Marine Lipids on Cardiovascular Diseases and Other Chronic Diseases Induced by Diet: An Insight Provided by Proteomics and Lipidomics
Abstract
1. Introduction
1.1. Chronic Diseases Induced by Diet: A World Health Problem
1.2. Marine Lipids as Bioactive Compounds against MetS and Chronic Diseases Induced by Diet
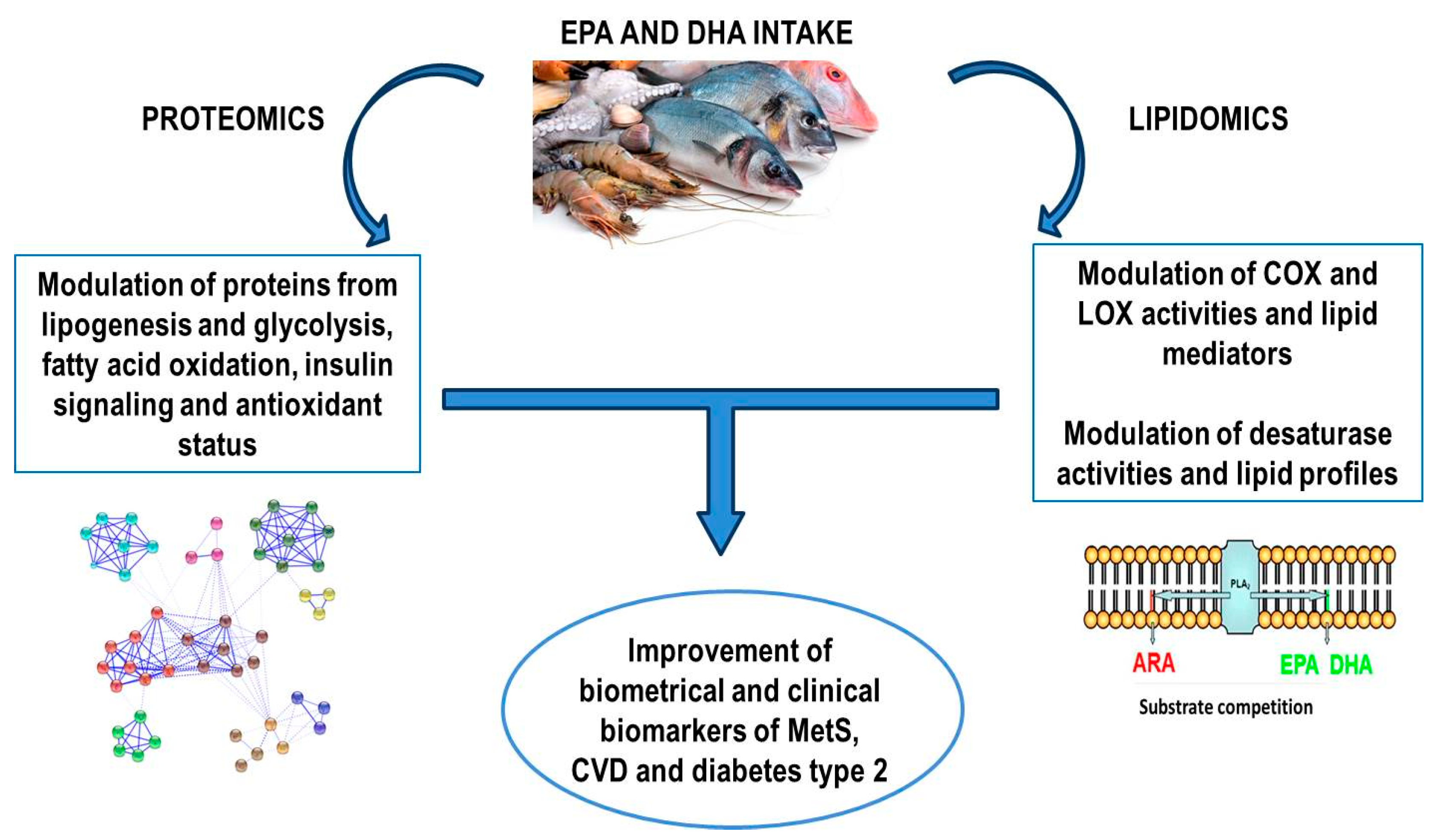
2. Omics for Unrevealing Mechanisms: Proteomics and Lipidomics
2.1. Beneficial Effects of Marine Lipids Intake Assayed by Proteomics
2.1.1. Proteomics in Clinical Trials
2.1.2. Proteomics in Animal Models and Cell Cultures
2.1.3. Proteomics for Studying Post-Translational Protein Modifications (PTMs)
2.2. Beneficial Effects of Marine Lipids Intake Assayed by Lipidomics
2.2.1. Lipidomics in Clinical Trials
2.2.2. Lipidomics in Animal Models
2.2.3. Lipidomics in Cell Cultures
2.2.4. Marine Lipids and Other Bioactive Compounds Assayed by Lipidomics
2.3. Beneficial Effects of Marine Lipids Intake Assayed by Both Proteomics and Lipidomics
3. Mechanisms behind the Beneficial Effects of Marine Lipids Assayed by Proteomics and Lipidomics
4. Concluding Remarks and Final Considerations
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [PubMed]
- Hutcheson, R.; Rocic, P. The metabolic syndrome, oxidative stress, environment, and cardiovascular disease: The great exploration. Exp. Diabetes Res. 2012, 2012, 271028. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Metabolic syndrome pandemic. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Eurostat. News Releases, Product Code: 3-24052016-AP, Published on 24 May 2016. Available online: http://ec.europa.eu/eurostat/documents/2995521/7335847/3-24052016-AP-EN.pdf/4dd0a8ad-5950-4425-9364-197a492d3648 (accessed on 17 August 2017).
- World Health Organization. The Top 10 Causes of Death. Available online: http://www.who.int/mediacentre/factsheets/fs310/en/index1.html (accessed on 17 August 2017).
