract. Saudi J. Biol. Sci. 2013, 20, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.; García, M.; Ruiz, D.; Autino, J.C.; Romanelli, G.; Blustein, G. Antifouling activity of green-synthesized 7-hydroxy-4-methylcoumarin. Mar. Environ. Res. 2016, 113, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Peres, R.S.; Armelin, E.; Alemán, C.; Ferreira, C.A. Modified tannin extracted from black wattle tree as an environmentally friendly antifouling pigment. Ind. Crops Prod. 2015, 65, 506–514. [Google Scholar] [CrossRef]
- Punitha, N.; Saravanan, P.; Mohan, R.; Ramesh, P.S. Antifouling activities of β-cyclodextrin stabilized peg based silver nanocomposites. Appl. Surf. Sci. 2017, 392, 126–134. [Google Scholar] [CrossRef]
- Kharchenko, U.; Beleneva, I.; Dmitrieva, E. Antifouling potential of a marine strain, Pseudomonas aeruginosa 1242, isolated from brass microfouling in Vietnam. Int. Biodeter. Biodegrad. 2012, 75, 68–74. [Google Scholar] [CrossRef]
- Ishimaru, N.; Tsukegi, T.; Wakisaka, M.; Shirai, Y.; Nishida, H. Effects of poly(l-lactic acid) hydrolysis on attachment of barnacle cypris larvae. Polym. Degrad. Stab. 2012, 97, 2170–2176. [Google Scholar] [CrossRef]
- Wang, K.; Xu, Y.; Lu, L.; Li, Y.; Han, Z.; Zhang, J.; Shao, C.; Wang, C.; Qian, P.Y. Low-toxicity diindol-3-ylmethanes as potent antifouling compounds. Mar. Biotechnol. 2015, 17, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ye, R.; Zhang, W.; Hu, C.; Zhou, B.; Peterson, D.R.; Au, D.W.T.; Lam, P.K.S.; Qian, P.Y. Endocrine disruption throughout the hypothalamus-pituitary-gonadal-liver (HPGL) axis in marine medaka (Oryzias melastigma) chronically exposed to 3,3′-diindolylmethane (DIM). Chem. Res. Toxicol. 2016, 29, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Au, D.W.T.; Hu, C.; Zhang, W.; Zhou, B.; Cai, L.; Giesy, J.P.; Qian, P.Y. Linking genomic responses of gonads with reproductive impairment in marine medaka (Oryzias melastigma) exposed chronically to the chemopreventive and antifouling agent, 3,3′-diindolylmethane (DIM). Aquat. Toxicol. 2017, 183, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Costas, E.; Gonzalez, R.; Lo’pez-Rodas, V.; Huertas, I.E. Mutation of microalgae from antifouling sensitivity to antifouling resistance allows phytoplankton dispersal through ships’ biofouling. Biol. Invasions 2013, 15, 1739–1750. [Google Scholar] [CrossRef]
- Lu, J.; Feng, J.; Cai, S.; Chen, Z. Metabolomic responses of Haliotis diversicolor to organotin compounds. Chemosphere 2017, 168, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Tosti, E. Adverse effect of antifouling compounds on the reproductive mechanisms of the ascidian Ciona intestinalis. Mar. Drugs 2013, 11, 3554–3568. [Google Scholar] [CrossRef] [PubMed]
- Cima, F.; Ballarin, L. Immunotoxicity in ascidians: Antifouling compounds alternative to organotins—IV. The case of zinc pyrithione. Comp. Biochem. Phys. C 2015, 169, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Higley, E.; Tompsett, A.R.; Giesy, J.P.; Hecker, M.; Wiseman, S. Effects of triphenyltin on growth and development of the wood frog (Lithobates sylvaticus). Aquat. Toxicol. 2013, 144–145, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Iyapparaj, P.; Revathi, P.; Ramasubburayan, R.; Prakash, S.; Anantharaman, P.; Immanuel, G.; Palavesam, A. Antifouling activity of the methanolic extract of Syringodium isoetifolium, and its toxicity relative to tributyltin on the ovarian development of brown mussel Perna indica. Ecotoxicol. Environ. Saf. 2013, 89, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Mai, H.; Cachot, J.; Brune, J.; Geffard, O.; Belles, A.; Budzinski, H.; Morin, B. Embryotoxic and genotoxic effects of heavy metals and pesticides on early life stages of pacific oyster (Crassostrea gigas). Mar. Pollut. Bull. 2012, 64, 2663–2670. [Google Scholar] [CrossRef] [PubMed]
