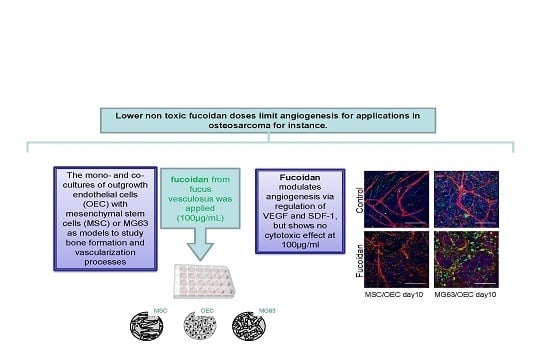
Crude Fucoidan Extracts Impair Angiogenesis in Models Relevant for Bone Regeneration and Osteosarcoma via Reduction of VEGF and SDF-1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval for the Use of Human Cells
2.2. Isolation and Culture of MSCs
2.3. Isolation and Expansion of OECs
2.4. Fucoidan Treatment of Mono and Co-Cultures
2.5. MTS Cell Metabolic Activity Assay
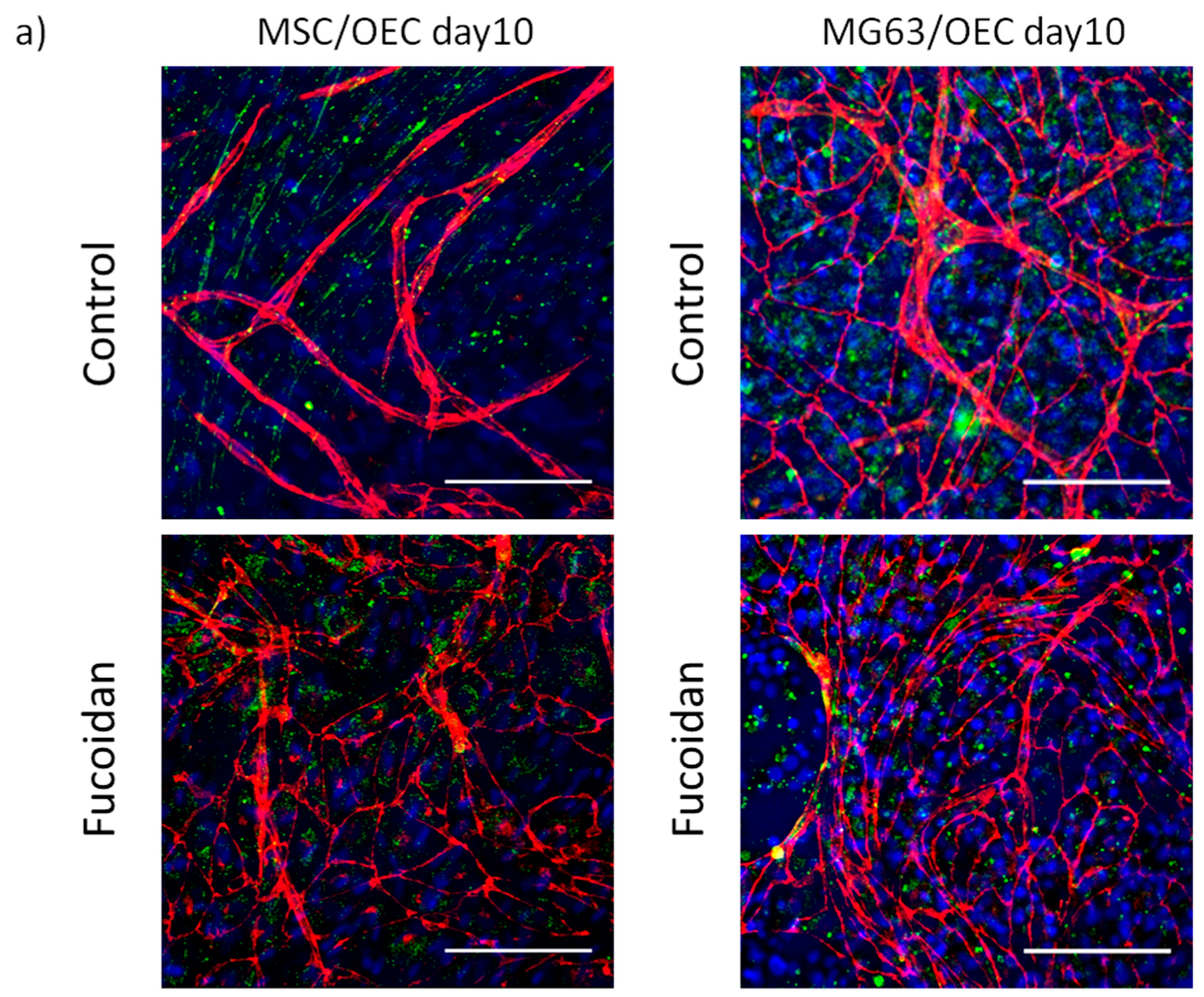
2.6. Immunofluorescence Staining and Visualization of Angiogenic Structures
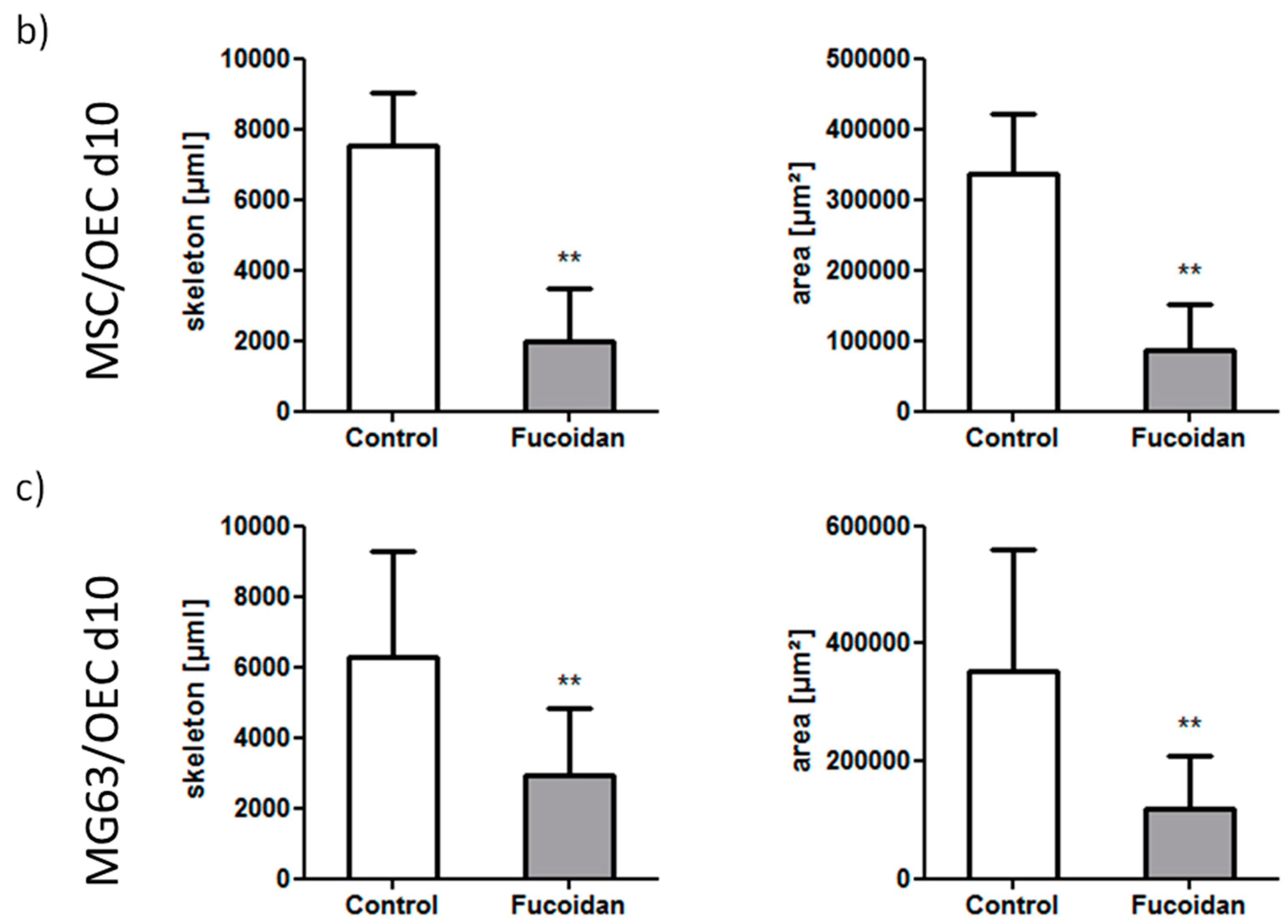
2.7. Quantification of Agiogenesis
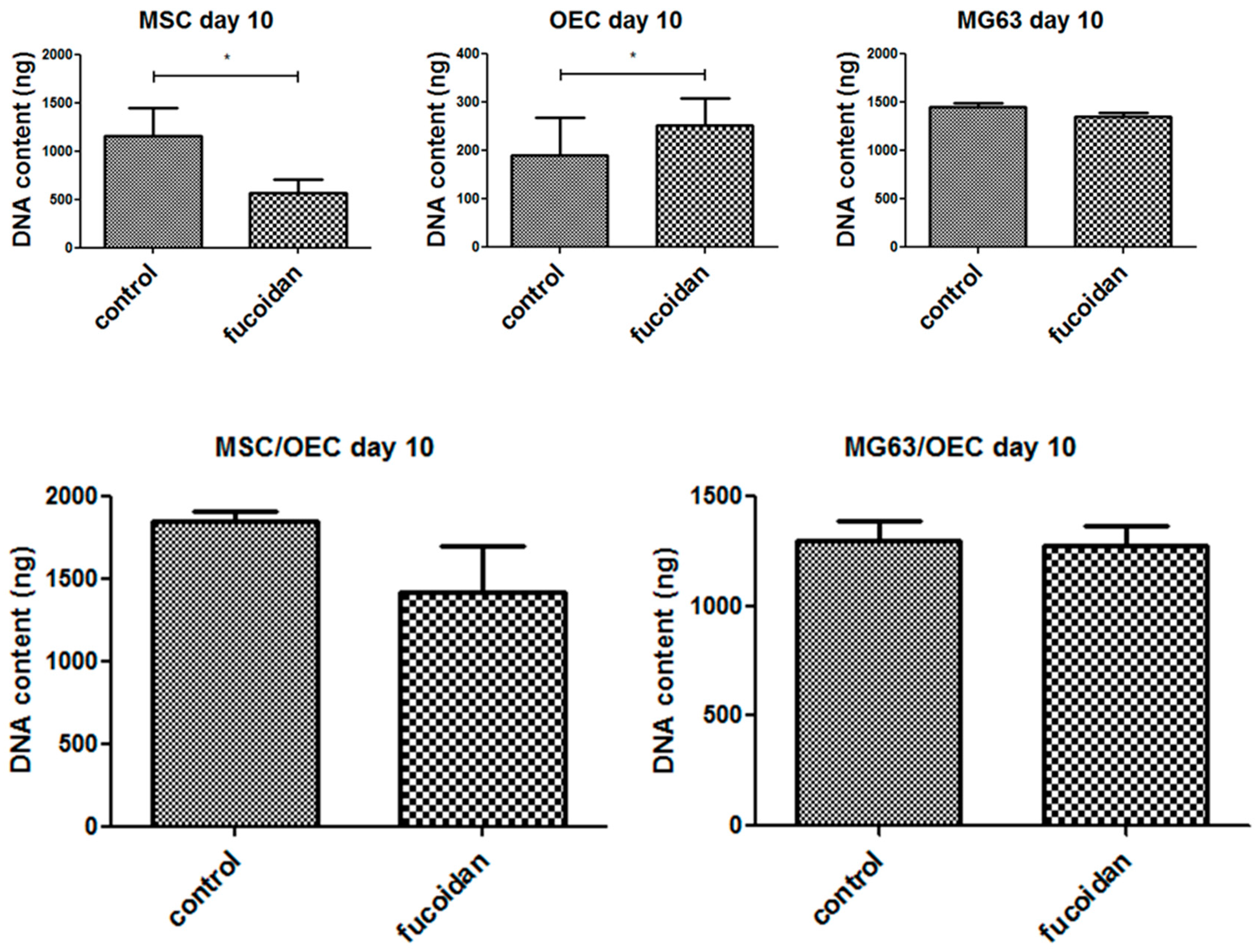
2.8. Quantification of DNA Content
2.9. Gene Expression Analysis
2.10. Enzyme Linked Immunosorbent Assay (ELISA)
2.11. Quantification of Mineralization by Alizarin Red Staining and Quantification
2.12. Statistical Analysis
3. Results
3.1. The Metabolic Activity of Individual Cell Types in Response to Fucoidan Dose
3.2. Angiogenic Structures of OECs in Co-Cultures
3.3. DNA Quantification
3.4. Quantitative Assessment of Gene Expression of Mono-/Co-Cultures
3.5. Analysis of Angiogenesis Relevant Factors in Culture Supernatants by Enzyme-Linked Immunosorbent Assay (ELISA)
3.6. Quantitative Analysis of Osteogenesis of MSC/OEC Co-Cultures
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ALP | alkaline phosphatase |
| ANGPT-1 | angiopoietin-1 |
| ANGPT-2 | angiopoietin-2 |
| BSA | bovine serum albumin |
| CXCR4 | C-X-C chemokine receptor type 4 receptor of SDF-1 |
| DMEM | Dulbecco’s Medium Essential Medium |
| EGM-2 | endothelial cell growth medium-2 |
| ELISA | enzyme linked immunosorbent assay |
| FBS | fetal bovine serum |
| MSC | mesenchymal stem cell |
| ODM | osteogenic differentiation medium |
| OEC | outgrowth endothelial cell |
| PBS | phosphate buffered saline |
| Pen/Strep | penicillin/ streptomycin |
| SDF-1 | stromal cell-derived factor-1 |
| VEGF | vascular endothelial growth factor |
| vwf | von willebrand factor |
References
- Rouwkema, J.; Rivron, N.C.; van Blitterswijk, C.A. Vascularization in tissue engineering. Trends Biotechnol. 2008, 26, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Sivaraj, K.K.; Adams, R.H. Blood vessel formation and function in bone. Development 2016, 143, 2706–2715. [Google Scholar] [CrossRef] [PubMed]
