Anti-Coagulant and Anti-Thrombotic Properties of Blacklip Abalone (Haliotis rubra): In Vitro and Animal Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Freeze Drying of AEC Pool 4 and Anti-Thrombotic Assessment In Vitro
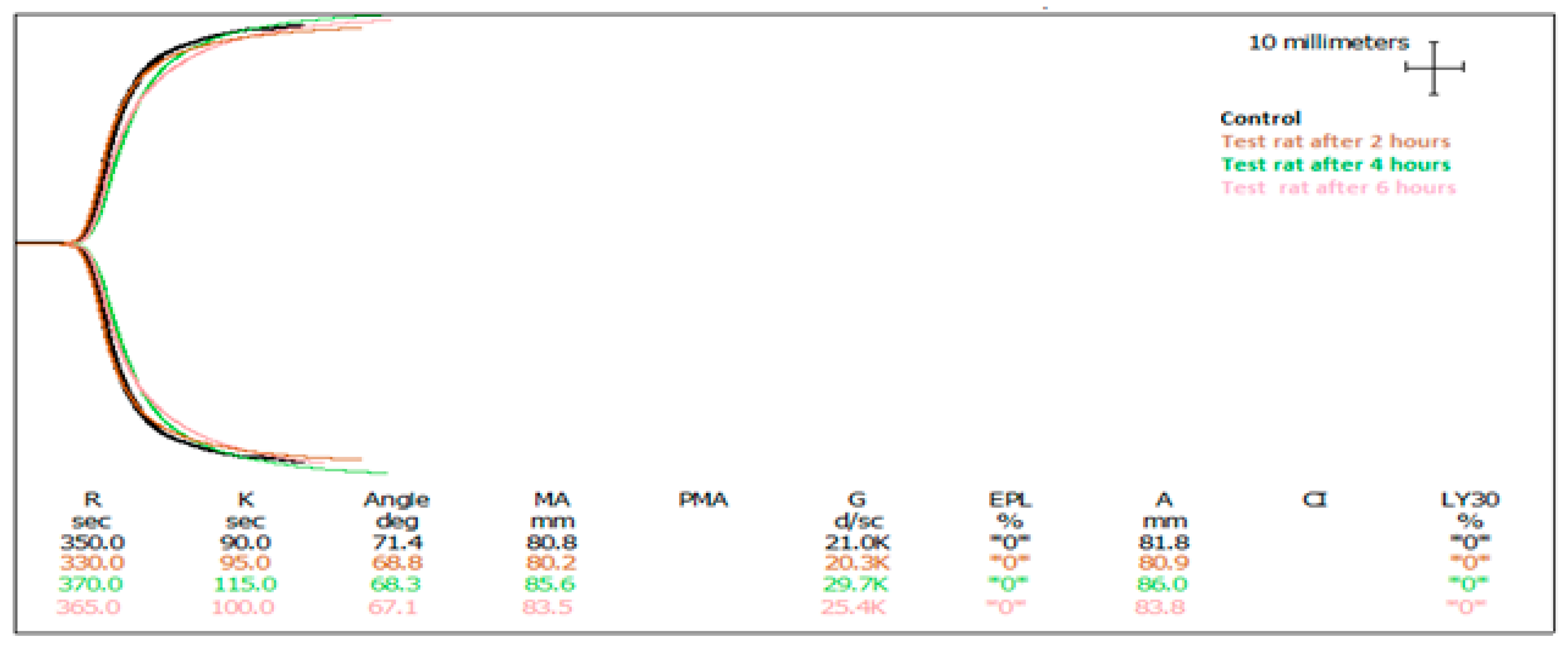
2.2. Oral Administration of Fractionated and Pooled Abalone Extract (AEC Pool 4) to Rats and Analysis of Whole Blood
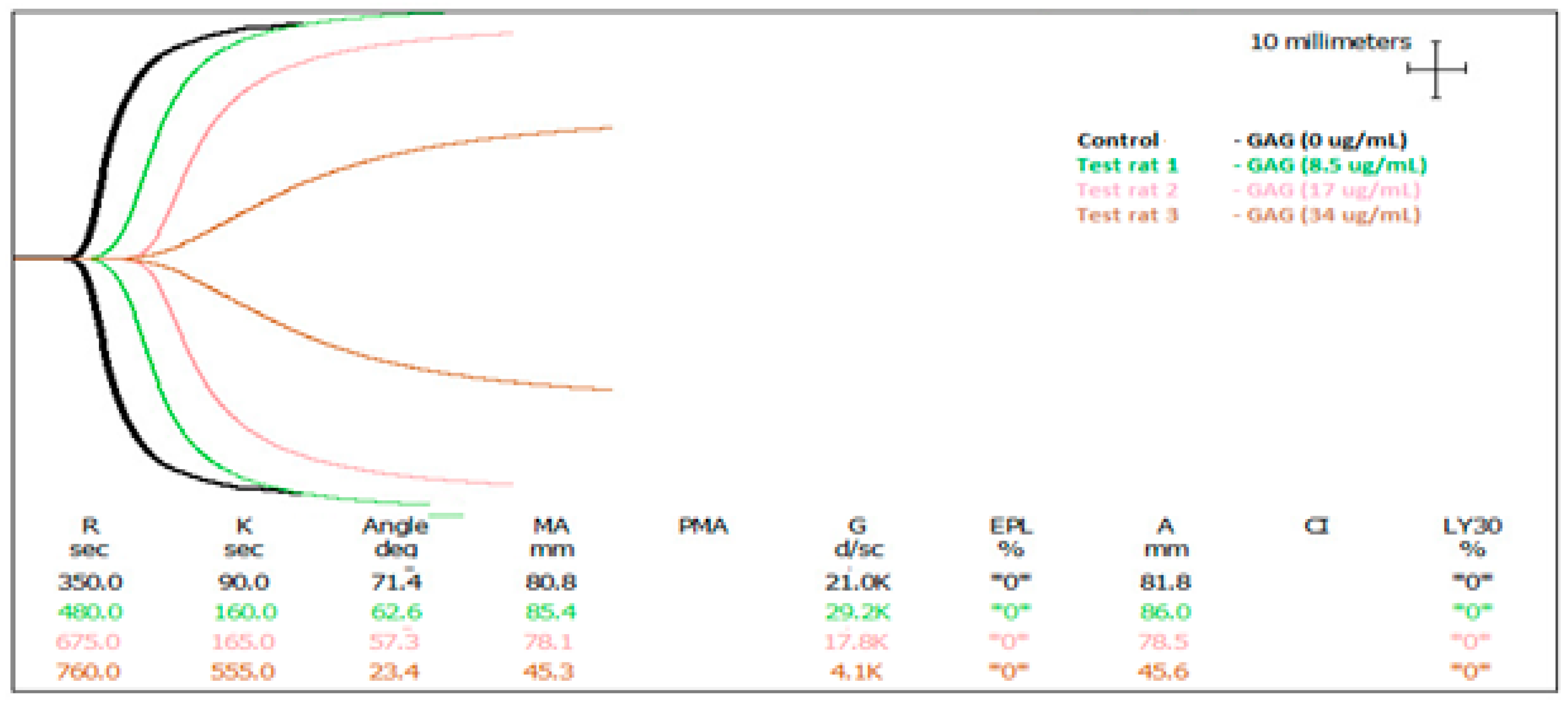
2.3. In Vitro Anti-Coagulant Assessment of AEC Pool 4 Dosed into Rat Blood
3. Material and Methods
3.1. Preparation of Fractionated Abalone Extract with Anti-Thrombotic and Anti-Coagulant Activity In Vitro
3.2. Freeze Drying of AEC Pool 4
3.3. In Vivo Anti-Coagulant Effect of AEC Pool 4 Following Oral Administration in a Rat Model
3.4. Histopathological and Hematological Parameters
3.5. Statistical Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pavão, M.S.G. Glycosaminoglycans analogs from marine invertebrates: Structure, biological effects, and potential as new therapeutics. Front. Cell Infect. Microbiol. 2014, 4, 123. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, U.L.F.; Kusche, M.; Lidholt, K.; Oscarsson, L.G. Biosynthesis of heparin and heparan sulfatea. Ann. N. Y. Acad. Sci. 1989, 556, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Fareed, J.; Hoppensteadt, D.A.; Bick, R.L. An update on heparins at the beginning of the new millennium. Semin. Thromb. Hemost. 2000, 26, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Hirsh, J.; Raschke, R. Heparin and low-molecular-weight heparin: The seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest 2004, 126, 188S–203S. [Google Scholar] [CrossRef] [PubMed]
- Mourao, P.A. Use of sulfated fucans as anti-coagulant and antithrombotic agents: Future perspectives. Curr. Pharm. Des. 2004, 10, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Aquino, R.S.; Pereira, M.S.; Vairo, B.C.; Cinelli, L.P.; Santos, G.R.; Fonseca, R.J.; Mourao, P.A. Heparins from porcine and bovine intestinal mucosa: Are they similar drugs? Thromb. Haemost. 2010, 103, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Hanley, J.P. Warfarin reversal. J. Clin. Pathol. 