- Johansson, P.; Eriksson, K.M.; Axelsson, L.; Blanck, H. Effects of seven antifouling compounds on photosynthesis and inorganic carbon use in sugar kelp Saccharina latissima (Linnaeus). Arch. Environ. Contam. Toxicol. 2012, 63, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ferandin, S.; Leroy, F.; Bouget, F.Y.; Joux, F. A new, sensitive marine microalgal recombinant biosensor using luminescence monitoring for toxicity testing of antifouling biocides. Appl. Environ. Microbiol. 2013, 79, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Gao, K.; Sun, J. Physiological and biochemical responses of Synechococcus sp. PCC7942 to Irgarol 1051 and diuron. Aquat. Toxicol. 2012, 122–123, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Arrhenius, Å.; Backhaus, T.; Hilvarsson, A.; Wendt, I.; Zgrundo, A.; Blanck, H. A novel bioassay for evaluating the efficacy of biocides to inhibit settling and early establishment of marine biofilms. Mar. Pollut. Bull. 2014, 87, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liang, B.; Li, L.; Liu, W. Induction of HepG2 cell apoptosis by Irgarol 1051 through mitochondrial dysfunction and oxidative stresses. Toxicol. In Vitro 2013, 27, 1771–1779. [Google Scholar] [CrossRef] [PubMed]
- Cima, F.; Ferrari, G.; Ferreira, N.G.C.; Rocha, R.J.M.; Serôdio, J.; Loureiro, S.; Calado, R. Preliminary evaluation of the toxic effects of the antifouling biocide Sea-Nine 211™ in the soft coral Sarcophyton cf. glaucum (Octocorallia, Alcyonacea) based on PAM fluorometry and biomarkers. Mar. Environ. Res. 2013, 83, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Mochida, K.; Ito, K.; Onduka, T.; Fujii, K. Induction of apoptosis in testis of the marine teleost mummichog Fundulus heteroclitus after in vivo exposure to the antifouling biocide 4,5-dichloro-2-n-octyl-3(2H)-isothiazolone (Sea-Nine 211). Chemosphere 2013, 90, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, W.; Ye, R.; Hu, C.; Wang, Q.; Seemann, F.; Au, D.W.T.; Zhou, B.; Giesy, J.P.; Qian, P.Y. Chronic exposure of marine medaka (Oryzias melastigma) to 4,5-dichloro-2-n-octyl-4-isothiazolin-3-one (DCOIT) reveals its mechanism of action in endocrine disruption via the hypothalamus-pituitary-gonadal-liver (HPGL) axis. Environ. Sci. Technol. 2016, 50, 4492–4501. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Au, D.W.T.; Hu, C.; Peterson, D.R.; Zhou, B.; Qian, P.Y. Identification of molecular targets for 4,5-dichloro-2-n-octyl-4-isothiazolin-3-one (DCOIT) in teleosts: New insight into mechanism of toxicity. Environ. Sci. Technol. 2017, 51, 1840–1847. [Google Scholar] [CrossRef] [PubMed]
- Khanam, M.R.M.; Shimasaki, Y.; Hosain, M.Z.; Mukai, K.; Tsuyama, M.; Qiu, X.; Tasmin, R.; Goto, H.; Oshima, Y. Diuron causes sinking retardation and physiochemical alteration in marine diatoms Thalassiosira pseudonana and Skeletonema marinoi-dohrnii complex. Chemosphere 2017, 175, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Ohlauson, C.; Blanck, H. A comparison of toxicant-induced succession for five antifouling compounds on marine periphyton in SWIFT microcosms. Biofouling 2014, 30, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Avelelas, F.; Martins, R.; Oliveira, T.; Maia, F.; Malheiro, E.; Soares, A.M.V.M.; Loureiro, S.; Tedim, J. Efficacy and ecotoxicity of novel anti-fouling nanomaterials in target and non-target marine species. Mar. Biotechnol. 2017, 19, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Tosti, E. Reprotoxicity of the antifoulant chlorothalonil in ascidians: An ecological risk assessment. PLoS ONE 2015, 10, e0123074. [Google Scholar] [CrossRef] [PubMed]