- Schipani, E.; Maes, C.; Carmeliet, G.; Semenza, G.L. Regulation of Osteogenesis-Angiogenesis Coupling by HIFs and VEGF. J. Bone Miner. Res. 2009, 24, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Brandi, M.L.; Collin-Osdoby, P. Vascular biology and the skeleton. J. Bone Miner. Res. 2006, 21, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Kuroda, R.; Mifune, Y.; Kawamoto, A.; Shoji, T.; Miwa, M.; Asahara, T.; Kurosaka, M. Circulating endothelial/skeletal progenitor cells for bone regeneration and healing. Bone 2008, 43, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Melero-Martin, J.M.; Dudley, A.C. Concise Review: Vascular Stem Cells and Tumor Angiogenesis. Stem Cells 2011, 29, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Versleijen-Jonkers, Y.M.H.; Vlenterie, M.; van de Luijtgaarden, A.C.M.; van der Graaf, W.T.A. Anti-angiogenic therapy, a new player in the field of sarcoma treatment. Crit. Rev. Oncol. Hematol. 2014, 91, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Papetti, M.; Herman, I.M. Mechanisms of normal and tumor-derived angiogenesis. Am. J. Physiol. Cell Physiol. 2002, 282, C947–C970. [Google Scholar] [CrossRef] [PubMed]
- Dohle, E.; Fuchs, S.; Kolbe, M.; Hofmann, A.; Schmidt, H.; Kirkpatrick, C.J. Comparative study assessing effects of sonic hedgehog and VEGF in a human co-culture model for bone vascularisation strategies. Eur. Cells Mater. 2011, 21, 144–156. [Google Scholar] [CrossRef]
- Laschke, M.W.; Schank, T.E.; Scheuer, C.; Kleer, S.; Shadmanov, T.; Eglin, D.; Alini, M.; Menger, M.D. In vitro osteogenic differentiation of adipose-derived mesenchymal stem cell spheroids impairs their in vivo vascularization capacity inside implanted porous polyurethane scaffolds. Acta Biomater. 2014, 10, 4226–4235. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Kramer, G.; Schroder, A.; Kirkpatrick, C.J.; Seekamp, A.; Schmidt, H.; Fuchs, S. Early endothelial progenitor cells as a source of myeloid cells to improve the pre-vascularisation of bone constructs. Eur. Cells Mater. 2014, 27, 64–79, discussion 79–80. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, F.; Tiwari, S.; Yesilbas, M.; Steubesand, N.; Weitkamp, J.T.; Kluter, T.; Lippross, S.; Eglin, D.; Seekamp, A.; et al. Role of myeloid early endothelial progenitor cells in bone formation and osteoclast differentiation in tissue construct based on hydroxyapatite poly(ester-urethane) scaffolds. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2016, 34, 1922–1932. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, C.J.; Fuchs, S.; Unger, R.E. Co-culture systems for vascularization—Learning from nature. Adv. Drug Deliv. Rev. 2011, 63, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Kolbe, M.; Dohle, E.; Katerla, D.; Kirkpatrick, C.J.; Fuchs, S. Enrichment of outgrowth endothelial cells in high and low colony-forming cultures from peripheral blood progenitors. Tissue Eng. Part C Methods 2010, 16, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Kolbe, M.; Xiang, Z.; Dohle, E.; Tonak, M.; Kirkpatrick, C.J.; Fuchs, S. Paracrine effects influenced by cell culture medium and consequences on microvessel-like structures in cocultures of mesenchymal stem cells and outgrowth endothelial cells. Tissue Eng. Part A 2011, 17, 2199–2212. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Nakasone, K.; Tomimori, K.; Ishikawa, C. Beneficial effects of fucoidan in patients with chronic hepatitis C virus infection. World J. Gastroenterol. 2012, 18, 2225–2230. [Google Scholar] [CrossRef] [PubMed]
- Prokofjeva, M.M.; Imbs, T.I.; Shevchenko, N.M.; Spirin, P.V.; Horn, S.; Fehse, B.; Zvyagintseva, T.N.; Prassolov, V.S. Fucoidans as potential inhibitors of HIV-1. Mar. Drugs 2013, 11, 3000–3014. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.S.; Lee, J.H.; Lee, S.H. Antitumor Effects of Fucoidan on Human Colon Cancer Cells via Activation of Akt Signaling. Biomol. Ther. 2015, 23, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.Y.; Lin, T.Y.; Wu, Y.C.; Tsao, S.M.; Hwang, P.A.; Shih, Y.W.; Hsu, J. Fucoidan inhibition of lung cancer in vivo and in vitro: Role of the Smurf2-dependent ubiquitin proteasome pathway in TGFbeta receptor degradation. Oncotarget 2014, 5, 7870–7885. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Ferreira, A.S.; Novoa-Carballal, R.; Nunes, C.; Pashkuleva, I.; Neves, N.M.; Coimbra, M.A.; Reis, R.L.; Martins, A.; Silva, T.H. The Key Role of Sulfation and Branching on Fucoidan Antitumor Activity. Macromol. Biosci. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Ruperez, P.; Ahrazem, O.; Leal, J.A. Potential antioxidant capacity of sulfated polysaccharides from the edible marine brown seaweed Fucus vesiculosus. J. Agric. Food Chem. 2002, 50, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Ye, Q.F. Fucoidan reduces inflammatory response in a rat model of hepatic ischemia-reperfusion injury. Can. J. Physiol. Pharmacol. 