Abstract
Nine new sulfated triterpene glycosides, magnumosides A1 (1), A2 (2), A3 (3), A4 (4), B1 (5), B2 (6), C1 (7), C2 (8) and C4 (9) as well as a known colochiroside B2 (10) have been isolated from the tropical Indo-West Pacific sea cucumber Neothynidium (=Massinium) magnum (Phyllophoridae, Dendrochirotida) collected in the Vietnamese shallow waters. The structures of new glycosides were elucidated by 2D NMR spectroscopy and mass-spectrometry. All the isolated new glycosides were characterized by the non-holostane type lanostane aglycones having 18(16)-lactone and 7(8)-double bond and differed from each other by the side chains and carbohydrate moieties structures. Magnumoside A1 (1) has unprecedented 20(24)-epoxy-group in the aglycone side chain. Magnumosides of the group A (1–4) contained disaccharide monosulfated carbohydrate moieties, of the group B (5, 6)—tetrasaccharide monosulfated carbohydrate moieties and, finally, of the group C (7–9)—tetrasaccharide disulfated carbohydrate moieties. The cytotoxic activities of the compounds 1–9 against mouse spleen lymphocytes, the ascites form of mouse Ehrlich carcinoma cells, human colorectal carcinoma DLD-1 cells as well as their hemolytic effects have been studied. Interestingly, the erythrocytes were more sensitive to the glycosides action than spleenocytes and cancer cells tested. The compounds 3 and 7 significantly inhibited the colony formation and decreased the size of colonies of DLD-1 cancer cells at non-cytotoxic concentrations. Moreover, the synergism of effects of radioactive irradiation and compounds 3 and 7–9 at subtoxic doses on proliferation of DLD-1 cells was demonstrated.
1. Introduction
The triterpene glycosides from sea cucumbers (class Holothurioidea) have a long history of investigation. These marine natural products are characterized by significant structural diversity [1,2,3] and taxonomic specificity which enables their use in resolving some systematic ambiguities [4,5,6]. Additionally, the triterpene glycosides exhibit a wide spectrum of biological activities [7,8], including anticancer effects against different cancer cell lines [9,10,11,12,13,14].
The glycosides from the sea cucumber Neothyonidium (=Massinium) magnum (Phyllophoridae, Dendrochirotida) have been previously investigated. The first studied sample of N. magnum was collected near the shores of New Caledonia [15]. The main component of glycosidic fraction, monosulfated tetraoside, “neothyоnidioside”, had the holostane-type aglycone with 9(11)- and 25(26)-double bonds and a 16-keto-group. Another sample of N. magnum was collected near Vietnam’s shore [16]. The main component of its glycosidic fraction, neothyonidioside C, was different from “neothyonidioside” and characterized by the C-16-acetylated holostane-type aglycone having 7(8)- and 25(26)-double bonds. The carbohydrate chain of neothyonidioside C had the same set of monosaccharide residues as “neothyonidioside” but was disulfated.
Herein we report the results of investigation of N. magnum also collected in Vietnamese shallow waters but having the glycosides, magnumosides A1–A4 (1–4), B1 (5), B2 (6), C1 (7), C2 (8) and C4 (9), which is significantly different from the compounds isolated previously. The structures of the glycosides were established based on 1H and 13C NMR spectra and 2D NMR (1H,1H-COSY, HMBC, HSQC, ROESY) and confirmed by HR-ESI mass spectrometry. The cytotoxic activities of 1–9 against mouse spleen lymphocytes, the ascites form of mouse Ehrlich carcinoma cells, mouse erythrocytes, and human colorectal adenocarcinoma DLD-1 cells were tested. The effects of compounds 1–9 on proliferation, colony formation of DLD-1 cells as well as the synergism of radioactive irradiation and compounds effects have been studied.
2. Results and Discussion
2.1. Structural Elucidation of the Glycosides
The sea cucumber Neothyonidium (=Massinium) magnum contains a very complicated mixture of glycosides, thus the isolation of individual compounds was rather labor-consuming and multistage. The concentrated ethanolic extract of N. magnum was chromatographed on a Polychrom-1 column (powdered Teflon, Biolar, Olaine, Latvia). The glycosides were eluted with 50% EtOH and separated by chromatography on Si gel column using CHCl3/EtOH/H2O (100:100:17) and (100:125:25) as mobile phases. The obtained fractions were subsequently subjected to HPLC on a silica-based Supelcosil LC-Si (4.6 × 150 mm) column, on a reversed-phase semipreparative Supelco Ascentis RP-Amide (10 × 250 mm) column or analytical Diasfer C-8 (4.6 × 250 mm) column to yield the magnumosides A1 (1) (3.6 mg), A2 (2) (5.0 mg), A3 (3) (3.7 mg), A4 (4) (8.0 mg), B1 (5) (2.6 mg), B2 (6) (1.8 mg), C1 (7) (5.7 mg) and C2 (8) (2.5 mg), C4 (9) (15 mg) and colochiroside B2 (10) (2.0 mg) (Figure 1). The known compound 10 was identified by comparison of its 1H and 13C NMR spectra with those reported for colochiroside B2 (10, 3β-O-[3-O-methyl-β-d-glucopyranosyl-(1→3)-β-d-xylopyranosyl-(1→4)-β-d-quinovopyranosyl-(1→2)-4-O-sodium sulfate-β-d-xylopyranosyl]-16β-acetoxyholosta-25-hydroxy-7,23E-diene) from Colochirus robustus [17].
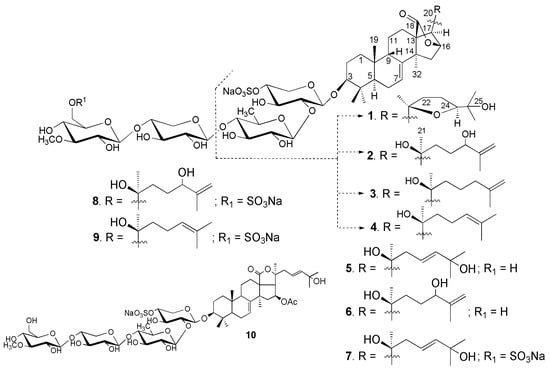
Figure 1.
Chemical structure of the glycosides 1–10 isolated from Neothyonidium magnum.
The 1H and 13C NMR spectra of carbohydrate parts of magnumosides A1–A4 (1–4) were coincident to each other, indicating the identity of carbohydrate chains of these glycosides. The presence of two characteristic doublets at δ(H) 4.66 (J = 7.0 Hz) and 5.00 (J = 7.6 Hz) in the 1H NMR spectra of the carbohydrate chains of 1–4 correlated by the HSQC spectra with the signals of anomeric carbons at δ(C) 104.8 and 105.2, correspondingly, were indicative of a disaccharide chain and β-configuration of glycosidic bonds. The 1H,1H-COSY and 1D TOCSY spectra of 1–4 showed the signals of two isolated spin systems assigned to the xylose and quinovose residues. The positions of interglycosidic linkages were confirmed by the ROESY and HMBC spectra of 1–4 (SM, Table 1) where the cross-peaks between H(1) of the xylose and H(3) (C(3)) of an aglycone and H(1) of the quinovose and H(2) (C(2)) of the xylose were observed. Thus, the carbohydrate chains of magnumosides of the group A (1–4) were identical to those of holothurins of the group B, that are characteristic glycosides for the representatives of the genus Holothuria and Actinopyga (Holothuriidae, Aspidochirotida) [1,18,19,20].

Table 1.
13C and 1H NMR chemical shifts of carbohydrate moieties of magnumosides of the group A (1–4), B (5, 6) and C (7–9) in C5D5N/D2O (5:1), δ in ppm, J in Hz.
The molecular formula of magnumoside A1 (1) was determined to be C41H63O16SNa from the [MNa − Na]− ion peak at m/z 843.3838 (calc. 843.3842) in the (−)HR-ESI-MS. Analysis of the 1H and 13C NMR spectra (Table 2 and Table 3) of the aglycone part of magnumoside A1 (1) suggested the presence of an 18(16)-lactone that was deduced from the characteristic signals of carbons C(18) (δ(C) 180.9) and C(20) (δ(C) 81.9) and characteristic signals of oxygen-bearing methine CH-O(16) (δ(C) 79.6; δ(H) 4.84 (s)). The availability of an 18(16)-lactone and (S)-configuration of C(16) asymmetric center was confirmed by the absence of coupling constant J17/16 for H(17) signal (δ(H) 2.48 (s)) in the 1H NMR spectrum of 1 as well as by the presence of correlations H(16)/C(18) in the HMBC spectrum (Figure 2) and H(16)/H(21) in the ROESY spectrum (Figure 3). The signals of olefinic methine group H-C(7) (δ(C) 122.4; δ(H) 5.61 (dt, J = 2.3, 7.4 Hz)) and quaternary carbon C(8) (δ(C) 147.6) in the 13C- and 1H NMR spectra were indicative of 7(8)-double bond in the aglycone nucleus. The signal of oxygenated tertiary asymmetric carbon C(20) assigned by the characteristic for the aglycones of sea cucumber glycosides HMBC correlation H(21)/C(20) was observed at δ(C) 81.9. Its δ(C) value was similar to those values in onekotanogenin [21] and in colochiroside E [22] having the 18(16)-lactone and acetylated C(20) position. However, the presence of acetoxy-group in the aglycone of 1 was excluded by ESI mass spectrometry.

Table 2.
1H NMR data for the aglycones of compounds 1–9 in C5D5N/D2O, δ in ppm, J in Hz.

Table 3.
13C NMR data for aglycones of compounds 1–8 in C5D5N/D2O, δ in ppm, mult.
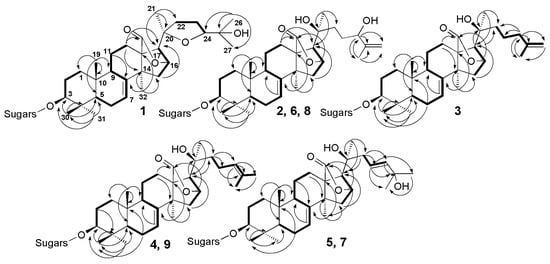
Figure 2.
1H,1H-COSY (—) and key HMBC (H→C) correlations for the aglycones of compounds 1–9.