- Simopoulos, A.P. Essential fatty acids in health and chronic disease. Am. J. Clin. Nutr. 1999, 70, 560S–569S. [Google Scholar] [PubMed]
- Moore, J.B.; Weeks, M.E. Proteomics and systems biology: Current and future applications in the nutritional sciences. Adv. Nutr. 2011, 2, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, V.; Hettiarachchy, N.S. Nutriproteomics: A promising tool to link diet and diseases in nutritional research. Biochim. Biophys. Acta BBA Proteins Proteom. 2012, 1824, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Kussmann, M.; Raymond, F.; Affolter, M. OMICS-driven biomarker discovery in nutrition and health. J. Biotechnol. 2006, 124, 758–787. [Google Scholar] [CrossRef] [PubMed]
- Tyers, M.; Mann, M. From genomics to proteomics. Nature 2003, 422, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Wasinger, V.C.; Cordwell, S.J.; Cerpa-Poljak, A.; Yan, J.X.; Gooley, A.A.; Wilkins, M.R.; Duncan, M.W.; Harris, R.; Williams, K.L.; Humphery-Smith, I. Progress with gene-product mapping of the Mollicutes: Mycoplasma genitalium. Electrophoresis 1995, 16, 1090–1094. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, M.; Gooley, A. Protein Identification in Proteome Projects. In Proteome Research: New Frontiers in Functional Genomics; Wilkins, M., Williams, K., Appel, R., Hochstrasser, D., Eds.; Springer: Berlin/Heidelberg, Germany, 1997; pp. 35–64. [Google Scholar]
- Sauer, S.; Luge, T. Nutriproteomics: Facts, concepts, and perspectives. Proteomics 2015, 15, 997–1013. [Google Scholar] [CrossRef] [PubMed]
- Camargo, A.; Rangel-Zúñiga, O.A.; Peña-Orihuela, P.; Marín, C.; Pérez-Martínez, P.; Delgado-Lista, J.; Gutierrez-Mariscal, F.M.; Malagón, M.M.; Roche, H.M.; Tinahones, F.J.; et al. Postprandial changes in the proteome are modulated by dietary fat in patients with metabolic syndrome. J. Nutr. Biochem. 2013, 24, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Zúñiga, O.A.; Camargo, A.; Marín, C.; Peña-Orihuela, P.; Pérez-Martínez, P.; Delgado-Lista, J.; González-Guardia, L.; Yubero-Serrano, E.M.; Tinahones, F.J.; Malagón, M.M.; et al. Proteome from patients with metabolic syndrome is regulated by quantity and quality of dietary lipids. BMC Genom. 2015, 16, 509. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Gómez, Y.; Cruz-Teno, C.; Rangel-Zúñiga, O.A.; Peinado, J.R.; Pérez-Martínez, P.; Delgado-Lista, J.; García-Ríos, A.; Camargo, A.; Vázquez-Martínez, R.; Ortega-Bellido, M.; et al. Effect of dietary fat modification on subcutaneous white adipose tissue insulin sensitivity in patients with metabolic syndrome. Mol. Nutr. Food Res. 2014, 58, 2177–2188. [Google Scholar] [CrossRef] [PubMed]
- De Roos, B.; Geelen, A.; Ross, K.; Rucklidge, G.; Reid, M.; Duncan, G.; Caslake, M.; Horgan, G.; Brouwer, I.A. Identification of potential serum biomarkers of inflammation and lipid modulation that are altered by fish oil supplementation in healthy volunteers. Proteomics 2008, 8, 1965–1974. [Google Scholar] [CrossRef] [PubMed]
- Burillo, E.; Mateo-Gallego, R.; Cenarro, A.; Fiddyment, S.; Bea, A.M.; Jorge, I.; Vázquez, J.; Civeira, F. Beneficial effects of omega-3 fatty acids in the proteome of high-density lipoprotein proteome. Lipids Health Dis. 2012, 11, 116. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.A.; Balogun, K.A.; Bykova, N.V.; Cheema, S.K. Novel regulatory roles of omega-3 fatty acids in metabolic pathways: A proteomics approach. Nutr. Metab. 2014, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Wrzesinski, K.; León, I.R.; Kulej, K.; Sprenger, R.R.; Bjørndal, B.; Christensen, B.J.; Berge, R.K.; Jensen, O.N.; Rogowska-Wrzesinska, A. Proteomics identifies molecular networks affected by tetradecylthioacetic acid and fish oil supplemented diets. J. Proteom. 2013, 84, 61–77. [Google Scholar] [CrossRef] [PubMed]
- De Roos, B.; Duivenvoorden, I.; Rucklidge, G.; Reid, M.; Ross, K.; Lamers, R.J.; Voshol, P.J.; Havekes, L.M.; Teusink, B. Response of apolipoprotein E*3-Leiden transgenic mice to dietary fatty acids: Combining liver proteomics with physiological data. FASEB J. 2005, 19, 813–815. [Google Scholar] [CrossRef] [PubMed]
- Méndez, L.; Ciordia, S.; Fernández, M.S.; Juárez, S.; Ramos, A.; Pazos, M.; Gallardo, J.M.; Torres, J.L.; Nogués, M.R.; Medina, I. Changes in liver proteins of rats fed standard and high-fat and sucrose diets induced by fish omega-3 PUFAs and their combination with grape polyphenols according to quantitative proteomics. J. Nutr. Biochem. 2017, 41, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Kalupahana, N.S.; Claycombe, K.; Newman, S.J.; Stewart, T.; Siriwardhana, N.; Matthan, N.; Lichtenstein, A.H.; Moustaid-Moussa, N. Eicosapentaenoic acid prevents and reverses insulin resistance in high-fat diet-induced obese mice via modulation of adipose tissue inflammation. J. Nutr. 2010, 140, 1915–1922. [Google Scholar] [CrossRef] [PubMed]