2015, 93, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.M.; Alves, L.G.; de Queiroz, K.C.; Santos, M.G.; Marques, C.T.; Chavante, S.F.; Rocha, H.A.; Leite, E.L. Partial characterization and anticoagulant activity of a heterofucan from the brown seaweed Padina gymnospora. Braz. J. Med. Biol. Res. 2005, 38, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.H.; Alves, A.; Popa, E.G.; Reys, L.L.; Gomes, M.E.; Sousa, R.A.; Silva, S.S.; Mano, J.F.; Reis, R.L. Marine algae sulfated polysaccharides for tissue engineering and drug delivery approaches. Biomatter 2012, 2, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef] [PubMed]
- Fitton, J.H.; Stringer, D.N.; Karpiniec, S.S. Therapies from Fucoidan: An Update. Mar. Drugs 2015, 13, 5920–5946. [Google Scholar] [CrossRef] [PubMed]
- Patankar, M.S.; Oehninger, S.; Barnett, T.; Williams, R.L.; Clark, G.F. A revised structure for fucoidan may explain some of its biological activities. J. Biol. Chem. 1993, 268, 21770–21776. [Google Scholar] [PubMed]
- Nishino, T.; Nishioka, C.; Ura, H.; Nagumo, T. Isolation and partial characterization of a novel amino sugar-containing fucan sulfate from commercial Fucus vesiculosus fucoidan. Carbohydr. Res. 1994, 255, 213–224. [Google Scholar] [CrossRef]
- Dithmer, M.; Fuchs, S.; Shi, Y.; Schmidt, H.; Richert, E.; Roider, J.; Klettner, A. Fucoidan Reduces Secretion and Expression of Vascular Endothelial Growth Factor in the Retinal Pigment Epithelium and Reduces Angiogenesis In Vitro. PLoS ONE 2014, 9, e89150. [Google Scholar] [CrossRef] [PubMed]
- Beamer, B.; Hettrich, C.; Lane, J. Vascular endothelial growth factor: An essential component of angiogenesis and fracture healing. HSS J. 2010, 6, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Olsen, B.R. The roles of vascular endothelial growth factor in bone repair and regeneration. Bone 2016, 91, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Li, B.; Winer, J.; Armanini, M.; Gillett, N.; Phillips, H.S.; Ferrara, N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 1993, 362, 841–844. [Google Scholar] [CrossRef] [PubMed]
- Sankar, M.J.; Sankar, J.; Mehta, M.; Bhat, V.; Srinivasan, R. Anti-vascular endothelial growth factor (VEGF) drugs for treatment of retinopathy of prematurity. Cochrane Database Syst. Rev. 2016, CD009734. [Google Scholar] [CrossRef]
- Schneider, T.; Ehrig, K.; Liewert, I.; Alban, S. Interference with the CXCL12/CXCR4 axis as potential antitumor strategy: Superiority of a sulfated galactofucan from the brown alga Saccharina latissima and fucoidan over heparins. Glycobiology 2015, 25, 812–824. [Google Scholar] [CrossRef] [PubMed]