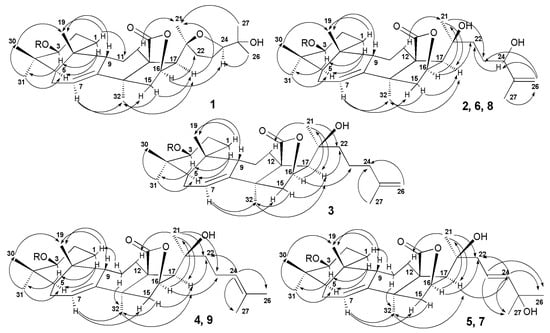
Figure 3.
Key ROESY correlations for the aglycones of compounds 1–9.
Additionally, there was another downshifted resonance in the 13C NMR spectrum of 1 at δ(C) 86.7 corresponding to the oxygen bearing methine carbon located at α-position to hydroxylated C-25. Its position as C(24) was deduced from the 1H,1H-COSY spectrum where the signals of isolated spin system from H(22) to H(24) were observed (Figure 2). Based on the NMR and ESI-MS data the presence of 20(24)-epoxy-group was suggested. The correlations from H-C(24) (δ(H) 3.97 (dd, J = 5.6; 9.3) to C(22), C(23), Me(26), Me(27); from H-C(22) to C(20), Me(21) and from Me(26)(27) to C(24) in the HMBC spectrum were in good agreement with this suggestion. Another downshifted signal (δ(C) 70.1) was assigned to the oxygen-bearing tertiary carbon positioned as C(25) that was deduced from the HMBC correlations Me(26)/C(25) and Me(27)/C(25). The multiplicity of the proton H(24), neighboring it, which was observed as doublet of doublets, confirmed this. The presence in the 1H–1H-COSY spectrum of the signals of four isolated spin systems confirmed the sequences of protons from H(1) to H(3); from H(5) to H(12); from H(15) to H(17) and finally from H(22) to H(24). The HMBC correlations Me(30)/C(4), Me(31)/C(4), Me(19)/C(10), Me(32)/C(13), C(14) and H(17)/C(20) allowed to elucidate the positions of quaternary carbons in the aglycone polycyclic nucleus.
The ROESY correlations (Figure 3) of 1 H(5)/H(3), H(5)/Me(31); H(9)/Me(19); H(17)/Me(21); (17)/Me(32) and Me(21)/H(12) confirmed the common elucidated earlier for the other sea cucumber glycosides (3S,5R,9S,10R,13S,14S,16S,17R,20S)-configurations [22,23,24] in magnumoside A1 (1). The ROE correlations H(16)/H(24); H(17)/H(24) and Me(21)/Me(27) observed in the ROESY spectrum of 1 could be realized in the case of (24S)-configuration only. Moreover, the coupling constants of H(24) (dd, J = 5.6; 9.3 Hz) in the 1H NMR spectrum were very close to the calculated ones, based on the dihedral angles values in MM2 optimized model of (20S),(24S)-isomer of 1.
The carbohydrate chain structure of 1 was confirmed by the (−)ESI-MS/MS of the [MNa − Na]− ion at m/z 843.4, where the peaks of fragment ions were observed at m/z 741.3 [MNa − Na − SO3Na + H]− and 697.3 [MNa − Na − Qui + H]−. The (+)ESI-MS/MS of the [MNa + Na]+ ion at m/z 889.4 demonstrated the peaks of ions at m/z: 769.4 [MNa + Na − NaHSO4]+, 623.4 [MNa + Na − NaSO4 − Qui]+, 491.3 [MNa + Na − NaSO4 − Qui − Xyl]+.
Additionally, an unusual fragmentation pattern, characteristic for 1–9, caused by the presence of 18(16)-lactone ring in their aglycones, was observed. In the (−)ESI-MS/MS of all biosides (magnumosides belonging to the group A) the ion peaks at m/z 497.2 appeared as a result of cleavage of B-ring of the aglycone, and 641.3 appeared as a result of D-ring cleavage, were observed (Figure 4).
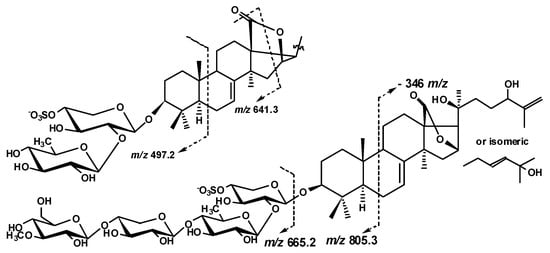
Figure 4.
The aglycones fragmentation observed in the (−)ESI-MS/MS of compounds 1–9.
Based on these results, the structure of magnumoside A1 (1) was determined as 3β-O-[β-d-quinovopyranosyl-(1→2)-4-O-sodium sulfate-β-d-xylopyranosyl]-9βH,20(S),24(S)-epoxy-25-hydroxylanosta-7-ene-18(16)-lactone.
The molecular formula of magnumoside A2 (2) was determined to be C41H63O16SNa from the [MNa − Na]− molecular ion peak at m/z 843.3838 (calc. 843.3842) in the (−)HR-ESI-MS. Extensive analysis of the 1H-, 13C- (Table 2 and Table 3) and 2D NMR spectra of the aglycone part of magnumoside A2 (2) showed similarity of its polycyclic system to that of 1. Indeed, the signals at δ(C) 182.6 (C(18)), 80.1 (C(16)), 62.2 (C(17)) as well as at δ(H) 5.12 (brs, H(16)) and 2.72 (s, H(17)) confirmed the presence of an 18(16)-lactone moiety, the signals at δ(C) 122.3 (C(7)) and 147.6 (C(8)) with corresponding proton signal at δ(H) 5.55–5.57 (m, H(7)) demonstrated the presence of 7(8)-double bond. However the signal of C(20) was high-shifted to δ(C) 71.3 when compared with that of 1 and was close to those of progenins obtained from cladoloside C by its alkaline treatment and having the identical to each other aglycone nuclei with hydroxylated C(20) position and non-shortened side chains [25]. The signals corresponding to the side chain of 2 formed the isolated spin system in the 1H,1H-COSY spectrum (Figure 2) from H(22) to H(24), indicating the latter signal was down-shifted to δ(H) 4.33 (brt, J = 6.0 Hz, H(24)) due to hydroxylation of this position and the multiplicity of the signal showed its vicinity to quaternary carbon C(25). The characteristic signals at δ(C) 148.4 (C(25)) and 110.6 (C(26)) with corresponding signals of olefinic protons at δ(H) 5.16 and 4.87 (each brs, H2(26)) indicated the presence of terminal 25(26)-double bond. The HMBC correlations from Me(27) to C(24), C(25) and Me(26) corroborated the structure of the side chain (Figure 2). The ROESY correlations (Figure 3) H-C(5)/H-C(3), Me(31); H-C(9)/Me(19); H-C(16)/Me(21) and H-C(17)/Me(21); H-C(17)/Me(32) confirmed the common (3S,5R,9S,10R,13S,14S,16S,17R,20S)-configurations in lanostane-type aglycone of magnumoside A2 (2) [22,23,24].
The absolute configuration of C(24) in 2 was determined by modified Mosher’s method. Treatment of compound 2 with (−)-(R)- and (+)-(S)-MTPA chlorides gave (S)- and (R)-MTPA esters, correspondingly, and their 1H,1H-COSY spectra were analyzed. The ∆SR signs were calculated and their values were negative for H(22) and H(23) and positive for H(26) and H(27), which allowed us to assign the (24S) configuration.
The (+)ESI-MS/MS of the [MNa + Na]+ ion at m/z 889.4 demonstrated the peaks of fragment ions at m/z: 769.4 [MNa + Na − NaHSO4]+, 623.4 [MNa + Na − NaSO4 − Qui]+, 491.3 [MNa + Na − NaSO4 − Qui − Xyl]+, analogous to those for 1, indicating the identity of their carbohydrate chains. The (−)ESI-MS/MS of 2 demonstrated the peak of fragment ion at m/z 697.3 [MNa − Na − Qui + H]− as well as the peaks at m/z 497.2 and 641.3 observed due to the aglycone cleavages mentioned above (Figure 4). Additionally, the peak [MNa − Na − C6H12O]− was observed at m/z 743.3 in the (−)ESI-MS/MS of 2 due to the cleavage of the aglycone side chain by C-20–C-22 single bond.
All these data indicate that magnumoside A2 (2) is 3β-O-[β-d-quinovopyranosyl-(1→2)-4-O-sodium sulfate-β-d-xylopyranosyl]-9βH,20(S),24(S)-dihydroxylanosta-7,25-diene-18(16)-lactone, having the new aglycone moiety.
Thus, applying Mosher’s method for the aglycone of 2 corroborated the same (24S)-configuration in 1 proposed by the ROESY spectrum.
The molecular formula of magnumoside A3 (3) was determined to be C41H63O15SNa from the [MNa − Na]− molecular ion peak at m/z 827.3892 (calc. 827.3893) in the (−)HR-ESI-MS. The comparison of 1H, 13C NMR spectra (Table 2 and Table 3) of its aglycone part with those of magnumoside A2 (2) showed the closeness of the signals of the polycyclic systems indicating their identity. The signal of C(20) was observed at δ(C) 71.5 indicating the hydroxylation of this position. The protons of the aglycone side chain formed an isolated spin system H(22)/H(23)/H(24)/H(26)/H(27) in the 1H,1H-COSY spectrum of 3 (Figure 2) that allowed us to elucidate its structure as having a terminal double bond, for which signals were observed at δ(C) 145.9 (C(25)) and 110.3 (C(26)) in the 13C NMR spectrum and at δ(H) 4.74 and 4.73 (each brs, H2(26)) in the 1H NMR spectrum. The ROESY correlations (Figure 3) confirmed the stereo structures of the aglycone polycyclic system and C(20)-chiral center of magnumoside A3 (3) to be the same as in 2.
The (+)ESI-MS/MS of 3 demonstrated the fragmentation of [MNa + Na]+ ion at m/z 873.4 resulting in the appearance of ion-peaks at m/z: 771.5 [MNa + Na − NaSO3 + H]+, 753.5 [MNa + Na − NaHSO4]+, 727.3 [MNa + Na − Qui + H]+, 607.4 [MNa + Na − NaSO4 − Qui]+, 475.3 [MNa + Na − NaSO4 − Qui − Xyl]+, analogous to those for 1 and 2, indicating the identity of their carbohydrate chains. The characteristic peaks of fragment ions at m/z 497.2 and 641.3 were also observed in the (−)ESI-MS/MS of 3 (Figure 4).