- Mavrommatis, Y.; Ross, K.; Rucklidge, G.; Reid, M.; Duncan, G.; Gordon, M.J.; Thies, F.; Sneddon, A.; De Roos, B. Intervention with fish oil, but not with docosahexaenoic acid, results in lower levels of hepatic soluble epoxide hydrolase with time in apoE knockout mice. Br. J. Nutr. 2010, 103, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.L.; Lalia, A.Z.; Dasari, S.; Pallauf, M.; Fitch, M.; Hellerstein, M.K.; Lanza, I.R. Eicosapentaenoic acid but not docosahexaenoic acid restores skeletal muscle mitochondrial oxidative capacity in old mice. Aging Cell 2015, 14, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Richard, D.; Kefi, K.; Barbe, U.; Bausero, P.; Visioli, F. Polyunsaturated fatty acids as antioxidants. Pharmacol. Res. 2008, 57, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Méndez, L.; Pazos, M.; Gallardo, J.M.; Torres, J.L.; Pérez-Jiménez, J.; Nogués, R.; Romeu, M.; Medina, I. Reduced protein oxidation in Wistar rats supplemented with marine omega-3 PUFAs. Free Radic. Biol. Med. 2013, 55, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Joumard-Cubizolles, L.; Gladine, C.; Gérard, N.; Chambon, C.; Brachet, P.; Comte, B.; Mazur, A. Proteomic analysis of aorta of LDLR-/- mice given omega-3 fatty acids reveals modulation of energy metabolism and oxidative stress pathway. Eur. J. Lipid Sci. Technol. 2013, 115, 1492–1498. [Google Scholar] [CrossRef]
- Wenk, M.R. The emerging field of lipidomics. Nat. Rev. Drug Discov. 2005, 4, 594–610. [Google Scholar] [CrossRef] [PubMed]
- Wymann, M.P.; Schneiter, R. Lipid signalling in disease. Nat. Rev. Mol. Cell Biol. 2008, 9, 162–176. [Google Scholar] [CrossRef] [PubMed]
- Shearer, G.C.; Harris, W.S.; Pedersen, T.L.; Newman, J.W. Detection of omega-3 oxylipins in human plasma and response to treatment with omega-3 acid ethyl esters. J. Lipid Res. 2010, 51, 2074–2081. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M. Lipid mediators in life science. Exp. Anim. 2011, 60, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Ottestad, I.; Hassani, S.; Borge, G.I.; Kohler, A.; Vogt, G.; Hyötyläinen, T.; Orešič, M.; Brønner, K.W.; Holven, K.B.; Ulven, S.M.; et al. Fish Oil Supplementation Alters the Plasma Lipidomic Profile and Increases Long-Chain PUFAs of Phospholipids and Triglycerides in Healthy Subjects. PLoS ONE 2012, 7, e42550. [Google Scholar] [CrossRef] [PubMed]
- Rudkowska, I.; Paradis, A.M.; Thifault, E.; Julien, P.; Tchernof, A.; Couture, P.; Lemieux, S.; Barbier, O.; Vohl, M.C. Transcriptomic and metabolomic signatures of an n-3 polyunsaturated fatty acids supplementation in a normolipidemic/normocholesterolemic Caucasian population. J. Nutr. Biochem. 2013, 24, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Nording, M.L.; Yang, J.; Georgi, K.; Hegedus Karbowski, C.; German, J.B.; Weiss, R.H.; Hogg, R.J.; Trygg, J.; Hammock, B.D.; Zivkovic, A.M. Individual variation in lipidomic profiles of healthy subjects in response to omega-3 fatty acids. PLoS ONE 2013, 8, e76575. [Google Scholar] [CrossRef] [PubMed]
- Mas, E.; Croft, K.D.; Zahra, P.; Barden, A.; Mori, T.A. Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clin. Chem. 2012, 58, 1476. [Google Scholar] [CrossRef] [PubMed]
- Barden, A.; Mas, E.; Croft, K.D.; Phillips, M.; Mori, T.A. Short-term n-3 fatty acid supplementation but not aspirin increases plasma proresolving mediators of inflammation. J. Lipid Res. 2014, 55, 2401–2407. [Google Scholar] [CrossRef] [PubMed]
- Keelan, J.A.; Mas, E.; D’Vaz, N.; Dunstan, J.A.; Li, S.; Barden, A.E.; Mark, P.J.; Waddell, B.J.; Prescott, S.L.; Mori, T.A. Effects of maternal n-3 fatty acid supplementation on placental cytokines, pro-resolving lipid mediators and their precursors. Reproduction 2015, 149, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Barden, A.E.; Mas, E.; Croft, K.D.; Phillips, M.; Mori, T.A. Specialized proresolving lipid mediators in humans with the metabolic syndrome after n-3 fatty acids and aspirin. Am. J. Clin. Nutr. 2015, 102, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Schuchardt, J.P.; Schmidt, S.; Kressel, G.; Willenberg, I.; Hammock, B.D.; Hahn, A.; Schebb, N.H. Modulation of blood oxylipin levels by long-chain omega-3 fatty acid supplementation in hyper- and normolipidemic men. Prostaglandins Leukot. Essent. Fat. Acids PLEFA 2014, 90, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Polus, A.; Zapala, B.; Razny, U.; Gielicz, A.; Kiec-Wilk, B.; Malczewska-Malec, M.; Sanak, M.; Childs, C.E.; Calder, P.C.; Dembinska-Kiec, A. Omega-3 fatty acid supplementation influences the whole blood transcriptome in women with obesity, associated with pro-resolving lipid mediator production. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2016, 1861, 1746–1755. [Google Scholar] [CrossRef] [PubMed]
- Lankinen, M.; Schwab, U.; Erkkilä, A.; Seppänen-Laakso, T.; Hannila, M.L.; Mussalo, H.; Lehto, S.; Uusitupa, M.; Gylling, H.; Orešič, M. Fatty fish intake decreases lipids related to inflammation and insulin signaling—A lipidomics approach. PLoS ONE 2009, 4, e5258. [Google Scholar] [CrossRef] [PubMed]
- Midtbø, L.K.; Borkowska, A.G.; Bernhard, A.; Rønnevik, A.K.; Lock, E.-J.; Fitzgerald, M.L.; Torstensen, B.E.; Liaset, B.; Brattelid, T.; Pedersen, T.L.; et al. Intake of farmed Atlantic salmon fed soybean oil increases hepatic levels of arachidonic acid-derived oxylipins and ceramides in mice. J. Nutr. Biochem. 2015, 26, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Dawczynski, C.; Massey, K.A.; Ness, C.; Kiehntopf, M.; Stepanow, S.; Platzer, M.; Grün, M.; Nicolaou, A.; Jahreis, G. Randomized placebo-controlled intervention with n-3 LC-PUFA-supplemented yoghurt: Effects on circulating eicosanoids and cardiovascular risk factors. Clin. Nutr. 2013, 32, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Padro, T.; Vilahur, G.; Sánchez-Hernández, J.; Hernández, M.; Antonijoan, R.M.; Pérez, A.; Badimon, L. Lipidomic changes of LDL in overweight and moderately hypercholesterolemic subjects taking phytosterol- and omega-3-supplemented milk. J. Lipid Res. 2015, 56, 1043–1056. [Google Scholar] [CrossRef] [PubMed]