- Eman, R.M.; Öner, F.C.; Kruyt, M.C.; Dhert, W.J.A.; Alblas, J. Stromal Cell-Derived Factor-1 Stimulates Cell Recruitment, Vascularization and Osteogenic Differentiation. Tissue Eng. Part A 2013, 20, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.; Verrier, S.; Alini, M. Strategies to Stimulate Mobilization and Homing of Endogenous Stem and Progenitor Cells for Bone Tissue Repair. Front. Bioeng. Biotechnol. 2015, 3, 79. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Lv, F.; Zhang, J.; Li, H. SDF-1 Expression is Associated with Poor Prognosis in Osteosarcoma. Ann. Clin. Lab. Sci. 2016, 46, 508–514. [Google Scholar] [PubMed]
- Qin, G.; Chen, Y.; Li, H.; Xu, S.; Li, Y.; Sun, J.; Rao, W.U.; Chen, C.; Du, M.; He, K.; et al. Melittin inhibits tumor angiogenesis modulated by endothelial progenitor cells associated with the SDF-1α/CXCR4 signaling pathway in a UMR-106 osteosarcoma xenograft mouse model. Mol. Med. Rep. 2016, 14, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.; Seshadri, M.; Komorowski, M.P.; Abrams, S.I.; Kozbor, D. Targeting CXCL12/CXCR4 signaling with oncolytic virotherapy disrupts tumor vasculature and inhibits breast cancer metastases. Proc. Natl. Acad. Sci. USA 2013, 110, E1291–E1300. [Google Scholar] [CrossRef] [PubMed]
- Katoh, H.; Hosono, K.; Ito, Y.; Suzuki, T.; Ogawa, Y.; Kubo, H.; Kamata, H.; Mishima, T.; Tamaki, H.; Sakagami, H.; et al. COX-2 and Prostaglandin EP3/EP4 Signaling Regulate the Tumor Stromal Proangiogenic Microenvironment via CXCL12-CXCR4 Chemokine Systems. Am. J. Pathol. 2010, 176, 1469–1483. [Google Scholar] [CrossRef] [PubMed]
- Liekens, S.; Schols, D.; Hatse, S. CXCL12-CXCR4 Axis in Angiogenesis, Metastasis and Stem Cell Mobilization. Curr. Pharm. Des. 2010, 16, 3903–3920. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Zhao, W.C.; Yuan, F. CXCR7/CXCR4/CXCL12 Axis Regulates the Proliferation, Migration, Survival and Tube Formation of Choroid-Retinal Endothelial Cells. Ophthalmic Res. 2013, 50, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Guerin, E.; Sheridan, C.; Assheton, D.; Kent, D.; Wong, D.; Grant, M.; Hiscott, P. SDF1-alpha is associated with VEGFR-2 in human choroidal neovascularisation. Microvasc. Res. 2008, 75, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Eklund, L.; Saharinen, P. Angiopoietin signaling in the vasculature. Exp. Cell Res. 2013, 319, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, S.; Hermanns, M.I.; Kirkpatrick, C.J. Retention of a differentiated endothelial phenotype by outgrowth endothelial cells isolated from human peripheral blood and expanded in long-term