All these data indicate that magnumoside A3 (3) is 3β-O-[β-d-quinovopyranosyl-(1→2)-4-O-sodium sulfate-β-d-xylopyranosyl]-9βH,20(S)-hydroxylanosta-7,25-diene-18(16)-lactone.
The molecular formula of magnumoside A4 (4) was determined to be the same as for 3 (C41H63O15SNa) from the [MNa − Na]− molecular ion peak at m/z 827.3892 (calc. 827.3893) in the (−)HR-ESI-MS. The comparison of 1H, 13C NMR spectra (Table 2 and Table 3) of the glycosides 3 and 4 revealed the coincidence of the major part of the signals, except the signals assigned to C(23)–C(27). Actually, in the spectra of 4 the signals at δ(C) 125.1 (C(24)), 131.0 (C(25)) with corresponding olefinic proton signal at δ(H) 5.27 (t, J = 7.1 Hz, H(24)) were observed indicating the presence of 24(25)-double bond, which was confirmed by the HMBC and ROESY correlations (Figure 2 and Figure 3). Thus, magnumosides A3 (3) and A4 (4) are the isomers by the double bond position that was corroborated by their ESI-MS spectra, demonstrating the presence of the ions with the same m/z values.
The fragmentation patterns in the (+) and (−)ESI-MS/MS of 4 were the same as for 3, corroborating the identity of their carbohydrate chains and the isomerism of the aglycones.
Thus, magnumoside A4 (4) is 3β-O-[β-d-quinovopyranosyl-(1→2)-4-O-sodium sulfate-β-d-xylopyranosyl]-9βH,20(S)-hydroxylanosta-7,24-diene-18(16)-lactone.
The 1H and 13C NMR spectra of carbohydrate parts of magnumosides B1 (5) and B2 (6) were coincident to each other showing the identity of carbohydrate chains of these glycosides. The presence of four characteristic doublets at δ(H) 4.65–5.21 (J = 7.3–8.0 Hz) in the 1H NMR spectra of the carbohydrate chains of 5, and 6 correlated by the HSQC spectra with the signals of anomeric carbons at δ(C) 104.4–104.8 were indicative of a tetrasaccharide chain and β-configuration of glycosidic bonds. The 1H,1H-COSY and 1D TOCSY spectra of 5 and 6 showed the signals of four isolated spin systems assigned to two xylose, one quinovose and 3-O-methylglucose residues. The positions of interglycosidic linkages were elucidated by the ROESY and HMBC spectra of 5 and 6 (Table 1) where the cross-peaks between H(1) of the first (xylose) residue and H(3) (C(3)) of an aglycone, H(1) of the second (quinovose) residue and H(2) (C(2)) of the first (xylose) residue, H(1) of the third (xylose) residue and H(4) (C(4)) of the second (quinovose) residue and H(1) of the terminal (3-O-methylglucose) residue and H(3) (C(3)) of the third (xylose) residue were observed. The signals of C(4) and C(5) of the first (xylose) residue were observed at δ(C) 76.0 and 63.9, correspondingly, indicating the presence of a sulfate group at C(4) of the sugar unit, analogically to the carbohydrate chains of magnumosides of the group A (1–4). The linear tetrasaccharide monosulfated carbohydrate chain of magnumosides of the group B (5, 6), having the xylose as third monosaccharide unit, were found earlier in “neothyonidioside” [15] isolated from another collection of N. magnum, also coincided with the sugar moiety of colochiroside B2 (9) [17] found in the investigated sample of N. magnum and are widely distributed in the triterpene glycosides of sea cucumbers of the orders Aspidochirotida [2] and Dendrochirotida [2,8,26,27,28].
The molecular formula of magnumoside B1 (5) was determined to be C53H83O25SNa from the [MNa − Na]− molecular ion peak at m/z 1151.4954 (calc. 1151.4950) in the (−)HR-ESI-MS. The polycyclic system and hydroxylated C(20)-position of 5 were identical to those of 2–4 that was deduced from the comparison of their 1H, 13C NMR spectra (Table 2 and Table 3). The olefinic carbons signals observed at δ(C) 142.8 (C(23)), 121.9 (C(24)) with corresponding protons signals at δ(H) 5.92 (d, J = 15.8 Hz, H(23)) and 6.13 (dt, J = 6.9; 8.0; 15.8 Hz, H(24)) and the signal of oxigenated tertiary carbon at δ(C) 69.9 (C(25)) in the 13C and 1H NMR spectra of 5, correspondingly, were indicative for 23(24)E-en-25-ol fragment in the aglycone side chain. This structure was confirmed by the HMBC correlations from H-C(22) to C(23) and C(24), from H-C(23) to C(22), C(25), Me(26) and Me(27) and from Me(26) and Me(27) to C(23), C(24), C(25) (Figure 2). The ROESY correlations also corroborated the aglycone structure (Figure 3).
The (−)ESI-MS/MS of 5 demonstrated the fragmentation of [MNa − Na]− ion at m/z 1151.4. The peaks of fragment ions were observed at m/z: 975.4 [MNa − Na − MeGlc + H]−, 843.4 [MNa − Na − MeGlc − Xyl]−, 697.3 [MNa − Na − MeGlc − Xyl − Qui + H]− corroborating the sequence of sugars in the carbohydrate chain of 5. The cleavage of the ring B of the aglycone led to the appearance of fragment ion-peak at m/z 805.3 [MNa − Na − C21H30O4]−. Its further fragmentation led to the consequent loss of monosaccharide units and the ion-peaks were observed at m/z 629.2 [MNa − Na − C21H30O4 − MeGlc + H]−, 497.2 [MNa − Na − C21H30O4 − MeGlc − Xyl]−, 351.1 [MNa − Na − C21H30O4 − MeGlc − Xyl − Qui + H]−.
All these data indicate that magnumoside B1 (5) is 3β-O-[3-O-methyl-β-d-glucopyranosyl-(1→3)-β-d-xylopyranosyl-(1→4)-β-d-quinovopyranosyl-(1→2)-4-O-sodium sulfate-β-d-xylopyranosyl]-9βH,20(S),25-dihydroxylanosta-7,23E-diene-18(16)-lactone.
The molecular formula of magnumoside B2 (6) was determined to be C53H83O25SNa from the [MNa − Na]− molecular ion peak at m/z 1151.4945 (calc. 1151.4950) in the (−)HR-ESI-MS that was coincident with the formula of magnumoside B1 (5). The analysis of the 1H, 13C NMR spectra of the aglycone part of 6 showed its identity to the aglycone part of magnumoside A2 (2) (Table 2 and Table 3). So, the glycosides 5 and 6 are the isomers by the positions of the double bond and hydroxyl group in the side chain.
The peaks of fragment ions in the (−)ESI-MS/MS of 6 were observed at the same m/z values as in the spectrum of 5, corroborating the identity of carbohydrate chain structures of these compounds. The consequent fragmentation of the ion [MNa − Na − C21H30O4]− at m/z 805.3 in the spectrum of 6 that led to the loss of the aglycone and the ion-peak [MNa − Na − C30H45O5]− was observed at m/z 665.2.
Thus, magnumoside B2 (6) is 3β-O-[3-O-methyl-β-d-glucopyranosyl-(1→3)-β-d-xylopyranosyl-(1→4)-β-d-quinovopyranosyl-(1→2)-4-O-sodium sulfate-β-d-xylopyranosyl]-9βH,20(S),24(S)-dihydroxylanosta-7,25-diene-18(16)-lactone.
The 1H and 13C NMR spectra of carbohydrate parts of magnumosides C1 (7), C2 (8) and C4 (9) were coincident to each other and to those of neothyonidioside C, isolated earlier from this species [16], that indicated the identity of carbohydrate chains of these glycosides. The presence of four characteristic doublets at δ(H) 4.65–5.12 (J = 7.0–7.9 Hz) in the 1H NMR spectra of the carbohydrate chains of 7–9 correlated by the HSQC spectra with the signals of anomeric carbons at δ(C) 104.3–104.8 were indicative of a tetrasaccharide chain and β-configuration of glycosidic bonds. The positions of interglycosidic linkages were elucidated based on the ROESY and HMBC spectra (SM, Table 1), where the cross-peaks analogical to 5, and 6 were observed. The differences between the 13C NMR spectra of magnumosides of the groups C (7–9) and B (5, 6) were in the signals of C(5) and C(6) of terminal monosaccharide residue that were observed in the 13C NMR spectra of 7–9 at δ(C) 75.5 and 67.1, correspondingly, due to the shifting effects of a sulfate group attached to C(6) of 3-O-methylglucose unit. Actually, magnumosides of the group C (7–8) are characterized by the linear tetrasaccharide disulfated carbohydrate chain, which have been found earlier in the glycosides of Mensamaria intercedens [29], Pseudocolochirus violaceus [30] and Colochirus robustus [17], representatives of the family Cucumariidae, order Dendrochirotida.
The molecular formula of magnumoside C1 (7) was determined to be C53H82O28S2Na2 from the [M2Na − Na]− molecular ion peak at m/z 1253.4328 (calc. 1253.4337) in the (−)HR-ESI-MS. The aglycones of magnumoside C1 (7) and B1 (5) were identical to each other due to the coincidence of their 13C NMR spectra (Table 2 and Table 3) and their carbohydrate chains differed only by the quantity of sulfate groups. The (−)ESI-MS/MS of 7 where the peaks of fragment ions were observed at m/z 1133.4 [M2Na − Na − NaHSO4]−, 957.4 [M2Na − Na − NaHSO4 − MeGlc]−,825.4 [M2Na − Na − NaHSO4 − MeGlc − Xyl]−, 679.3 [M2Na − Na − NaHSO4 −MeGlc − Xyl − Qui]− corroborated the structure of carbohydrate chain. The cleavage of the ring B of the aglycone as well as the loss of one of the sulfate groups led to the appearance of fragment ion-peak at m/z 805.3 [M2Na − Na − NaSO3 − C21H30O4 + H]−. Its further fragmentation resulted in the loss of the aglycone, the ion-peak at m/z 665.2 [M2Na − Na − NaSO3 − C30H45O5]−, followed by the loss of sugar units, m/z: 533.1 [M2Na − Na − NaSO3 − C30H45O5 − Xyl]−, 387.1 [M2Na − Na − NaSO3 − C30H45O5 − Xyl − Qui]−, 255 [M2Na − Na − NaSO3 − C30H45O5 − Xyl − Qui − Xyl]−. The ion-peak at m/z 255 [C7H12O8SNa − Na − H]− is also corresponded to the sulfated 3-O-methyl glucose residue.