- Dasilva, G.; Pazos, M.; García-Egido, E.; Gallardo, J.M.; Rodríguez, I.; Cela, R.; Medina, I. Healthy effect of different proportions of marine ω-3 PUFAs EPA and DHA supplementation in Wistar rats: Lipidomic biomarkers of oxidative stress and inflammation. J. Nutr. Biochem. 2015, 26, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Dasilva, G.; Pazos, M.; García-Egido, E.; Pérez-Jiménez, J.; Torres, J.L.; Giralt, M.; Nogués, M.R.; Medina, I. Lipidomics to analyze the influence of diets with different EPA:DHA ratios in the progression of Metabolic Syndrome using SHROB rats as a model. Food Chem. 2016, 205, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Cipollina, C.; Salvatore, S.R.; Muldoon, M.F.; Freeman, B.A.; Schopfer, F.J. Generation and dietary modulation of anti-inflammatory electrophilic omega-3 fatty acid derivatives. PLoS ONE 2014, 9, e94836. [Google Scholar] [CrossRef] [PubMed]
- Balogun, K.A.; Albert, C.J.; Ford, D.A.; Brown, R.J.; Cheema, S.K. Dietary omega-3 polyunsaturated fatty acids alter the fatty acid composition of hepatic and plasma bioactive lipids in C57BL/6 mice: A lipidomic approach. PLoS ONE 2013, 8, e82399. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, R.C.; Gotlinger, K.H.; Serhan, C.N.; Kruger, M.C. Identification of inflammatory and proresolving lipid mediators in bone marrow and their lipidomic profiles with ovariectomy and omega-3 intake. Am. J. Hematol. 2008, 83, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Taltavull, N.; Ras, R.; Mariné, S.; Romeu, M.; Giralt, M.; Méndez, L.; Medina, I.; Ramos-Romero, S.; Torres, J.L. Protective effects of fish oil on pre-diabetes: A lipidomic analysis of liver ceramides in rats. Food Funct. 2016, 7, 3981–3988. [Google Scholar] [CrossRef] [PubMed]
- Caesar, R.; Nygren, H.; Orešic, M.; Bäckhed, F. Interaction between dietary lipids and gut microbiota regulates hepatic cholesterol metabolism. J. Lipid Res. 2016, 57, 474–781. [Google Scholar] [CrossRef] [PubMed]
- Kuda, O.; Rombaldova, M.; Janovska, P.; Flachs, P.; Kopecky, J. Cell type-specific modulation of lipid mediator’s formation in murine adipose tissue by omega-3 fatty acids. Biochem. Biophys. Res. Commun. 2016, 469, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Flachs, P.; Ruhl, R.; Hensler, M.; Janovska, P.; Zouhar, P.; Kus, V.; Macek Jilkova, Z.; Papp, E.; Kuda, O.; Svobodova, M.; et al. Synergistic induction of lipid catabolism and anti-inflammatory lipids in white fat of dietary obese mice in response to calorie restriction and n-3 fatty acids. Diabetologia 2011, 54, 2626–2638. [Google Scholar] [CrossRef] [PubMed]
- González-Périz, A.; Horrillo, R.; Ferré, N.; Gronert, K.; Dong, B.; Morán-Salvador, E.; Titos, E.; Martínez-Clemente, M.; López-Parra, M.; Arroyo, V.; et al. Obesity-induced insulin resistance and hepatic steatosis are alleviated by ω-3 fatty acids: A role for resolvins and protectins. FASEB J. 2009, 23, 1946–1957. [Google Scholar] [CrossRef] [PubMed]
- Kalish, B.T.; Le, H.D.; Fitzgerald, J.M.; Wang, S.; Seamon, K.; Gura, K.M.; Gronert, K.; Puder, M. Intravenous fish oil lipid emulsion promotes a shift toward anti-inflammatory proresolving lipid mediators. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G818–G828. [Google Scholar] [CrossRef] [PubMed]
- González-Périz, A.; Planagumà, A.; Gronert, K.; Miquel, R.; López-Parra, M.; Titos, E.; Horrillo, R.; Ferré, N.; Deulofeu, R.; Arroyo, V.; et al. Docosahexaenoic acid (DHA) blunts liver injury by conversion to protective lipid mediators: Protectin D1 and 17S-hydroxy-DHA. FASEB J. 2006, 20, 2537–2539. [Google Scholar] [CrossRef] [PubMed]
- Aukema, H.M.; Lu, J.; Borthwick, F.; Proctor, S.D. Dietary fish oil reduces glomerular injury and elevated renal hydroxyeicosatetraenoic acid levels in the JCR:LA-cp rat, a model of the metabolic syndrome. Br. J. Nutr. 2013, 110, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Gladine, C.; Newman, J.W.; Durand, T.; Pedersen, T.L.; Galano, J.M.; Demougeot, C.; Berdeaux, O.; Pujos-Guillot, E.; Mazur, A.; Comte, B. Lipid profiling following intake of the omega 3 fatty acid DHA identifies the peroxidized metabolites F4-neuroprostanes as the best predictors of atherosclerosis prevention. PLoS ONE 2014, 9, e89393. [Google Scholar] [CrossRef] [PubMed]
- Skorve, J.; Hilvo, M.; Vihervaara, T.; Burri, L.; Bohov, P.; Tillander, V.; Bjørndal, B.; Suoniemi, M.; Laaksonen, R.; Ekroos, K.; et al. Fish oil and krill oil differentially modify the liver and brain lipidome when fed to mice. Lipids Health Dis. 2015, 14. [Google Scholar] [CrossRef] [PubMed]
- Polus, A.; Kiec-Wilk, B.; Razny, U.; Gielicz, A.; Schmitz, G.; Dembinska-Kiec, A. Influence of dietary fatty acids on differentiation of human stromal vascular fraction preadipocytes. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2015, 1851, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Capel, F.; Acquaviva, C.; Pitois, E.; Laillet, B.; Rigaudière, J.P.; Jouve, C.; Pouyet, C.; Gladine, C.; Comte, B.; Vianey Saban, C.; et al. DHA at nutritional doses restores insulin sensitivity in skeletal muscle by preventing lipotoxicity and inflammation. J. Nutr. Biochem. 2015, 26, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Ting, H.-C.; Chao, Y.-J.; Hsu, Y.-H.H. Polyunsaturated fatty acids incorporation into cardiolipin in H9c2 cardiac myoblast. J. Nutr. Biochem. 