| Proposed Molecular Mechanism and Targets | Compounds | Activity | Category | Sources | Toxicity | References |
|---|---|---|---|---|---|---|
| Inhibitors of Transmembrane Transport | ||||||
| Blocking selectively the sodium channel to paralyze the peripheral neuromuscular system | Crude toxin extracts | Antifouling in paint | Natural product | Puffer fish Amblyrhynchotes hypselogenion and Lagocephalus sceleratus | Toxic | [9] |
| Triggering algal cellular Ca2+ efflux | Gramine, 6-chloroindole, 7-chloroindole, 6-bromoindole | Antibacterial and anti-algae | Shelf-stable | Halogenated indole derivatives | Non-toxic | [10,11] |
| Removing Ca2+ from the cell membrane and causing cell death | Polyphosphate | Antibacterial | Shelf-stable | Orthophosphate polymer | Non-toxic | [12] |
| Affecting tryptophan amino acid import through membrane | Alkylated guanidinium compounds | Antimicrobial (yeast Saccharomyces cerevisae) | Shelf-stable | Synthetic in lab | Non-toxic | [13] |
| Quorum Sensing Inhibitors | ||||||
| Inhibiting quorum sensing | Furanosesterterpenes | Antibacterial | Natural product | Spong Ircinia felix | Non-toxic | [14] |
| Quorum sensing inhibition | 2-Dodecanoyloxyethanesulfonate | Antibacterial | Natural product | Red alga Asparagopsis taxiformis | Non-toxic | [15] |
| Inhibiting biofilm formation through interference with quorum sensing | Secochiliolide acid | Antifouling (diatom, algae, bryozoan, tubeworm, ascidian) | Natural product | Patagonian shrub Nardophyllum bryoides | Non-toxic | [16] |
| Quorum sensing inhibition | Crude extract | Antibacterial; antifouling (diatom, bryozoan Bugula neritina) | Natural product | Invasive brown macroalga Sargassum muticum | Non-toxic | [17] |
| Inhibiting quorum sensing | Crude extract | Antibacterial | Natural product | Macroalgae from the Brazilian coast | Non-toxic | [18] |
| Bacteiral quorum-sensing inhibitory activity | Diketopiperazines | Antibacterial | Natural product | Microorganism Marinobacter sp. SK-3 and Rheinheimera japonica KMM 9513T | Non-toxic | [19,20] |
| Potent quorum-sensing attenuation to inhibit the growth of biofilms | A low molecular mass compound | Antibacterial | Natural product | Coral-associated bacterial isolates | Non-toxic | [21] |
| Quorum-sensing inhibitory activity | A combination of fungal secondary metabolites and fatty acids | Antibacterial | Natural product | Marine endophytic fungal isolates from coral Diploria strigosa | Non-toxic | [22] |
| Quorum-sensing inhibition | Ethanol extracts | Antibacterial | Natural product | Gorgonian corals Pseudopterogorgia americana, P. acerosa, and Pseudoplexuara flexuosa | Non-toxic | [23] |
| Quorum-sensing inhibition and biofilm inhibition | Cembranoid diterpenes | Antibacterial | Natural product | Caribbean gorgonian Eunicea knighti | Non-toxic | [24] |
| Non-toxic quorum sensing disruptors | Alkyl triphenylphosphonium Salts | Antimicrobial (marine bacteria, fungi, diatom); Antifouling (macroalgae Gayralia oxysperma, mussel Mytilus galloprovincialis) | Shelf-stable | Synthetic in lab | Non-toxic | [25] |
| Hydrolysis of N-acyl homoserine lactone (AHL) autoinducers | Acylase | Antibacterial | Shelf-stable | Enzymes | Non-toxic | [26] |