Therefore, magnumoside C1 (7) is 3β-O-[6-O-sodium sulfate-3-O-methyl-β-d-glucopyranosyl-(1→3)-β-d-xylopyranosyl-(1→4)-β-d-quinovopyranosyl-(1→2)-4-O-sodium sulfate-β-d-xylopyranosyl]-9βH,20(S),25-dihydroxylanosta-7,23E-diene-18(16)-lactone.
The molecular formula of magnumoside C2 (8) coincided with that of 7 (C53H82O28S2Na2) that was deduced from the [M2Na − Na]− molecular ion peak at m/z 1253.4341 (calc. 1253.4337) in the (−)HR-ESI-MS. The aglycone of magnumoside C2 (8) was identical to those of the magnumosides A2 (2) and B2 (6) that was suggested based on the coincidence of their 13C NMR spectra (Table 2 and Table 3). So, magnumoside C2 (8) is additionally sulfated, by C(6) of 3-O-methylglucose residue, analog of magnumoside B2 (6). The (−)ESI-MS/MS of 8 corroborated its isomerism to the magnumoside C1 (7) since their fragmentation patterns were the same and the ion-peaks were characterized by the identical m/z values.
Thus, magnumoside C2 (8) is 3β-O-[6-O-sodium sulfate-3-O-methyl-β-d-glucopyranosyl-(1→3)-β-d-xylopyranosyl-(1→4)-β-d-quinovopyranosyl-(1→2)-4-O-sodium sulfate-β-d-xylopyranosyl]-9βH,20(S),24(S)-dihydroxylanosta-7,25-diene-18(16)-lactone.
The NMR spectra of the aglycone moiety of magnumoside C4 (9) were coincident to those of magnumoside A4 (4) (Table 2 and Table 3).
The molecular formula of magnumoside C4 (9) was determined to be C53H82O27S2Na2 from the [M2Na − Na]− molecular ion peak at m/z 1237.4390 (calc. 1237.4388) in the (−)HR ESIMS. The peaks of fragment ions in the (−) ESI MS/MS of 9 were observed at m/z 1135.4 [M2Na − Na − NaSO3 + H]−, 1117.4 [M2Na − Na − NaHSO4]−, 959.4 [M2Na − Na − NaSO4 − MeGlc]−, 827.4 [M2Na − Na − NaSO4 − MeGlc − Xyl]−, 681.3 [M2Na − Na − NaSO4 −MeGlc − Xyl − Qui]− corroborated the structure of carbohydrate chain. The loss of the aglycone from the ion at m/z 1135.4 [M2Na − Na − NaSO3 + H]− led to the appearance of the ion-peak at m/z 665.2 [M2Na − Na − NaSO3 − C30H45O4]−, its further fragmentation resulted in the consequent loss of sugar units, m/z: 533.1 [M2Na − Na − NaSO3 − C30H45O4 − Xyl]−, 387.1 [M2Na − Na − NaSO3 − C30H45O4 − Xyl − Qui]−, 255 [M2Na − Na − NaSO3 − C30H45O4 − Xyl − Qui − Xyl]−. The ion-peak at m/z 255 [C7H12O8SNa − Na − H]− is also corresponded to the sulfated 3-O-methyl glucose residue.
All these data indicate that magnumoside C4 (9) is 3β-O-[6-O-sodium sulfate-3-O-methyl-β-d-glucopyranosyl-(1→3)-β-d-xylopyranosyl-(1→4)-β-d-quinovopyranosyl-(1→2)-4-O-sodium sulfate-β-d-xylopyranosyl]-9βH,20(S)-hydroxylanosta-7,24-diene-18(16)-lactone.
All the new glycosides, magnumosides A1 (1), A2 (2), A3 (3), A4 (4), B1 (5), B2 (6), C1 (7), C2 (8) and C4 (9), isolated from N. magnum, are characterized by the presence of non-holostane-type aglycones with 18(16)-lactone moieties and non-shortened side chains. The aglycone of 1 having 20(24)-epoxy group in the side chain was found first in the triterpene glycosides of sea cucumbers. The aglycones, having 18(16)-lactone, are of rare occurrence in the sea cucumber glycosides. Such type aglycones were found in two variants: with shortened side chains, like in some glycosides from Eupentacta fraudatrix [31,32,33] and Pentamera calcigera [34]; and, that is more rare, with non-shortened side chains, like in psolusoside B from Psolus fabricii [21], colochiroside E from Colochirus robustus [22] and in magnumosides A1 (1), A2 (2), A3 (3), A4 (4), B1 (5), B2 (6), C1 (7), C2 (8) and C4 (9).
These aglycones are strongly differing from the holostane-type aglycones found in isolated earlier glycosides, “neothyonidioside” and neothyonidioside C [15,16], from New-Caledonian and Vietnamese collections of N. magnum, correspondingly. In contrast, the aglycone of colochiroside B2 (10) found in the investigated sample of N. magnum is biogenetically very close to that of neothyonidioside C which also has the holostane-type aglycone with 16-acetoxygroup and differs in the side chain structure. Furthermore, the monosulfated tetrasaccharide carbohydrate chain of 10 was identical to those of “neothyonidioside” and magnumosides of the group B (5, 6). The disulfated tetrasaccharide chain of magnumosides of the group C (7–9) was identical to that of neothyonidioside C. The finding of compound 10 in recent Vietnamese collection of N. magnum structurally close to found earlier glycosides from this species confirms the correctness of biological identification of the all studied samples. The predominance of non-holostane-type glycosides and the fact that neither “neothyonidioside” nor neothyonidioside C have been found in the studying sample of the animal could be explained by the changes in the quantitative and qualitative composition of different components of glycosidic fraction in the samples of one species collected in different places and seasons. The representative example of such changes demonstrated the components of glycosidic fraction of Psolus fabricii. Psolusoside A, a holostane glycoside was predominant in P. fabricii collected near the shore of the Onekotan Island of Kuril Ridge and non-holostane psolusoside B having a 18(16)-lactone was the minor one [35]. The opposite situation was observed in samples of P. fabricii collected in the Kraternaya Bay of Ushishir Islands of the Kuril Archipelago, where psolusoside A was found only in trace amount and psolusoside B was the predominant component [36].
The sets of magnumosides A2→B2→C2 and B1→C1, in which the glycosides were characterized by the same aglycones and different carbohydrate chains within the set, demonstrated the biosynthetic transformations of the carbohydrate chains resulting in their elongation and additional sulfation. The biosynthetic modifications of the aglycones are mainly concerned with side chains transformations, when the double bonds and hydroxyls are introduced in different positions. The aglycone of magnumosides B1 (5) and C1 (7) with 23(24)-double bond and 20,25-hydroxyls is obviously could be the precursor of the unusual aglycone of magnumoside A1 (1) with 20(24)-epoxy-25-hydroxy-fragment. The similar conversion of the aglycone having a linear side chain with a double bond and hydroxyl group to the aglycone having epoxy-group was observed in the process of obtaining the genins with 18(16)-lactone moieties by chemical transformations of the holostane aglycone of cladoloside C [25]. It was considered as a biomimetic reaction, modeling the biosynthetic process in the glycoside aglycones. The aglycone structure of colochiroside B2 (10) is biogenetically related with the aglycones of 1, 5 and 7. It is known that the formation of 18(16)-lactone biosynthetically occurs in C(18)-carboxylated derivatives having both hydroxylated C(16) and C(20) positions. However, when the further oxidation (acetylation or carboxylation) of C(16) precedes the carboxylation of C(18) the holostane-type aglycones with functionality at C(16) are biosynthesized [37] (Figure 5). In the case of 10, this process took place. Interestingly, the oxidative transformations of the side chain in biosynthesis of 10 may precede the lactonization.
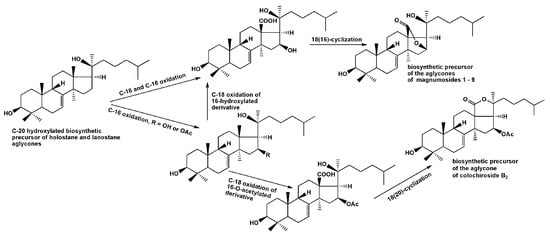
Figure 5.
The hypothetic scheme of the aglycones biosynthesis of glycosides of N. magnum.
2.2. Biological Activities of Glycosides
2.2.1. Hemolytic and Cytotoxic Activities of the Glycosides 1–9 against Mouse Spleenocites and the Ascites Form of Mouse Ehrlich Carcinoma Cells
The cytotoxic action of the compounds 1–9 against mouse spleenocytes and the ascites form of mouse Ehrlich carcinoma cells as well as hemolytic action against mouse erythrocytes have been studied (Table 4). Magnumoside A1 (1) was the only glycoside inactive in all tests that was caused by unusual aglycone structure having 18(16)-lactone in combination with 20(24)-epoxy-25-hydroxy-fragment. Magnumosides A2 (2), B1 (5) and B2 (6) demonstrated moderate hemolytic activity and were non-cytotoxic up to the ultimate investigated concentration of 100 μM, that was explained by the presence of hydroxyls in their side chains [17,32,33]. However, magnumosides C1 (7) and C2 (8) that had aglycones identical to those of magnumosides B1 (5) and B2 (6) and differed from the latter compounds by the presence of the additional sulfate group were the exclusions from this relation and demonstrated significant effects in all tests. Moreover, magnumosides C1 (7) and C4 (9) turned out to be the most active compounds in this series. So, the activity-decreasing influence of hydroxyl-group in the side chain of 7 and 8 was counterbalanced by the additional sulfate group attached to C-6 of terminal monosaccharide residue of these compounds. On the whole, the erythrocytes were more sensitive to the glycosides action than the spleenocytes and cancer cells studied.

Table 4.
Hemolytic activity of the glycosides 1–9 against mouse erythrocytes and cytotoxic activity against mouse spleenocytes and the ascites form of mouse Ehrlich carcinoma cells.