2015, 26, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Lankinen, M.; Schwab, U.; Kolehmainen, M.; Paananen, J.; Poutanen, K.; Mykkänen, H.; Seppänen-Laakso, T.; Gylling, H.; Uusitupa, M.; Orešič, M. Whole grain products, fish and bilberries alter glucose and lipid metabolism in a randomized, controlled trial: The Sysdimet study. PLoS ONE 2011, 6, e22646. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Kato, T.S.; Ji, R.; Zizola, C.; Brunjes, D.L.; Deng, Y.; Akashi, H.; Armstrong, H.F.; Kennel, P.J.; Thomas, T.; et al. Supplementation of l-Alanyl-l-Glutamine and fish oil improves body composition and quality of life in patients with chronic heart failure. Circ. Heart Fail. 2015, 8, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Mas, E.; Barden, A.; Burke, V.; Beilin, L.J.; Watts, G.F.; Huang, R.C.; Puddey, I.B.; Irish, A.B.; Mori, T.A. A randomized controlled trial of the effects of n-3 fatty acids on resolvins in chronic kidney disease. Clin. Nutr. 2016, 35, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Bondia-Pons, I.; Pöhö, P.; Bozzetto, L.; Vetrani, C.; Patti, L.; Aura, A.M.; Annuzzi, G.; Hyötyläinen, T.; Rivellese, A.A.; Orešič, M. Isoenergetic diets differing in their n-3 fatty acid and polyphenol content reflect different plasma and HDL-fraction lipidomic profiles in subjects at high cardiovascular risk. Mol. Nutr. Food Res. 2014, 58, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- Dasilva, G.; Pazos, M.; García-Egido, E.; Gallardo, J.M.; Ramos-Romero, S.; Torres, J.L.; Romeu, M.; Nogués, M.-R.; Medina, I. A lipidomic study on the regulation of inflammation and oxidative stress targeted by marine ω-3 PUFA and polyphenols in high-fat high-sucrose diets. J. Nutr. Biochem. 2017, 43, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Bakker, G.C.; van Erk, M.J.; Pellis, L.; Wopereis, S.; Rubingh, C.M.; Cnubben, N.H.; Kooistra, T.; van Ommen, B.; Hendriks, H.F. An antiinflammatory dietary mix modulates inflammation and oxidative and metabolic stress in overweight men: A nutrigenomics approach. Am. J. Clin. Nutr. 2010, 91, 1044–1059. [Google Scholar] [CrossRef] [PubMed]
- Pellis, L.; van Erk, M.J.; van Ommen, B.; Bakker, G.C.M.; Hendriks, H.F.J.; Cnubben, N.H.P.; Kleemann, R.; van Someren, E.P.; Bobeldijk, I.; Rubingh, C.M.; et al. Plasma metabolomics and proteomics profiling after a postprandial challenge reveal subtle diet effects on human metabolic status. Metabolomics 2012, 8, 347–359. [Google Scholar] [CrossRef] [PubMed]
- López, E.F.; Kabarowski, J.H.; Ingle, K.A.; Kain, V.; Barnes, S.; Crossman, D.K.; Lindsey, M.L.; Halade, G.V. Obesity superimposed on aging magnifies inflammation and delays the resolving response after myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H269–H280. [Google Scholar] [CrossRef] [PubMed]
- Warensjö, E.; Riserus, U.; Vessby, B. Fatty acid composition of serum lipids predicts the development of the metabolic syndrome in men. Diabetologia 2005, 48, 1999–2005. [Google Scholar] [CrossRef] [PubMed]
- Filep, J.G. Resolution pathways in inflammation: The devil in the adipose tissues and in the details. Focus on “Diversity of lipid mediators in human adipose tissue depots”. Am. J. Physiol. Cell Physiol. 2013, 304, C1127–C1128. [Google Scholar] [CrossRef] [PubMed]
- Sears, B. The Anti-Inflammation Zone; Regan Books: New York, NY, USA, 2005. [Google Scholar]
- McDaniel, J.C.; Massey, K.; Nicolaou, A. Fish oil supplementation alters levels of lipid mediators of inflammation in microenvironment of acute human wounds. Wound Repair Regen. 2011, 19, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Bouwens, M.; van de Rest, O.; Dellschaft, N.; Bromhaar, M.G.; de Groot, L.C.; Geleijnse, J.M.; Müller, M.; Afman, L.A. Fish-oil supplementation induces antiinflammatory gene expression profiles in human blood mononuclear cells. Am. J. Clin. Nutr. 2009, 90, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.H. Mechanisms for the Hypotriglyceridemic Effect of Marine Omega-3 Fatty Acids. Am. J. Cardiol. 2006, 98 (Suppl. 1), 27–33. [Google Scholar] [CrossRef] [PubMed]
- Krammer, J.; Digel, M.; Ehehalt, F.; Stremmel, W.; Fullekrug, J.; Ehehalt, R. Overexpression of CD36 and acyl-CoA synthetases FATP2, FATP4 and ACSL1 increases fatty acid uptake in human hepatoma cells. Int. J. Med. Sci. 2011, 8, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Dobler, D.; Dean, M.; Thornalley, P.J. Peptide mapping identifies hotspot site of modification in human serum albumin by methylglyoxal involved in ligand binding and esterase activity. J. Biol. Chem. 2005, 280, 5724–5732. [Google Scholar] [CrossRef] [PubMed]
- Ruskovska, T.; Bernlohr, D.A. Oxidative stress and protein carbonylation in adipose tissue—Implications for insulin resistance and diabetes mellitus. J. Proteom. 2013, 92, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Keaney, J.F.; Larson, M.G.; Vasan, R.S.; Wilson, P.W.F.; Lipinska, I.; Corey, D.; Massaro, J.M.; Sutherland, P.; Vita, J.A.; Benjamin, E.J. Obesity and Systemic Oxidative Stress: Clinical Correlates of Oxidative Stress in The Framingham Study. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 434–439. [Google Scholar] [CrossRef] [PubMed]
| Reference | Marine Lipids Intervention | Experimental Model | Proteomics Tools | Target Proteome | Main Effects |
|---|---|---|---|---|---|
| Camargo et al., 2013 [14] | Acute intake of EPA/DHA (1.4:1) | Human suffering MetS | Quantitative 2-DE-MS/MS | PBMCs | 5 proteins regulated from cell signaling and interaction, DNA repair, cellular assembly and organization and cell morphology |