| Quorum sensing inhibition by modulating AHL activity and synthesis | Allylisothiocyanate, benzylisothiocyanate and 2-phenylethylisothiocyanate | Antibacterial | Shelf-stable | Isothiocyanates | Non-toxic | [27] |
| Inhibitory effect on luxS-encoded autoinducer 2 signaling | Patulin and penicillic acid | Antibacterial | Shelf-stable | Mycotoxin | Toxic | [28] |
| Neurotransmission Blockers | ||||||
| Strong inhibitor of acetylcholine esterase (AChE) | Territrem derivatives | Antifouling (Balanus amphitrite) | Natural product | Marine-derived fungus Aspergillus terreus SCSGAF0162 | Non-toxic | [29] |
| Reversible and noncompetitive AChE inhibitors | Pulmonarins A and B | Antibacterial | Natural product | Sub-Arctic ascidian Synoicum pulmonaria | Non-toxic | [30,31] |
| Interruption of cholinergic system through AChE inhibition | 3-Alkylpyridinium oligomers and polymers (3-APS) | Antimicrobial (bacteria, fungi); antifouling | Shelf-stable | Cholinergic antagonist | Non-toxic | [32] |
| Competition with acetylcholine to receptors and inhibition of the cholinergic system | Poly-APS analog APS8 | Antifouling (B. amphitrite) | Shelf-stable | Synthetic in lab | Non-toxic | [33] |
| Influencing histamine neurotransmitter signaling for photoreceptors | Triprolidine and cetirizine | Antifouling (B. amphitrite) | Shelf-stable | Histamine receptor antagonist | Non-toxic | [34,35,36] |
| Adhesive Production/Release Inhibitors | ||||||
| Strong inhibitors of blue mussel phenoloxidase | Bromotyrosine derivative ianthelline | Antibacterial; antifouling (microalgae, barnacle B. improvisus, blue mussel M. edulis) | Natural product | Arctic marine sponge Stryphnus fortis | Non-toxic | [37] |
| Potent inhibitors of blue mussel phenoloxidase | Hemibastadin derivatives | Antifouling (blue mussel M. edulis) | Shelf-stable | Synthetic in lab | Non-toxic | [38] |
| Inhibitory activity on tyrosinase for mussel byssal production | Alkyl triphenylphosphonium salts | Antimicrobial (marine bacteria, fungi, diatom); Antifouling (macroalgae G. oxysperma, mussel M. galloprovincialis) | Shelf-stable | Synthetic in lab | Non-toxic | [25] |
| Enzyme/Protein Inhibitors | ||||||
| Inhibiting target DNA modulating enzymes to block bacterial growth | Red pigment prodigiosin | Antibacterial; antifouling (cyanobacteria Synechococcus sp.; B. amphitrite) | Natural product | Serratia marcescens CMST 07 | Non-toxic | [39] |
| Interference with HSP-90 to inhibit metamorphosis | Radicicol and polygodial | Antifouling (ascidian Ciona savignyi) | Shelf-stable | Allelochemicals | Non-toxic | [40] |
| Glucosidase inhibition to affect energy production | Dibutylphthalate | Antibacterial | Natural product | Marine bacterium R. japonica KMM 9513T | Non-toxic | [20] |
| Enzymatic inhibitory activities towards Src homology 2 domain-containing phosphotyrosine phosphatase and inosine monophosphate dehydrogenase | Dicitrinin A | Antifouling (B. neritina) | Natural product | Marine gorgonian-derived fungal strain Xylariaceae sp. SCSGAF0086 | Non-toxic | [41] |
| Inhibitory activity towards cathepsin B | Phenol A acid | Antifouling (B. neritina) | Natural product | Marine gorgonian-derived fungal strain Xylariaceae sp. SCSGAF0086 | Non-toxic | [41] |
| Proposed Molecular Mechanism and Targets | Compounds | Activity | Category | Sources | Toxicity | References |
|---|---|---|---|---|---|---|
| Protein Expression/Metabolic Activity Regulators | ||||||
| Leading to global stress on cells and favoring the expression of quorum-sensing and flagella synthesis | Zosteric acid sodium salt | Antibacterial | Shelf-stable | Synthetic in lab | Non-toxic | [42] |