2.2.2. The Effect of the Glycosides on Cell Viability of Human Colorectal Adenocarcinoma DLD-1 Cells
To determine cytotoxic effect of the compounds against human colorectal adenocarcinoma, DLD-1 cells were treated with various concentrations of 1–9 (0–100 µM) for 24 h and then cell viability was assessed by the MTS assay. It was showed that 1, 2, 4–6 did not possessed cytotoxic effect against DLD-1 cells that is in good correlation with the data on Echrlich carcinoma cells. The magnumoside A3 (3), magnumoside C1 (7), magnumoside C2 (8) and magnumoside C4 (9) decreased cell viability with IC50 value of 30.3, 34.3, 32.9, 37.1, and 33.9 µM, respectively (Table 5). Thus, for further experiments we chose the concentrations of investigated compounds lower than IC50, at which no significant cytotoxic effect on DLD-1 cells was observed.

Table 5.
The cytotoxic activity of the glycosides 1–9 against DLD-1 cells.
2.2.3. The Effect of the Glycosides on Formation and Growth of Colonies of Human Colorectal Adenocarcinoma DLD-1 Cells
The effect of 3, 7–9 on the colony formation of DLD-1 cells using soft agar assay has been studied. The magnumoside A3 (3) and magnumoside C1 (7) inhibited spontaneous colony formation by 22% and 26%, respectively, (Figure 6A) and, at the same time, they reduced colonies size of DLD-1 cancer cells by 49% and 43%, respectively, (Figure 6B). On the other hand, magnumosides C2 (8) and C4 (9) possessed slight inhibitory activity in this experiment (the percentages of inhibition were less than 20%) (Figure 6A,B).
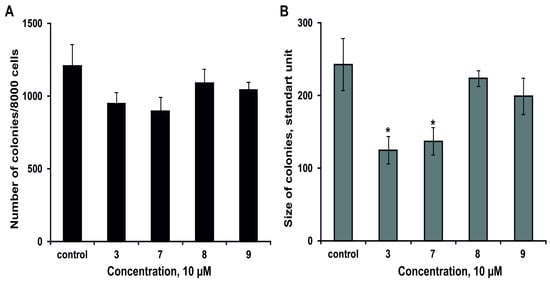
Figure 6.
The effect of the glycosides 3, 7–9 on colony formation of DLD-1 cells. (A) The compounds decreased the number of colonies of cancer cells. (B) The compounds decreased the size of colonies of cancer cells. Data are shown as means ± standard deviation and the asterisks (* p < 0.05) indicates a significant decrease in colony formation of cells treated with the compounds compared with the control.
2.2.4. The Synergism of Radioactive Irradiation and the Compounds Effects on Proliferation and Colony Formation of Human Colorectal Adenocarcinoma Cells
At first, the individual effect of radiation or the compounds at concentration 2 µM on colony formation of DLD-1 cells was checked. The tested compounds did not influence the process of colonies formation at the dose of 2 µM. The number and size of colonies of DLD-1 cells were found to be decreased by 7% and 67%, respectively, after radiation exposure at dose of 4 Gy. Moreover, the synergism of effects of radioactive irradiation (4 Gy) and the compounds 3, 7–9 (2 µM) was not observed (data not shown).
Nevertheless, the investigated compounds (2 µM) enhanced the antiproliferative effect from radioactive irradiation (4 Gy). Magnumoside C4 (9) possessed the highest activity in this experiment; it increased the inhibitory effect from radiation on proliferation of DLD-1 cancer cells by 45%. Magnumoside A3 (3), magnumoside C1 (7) and magnumoside C2 (8) enhanced the effect from radiation by more than 30% (Figure 7). Recently, it was reported that ginsenoside Rg3 isolated from the roots of Panax ginseng sensitized human lung carcinoma A549 and H1299 cells to γ-radiation and significantly enhanced the efficacy of radiation therapy in C57BL/6 mice bearing a Lewis lung carcinoma cell xenograft tumor [38]. Nevertheless, our finding of a synergism of the antiproliferative effect of the radioactive irradiation and a series of glycosides from Neothyonidium magnum on human tumor cells is the first study on sea cucumber triterpene glycosides.
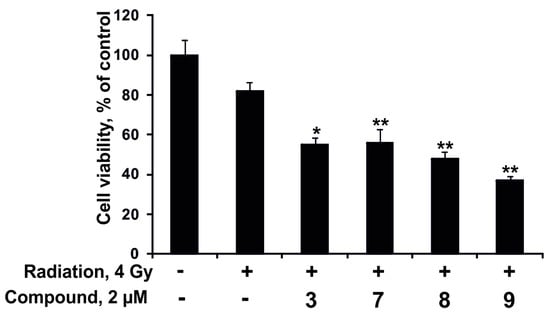
Figure 7.
The effect of radioactive irradiation and a combination of radioactive irradiation and the compounds 3, 7–9 on DLD-1 cancer cell proliferation. DLD-1 cells (8.0 × 103) were treated with radiation 4 Gy and the compounds 3, 7–9 (2 µM) for 96 h. Cell viability was estimated using the MTS assay. Data are represented as the mean ± SD as determined from triplicate experiments. A Student’s t-test was used to evaluate the data with the following significance levels: * p < 0.05, ** p < 0.01.
3. Experimental Section
3.1. General Experimental Procedures
Specific rotations were measured on Perkin-Elmer 343 polarimeter. NMR spectra were obtained on an AVANCE III-700 Bruker spectrometer (Bruker BioSpin, Fällanden, Switzerland) at 700.13 (1H) and 176.04 (13C) MHz, δ in ppm rel. to Me4Si, J in Hz. ESI-MS/MS and HR-ESI-MS were run on an Agilent 6510 Q-TOF apparatus (Agilent Technologies, Santa Clara, CA, USA), sample concentration 0.01 mg/mL, in m/z. HPLC was carried out on an Agilent 1100 chromatograph equipped with a differential refractometer using Supelcosil LC-Si (4.6 × 150 mm, 5 μm) (Agilent Technologies, Santa Clara, CA, USA), Supelco Ascentis RP-Amide (10 × 250 mm, 5 μm) (Supelco Analytical, Bellefonte, PA, USA) and Diasfer C-8 (4 × 250 mm, 5 μm) (Supelco Analytical, Bellefonte, PA, USA) columns. Polychrom-1 (powdered Teflon, 0.25–0.50 mm; Biolar, Olaine, Latvia), silica gel KSK (50–160 μM, Sorbpolimer, Krasnodar, Russia) were used for column chromatography.
3.2. Animal Material
Specimens of the sea cucumber Neothyonidium (=Massinium) magnum (family Phyllophoridae; order Dendrochirotida) were collected during the expedition of Joint Russia-Vietnam laboratory of Marine Biology RAS-VAST in South China Sea in Nha Trang bay near Tam Island. Sampling was performed by SCUBA in July 2015 (collector T. Dautova) at a depth of 8 m. Sea cucumbers were identified by T. Dautova; voucher specimens are preserved in the collection of the Museum of A.V. Zhirmunsky Institute of Marine Biology, Vladivostok, Russia.
3.3. Extraction and Isolation
The sea cucumbers were minced and extracted twice with refluxing 60% EtOH. The dry weight of the residue was about 59.2 g. The combined extracts were concentrated to dryness in vacuum, dissolved in H2O, and chromatographed on a Polychrom-1 column (powdered Teflon, Biolar, Latvia). Eluting first of the inorganic salts and impurities with H2O and then the glycosides with 50% EtOH gave 1800 mg of crude glycoside fraction. Then it was chromatographed on Si gel column using CHCl3/EtOH/H2O (100:100:17) followed by (100:125:25) as mobile phase to give fractions 1 (332 mg) and 2 (340 mg). The fraction 1 was additionally chromatographed on Si gel column with CHCl3/EtOH/H2O (100:75:10) as mobile phase to give three subfractions: 1.1 (95 mg), 1.2 (127 mg) and 1.3 (46 mg). Subfraction 1.1 was submitted to HPLC on silica-based column Supelcosil LC-Si (4.6 × 150 mm) with CHCl3/MeOH/H2O (65:20:2) as mobile phase to obtain fractions 1.1.1 (45 mg) and 1.1.2 (5 mg) that were sequentially submitted to HPLC on a reversed-phase semipreparative Supelco Ascentis RP-Amide column (10 × 250 mm) with different ratios of MeOH/H2O/NH4OAc (1 M water solution) as mobile phase. Fraction 1.1.1 chromatographed with the ratio (65/34/1) followed by the ratios (63/36/1) gave magnumosides A3 (3) (3.7 mg) and A4 (4) (8.0 mg); (58/41/1)—magnumoside A1 (1) (3.6 mg). Fraction 1.1.2 chromatographed with the ratio (55/44/1) gave magnumoside A2 (2) (5.0 mg).
Subfraction 1.3, obtained after chromatography on Si gel, was submitted to HPLC on Supelco Ascentis RP-Amide column (10 × 250 mm) with MeOH/H2O/NH4OAc (1 M water solution) (57/41/2) followed by (55/43/2) as mobile phase to give magnumosides B1 (5) (2.6 mg), B2 (6) (1.8 mg) and colochiroside B2 (10) (2.0 mg).
The most polar fraction 2, obtained after the separation of crude glycoside fraction of Si gel column, was submitted to HPLC on Supelco Ascentis RP-Amide column (10 × 250 mm) with MeOH/H2O/NH4OAc (1 M water solution) (57/41/2) to give a set of subfractions. Subfraction 2.1 was submitted to HPLC on Diasfer C-8 (4.6 × 250 mm) column with AcN/H2O/ NH4OAc (1 M water solution) (24/75/1) to obtain magnumoside C1 (7) (5.7 mg). Subfraction 2.2 was submitted to HPLC in the same conditions to obtain magnumoside C2 (8) (2.5 mg) and C4 (9) (15 mg).
3.3.1. Magnumoside A1 (1)
Colorless powder; −48 (c 0.1, 50% MeOH); 1H NMR data see Table 1 and Table 2; 13C NMR data see Table 1 and Table 3; (−)HR-ESI-MS, m/z: 843.3838 [MNa − Na]− (calcd for C41H63O16S−, 843.3842); (−)ESI-MS/MS, m/z: 843.4 [MNa − Na]−, 741.3 [MNa − Na − SO3Na + H]−, 697.3 [MNa − Na − Qui + H]−, 641.3 [MNa − Na − C10H18O4]−, 497.2 [MNa − Na − C21H30O4]−. (+)ESI-MS/MS, m/z: 889.4 [MNa + Na]+, 769.4 [MNa + Na − NaHSO4]+, 623.4 [MNa + Na − NaSO4 − Qui]+, 491.3 [MNa + Na − NaSO4 − Qui − Xyl]+.