cultures. Cell Tissue Res. 2006, 326, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, S.; Jiang, X.; Schmidt, H.; Dohle, E.; Ghanaati, S.; Orth, C.; Hofmann, A.; Motta, A.; Migliaresi, C.; Kirkpatrick, C.J. Dynamic processes involved in the pre-vascularization of silk fibroin constructs for bone regeneration using outgrowth endothelial cells. Biomaterials 2009, 30, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Moens, S.; Goveia, J.; Stapor, P.C.; Cantelmo, A.R.; Carmeliet, P. The multifaceted activity of VEGF in angiogenesis—Implications for therapy responses. Cytokine Growth Factor Rev. 2014, 25, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Dohle, E.; Fuchs, S.; Kolbe, M.; Hofmann, A.; Schmidt, H.; Kirkpatrick, C.J. Sonic hedgehog promotes angiogenesis and osteogenesis in a coculture system consisting of primary osteoblasts and outgrowth endothelial cells. Tissue Eng. Part A 2010, 16, 1235–1237. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.-Y.; Sanghani, A.; Hua, J.; Coathup, M.; Kalia, P.; Blunn, G. Mesenchymal Stem Cells with Increased Stromal Cell-Derived Factor 1 Expression Enhanced Fracture Healing. Tissue Eng. Part A 2014, 21, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, K.; Greenfield, S.; Pan, H.; Vasanji, A.; Kumagai, K.; Midura, R.J.; Kiedrowski, M.; Penn, M.S.; Muschler, G.F. Stromal cell-derived factor-1 and monocyte chemotactic protein-3 improve recruitment of osteogenic cells into sites of musculoskeletal repair. J. Orthop. Res. 2011, 29, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Kimura, R.; Rokkaku, T.; Takeda, S.; Senba, M.; Mori, N. Cytotoxic effects of fucoidan nanoparticles against osteosarcoma. Mar. Drugs 2013, 11, 4267–4278. [Google Scholar] [CrossRef] [PubMed]
- Jerez, S.; Araya, H.; Thaler, R.; Charlesworth, M.C.; López-Solís, R.; Kalergis, A.M.; Céspedes, P.F.; Dudakovic, A.; Stein, G.S.; van Wijnen, A.J.; et al. Proteomic Analysis of Exosomes and Exosome-Free Conditioned Media From Human Osteosarcoma Cell Lines Reveals Secretion of Proteins Related to Tumor Progression. J. Cell. Biochem. 2017, 118, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Benslimane-Ahmim, Z.; Pereira, J.; Lokajczyk, A.; Dizier, B.; Galy-Fauroux, I.; Fischer, A.-M.; Heymann, D.; Boisson-Vidal, C. Osteoprotegerin regulates cancer cell migration through SDF-1/CXCR4 axis and promotes tumour development by increasing neovascularization. Cancer Lett. 2017, 395, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, Z.