| Initiating detoxifying systems to ensure fast elimination from organisms and lower non-specific toxicity | Butenolide | Antibacterial; antifouling (barnacle Balanus amphitrite; tubeworm Hydroides elegans; bryozoan Bugula neritina) | Natural product | Streptomyces albidoflavus strain UST040711-291 | Non-toxic | [43,44,45,46,47] |
| Affecting protein expression related to nucleotide metabolism, the glyoxylate cycle, and stress responses | Poly-ether B | Antibacterial | Natural product | Sponge-associated bacterium Winogradskyella poriferorum | Non-toxic | [48] |
| Binding with thiol groups of DNA and RNA and affect the protein biosynthesis of bacteria | Biogenic silver nanoparticles | Antibacterial; antifouling (barnacle B. amphitrite) | Natural product | Brown alga Turbinaria ornata and T. conoides | Non-toxic | [49,50] |
| Affected the cytochrome P450, glutathione S-transferase (GST) and NO/cGMP pathways | Cochliomycin A | Antifouling (barnacle B. amphitrite) | Natural product | Fungus Cochliobolus | Non-toxic | [51] |
| Reducing the expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase 2 (COX-2) | Diterpenes: (−)14-deoxycrassin | Antifouling (bryozoan B. neritina, barnacle B. albicostatus) | Natural product | Soft coral Sinularia flexibilis | Non-toxic | [52] |
| Inhibitory activities of cell division and growth | Eunicellin-type diterpenoids | Antifouling (barnacle B. amphitrite) | Natural product | Chinese gorgonian Astrogorgia sp. | Non-toxic | [53] |
| Increasing metabolic activity, depleting energy reserve of cyprids and retarding settlement | Atrovastatin | Antifouling (barnacle B. amphitrite) | Shelf-stable | Lipid-regulating compound | Non-toxic | [36] |
| Lowering pH values and releasing sorbic acid into the cytoplasm to inhibit many metabolic functions | Ferric sorbate and aluminum sorbate | Antifouling in paint (diatom, seaweed, barnacle, tubeworm, bryozoan, ascidian) | Shelf-stable | Synthetic in lab | Non-toxic | [54] |
| Acting in the oil cell region and attaching to the carapace surface to induce agglutination of cyprids | Fluorescent probes | Antifouling (barnacle B. amphitrite) | Shelf-stable | Synthetic in lab | Non-toxic | [55] |
| Able to inhibit RNA transcription | Usnic acid | Antibacterial | Shelf-stable | Dibenzofuran derivative | Non-toxic | [56] |
| Oxidative Stress Inducers | ||||||
| Enzymatic generation of hydrogen peroxide (H2O2) by hexose oxidase | Crude extract | Antibacterial | Natural product | Red seaweed Chondrus crispus | Non-toxic | [56] |
| Reacting with seawater to create H2O2 | Zinc peroxide (ZnO2) | Antibacterial and antifouling | Shelf-stable | Strong oxidizing agent | Non-toxic | [57,58] |
| Production of H2O2 on the surface of the coating | Zinc oxide nanorod (ZnO) | Antibacterial; antifouling (algae, barnacle) in field | Shelf-stable | Synthetic in lab | Non-toxic | [59] |
| Photocatalytic generation of reactive oxygen species by ZnO nanoparticles | Chitosan/ZnO nanocomposite | Antimicrobial (bacteria, fungi, microalgae) | Shelf-stable | Synthetic in lab | Non-toxic | [60] |
| Formation of reactive oxygen species resulting in cell death | Chitosan-decorated copper nanoparticles | Antibacterial | Shelf-stable | Synthetic in lab | Non-toxic | [61] |
| Producing reactive oxygen species to selectively kill microorganisms | Chitosan-porphyrin films | Antibacterial (Listeria innocua) | Shelf-stable | Synthetic in lab | Non-toxic | [62] |
| Attacking the sulfhydryl groups of biomolecules | Chlorine dioxide | Antibacterial; antifouling (barnacle B. reticulatus) | Shelf-stable | Potent oxidant | Toxic | [63] |