| Rangel-Zúñiga et al., 2015 [15] | EPA/DHA (1.4:1) for 12 weeks | Human suffering MetS | Quantitative 2-DE-MS/MS | PBMCs | 17 proteins regulated from immunological diseases and inflammatory response, oxidative stress, inflammation, endoplasmic reticulum stress and DNA repair |
| Jiménez-Gómez et al., 2014 [16] | EPA/DHA (1.4:1) for 12 weeks | Human suffering MetS | Quantitative 2-DE-MS/MS | White adipose tissue | 3 proteins regulated from glucose metabolism |
| De Roos et al., 2008 [17] | EPA/DHA (2:1) for 6 weeks | Healthy humans | Quantitative 2-DE-MS/MS | Serum | 10 proteins regulated from lipoprotein metabolism and inflammation |
| Burillo et al., 2012 [18] | 0.6 g/d EPA and DHA for 5 weeks | Healthy smokers humans | 2-DIGE-MS/MS | HDL | 12 proteins regulated related to antioxidant, anti-inflammatory and anti-atherosclerotic properties, regulation of complement activation and acute phase response |
| Ahmed et al., 2014 [19] | EPA/DHA (1:1) for 4 months | Healthy C57BL/6 mice | Quantitative 2-DE-MS/MS | Liver | 11 proteins regulated from lipid, carbohydrate, one-carbon, citric acid cycle and protein metabolisms |
| Wrzesinski et al., 2013 [20] | EPA/DHA (2:1) for 50 weeks | Wistar rats fed HFHS diet | Quantitative 2-DE-MS/MS | Liver mitochondria | 54 proteins regulated from fatty acid and amino acid metabolisms, fatty acid oxidation and oxidative phosphorylation |
| De Roos et al., 2005 [21] | EPA/DHA (2:1) for 3 weeks | APOE*3 Leiden transgenic mice fed HFC diet | Quantitative 2-DE-MS/MS | Liver | 44 proteins regulated from glucose and lipid metabolism, oxidation and aging processes |
| Méndez et al., 2017 [22] | EPA/DHA (1:1) for 28 weeks | Wistar Kyoto rats fed HFHS diet or STD diet | 2-DIGE-MS/MS iTRAQ-nanoLC-MS/MS | Liver | 6 proteins regulated in STD diet 31 proteins regulated in HFHS diet from lipogenesis and glycolysis, fatty acid beta-oxidation, insulin signaling, oxidative stress and ameliorating endoplasmic reticulum stress |
| Kalupahana et al., 2010 [23] | EPA | Cell culture | 2-DIGE-MS/MS | 3T3-L1 adipocytes | 27 proteins regulated from carbohydrate and fatty acid and cell metabolism, response to stress, lipogenesis, cytoskeleton organization and biogenesis |
| Mavrommatis et al., 2010 [24] | EPA/DHA (1.4:1) or DHA for 2 weeks | apoE knockout mice fed HFC diet | Quantitative 2DE-MS/MS | Liver | 35 proteins regulated from of lipoproteins metabolism and oxidative stress; 4 of them different between DHA and fish oil |
| Johnson et al., 2015 [25] | 0.5% EPA or 0.5% DHA for 10 weeks | 6- or 24-months C57BL/6 mice | Quantitative untargeted nanoLC-MS/MS | Quadriceps muscle | 39 proteins regulated by EPA-treated and 32 proteins regulated by DHA-treated old mice related to anticoagulation, anti-inflammatory, reduced FXR/RXR activation EPA decrease protein carbamylation |
| Méndez et al., 2013 [27] | EPA:DHA 1:1 or 2:1 or 1:2 for 13 weeks | Wistar Kyoto rats | FTSC-carbonyl protein labeling Quantitative 1DE- and 2DE-MS/MS | Plasma, kidney, skeletal muscle, and liver | 6 carbonylated protein targets regulated by 1:1 EPA:DHA in plasma and liver |
| Jourmard-Cubizolles et al., 2013 [28] | 2% DHA for 20 weeks | LDLR−/− mice fed atherosclerotic diet | Quantitative 2DE-MS/MS | Aorta | 19 proteins regulated from glucose and lipid metabolisms and oxidative stress 12 identified 4-HNE-proteins |
| Family | Lipid Mediators from ARA | Lipid Mediators from EPA | Lipid Mediators from DHA | |||
|---|---|---|---|---|---|---|
| Nomenclature | Isomers | Nomenclature | Isomers | Nomenclature | Isomers | |
| Monohydroxys | HETE | 3-, 5-, 8-, 9-, 11-, 12-, 15, 18-, 19- and 20HETE | HEPE | 5-, 8-, 9-, 11-, 12-, 15 and 18HEPE | HDoHE | 4-, 7-, 8-, 10-, 11-, 13-, 14-, 16-, 17- and 20HDoHE |
| Dihydroxys | DiHET (DiHETrE) | 5,6-,8,9-, 11,12- and 14,15 DiHETrE | DiHETE | 5,6-, 5,12-, 5,15-, 8,15-, 14,15- and 17,18DiHETE | DiHDPA | 10,11-, 14,21- and 19,20DiHDPA |
| Leukotrienes | LT-4 | LTA4, -B4, -C4, -D4 and –E4 | LT-5 | LTA5, -B5, -C5, -D5 and -E5 | ||
| Trihydroxys (lipoxins) | LX-4 | LXA4 and –B4 | LX-5 | LXA5 | ||
| Hydroperoxides | HpETE | 5-, 8-, 9-, 11-, 12-, 15-, 19- and 20HpETE | HpEPE | 5-, 8-, 9-, 11-, 12-, 15 and 18HpEPE | HpDoHE | 4-, 7-, 8-, 10-, 11-, 13-, 14-, 16-, 17- and 20HpDoHE |
| Epoxides | EET (EpETrE) | 5,6-, 8,9-, 11,12- and 14,1EET | EEQ (EpETE) | 8,9-, 11,12-, 14,15- and 17,18EEQ | EDP (EpDPA) | 7,8-,10,11-, 13,14, 16,17- and 19,20EDP |
| Thromboxanes | TX-2 | TXA2 and -B2 | TX-3 | TXA3 and -B3 | ||
| Prostaglandins | PG-2 | PGA2, -B2, -D2, -E2, -G2, -H2, -I2, -J2 and –F2α | PG-3 | PGA3, -B3, -C3, -D3, -E3, -I3, -H3 and –F3α | ||
| Isoprostanes | IsoP-2 | 8isoPGJ2, -A2, -E2 and-D2 | IsoP-3 | 8-, 5-, 11-, 12-, 15- and 18isoPGF3α | ||
| Resolvins | 8-, 5-, 12 and 15isoPGF2α | RvE | RvE1, -E2 and -E3 | RvD | RvD1, -2, -3 and -4 | |
| Neuroprotectins | PD | PD1 | ||||
| Maresins | MaR | MaR2 (13,14DiHDPA) 7-MaR1 | ||||
| Keto-derivatives | ||||||
| Keto-PG | oxoETE | 5-, 8-, 9-, 11-, 12-, 15, 19- and 20 oxoETE | ||||
| Reference | Marine Lipids Intervention | Experimental Model | Lipidomics Tools | Target Lipidome | Main Effects |
|---|---|---|---|---|---|
| Ottestad et al., 2012 [34] | 0.7 g/day EPA and 0.9 g/day DHA for 7 weeks | Healthy humans | UPLC-MS | Plasma | Decreased 23 lipids Increased PLs and TGs containing EPA and DHA |
| Rudkowska et al., 2013 [35] | 1.9 g/day EPA and 1.1 g/day DHA for 6 weeks | Healthy humans | MS assay kit | Plasma | Increased glyPCs in unsaturated FA |
| Nording et al., 2013 [36] | 1.9 g/day EPA and 1.5 g/day DHA for 6 weeks | Healthy humans | HPLC-GS-MS SPE-LC-MS/MS | Plasma | Increased incorporation of EPA and DHA into 7 lipid classes High variability in 87 lipid mediators measured |