3.3.2. Magnumoside A2 (2)
Colorless powder; −12 (c 0.1, 50% MeOH); 1H NMR data see Table 1 and Table 2; 13C NMR data see Table 1 and Table 3; (−)HR-ESI-MS, m/z: 843.3838 [MNa − Na]−, (calcd for C41H63O16S−, 843.3842), (+)ESI-MS/MS, m/z: 889.4 [MNa + Na]+, 769.4 [MNa + Na − NaHSO4]+, 623.4 [MNa + Na − NaSO4 − Qui]+, 491.3 [MNa + Na − NaSO4 − Qui − Xyl]+; (−)ESI-MS/MS, m/z: 843.4 [MNa − Na]−, 697.3 [MNa − Na − Qui + H]−, 743.3 [MNa − Na − C6H12O]−, 641.3 [MNa − Na − C10H18O4]−, 497.2 [MNa − Na − C21H30O4]−.
3.3.3. Magnumoside A3 (3)
Colorless powder; −63 (c 0.1, 50% MeOH); 1H NMR data see Table 1 and Table 2; 13C NMR data see Table 1 and Table 3. (−)HR-ESI-MS, m/z: 827.3892 [MNa − Na]− (calcd for C41H63O15S−, 827.3893); (+)ESI-MS/MS, m/z: 873.4 [MNa + Na]+, 771.5 [MNa + Na − NaSO3 + H]+, 753.5 [MNa + Na − NaHSO4]+, 727.3 [MNa + Na − Qui + H]+, 607.4 [MNa + Na − NaSO4 − Qui]+, 475.3 [MNa + Na − NaSO4 − Qui − Xyl]+; (−)ESI-MS/MS, m/z 641.3 [MNa − Na − C10H18O3]−, 497.2 [MNa − Na − C21H30O3]−.
3.3.4. Magnumoside A4 (4)
Colorless powder; −98 (c 0.1, 50% MeOH); 1H NMR: Table 1 and Table 2; 13C NMR data see Table 1 and Table 3; (−)HR-ESI-MS, m/z: 827.3892 [MNa − Na]− (calcd for C41H63O15S−, 827.3893); (+)ESI-MS/MS, m/z: 873.4 [MNa + Na]+, 771.5 [MNa + Na − NaSO3 + H]+, 753.5 [MNa + Na − NaHSO4]+, 727.3 [MNa + Na − Qui + H]+, 607.4 [MNa + Na − NaSO4 − Qui]+, 475.3 [MNa + Na − NaSO4 − Qui − Xyl]+; (−)ESI-MS/MS, m/z 641.3 [MNa − Na − C10H18O3]−, 497.2 [MNa − Na − C21H30O3]−.
3.3.5. Magnumoside B1 (5)
Colorless powder; −74 (c 0.1, 50% MeOH); 1H NMR data see Table 1 and Table 2; 13C NMR data see Table 1 and Table 3; (−)HR-ESI-MS, m/z: 1151.4954 [MNa − Na]− (calcd for C53H83O25S−, 1151.4950); (−)ESI-MS/MS, m/z: 1151.4 [MNa − Na]−, 975.4 [MNa − Na − MeGlc + H]−, 843.4 [MNa − Na − MeGlc − Xyl]−, 697.3 [MNa − Na − MeGlc − Xyl − Qui + H]−, 805.3 [MNa − Na − C21H30O4]−, 629.2 [MNa − Na − C21H30O4 − MeGlc + H]−, 497.2 [MNa − Na − C21H30O4 − MeGlc − Xyl]−, 351.1 [MNa − Na − C21H30O4 − MeGlc − Xyl − Qui + H]−.
3.3.6. Magnumoside B2 (6)
Colorless powder; −57 (c 0.1, 50% MeOH); 1H NMR: Table 1 and Table 2; 13C NMR data see Table 1 and Table 3; (−)HR-ESI-MS, m/z: 1151.4954 [MNa − Na]− (calcd for C53H83O25S−, 1151.4950); (−)ESI-MS/MS, m/z: 1151.4 [MNa − Na]−, 975.4 [MNa − Na − MeGlc + H]−, 843.4 [MNa − Na − MeGlc − Xyl]−, 697.3 [MNa − Na − MeGlc − Xyl − Qui + H]−, 805.3 [MNa − Na − C21H30O4]−, 665.2 [MNa − Na − C30H45O5]−.
3.3.7. Magnumoside C1 (7)
Colorless powder; −22 (c 0.1, 50% MeOH); 1H NMR: Table 1 and Table 2 (for carbohydrate chain); 13C NMR data see Table 1 and Table 3; (−)HR-ESI-MS, m/z: 1253.4328 [M2Na − Na]− (calcd for C53H82O28S2Na−, 1253.4337); (−)ESI-MS/MS, m/z: 1133.4 [M2Na − Na − NaHSO4]−, 957.4 [M2Na − Na − NaHSO4 − MeGlc]−,825.4 [M2Na − Na − NaHSO4 − MeGlc − Xyl]−, 679.3 [M2Na − Na − NaHSO4 −MeGlc − Xyl − Qui]−, 805.3 [M2Na − Na − NaSO3 − C21H30O4 + H]−, 665.2 [M2Na − Na − NaSO3 − C30H45O5]−, 533.1 [M2Na − Na − NaSO3 − C30H45O5 − Xyl]−, 387.1 [M2Na − Na − NaSO3 − C30H45O5 − Xyl − Qui]−, 255 [M2Na − Na − NaSO3 − C30H45O5 − Xyl − Qui − Xyl]−.
3.3.8. Magnumoside C2 (8)
Colorless powder; −64 (c 0.1, 50% MeOH); 1H NMR data see Table 1 and Table 2; 13C NMR data see Table 1 and Table 3; (−)HR-ESI-MS, m/z: 1253.4328 [M2Na − Na]− (calcd for C53H82O28S2Na−, 1253.4337); (−)ESI-MS/MS, m/z: 1133.4 [M2Na − Na − NaHSO4]−, 957.4 [M2Na − Na − NaHSO4 − MeGlc]−,825.4 [M2Na − Na − NaHSO4 − MeGlc − Xyl]−, 679.3 [M2Na − Na − NaHSO4 −MeGlc − Xyl − Qui]−, 805.3 [M2Na − Na − NaSO3 − C21H30O4 + H]−, 665.2 [M2Na − Na − NaSO3 − C30H45O5]−, 533.1 [M2Na − Na − NaSO3 − C30H45O5 − Xyl]−, 387.1 [M2Na − Na − NaSO3 − C30H45O5 − Xyl − Qui]−, 255 [M2Na − Na − NaSO3 − C30H45O5 − Xyl − Qui − Xyl]−.
3.3.9. Magnumoside C4 (9)
Colorless powder. −52 (c 0.1, H2O). NMR: See Table 2 and Table 3. HR ESI MS (−) m/z: 1237.4390 (calc. 1237.4388) [M2Na − Na]−; ESI MS/MS (−) m/z: 1135.4 [M2Na − Na − NaSO3 + H]−, 1117.4 [M2Na − Na − NaHSO4]−, 959.4 [M2Na − Na − NaSO4 − MeGlc]−, 827.4 [M2Na − Na − NaSO4 − MeGlc − Xyl]−, 681.3 [M2Na − Na − NaSO4 −MeGlc − Xyl − Qui]−, 665.2 [M2Na − Na − NaSO3 − C30H45O4]−, 533.1 [M2Na − Na − NaSO3 − C30H45O4 − Xyl]−, 387.1 [M2Na − Na − NaSO3 − C30H45O4 − Xyl − Qui]−, 255 [M2Na − Na − NaSO3 − C30H45O4 − Xyl − Qui − Xyl]−.
3.4. Preparation of the MTPA Esters of Compound 2
Two aliquots (0.6 mg) of compound 2 were treated with (−)-(R)- and (+)-(S)-α-methoxy-α-(trifluoromethyl)-phenylacetyl (MTPA) chloride (10 μL) in dry C5D5N (600 μL) for 2 h at r.t. in NMR tubes to give corresponding (S)- and (R)-MTPA esters. Data of 24-(S)-MTPA Ester of 2. 1H NMR (700 MHz): 1.66 (m, 1H of CH2(22)), 1.79 (s, Me(27)), 1.85 (m, 1H of CH2(22)), 2.07 (m, 1H of CH2(23)), 2.23 (m, 1H of CH2(23)), 5.04 (brs, 1H of CH2(26)), 5.24 (brs, 1H of CH2(26)), 5.75 (m, H-C(24)). Data of 24-(R)-MTPA Ester of 2. 1H NMR (700 MHz): 1.67 (s, Me(27)), 1.80 (m, 1H of CH2(22)), 1.98 (m, 1H of CH2(22)), 2.13 (m, 1H of CH2(23)), 2.27 (m, 1H of CH2(23)), 4.99 (brs, 1H of CH2(26)), 5.14 (brs, 1H of CH2(26)), 5.72 (m, H-C(24)).
3.5. Bioassay
3.5.1. Cell Culture
The spleenocytes from CD-1 line mice were used. The spleen was isolated from mice and homogenized. The spleenocytes were washed thrice and resuspended with RPMI-1640 medium contained gentamicine 8 μg/mL (Biolot, Saint Petersburg, Russia). The museum tetraploid strain of murine ascites Ehrlich carcinoma cells from the All-Russian cancer center RAMS (Moscow, Russia) was used. The cells of the ascites Ehrlich carcinoma were separated from ascites, which were collected on day 7 after inoculation in mouse CD-1 line. The cells were washed of ascites thrice and then resuspended in liquid media DMEM with L-Glutamine (Biolot, Saint Petersburg, Russia).