| Interfering with vital cell processes | Juglone | Antibacterial | Shelf-stable | Potent oxidant | Non-toxic | [56] |
| Neurotransmission Blockers | ||||||
| Interacting with multiple neurotransmitter systems | Oleamide | Antifouling (algae Porphyra suborbiculata) | Natural product | Marine mussels (Mytilus edulis) | Non-toxic | [64] |
| Affecting the concentration of methyl farnesoate, a potential crustacean hormone | Atrovastatin | Antifouling (barnacle B. amphitrite) | Shelf-stable | Lipid-regulating compound | Non-toxic | [36] |
| Surface Modifiers | ||||||
| Nonionic surfactant properties to disrupt the cell membrane | Polygodial | Antibacterial; antifouling (microalgae, Ascidian Ciona savignyi, barnacle B. improvisus, mussel, tubeworm) | Natural product | Canelo tree Drimys winteri | Non-toxic | [65,66] |
| Surfactant and lysis of cell membrane and microbes | 3-Alkylpyridinium oligomers and polymers (3-APS) | Antimicrobial (bacteria, fungi); antifouling | Shelf-stable | Synthetic in lab | Non-toxic | [32] |
| Detergent properties at high concentrations to solubilize the membrane | Linoleic acid | Antibacterial | Natural product | Semi-evergreen plant Dryopteris crassirhizoma | Non-toxic | [67] |
| Interacting with bacterial membrane to allow for membrane insertion | Cationic micropeptides | Antibacterial; antifouling (algae, barnacle B. improvisus) | Shelf-stable | Synthetic in lab | Non-toxic | [68] |
| Selective lysis of microbial membranes and subsequent killing of bacteria | Natural resin acid-derived cationic compounds and polymers | Antibacterial | Shelf-stable | Synthetic in lab | Non-toxic | [69] |
| Interacting with the negative charges of the microbial cell membrane due to cationic nature of chitosan | Chitosan/ZnO nanocomposite | Antimicrobial (bacteria, fungi, microalgae) | Shelf-stable | Synthetic in lab | Non-toxic | [60] |
| Interacting and decomposing the negatively-charged cell membrane | Polyhexamethylene guanidine molybdate | Antibacterial; antifouling (bryozoan, Dreissenidae mollusk) | Shelf-stable | Synthetic in lab | Non-toxic | [70] |
| Cationic binding to negatively charged bacterial cell walls | Chlorhexidine | Antibacterial and antifouling | Shelf-stable | Cationic molecule | Non-toxic | [57,58] |
| Interacting with the lipid bilayer of cytoplasmic membranes and causing loss of integrity | Thymol and eugenol | Antifouling (barnacle, tubeworm, bryozoan, ascidian, algae) | Shelf-stable | Lipophilic phenolic compounds | Non-toxic | [71] |
| Altering the roughness of surfaces and the contacts of cyprid antennular discs | Nano-sized carbon black | Antifouling (barnacle B. amphitrite) | Shelf-stable | Carbon-based nanomaterials | Non-toxic | [72] |
| Increasing hydrophilic surface and thereby reducing the adhesion of microorganisms | Tween 85 | Antibacterial and antifouling | Shelf-stable | Non-ionic surfactant | Non-toxic | [57,58] |
| Affecting the EPS production, growth and the surface hydrophobicity of the biofilm-forming bacteria | Coconut husk extract (phenolic compounds) | Antibacterial | Natural product | Coconut Cocos nucifera L. | Non-toxic | [73] |
| Biofilm Inhibitors | ||||||
| Inhibition of bacterial nucleic acid synthesis and reduce biofilm formation via quorum sensing inhibition | 7-Hydroxy-4-methylcoumarin | Antibacterial; antifouling (diatom, algae, bryozoan, tubeworm, ascidian, mussel) | Shelf-stable | Synthetic in lab | Non-toxic | [74] |
| Removing metals essential for the growth of microorganisms | Modified black wattle tannin | Antibacterial; Antifouling in field | Shelf-stable | Chemically modified in lab | Non-toxic | [75] |