| Mas et al., 2012 [37] | 4 g fish oil/day (35% EPA and 25% DHA) for 3 weeks | Healthy humans | SPE-LC-MS/MS | Plasma/serum | Measured for first time 17R/SHDHA, RvD1, and RvD2 concentrations RvD1 and RvD2 into anti-inflammatory and pro-resolving concentration range |
| Barden et al., 2014 [38] | 4 g fish oil/day (35% EPA and 25% DHA) for 5 days | Healthy humans | SPE-LC-MS/MS | Plasma | Increased RvE1, 18R/S-HEPE, 17R/S-HDHA and 14R/S-HDHA |
| Keelan et al., 2015 [39] | 3.7 g/day (27.7% EPA and 56.% DHA) from 20 pregnancy-week | Healthy pregnant women | GC SPE-LC-MS/MS | Placenta | Increased DHA Increased 18-HEPE and 17-HDHA |
| Barden et al., 2015 [40] | 1.4 g EPA/day and 1 g DHA/day in the form of triglycerides for 3 weeks. | Human suffering metabolic syndrome | SPE-LC-MS/MS | Plasma | Increased E-series resolvins in MetS patients and controls, in which also increased D-series resolvin precursors and 14-HDHA |
| Schuchardt et al., 2014 [41] | 1.14 g/day DHA and 1.56 g/day EPA for 12 weeks | Hyperlipidemic men | SPE-LC-MS/MS | Plasma | Increased EPA-derived lipid mediators Less increased DHA-derived lipid mediators |
| Polus et al., 2016 [42] | 3× (430 mg of DHA and 90–150 mg of EPA)/day for 3 months | Obese women | GC-MS LC-MS/MS | Plasma | Increased pro-resolving DHA derivatives |
| Lankinen et al., 2009 [43] | Fatty or lean fish for 8 weeks | Coronary heart disease patients | GC-MS UPLC-ESI-MS | Plasma | Decreased 59 bioactive lipid species (ceramides, lysoPCs and DGs) by fatty fish Increased cholesterol esters and specific long-chain TGs by lean fish |
| Midtbø et al., 2015 [44] | Farmed salmon fed with a reduced ratio of ω-3/ω-6 for 10 weeks | C57BL/6J mice fed western diets | LC-MS/MS | Liver | Increased ARA in PLs Increased ceramides Increased ARA-derived pro-inflammatory mediators Decreased lipid mediators derived from EPA and DHA |
| Dawczynski et al., 2013 [45] | 3 g of EPA and DHA (in 1:1 ratio)/day for 10 weeks | Mildly hypertriacylglycerolemic subjects | LC-MS/MS | Plasma Red blood cells | Increased EPA and DHA levels in plasma and red blood cells Increased plasma EPA-derived mediators (PGE3, and 12-, 15- and 18-HEPE) |
| Padro et al., 2015 [46] | 0.375 EPA and DHA g/day for 28 days | Overweight and moderately hypercholesterolemic subjects | LC-MS/MS | LDL | Increased long-chain polyunsaturated CEs Increased ratio PC36:5/lysoPC16:0 |
| Dasilva et al., 2015 [47] | EPA:DHA 1:1 or 2:1 or 1:2 for 13 weeks | Wistar Kyoto rats | SPE-LC-MS/MS | Plasma | Decreased pro-inflammatory ARA eicosanoids by 1:1 and 2:1 ratios |
| Dasilva et al., 2016 [48] | EPA:DHA 1:1 or 2:1 or 1:2 for weeks | SHROB rats | SPE-LC-MS/MS | Plasma | Decreased pro-inflammatory ARA eicosanoids by 1:1 and 2:1 ratios |
| Cipollina et al., 2014 [49] | 1 g/day EPA and 0.4 g/day DHA for 4 months | Healthy humans | BME reaction | Blood neutrophils | Increased 7-oxo-DHA and 5-oxo-EPA |
| Balogun et al., 2013 [50] | EPA:DHA 1:1 for 4 months | C57BL/6 mice | LC-MS | Plasma Liver | Increased EPA containing PCs, LPCs, and CEs Increased free ω-3 PUFAs |
| Poulsen et al., 2008 [51] | 0.5 g DHA or EPA ethyl ester/kg body weight/day 4 months | Sprague–Dawley rats | LC-MS/MS | Bone marrow | Increased EPA and DHA Increased LOX mediators biosynthesized from DHA and EPA (lipoxins, resolving D1, resolvin E1 and protectin D1) |
| Taltavull et al., 2016 [52] | EPA/DHA (1:1) for 24 weeks | Wistar Kyoto rats fed HFHS diet | GS-MS SPE-LC-MS/MS | Liver | Decreased total ceramides Decreased long chain ceramide 18:1/18:0 Increased very long chain ceramides 18:1/24:0 and 18:1/20:0 |
| Caesar et al., 2016 [53] | Menhaden fish oil (25.2g EPA and 18.2 g DHA/100 g) for 11 weeks | C57BL/6 mice fed HF diet | UPLC-MS | Serum Liver | Interaction with gut microbiota increased hepatic levels of cholesterol and cholesteryl esters by lard but not by fish oil |
| Kuda et al., 2016 [54] | 4.3 mg EPA and 14.7 mg DHA/g diet for 5 weeks | C57BL/6J mice fed obesogenic HF diet | SPE-LC-MS/MS | White adipose tissue | Increased anti-inflammatory lipid mediators (endocannabinoid-related Ndocosahexaenoylethanolamine) and pro-resolving lipid mediator protectin D1 |
| Flachs et al., 2011 [55] | 46% DHA and 14% EPA for 5 weeks | Mice fed obesogenic MF diet | LC-MS/MS | White adipose tissue | Increased anti-inflammatory lipid mediators (15-deoxy-Δ(12,15)-prostaglandin J2 and protectin D1) in epididymal fat |
| González-Périz et al., 2009 [56] | 6 g/100 g ω-3 PUFAs for 5 weeks | ob/ob mice (B6.VLep/J) | SPE-LC-MS/MS | Liver | Inhibited formation of ω-6 PUFAs derived eicosanoids Induced formation of ω-3 PUFAs derived resolvins and protectins |
| Kalish et al., 2013 [57] | Parental nutrition with fish oil-based lipid emulsions | C57BL6/J mice high-carbohydrate diet | LC-MS/MS | Liver | Induced production of anti-inflammatory and pro-resolving lipid mediators |
| González-Périz et al., 2006 [58] | 1.37% DHA or 1.37% EPA and DHA for 5 weeks | 129S2/SvPasCrl mice fed high saturated fat diets | HPLC-GC/MS | Liver | Increased DHA-derived lipid mediators (17S-hydroxy-DHA (17S-HDHA) and protectin D1 by both supplementations |
| Aukema et al., 2013 [59] | 5% or 10% fish oil for 16 weeks | JCR:LA-cp rats | LC-MS/MS | Kidney | Decreased 5-, 9- 11-, 12- and 15-HETE Decreased endogenous renal levels of 6-keto PGF1α, TXB2, PGF2α and PGD2 |