-R.; Zhao, N.; Ma, B.-A.; Fan, Q.-Y. VEGF Silencing Inhibits Human Osteosarcoma Angiogenesis and Promotes Cell Apoptosis via PI3K/AKT Signaling Pathway. Cell Biochem. Biophys. 2015, 73, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.E.; de Palma, M.; Naldini, L. Tie2-Expressing Monocytes and Tumor Angiogenesis: Regulation by Hypoxia and Angiopoietin-2. Cancer Res. 2007, 67, 8429–8432. [Google Scholar] [CrossRef] [PubMed]
- DuBois, S.; Demetri, G. Markers of angiogenesis and clinical features in patients with sarcoma. Cancer 2007, 109, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Tait, C.R.; Jones, P.F. Angiopoietins in tumours: the angiogenic switch. J. Pathol. 2004, 204, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-C.; Hsu, W.-L.; Hwang, P.-A.; Chou, T.-C. Low Molecular Weight Fucoidan Inhibits Tumor Angiogenesis through Downregulation of HIF-1/VEGF Signaling under Hypoxia. Mar. Drugs 2015, 13, 4436–4451. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-H.; Chiu, Y.-H.; Chan, Y.-L.; Chiu, Y.-H.; Wang, H.; Huang, K.-C.; Li, T.-L.; Hsu, K.-H.; Wu, C.-J. Prophylactic Administration of Fucoidan Represses Cancer Metastasis by Inhibiting Vascular Endothelial Growth Factor (VEGF) and Matrix Metalloproteinases (MMPs) in Lewis Tumor-Bearing Mice. Mar. Drugs 2015, 13, 1882–1990. [Google Scholar] [CrossRef] [PubMed]
- Ustyuzhanina, N.E.; Bilan, M.I.; Ushakova, N.A.; Usov, A.I.; Kiselevskiy, M.V.; Nifantiev, N.E. Fucoidans: Pro- or antiangiogenic agents? Glycobiology 2014, 24, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Purnama, A.; Aid-Launais, R.; Haddad, O.; Maire, M.; Mantovani, D.; Letourneur, D.; Hlawaty, H.; le Visage, C. Fucoidan in a 3D scaffold interacts with vascular endothelial growth factor and promotes neovascularization in mice. Drug Deliv. Transl. Res. 2015, 5, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Boisson-Vidal, C.; Zemani, F.; Caligiuri, G.; Galy-Fauroux, I.; Colliec-Jouault, S.; Helley, D.; Fischer, A.M. Neoangiogenesis Induced by Progenitor Endothelial Cells: Effect of Fucoidan from Marine Algae. Cardiovasc. Hematol. Agents Med. Chem. 2007, 5, 67–77. [Google Scholar] [CrossRef] [PubMed]
- De Azevedo, T.C.G.; Bezerra, M.E.B.; Santos, M.d.G.d.L.; Souza, L.A.; Marques, C.T.; Benevides, N.M.B.; Leite, E.L. Heparinoids algal and their anticoagulant, hemorrhagic activities and platelet aggregation. Biomed. Pharm. 2009, 63, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Lake, A.C.; Vassy, R.; di Benedetto, M.; Lavigne, D.; le Visage, C.; Perret, G.Y.; Letourneur, D. Low Molecular Weight Fucoidan Increases VEGF165-induced Endothelial Cell Migration by Enhancing VEGF165 Binding to VEGFR-2 and NRP1. J. Biol. Chem. 2006, 281, 37844–37852. [Google Scholar] [CrossRef] [PubMed]