2004, 57, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Alving, B.M. How I treat heparin-induced thrombocytopenia and thrombosis. Blood 2003, 101, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Awad, N.I.; Cocchio, C. Activated prothrombin complex concentrates for the reversal of anti-coagulant-associated coagulopathy. Pharmacol. Ther. 2013, 38, 696–701. [Google Scholar]
- Suleria, H.A.; Masci, P.P.; Gobe, G.C.; Osborne, S.A. Current and potential uses of bioactive molecules frommarine processing waste. J. Sci. Food Agric. 2015, 15, 1064–1067. [Google Scholar]
- Suleria, H.A.; Masci, P.P.; Gobe, G.C.; Osborne, S.A. Therapeutic potential of abalone and status of bioactive molecules: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1742–1748. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H.; Mourao, P.A. Structure, biology, evolution, and medical importance of sulfated fucans and galactans. Glycobiology 2008, 18, 1016–1027. [Google Scholar] [CrossRef] [PubMed]
- Cassaro, C.M.; Dietrich, C.P. Distribution of sulfated mucopolysaccharides in invertebrates. J. Biol. Chem. 1977, 252, 2254–2261. [Google Scholar] [PubMed]
- Cui, Q.; Wang, H.; Yuan, C. The preliminary study on the antithrombotic mechanism of glycosaminoglycan from mactra veneriformis. Blood Coagul. Fibrinolysis 2014, 25, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive peptides from marine processing waste and shellfish: A review. J. Funct. Foods 2012, 4, 6–24. [Google Scholar] [CrossRef]
- Li, G.; Chen, S.; Wang, Y.; Xue, Y.; Chang, Y.; Li, Z.; Xue, C. A novel glycosaminoglycan-like polysaccharide from abalone Haliotis discus hannai Ino: Purification, structure identification and anti-coagulant activity. Int. J. Biol. Macromol. 2011, 49, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Ko, D.O.; Kang, S.G. Antithrombotic potential of extracts from abalone, Haliotis discus hannai Ino: In vitro and animal studies. Food Sci. Biotechnol. 2013, 22, 471–476. [Google Scholar] [CrossRef]
- World Health Organization. The Global Burden of Disease: 2004 Update; WHO: Geneva, Switzerland, 2008; 146p. [Google Scholar]