| Gladine et al., 2014 [60] | DHA (0%, 0.1%, 1% or 2% of energy) for 20 weeks | LDLR−/− mice | GC-MS SPE-LC-MS/MS | Plasma Liver | Increased DHA Increased F4-neuroprostanes (DHA peroxidized metabolites) |
| Skorve et al., 2015 [61] | Fish oil or krill oil for 6 weeks | C57BL/6 J mice fed HF diet | GC-MS UPLC-MS/MS | Liver Brain | Decreased unsaturated fatty acids by fish and krill oils Decreased ceramides and DGs in liver and brain by krill oil Increased CEs by krill oil in liver Decreased plasmalogens by fish oil in liver Increased hepatic sphingolipids and ARA fatty acid levels more by krill than fish oil in liver Increased ceramides and lactosylceramides more by fish than krill oil in brain |
| Polus et al., 2015 [62] | EPA | Cell culture | GS-MS LC-MS/MS | Human subcutaneous adipose tissue stromal vascular fraction cells | Decreased pro-inflammatory mediators from ARA Increased anti-inflammatory eicosanoid from EPA |
| Capel et al., 2015 [63] | DHA | Cell culture | GC-FID LC-MS/MS | C2C12 myotubes | Restoring cellular acylcarnitine profile |
| Ting et al., 2015 [64] | EPA or DHA | Cell culture | LC-MS/MS | H9c2 cardiac myoblast | Elevation of less unsaturated and ω-3 cardiolipin species mainly by DHA |
| Lankinen et al., 2011 [65] | Fatty fish and other bioactive compounds for 12 weeks | Metabolic syndrome patients | UPLC-ESI-MS | Plasma | 25 altered lipids, including multiple TGs incorporating the long chain ω-3 PUFAs |
| Wu et al., 2015 [66] | ω-3 PUFA (6.5 g/day) and l-alanyl-l-glutamine (8 g/day) for 3 months | Patients with chronic heart failure | LC-MS | Plasma Skeletal muscle | Increased uptake EPA and DHA Decreased total ceramides and ceramides 22:1 and 20:1 |
| Mas et al., 2016 [67] | ω-3 fatty acids (4 g), Coenzyme Q10 (CoQ) (200 mg) or both for 8 weeks | Patients with chronic kidney disease | LC-MS/MS | Plasma | Increased 8-HEPE, 17-HDHA and RvD1 by ω-3 PUFAs |
| Bondia-Pons et al., 2014 [68] | 0.5% or 1.5% total energy intake EPA and DHA 365 mg or 2900 mg of polyphenols | Patients with metabolic syndrome | UPLC-QTOF-MS | Plasma and HDL fraction | Increased plasma highly unsaturated long-chain TGs and EPA and DHA-containing PLs by ω-3 diets Decreased plasma low unsaturated PLs, PCes, LysoPCs and PCps containing ARA by ω-3 diets Increased PCs and TGs containing DHA or EPA by ω-3 diets in HDL fraction Decreased PCes and PCps containing ARA and medium-chain PCs by ω-3 diets in HDL fraction Decreased PCs and Pes, several alkyl and alkenyl etherlipids containing 16:0 and saturated and low-unsaturated PCs and PEs by both ω-3 and polyphenols diet |
| Dasilva et al., 2017 [69] | EPA/DHA (1:1) Grape polyphenols for 24 weeks | Wistar Kyoto rats fed HFHS diet or STD diet | GS-MS SPE-LC-MS/MS | Plasma Liver Adipose tissue | Decreased ω-6/ω-3 index in plasma and membranes by ω-3 diets Decreased ARA pro-inflammatory lipid mediators by ω-3 diets Increased desaturases related to EPA and DHA synthesis by ω-3 diets Decreased desaturases related to ARA synthesis by ω-3 diets Combination ω-3&polyphenols cooperative down-regulated Δ5D related with ARA synthesis, decreased COX activity on ARA and total FFA in plasma into STD and HFHS diets |
| Reference | Marine Lipids Intervention | Experimental Model | Proteomics and Lipidomics Tools | Target Proteome and Lipidome | Main Effects |
|---|---|---|---|---|---|
| Bakker et al., 2010 [70] | 380 mg EPA and 260 mg DHA and other anti-inflammatory compounds for 5 weeks | Healthy overweight men | HumanMAP GS-MS LC-MS/MS | Plasma | Regulated plasma proteins and plasma metabolites (lipids, free fatty acids, and polar compounds) related to modulation of inflammation, improved endothelial function, oxidative stress and increased fatty acid oxidation. |
| Pellis et al., 2012 [71] | Postprandial response in anti-inflammatory mix-supplemented men Acute intake | Healthy overweight men | HumanMAP GS-MS | Plasma | 31 regulated proteins and lipids involved in amino acid metabolism, oxidative stress, inflammation and endocrine metabolism. |
| López et al., 2015 [72] | ω-6:ω-3 in 442:1 ratio for 5 months | Aging C57BL/6J mice previously suffered myocardial infarction | Protein immunoblot analysis LC-MS/MS | Plasma | Increased VCAM-1, macrophage inflammatory protein-1, D40 and myeloperoxidase Increased ARA and 12(S)-HETE and altered levels of inflammation-resolving enzymes 5-LOX, COX-2, and heme oxygenase-1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Méndez, L.; Dasilva, G.; Taltavull, N.; Romeu, M.; Medina, I. Marine Lipids on Cardiovascular Diseases and Other Chronic Diseases Induced by Diet: An Insight Provided by Proteomics and Lipidomics. Mar. Drugs 2017, 15, 258. https://doi.org/10.3390/md15080258
Méndez L, Dasilva G, Taltavull N, Romeu M, Medina I. Marine Lipids on Cardiovascular Diseases and Other Chronic Diseases Induced by Diet: An Insight Provided by Proteomics and Lipidomics. Marine Drugs. 2017; 15(8):258. https://doi.org/10.3390/md15080258
Chicago/Turabian StyleMéndez, Lucía, Gabriel Dasilva, Nùria Taltavull, Marta Romeu, and Isabel Medina. 2017. "Marine Lipids on Cardiovascular Diseases and Other Chronic Diseases Induced by Diet: An Insight Provided by Proteomics and Lipidomics" Marine Drugs 15, no. 8: 258. https://doi.org/10.3390/md15080258
APA StyleMéndez, L., Dasilva, G., Taltavull, N., Romeu, M., & Medina, I. (2017). Marine Lipids on Cardiovascular Diseases and Other Chronic Diseases Induced by Diet: An Insight Provided by Proteomics and Lipidomics. Marine Drugs, 15(8), 258. https://doi.org/10.3390/md15080258