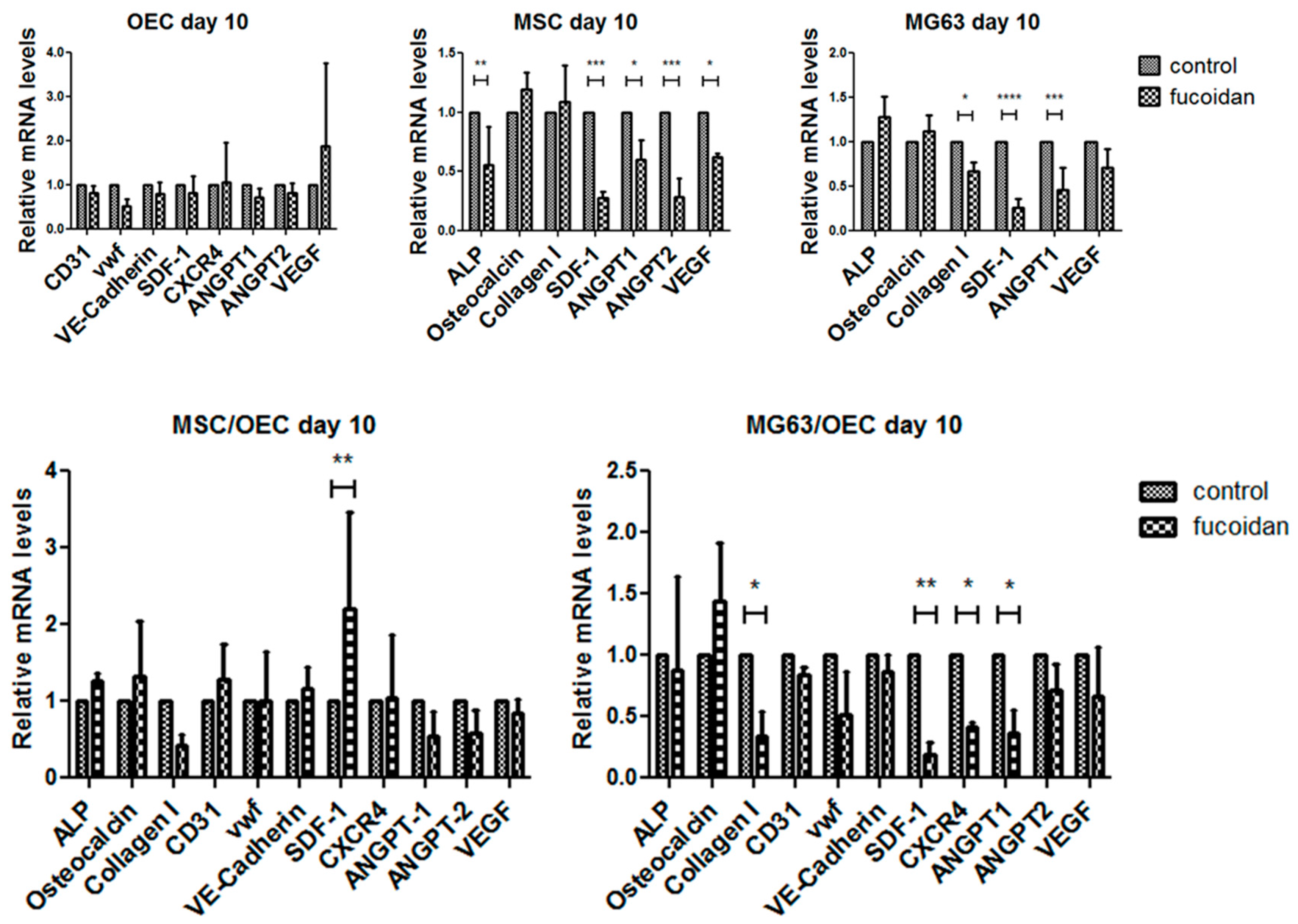
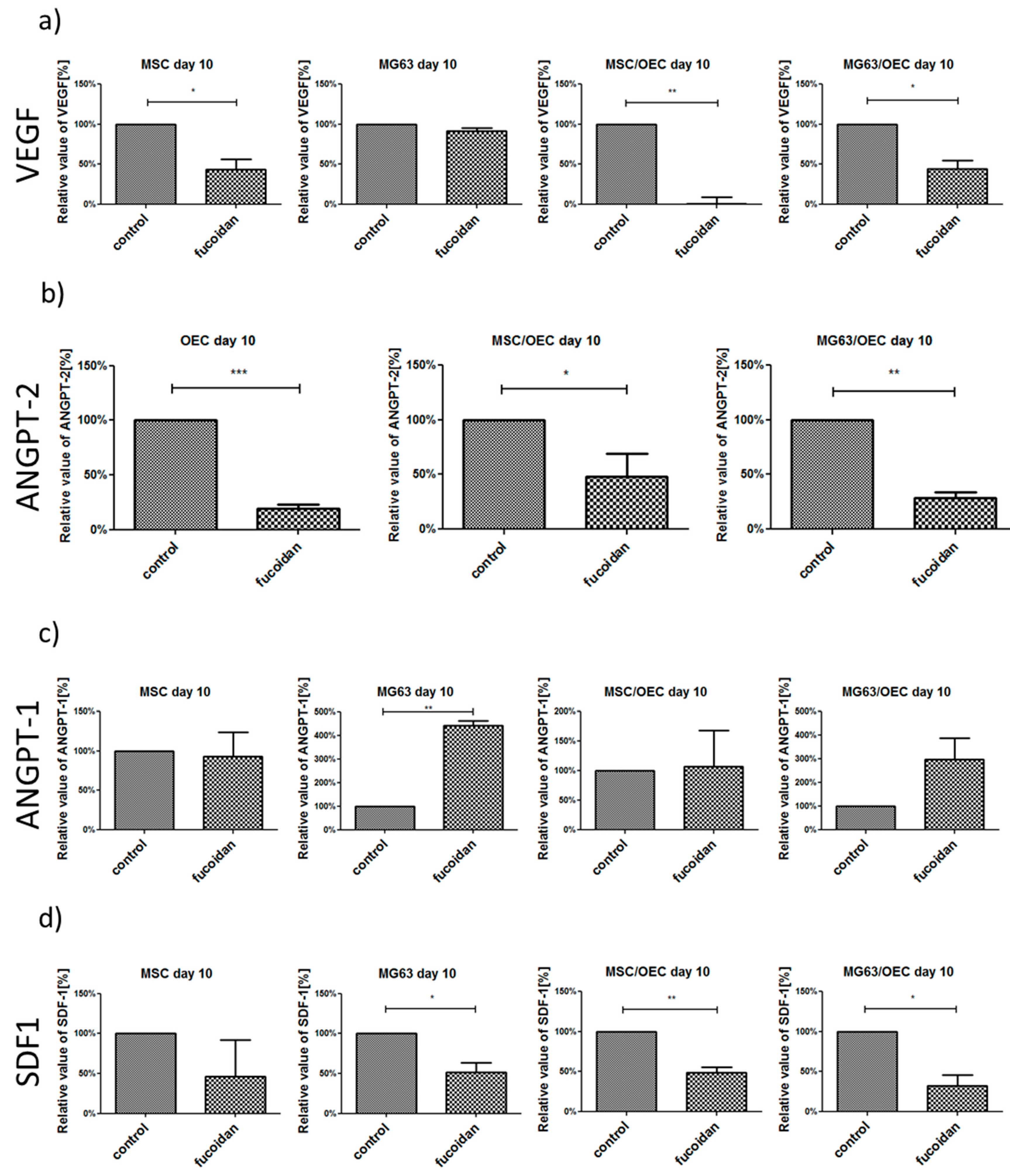
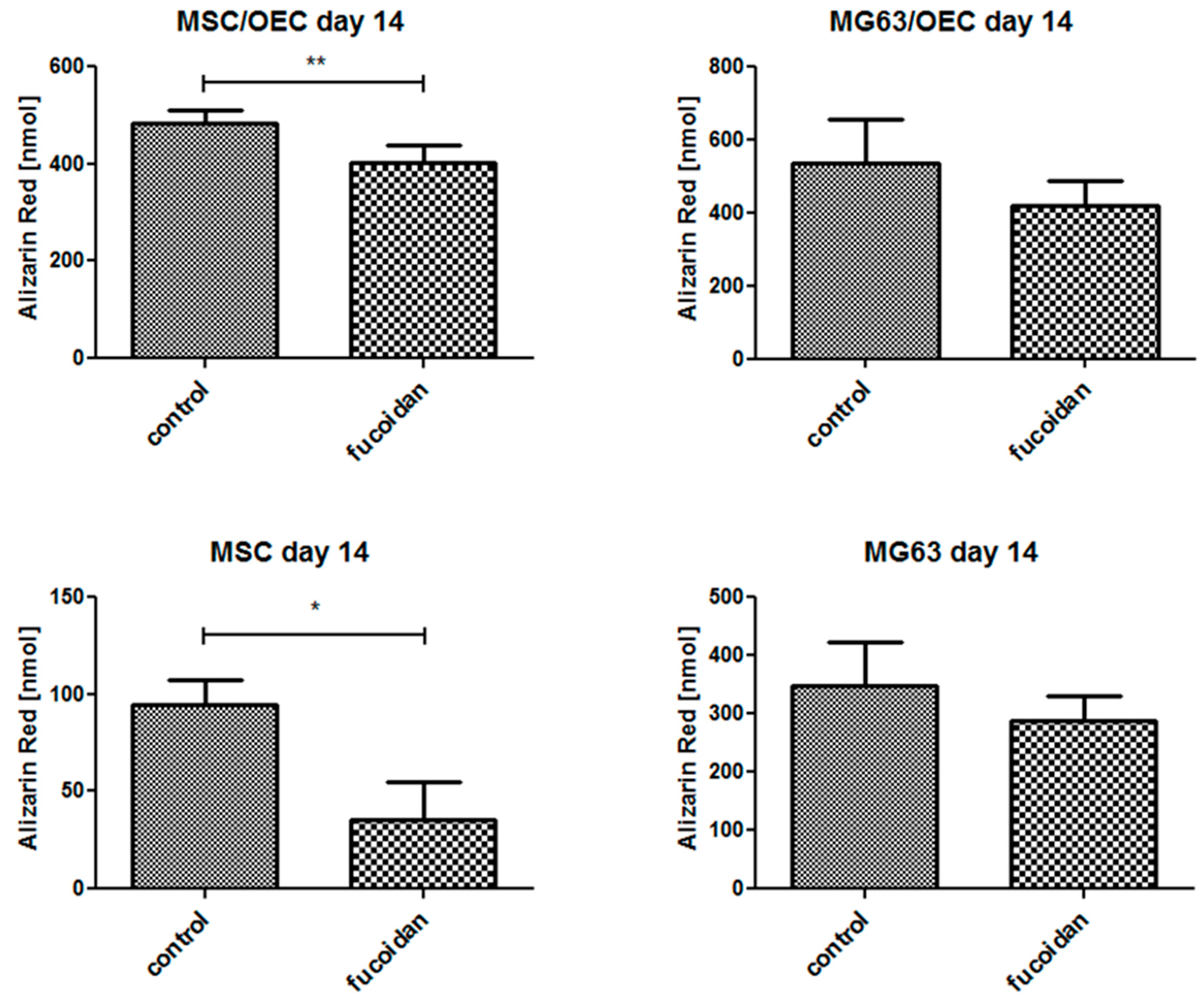
| Gene Name | Primer Assay Name | Catalogue Number |
|---|---|---|
| ALP | Hs_ALPL_1_SG QuantiTect Primer Assay | QT00012957 |
| Osteocalcin | Hs_BGLAP_1_SG QuantiTect Primer Assay | QT00232771 |
| Collagen I | Hs_COL1A1_1_SG QuantiTect Primer Assay | QT00037793 |
| vwf | Hs_VWF_1_SG QuantiTect Primer Assay | QT00051975 |
| VE-Cadherin | Hs_CDH5_1_SG QuantiTect Primer Assay | QT00013244 |
| SDF-1 | HS_CXCL12_1_SG QuantiTect Primer Assay | QT00087591 |
| CXCR4 | Hs_CXCR4_2_SG QuantiTect Primer Assay | QT02311841 |
| ANGPT-1 | Hs_ANGPT1_1_SG QuantiTect Primer Assay | QT00046865 |
| ANGPT-2 | Hs_ANGPT2_1_SG QuantiTect Primer Assay | QT00100947 |
| VEGF | Hs_VEGFA_2_SG QuantiTech Primer Assay | QT01036861 |
| RPL13a | Hs_RPL13A_1_SG QuantiTect Primer Assay | QT00089915 |
| Gene name | Sequence | Annealing temperature |
| CD 31 | for 5′- CCGGATCTATGACTCAGGGACCAT-3′ rev 5′-GGATGGCCTCTTTCTTGTCCAG-3′ | 55 °C |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Schmidt, H.; Pavleska, D.; Wermann, T.; Seekamp, A.; Fuchs, S. Crude Fucoidan Extracts Impair Angiogenesis in Models Relevant for Bone Regeneration and Osteosarcoma via Reduction of VEGF and SDF-1. Mar. Drugs 2017, 15, 186. https://doi.org/10.3390/md15060186
Wang F, Schmidt H, Pavleska D, Wermann T, Seekamp A, Fuchs S. Crude Fucoidan Extracts Impair Angiogenesis in Models Relevant for Bone Regeneration and Osteosarcoma via Reduction of VEGF and SDF-1. Marine Drugs. 2017; 15(6):186. https://doi.org/10.3390/md15060186
Chicago/Turabian StyleWang, Fanlu, Harald Schmidt, Dijana Pavleska, Thees Wermann, Andreas Seekamp, and Sabine Fuchs. 2017. "Crude Fucoidan Extracts Impair Angiogenesis in Models Relevant for Bone Regeneration and Osteosarcoma via Reduction of VEGF and SDF-1" Marine Drugs 15, no. 6: 186. https://doi.org/10.3390/md15060186
APA StyleWang, F., Schmidt, H., Pavleska, D., Wermann, T., Seekamp, A., & Fuchs, S. (2017). Crude Fucoidan Extracts Impair Angiogenesis in Models Relevant for Bone Regeneration and Osteosarcoma via Reduction of VEGF and SDF-1. Marine Drugs, 15(6), 186. https://doi.org/10.3390/md15060186