- Chen, X.; Yang, S.; Wang, J.; Song, L.; Xing, R.; Liu, S.; Li, P. Sulfated polysaccharides isolated from cloned grateloupia filicina and their anti-coagulant activity. Biomed. Res. Int. 2015, 2015, 5. [Google Scholar]
- Rocha, H.A.O.; Leite, E.L.; Medeiros, V.P.; Lopes, C.C.; Nascimento, F.D.; Tersariol, I.L.S.; Sampaio, L.O.; Nader, H.B. Natural sulfated polysaccharides as antithrombotic compounds. Structural characteristics and effects on the coagulation cascade. In Insight into Carbohydrate Structure and Biological Function; Verli, H., Guimaraes, J.A., Eds.; Transworld Research Network: Kerala, India, 2006; pp. 51–67. [Google Scholar]
- Suleria, H.A.R.; Gobe, G.; Masci, P.; Osborne, S.A. Marine bioactive compounds and health promoting perspectives; innovation pathways for drug discovery. Trends Food Sci. Technol. 2016, 50, 44–55. [Google Scholar] [CrossRef]
- Suleria, H.A.R.; Osborne, S.; Masci, P.; Gobe, G. Marine-based nutraceuticals: An innovative trend in the food and supplement industries. Mar. Drugs 2015, 13, 6336–6351. [Google Scholar] [CrossRef] [PubMed]
- Hirsh, J.; Anand, S.S.; Halperin, J.L.; Fuster, V. Guide to anti-coagulant therapy: Heparin: A statement for healthcare professionals from the American Heart Association. Circulation 2001, 103, 2994–3018. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.M.; Kozlowski, E.O.; Pomin, V.H.; de Barros, C.M.; Zaganeli, J.L.; Pavao, M.S. Unique extracellular matrix heparan sulfate from the bivalve Nodipecten nodosus (Linnaeus, 1758) safely inhibits arterial thrombosis after photochemically induced endothelial lesion. J. Biol. Chem. 2010, 285, 7312–7323. [Google Scholar] [CrossRef] [PubMed]
- Suleria, H.A.R.; Hines, B.M.; Addepalli, R.; Chen, W.; Masci, P.; Gobe, G.; Osborne, S.A. In vitro anti-thrombotic activity of extracts from blacklip abalone (Haliotis rubra) processing waste. Mar. Drugs 2017, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- Suleria, H.A.R.; Masci, P.P.; Addepalli, R.; Chen, W.; Gobe, G.C.; Osborne, S.A. In vitro anti-thrombotic and anti-coagulant properties of blacklip abalone (Haliotis rubra) viscera hydrolysate. Anal. Bioanal. Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.H.; Tefferi, A.; Pruthi, R.K. How to interpret and pursue an abnormal prothrombin time, activated partial thromboplastin time, and bleeding time in adults. Mayo Clin. Proc. 2007, 82, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Koch, E.; Biber, A. Treatment of rats with the Pelargonium sidoides extract EPs 7630 has no effect on blood coagulation parameters or on the pharmacokinetics of warfarin. Phytomedicine 2007, 14, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Holster, I.L.; Valkhoff, V.E.; Kuipers, E.J.; Tjwa, E.T. New oral anti-coagulants increase risk for gastrointestinal bleeding: A systematic review and meta-analysis. Gastroenterol 2013, 145, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Michel, C.; Lahaye, M.; Bonnet, C.; Mabeau, S.; Barry, J.L. In vitro fermentation by human faecal bacteria of total and purified dietary fibres from brown seaweeds. Br. J. Nutr. 1996, 75, 263–280. [Google Scholar] [CrossRef] [PubMed]
- Li, P.L.; Li, C.X.; Xue, Y.T.; Li, H.H.; Liu, H.B.; He, X.X.; Yu, G.L.; Guan, H.S. An HPLC method for microanalysis and pharmacokinetics of marine sulfated polysaccharide PSS-loaded Poly Lactic-co-Glycolic Acid (PLGA) nanoparticles in rat plasma. Mar. Drugs 2013, 11, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Lam, U.I. Chitosan/Fucoidan pH sensitive nanoparticles for oral delivery system. J. Chin. Chem. Soc. 2011, 58, 779–785. [Google Scholar] [CrossRef]
- Suleria, H.A.R.; Addepalli, R.; Masci, P.; Gobe, G.; Osborne, S.A. In vitro anti-inflammatory activities of blacklip abalone (Haliotis rubra) in RAW 264.7 macrophages. Food Agric. Immunol. 2017, 28, 711–724. [Google Scholar] [CrossRef]
- Dupouy, D.; Sié, P.; Dol, F.; Boneu, B. A simple method to measure dermatan sulfate at sub-microgram concentrations in plasma. Thromb. Haemost. 1988, 60, 236–239. [Google Scholar] [PubMed]
- Hines, B.M.; Suleria, H.A.R.; Osborne, S.A. Automated screening potential thrombin inhibitors using the epMotion® 5075. Eppendorf 2016, 377, 1–6. [Google Scholar]
- Du, Q. A Study of the Anti-Coagulant Mechanisms of Three Biological Compounds In Vitro and In Vivo. Ph.D. Thesis, The University of Queensland, Brisbane, Australia, 2014. [Google Scholar] [CrossRef]