3.5.2. Cytotoxic Activity
The solutions of tested substances in different concentrations (20 µL) and cell suspension (200 µL) were added in wells of 96-well plate and incubated over night at 37 °C and 5% CO2. After incubation the cells were sedimented by centrifugation, 200 µL of medium from each well were collected and 100 µL of pure medium were added. Then 10 µL of MTT solution 5 µg/mL (Sigma, St. Louis, MO, USA) were added in each well. Plate was incubated 4 h, after that 100 µL SDS-HCl were added to each well and plate was incubated at 37 °C 4–18 h. Optical density was measured at 570 nm and 630–690 nm. The activity of the substances was calculated as the ratio of the dead cells to general cells amount (ED50). Typicoside A1 [28] and cucumarioside A2-2 [11] were used as positive controls.
3.5.3. Hemolytic Activity
Blood was taken from a CD-1 mouse. The erythrocytes were washed thrice with 0.9% NaCl, centrifuged (450 g) on a centrifuge LABOFUGE 400R (Heraeus, Hanau, Germany) for 5 min followed by re-suspending in phosphate-buffered saline (PBS), pH 7.2–7.4. Erythrocytes were used at a concentration provided an optical density of 1.5 at 700 nm for a non-hemolyzed sample. 20 μL of a water solution of test substance with a fixed concentration (0.12–100.00 μM) were added to a well of a 96-well plate containing 180 mL of the erythrocyte suspension and incubated for 1 h at 37 °C. The plates were centrifuged (900× g) on a LMC-3000 laboratory centrifuge (Biosan, Riga, Latvia) for 10 min. 10 μL of the supernatant were placed to special microplate with plate bottom for determination of the optical density on a spectrofotometer Multiskan FC at λ = 570 nm. ED50 was calculated using SigmaPlot 3.02 software (Jandel Scientific, San Rafael, CA, USA). Triton X-100 (Biolot, Saint Petersburg, Russia) at concentration 1%, caused the hemolysis of 100% cells was used as positive control. The erythrocyte suspension in phosphate-buffered saline, pH 7.2–7.4 (PBS) with 20 μL of the solvent without a tested compound was used as negative control.
3.5.4. DLD-1 Human Colorectal Adenocarcinoma Cell Culture
Human colorectal adenocarcinoma cells DLD-1 were cultured in RPMI-1640 medium. Culture media was supplemented with 10% fetal bovine serum (FBS) and penicillin—streptomycin solution. Cells were maintained in a sterile environment and kept in an incubator at 5% CO2 and 37 °C to promote growth. DLD-1 cells were sub-cultured every 3–4 days by their rinsing with phosphate buffered saline (PBS), adding trypsin to detach the cells from the tissue culture flask, and transferring 10–20% of the harvested cells to a new flask containing fresh growth media.
3.5.5. Cytotoxicity against DLD-1 Cells Assay
DLD-1 cells (1.0 × 104/well) were seeded in 96-well plates for 24 h at 37 °C in 5% CO2 incubator. The cells were treated with the compounds 1–9 at concentrations range from 0 to 100 µM for additional 24 h. Subsequently, cells were incubated with 15 µL MTS reagent for 3 h, and the absorbance of each well was measured at 490/630 nm using microplate reader “Power Wave XS” (Bio Tek, Winooski, VT, USA). All the experiments were repeated three times, and the mean absorbance values were calculated. The results are expressed as the percentage of inhibition that produced a reduction in absorbance by compound’s treatment compared to the non-treated cells (control).
3.5.6. Soft Agar Assay
DLD-1 cells (8.0 × 103) were seeded in 6-well plate and treated with the 3, 7–9 (10 µM) in 1 mL of 0.3% Basal Medium Eagle (BME) agar containing 10% FBS, 2 mM l-glutamine, and 25 µg/mL gentamicin. The cultures were maintained at 37 °C in a 5% CO2 incubator for 14 days, and the cell’s colonies were scored using a microscope “Motic AE 20” (Scientific Instrument Company, Campbell, CA, USA) and the Motic Image Plus computer program (Scientific Instrument Company, Campbell, CA, USA) [39].
3.5.7. DLD-1 Cell Proliferation Assay
DLD-1 cells (8 × 103) cells were seeded in 96-well plates in 200 mL of RPMI-1640/10%FBS medium at 37 °C in a 5% CO2 incubator for 24 h. Then the cells were treated with 2 µM of 3, 7–9 for additional 24, 48, 72 or 96 h at 37 °C in a 5% CO2 atmosphere. The reaction was terminated by adding MTS reagent to each well as described in the Section 3.5.5.
3.5.8. Radiation Exposure
Irradiation was delivered at room temperature using single doses of X-ray system XPERT 80 (KUB Technologies, Inc, Milford, CT, USA). The doses were from 4 to 8 Gy for colony formation assay. The absorber dose was measured using X-ray radiation clinical dosimeter DRK-1 (Akselbant, Moscow, Russia).
3.5.9. Cell Irradiation
DLD-1 cells (5.0 × 105) were plated at 60 mm dishes and incubated for 24 h. After the incubation, the cells were cultured in the presence or absence of 2 μM 3, 7–9 for additional 24 h before irradiation at the dose of 4 Gy. Immediately after irradiation, cells were returned to the incubator for recovery. Three hour later, the cells were harvested and used for soft agar assay or proliferation assay to establish the synergism of radioactive irradiation and investigated compounds effects on colony formation or proliferation of tested cells.
3.5.10. Statistical Analysis
All assays were performed in triplicate. The results are expressed as the means ± standard deviation (SD). A Student’s t-test was used to evaluate the data with the significance level of p < 0.05. The mean and standard deviation were calculated and plotted using SigmaPlot 3.02 Software (Jandel Scientific, San Rafael, CA, USA).
4. Conclusions
Summarizing the obtained data, three types of previously known carbohydrate chains, five new aglycones as well as one known aglycone have been found in the isolated glycosides 1–9. Magnumoside A1 (1) has uncommon non-holostane aglycone with a unique for sea cucumber triterpene glycosides 20(24)-epoxy-25-hydroxy-fragment in the side chain. The biosythesis of the glycosides found in N. magnum has mosaic character, i.e., transformation in cyclic systems of aglycones and in their side chains as well as in carbohydrate chains (elongation and sulfation) proceed parallel and independently and they have the characteristics of a biosynthetic network. Disulfated glycosides 7 and 8 showed surprisingly high hemolytic and cytotoxic actions in spite of the presence of hydroxyl-groups in their side chains. The data concerning cytotoxic activities on DLD-1 human colorectal adenocarcinoma cells good correlated with the data on mouse ascites Echrlich carcinoma cells that confirmed the usefulness of the last model tumor for screening of substances that are cytotoxic against human tumor cells. The data concerning synergy of the activities of the glycosides 3 and 7–9 in subcytotoxic doses and subtoxic doses of radiation were obtained for the first time where magnumoside C4 (9) revealed the highest increase of the inhibitory effect of radiation on cell proliferation of 45%. The substances having such effects allow a decrease in the effective doses of radiation that may be used for radiation therapy of human tumors. The search for substances with a similar mode of action among sea cucumber triterpene glycosides should be continued.
Acknowledgments
The chemical structure and part of bioassay were carried out at partial financial support of the Grant of the Russian Foundation of Basic Research No. 16-04-00010 for partial financial support. The studies of cytotoxic activities on a series of human cancer cell lines and it synergy with radioactive irradiation were supported by the Grant of the Russian Science Foundation No. 16-14-10131. The field work and identification processing of the animal samples were supported by APN (Asia Pacific Network for the Global Change Research) CAF2016-RR08-CMY-Dautova. The authors are very appreciative to Valentin A. Stonik (G.B. Elyakov Pacific Institute of Bioorganic Chemistry of the FEB RAS, Vladivostok, Russia) for reading, checking and discussion of the manuscript.