| Binding to sulfur and phosphorus containing biomolecules and causing cell damage | Poly ethylene glycol based silver nanocomposites | Antibacterial | Shelf-stable | Synthetic in lab | Non-toxic | [76] |
| Adhesive Production/Release Inhibitors | ||||||
| Proteolytic and amylase enzyme activity on the adhesives of settling organisms | Bacterial immobilization in paint (“living paint”) | Antibacterial; antifouling (diatom, polychaete, bryozoan, algae) | Natural product | Marine strain Pseudomonas aeruginosa 1242 | Non-toxic | [77] |
| Inhibiting cross-linking reactions of cement proteins due to acidity | Poly(l-lactic acid) | Antifouling (barnacle B. amphitrite) in lab and field | Shelf-stable | Synthetic in lab | Non-toxic | [78] |
| Toxic Killing | ||||||
| Strong endocrine disruptor | 3,3′-Diindolylmethane | Antifouling (barnacle B. Amphitrite, bryozoan B. neritina) | Natural product | Pseudovibrio denitrificans UST4-50 | Toxic | [79,80,81] |
| Disturbing energy metabolism and osmotic balance; induce oxidative stress; immunosuppression; reproductive impairment; disrupting signaling transduction | Organotin | Antifouling | Heavy metal | Organometallics | Toxic | [82,83,84,85,86,87] |
| Increasing larval abnormalities and DNA damage | Copper; cadmium | Antifouling | Heavy metal | Toxic | [88] | |
| Inhibiting the photosynthesis; genotoxic; oxidative stress; inhibiting cell cycle and inducing apoptosis | Irgarol 1051 | Antifouling | Booster biocide | Herbicide | Toxic | [88,89,90,91,92,93] |
| Inhibiting the photosynthesis; oxidative stress; endocrine disruption and reproductive impairment | Sea-Nine 211 | Antifouling | Booster biocide | Isothiazolone compound | Toxic | [45,46,47,89,92,94,95,96,97] |
| Inhibiting the photosynthesis; oxidative stress; inhibiting cell cycle and hatching; reproductive impairment | Diuron | Antifouling | Booster biocide | Herbicide | Toxic | [84,89,90,91,98] |
| Disrupting the cell membrane through apoptosis | Copper pyrithione | Antialgae | Booster biocide | Fungicide | Toxic | [92] |
| Changing the composition of the periphyton community; immunosuppressive toxicity; oxidative stress | Zinc pyrithione | Antifouling | Booster biocide | Bactericide; fungicide; algicide | Toxic | [85,99,100] |
| Inhibition of photosynthesis and carbon incorporation | Dichlofluanid | Antialgae | Booster biocide | Fungicide | Toxic | [89] |
| Inhibition of photosynthesis and carbon incorporation; disrupting folate synthesis and inhibiting thiol-containing enzymes | Tolyfluanid | Antialgae | Booster biocide | Fungicide | Toxic | [89,92] |
| Inhibiting the photosynthesis; reproductive impairment and teratogenic | Chlorothalonil | Antifouling | Booster biocide | Fungicide | Toxic | [89,101] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Qian, P.-Y. Review on Molecular Mechanisms of Antifouling Compounds: An Update since 2012. Mar. Drugs 2017, 15, 264. https://doi.org/10.3390/md15090264
Chen L, Qian P-Y. Review on Molecular Mechanisms of Antifouling Compounds: An Update since 2012. Marine Drugs. 2017; 15(9):264. https://doi.org/10.3390/md15090264
Chicago/Turabian StyleChen, Lianguo, and Pei-Yuan Qian. 2017. "Review on Molecular Mechanisms of Antifouling Compounds: An Update since 2012" Marine Drugs 15, no. 9: 264. https://doi.org/10.3390/md15090264
APA StyleChen, L., & Qian, P.-Y. (2017). Review on Molecular Mechanisms of Antifouling Compounds: An Update since 2012. Marine Drugs, 15(9), 264. https://doi.org/10.3390/md15090264