- Wunnapuk, K.; Liu, X.; Gobe, G.C.; Endre, Z.H.; Peake, P.W.; Grice, J.E.; Roberts, M.S.; Buckley, N.A. Kidney biomarkers in MCPA-induced acute kidney injury in rats: Reduced clearance enhances early biomarker performance. Toxicol. Lett. 2014, 225, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Korenkova, V.; Jones, A.; Hoy, W.E.; Morais, C.; Cooper, M.A.; Gobe, G.C. Urinary biomarkers for detection of early and advanced chronic kidney disease—A pilot study. Med. Chem. 2015, 5, 96–103. [Google Scholar] [CrossRef]
| Sample Descriptions | SP (µg/mL) | Time (s) | INR |
|---|---|---|---|
| PT | |||
| Rat plasma (Before freeze drying) | 0 | 9.7 ± 0.02 | 1.0 |
| 50 | 12.1 ± 0.08 * | 1.2 | |
| 100 | 13.3 ± 0.04 * | 1.4 | |
| 150 | 15.2 ± 0.01 ** | 1.6 | |
| Rat plasma (After freeze drying) | 0 | 9.1 ± 0.01 | 1.0 |
| 50 | 11.2 ± 0.06 * | 1.2 | |
| 100 | 12.4 ± 0.08 * | 1.4 | |
| 150 | 14.8 ± 0.06 ** | 1.6 | |
| aPTT | |||
| Rat plasma (Before freeze drying) | 0 | 31.4 ± 0.3 | 1.0 |
| 5 | 39.2 ± 0.7 * | 1.2 | |
| 10 | 50.1 ± 0.2 ** | 1.6 | |
| 20 | 71.5 ± 0.3 *** | 2.3 | |
| Rat plasma (After freeze drying) | 0 | 30.4 ± 0.01 | 1.0 |
| 5 | 38.4 ± 0.2 * | 1.3 | |
| 10 | 52.0 ± 0.2 ** | 1.7 | |
| 20 | 73.8 ± 0.03 *** | 2.4 | |
| Blood Parameters | Normal Range | Control | Test, 2 h Post-Gavage | Test, 4 h Post-Gavage | Test, 6 h Post-Gavage |
|---|---|---|---|---|---|
| White blood cells (10 × 9/L) | 5.10–12.16 | 8.66 | 11.19 | 9.2 | 10.09 |
| Red blood cells (10 × 12/L) | 5.79–7.14 | 6.46 | 7.54 | 6.4 | 7.36 |
| Hemoglobin (g/L) | 122–148 | 135 | 142 | 131 | 139 |
| Hematocrit (Ratio) | 0.30–0.50 | 0.36 | 0.479 | 0.37 | 0.384 |
| Mean corpuscular volume (fL) | 53–59 | 56 | 63.5 | 57.8 | 52.2 |
| Mean corpuscular hemoglobin (pg) | 18–22 | 20 | 18.8 | 20.5 | 18.9 |
| Mean corpuscular hemoglobin concentration (g/L) | 330–410 | 370 | 296 | 354 | 362 |
| Platelet count (10 × 9/L) | 600–700 | 700 | 603 | 641 | 680 |
| Platelet large cell ratio (%) | 3.8–4.5 | 4.1 | 4.6 | 3.6 | 4.4 |
| Platelet count (%) | 0.4–0.6 | 0.47 | 0.52 | 0.42 | 0.45 |