Author Contributions
Alexandra S. Silchenko and Vladimir I. Kalinin wrote the paper. Aleksandra S. Silchenko, Sergey A. Avilov conceived, designed and performed the experiments concerning isolation of the glycosides. Pelageya V. Andrijaschenko carried out some procedures of glycosides isolation. Anatoly I. Kalinovsky obtained and analyzed the NMR data. Alexandra S. Silchenko analyzed the NMR data. Pavel S. Dmitrenok performed the mass-spectrometry experiments and analyzed their results; Ekaterina A. Chingizova performed the bioassays; Olesya S. Malyarenko and Svetlana P. Ermakova performed the bioassays on DLD-1 cells; Tatiana N. Dautova collected and identified the animal material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stonik, V.A.; Kalinin, V.I.; Avilov, S.A. Toxins from the sea cucumbers (Holothuroids): Chemical structures, properties, taxonomic distribution, biosynthesis and evolution. J. Nat. Toxins 1999, 8, 235–248. [Google Scholar] [PubMed]
- Kalinin, V.I.; Silchenko, A.S.; Avilov, S.A.; Stonik, V.A.; Smirnov, A.V. Sea cucumbers triterpene glycosides, the recent progress in structural elucidation and chemotaxonomy. Phytochem. Rev. 2005, 4, 221–236. [Google Scholar] [CrossRef]
- Bahrami, Y.; Franko, C.M.M. Acetylated triterpene glycosides and their biological activity from Holothurioidea reported in the past six decades. Mar. Drugs 2016, 14, 147. [Google Scholar] [CrossRef] [PubMed]
- Kalinin, V.I.; Avilov, S.A.; Silchenko, A.S.; Stonik, V.A. Triterpene glycosides of sea cucumbers (Holothuroidea, Echinodermata) as taxonomic markers. Nat. Prod. Commun. 2015, 10, 21–26. [Google Scholar] [PubMed]
- Kalinin, V.I.; Silchenko, A.S.; Avilov, S.A. Taxonomic significance and ecological role of triterpene glycosides from Holothurians. Biol. Bull. 2016, 43, 532–540. [Google Scholar] [CrossRef]
- Honey-Escandon, M.; Arreguin-Espinosa, R.; Solis-Martin, F.A.; Samyn, Y. Biological and taxonomic perspective of triterpenoid glycosides of sea cucumbers of the family Holothuriidae (Echinodermata, Holothuroidea). Comp. Biochem. Physiol. 2015, 180B, 16–39. [Google Scholar] [CrossRef] [PubMed]
- Kalinin, V.I.; Aminin, D.L.; Avilov, S.A.; Silchenko, A.S.; Stonik, V.A. Triterpene glycosides from sea cucucmbers (Holothurioidae, Echinodermata), biological activities and functions. In Studies in Natural Product Chemistry (Bioactive Natural Products); Atta-ur-Rahman, Ed.; Elsevier Science Publisher: Amsterdam, The Netherlands, 2008; Volume 35, pp. 135–196. [Google Scholar]
- Kim, S.K.; Himaya, S.W.A. Triterpene glycosides from sea cucucmbers and their biological activities. Adv. Food Nutr. Res. 2012, 63, 297–319. [Google Scholar]
- Aminin, D.L.; Pislyagin, E.A.; Menchinskaya, E.S.; Silchenko, A.S.; Avilov, S.A.; Kalinin, V.I. Immunomodulatory and anticancer activity of sea cucumber triterpene glycosides. In Studies in Natural Products Chemistry (Bioactive Natural Products); Atta-ur-Rahman, Ed.; Elsevier Science Publisher: Amsterdam, The Netherlands, 2014; Volume 41, pp. 75–94. [Google Scholar]
- Careaga, V.P.; Maier, M.S. Cytotoxic triterpene glycosides from sea cucumbers. In Handbook of Anticancer Drugs from Marine Origin; Kim, S.-K., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 515–528. [Google Scholar]
- Aminin, D.L.; Menchinskaya, E.S.; Pislyagin, E.A.; Silchenko, A.S.; Avilov, S.A.; Kalinin, V.I. Anticancer activity of sea cucumber triterpene glycosides. Mar. Drugs 2015, 13, 1202–1223. [Google Scholar] [CrossRef] [PubMed]
- Janakiram, N.B.; Mohammed, A.; Rao, C. Sea cucumbers metabolites as potent anti-cancer agents. Mar. Drugs 2015, 13, 2909–2923. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, S.N.; Dyshlovoy, S.A.; Kuzmich, A.S.; Shubina, L.K.; Avilov, S.A.; Silchenko, A.S.; Bode, A.M.; Dong, Z.; Stonik, V.A. In vitro anticancer activities of some triterpene glycosides from holothurians of Cucumariidae, Stichopodidae, Psolidae, Holothuriidae and Synaptidae families. Nat. Prod. Commun. 2016, 11, 1239–1242. [Google Scholar]
- Aminin, D.L.; Menchinskaya, E.S.; Pisliagin, E.A.; Silchenko, A.S.; Avilov, S.A.; Kalinin, V.I. Sea cucumber triterpene glycosides as anticancer agents. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier Science Publisher: Amsterdam, The Netherlands, 2016; Volume 49, pp. 55–105. [Google Scholar]
- Zurita, M.B.; Ahond, A.; Poupat, C.; Potier, P. Invertebres marins du lagon Neo-Caledonien, VII. Etude structurale d’un nouveau saponoside sulfate extrait de l’holothurie Neothyonidium magnum. J. Nat. Prod. 1986, 49, 809–813. [Google Scholar] [CrossRef]
- Avilov, S.A.; Kalinovskii, A.I.; Stonik, V.A. New triterpene glycoside from the holothurian Neothyonidium magnum. Chem. Nat. Comp. 1990, 26, 42–45. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Kalinin, V.I.; Yurchenko, E.A.; Dolmatov, I.Y. Colochirosides B1, B2, B3 and C, novel sulfated triterpene glycosides from the sea cucumber Colochirus robustus (Cucumariidae, Dendrochirotida). Nat. Prod. Commun. 2015, 10, 1687–1694. [Google Scholar] [PubMed]
- Silchenko, A.S.; Stonik, V.A.; Avilov, S.A.; Kalinin, V.I.; Kalinovsky, A.I.; Zaharenko, A.M.; Smirnov, A.V.; Mollo, E.; Cimino, G. Holothurins B2, B3 and B4, New triterpene glycosides from Mediteranean sea cucumbers of the genus Holothuria. J. Nat. Prod. 2005, 68, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.L.; Li, L.; Sun, P.; Yi, Y.H. Lecanorosides A and B, two new triterpene glycosides from the sea cucumber Actinopyga lecanora. J. Asian Nat. Prod. Res. 2008, 10, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Zhang, W.; Liu, B.-S.; Pan, M.-X.; Wang, X.-H. A novel sulfated holostane glycoside from sea cucumber Holothuria leucospilota. Chem. Biodivers. 2010, 7, 1764–1769. [Google Scholar] [CrossRef] [PubMed]
- Kalinin, V.I.; Kalinovskii, A.I.; Stonik, V.A. Onekotanogenin—A new triterpene genin from the holothurian Psolus fabricii. Chem. Nat. Comp. 1987, 23, 560–563. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Yurchenko, E.A.; Dolmatov, I.Y.; Dautov, S.S.; Stonik, V.A.; Kalinin, V.I. Colochiroside E, an usual non-holostane triterpene sulfated trioside from the sea cucumber Colochirus robustus and evidence of the impossibility of a 7(8)-double bond migration in lanostane derivatives having an 18(16)-lactone. Nat. Prod. Commun. 2016, 11, 741–746. [Google Scholar] [PubMed]
- Ilyin, S.G.; Reshetnyak, M.V.; Afiyatullov, S.S.; Stonik, V.A.; Elyakov, G.B. The crystal and molecular structure of diacetate of holost-8(9)-en-3α,16β-diol. Rep. USSR Acad. Sci. 1985, 284, 356–359. [Google Scholar]
- Ilyin, S.G.; Sharypov, V.F.; Stonik, V.A.; Antipin, M.Y.; Struchkov, Y.T.; Elyakov, G.B. The crystal and molecular structure of (23S)-acetoxy-9β-holost-7-en-3β-ol and stereochemical peculiarities of the double bond migration from 7(8) to 8(9) and 9(11)-positions in holostane-type triterpenoids. Bioorg. Chem. 1991, 17, 1123–1128. [Google Scholar]
- Kalinovsky, A.I.; Silchenko, A.S.; Avilov, S.A.; Kalinin, V.I. The assignment of the absolute configuration of C-22 chiral center in the aglycones of triterpene glycosides from the sea cucumber Cladolabes schmeltzii and chemical transformations of cladoloside C. Nat. Prod. Commun. 2015, 10, 1167–1170. [Google Scholar] [PubMed]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Kalinin, V.I.; Yurchenko, E.A.; Dolmatov, I.Y. Colochirosides A1, A2, A3 and D, four novel sulfated triterpene glycosides from the sea cucumber Colochirus robustus (Cucumariidae, Dendrochirotida). Nat. Prod. Commun. 2016, 11, 381–387. [Google Scholar] [PubMed]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Kalinin, V.I.; Yurchenko, E.A.; Dautov, S.S. Structures of violaceusosides C, D, E and G, sulfated triterpene glycosides from the sea cucumber Pseudocolochirus violaceus (Cucumariidae, Denrochirotida). Nat. Prod. Commun. 2014, 9, 391–399. [Google Scholar] [PubMed]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Martyyas, E.A.; Kalinin, V.I.; Jayasandhya, P.; Rajan, G.C.; Padmakumar, K.P. Structures and biological activities of typicosides A1, A2, B1, C1 and C2, triterpene glycosides from the sea cucumbers Actinocucumis typica. Nat. Prod. Commun. 2013, 8, 301–310. [Google Scholar] [PubMed]
- Zou, Z.R.; Yi, Y.H.; Xu, Q.Z.; Wu, H.M.; Lin, H.W. A new disulfated triterpene glycoside from the sea cucumber Mensamaria intercedens Lampert. Chin. Chem. Lett. 2003, 14, 585–587. [Google Scholar]
- Zhang, S.-Y.; Yi, Y.-H.; Tang, H.-F. Cytotoxic sulfated triterpene glycosides from the sea cucmber Pseudocolochirus violaceus. Chem. Biodivers. 2006, 3, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Avilov, S.A.; Kalinin, V.I.; Makarieva, T.N.; Stonik, V.A.; Kalinovskii, A.I. Structure of Cucumarioside G2, a novel nonholostane glycoside from the sea cucumber Eupentacta fraudatrix. J. Nat. Prod. 1994, 57, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Yurchenko, E.A.; Kalinin, V.I. Structures and cytotoxic properties of cucumariosides H2, H3 and H4 from the sea cucumber Eupentacta fraudatrix. Nat. Prod. Res. 2012, 26, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Martyyas, E.A.; Kalinin, V.I. Triterpene glycosides from the sea cucumber Eupentacta fraudatrix. Structure and cytotoxic action of cucumariosides A2, A7, A9, A10, A11, A13 and A14, seven new minor non-sulated tetraosides and an aglycone with an uncommon 18-hydroxy group. Nat. Prod. Commun. 2012, 7, 845–852. [Google Scholar] [PubMed]
- Avilov, S.A.; Antonov, A.S.; Drozdova, O.A.; Kalinin, V.I.; Kalinovsky, A.I.; Stonik, V.A.; Riguera, R.; Lenis, L.A.; Jimenez, C. Triterpene glycosides from the Far-Eastern sea cucumber Pentamera calcigera. 1. Monosulfated glycosides and cytotoxicity of their unsulfated derivatives. J. Nat. Prod. 2000, 63, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Kalinin, V.I.; Kalinivskii, A.I.; Stonik, V.A. Psolusoside A—The main triterpene glycoside from the holothurian Psolus fabricii. Chem. Nat. Compd. 1985, 21, 197–202. [Google Scholar] [CrossRef]
- Kalinin, V.I.; Kalinovskii, A.I.; Stonik, V.A.; Dmitrenok, P.S.; El’kin, Y.N. Structure of psolusoside B—A nonholostane triterpene glycoside of the holothurian genus Psolus. Chem. Nat. Compd. 1989, 25, 311–317. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjashenko, P.V.; Dmitrenok, P.S.; Kalinin, V.I.; Stonik, V.A. 3β-O-Glycosylated 16β-acetoxy-9β-H-lanosta-7,24-diene-3β,18,20β-triol, an intermediate metabolite from the sea cucumber Eupentacta fraudatrix and its biosynthetic significance. Biochem. Syst. Ecol. 2012, 44, 53–60. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Song, Y.M.; Wang, B.; Zhang, F.R.; Yang, R.; Wang, H.Q.; Zhang, G.J. Ginsenoside Rg3 sensitizes human non-small cell lung cancer cells to γ-radiation by targeting the nuclear factor-κB pathway. Mol. Med. Rep. 2015, 12, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Vishchuk, O.S.; Ermakova, S.P.; Zvyagintseva, T.N. The fucoidans from brown algae of Far-Eastern seas: Anti-tumor activity and structure-function relationship. Food Chem. 2013, 141, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).