| Sample Description | SP (mg) | R (Sec) | Angle (α) | MA (mm) |
|---|---|---|---|---|
| Control | 0 | 355 ± 5 | 66.1 ± 2.2 | 71.9 ± 13.7 |
| Test 1—2 h post gavage | 40 | 335 ± 7.5 | 67.1 ± 1.2 | 83.5 ± 14.7 |
| Test 2—4 h post gavage | 40 | 380 ± 10 * | 71.1 ± 3 * | 60.1 ± 2.4 |
| Rat 3—6 h post gavage | 40 | 360 ± 15 | 80.2 ± 0.2 ** | 75.1 ± 14.5 |
| Sample Description | SP (µg/mL) | R (Sec) | Angle (α) | MA (mm) |
|---|---|---|---|---|
| Control | 0 | 375 ± 5 | 66.1 ± 2.2 | 71.9 ± 13.7 |
| Test 1—2 h post-gavage | 8.5 | 427 ± 2.2 ** | 64.2 ± 4.1 | 69.8 ± 7.5 |
| 17 | 552.5 ± 3.5 *** | 62.25 ± 6.6 | 57.1 ± 10.1 | |
| 34 | 810 ± 5 *** | 29 ± 1 ** | 37.75 ± 3.4 ** | |
| Test 2—4 h post-gavage | 17 | 357.5 ± 7.5 | 70.65 ± 0.7 | 67.35 ± 13.4 |
| 34 | 720 ± 15 ** | 28 ± 4.6 ** | 52.4 ± 7.1 * | |
| Test 3—6 h post-gavage | 17 | 467.5 ± 12.5 * | 64.85 ± 2.2 | 73.65 ± 10.8 |
| 34 | 667.5 ± 7.5 *** | 50.4 ± 6.9 | 72.85 ± 5.2 | |
| 50 | No blood clot | |||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suleria, H.A.R.; Masci, P.P.; Zhao, K.-N.; Addepalli, R.; Chen, W.; Osborne, S.A.; Gobe, G.C. Anti-Coagulant and Anti-Thrombotic Properties of Blacklip Abalone (Haliotis rubra): In Vitro and Animal Studies. Mar. Drugs 2017, 15, 240. https://doi.org/10.3390/md15080240
Suleria HAR, Masci PP, Zhao K-N, Addepalli R, Chen W, Osborne SA, Gobe GC. Anti-Coagulant and Anti-Thrombotic Properties of Blacklip Abalone (Haliotis rubra): In Vitro and Animal Studies. Marine Drugs. 2017; 15(8):240. https://doi.org/10.3390/md15080240
Chicago/Turabian StyleSuleria, Hafiz Ansar Rasul, Paul P. Masci, Kong-Nan Zhao, Rama Addepalli, Wei Chen, Simone A. Osborne, and Glenda C. Gobe. 2017. "Anti-Coagulant and Anti-Thrombotic Properties of Blacklip Abalone (Haliotis rubra): In Vitro and Animal Studies" Marine Drugs 15, no. 8: 240. https://doi.org/10.3390/md15080240
APA StyleSuleria, H. A. R., Masci, P. P., Zhao, K.-N., Addepalli, R., Chen, W., Osborne, S. A., & Gobe, G. C. (2017). Anti-Coagulant and Anti-Thrombotic Properties of Blacklip Abalone (Haliotis rubra): In Vitro and Animal Studies. Marine Drugs, 15(8), 240. https://doi.org/10.3390/md15080240






