Compositional Characteristics and In Vitro Evaluations of Antioxidant and Neuroprotective Properties of Crude Extracts of Fucoidan Prepared from Compressional Puffing-Pretreated Sargassum crassifolium
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of Compressional-Puffing Parameters on the Characteristics of Puffed Algal Samples
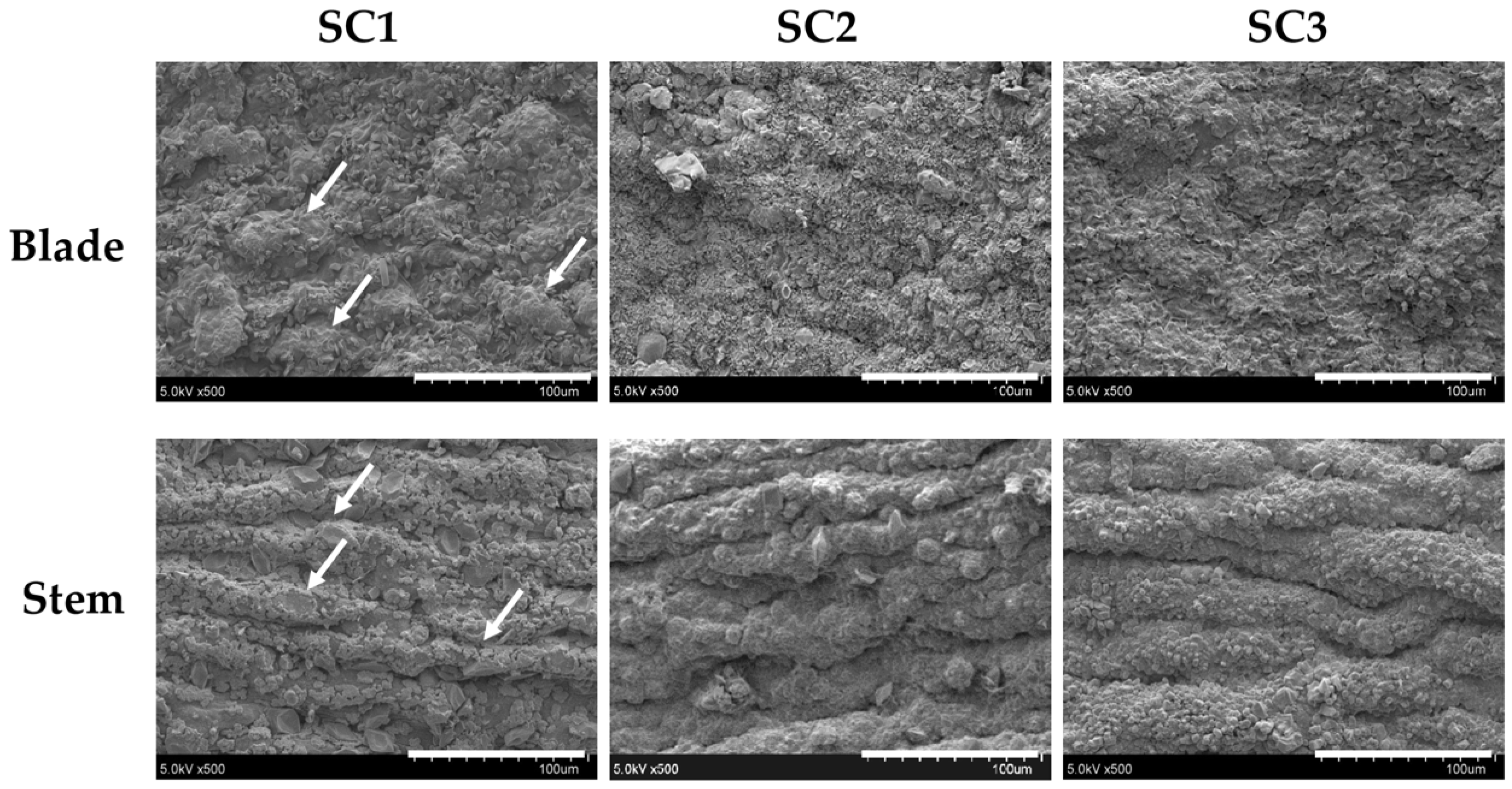
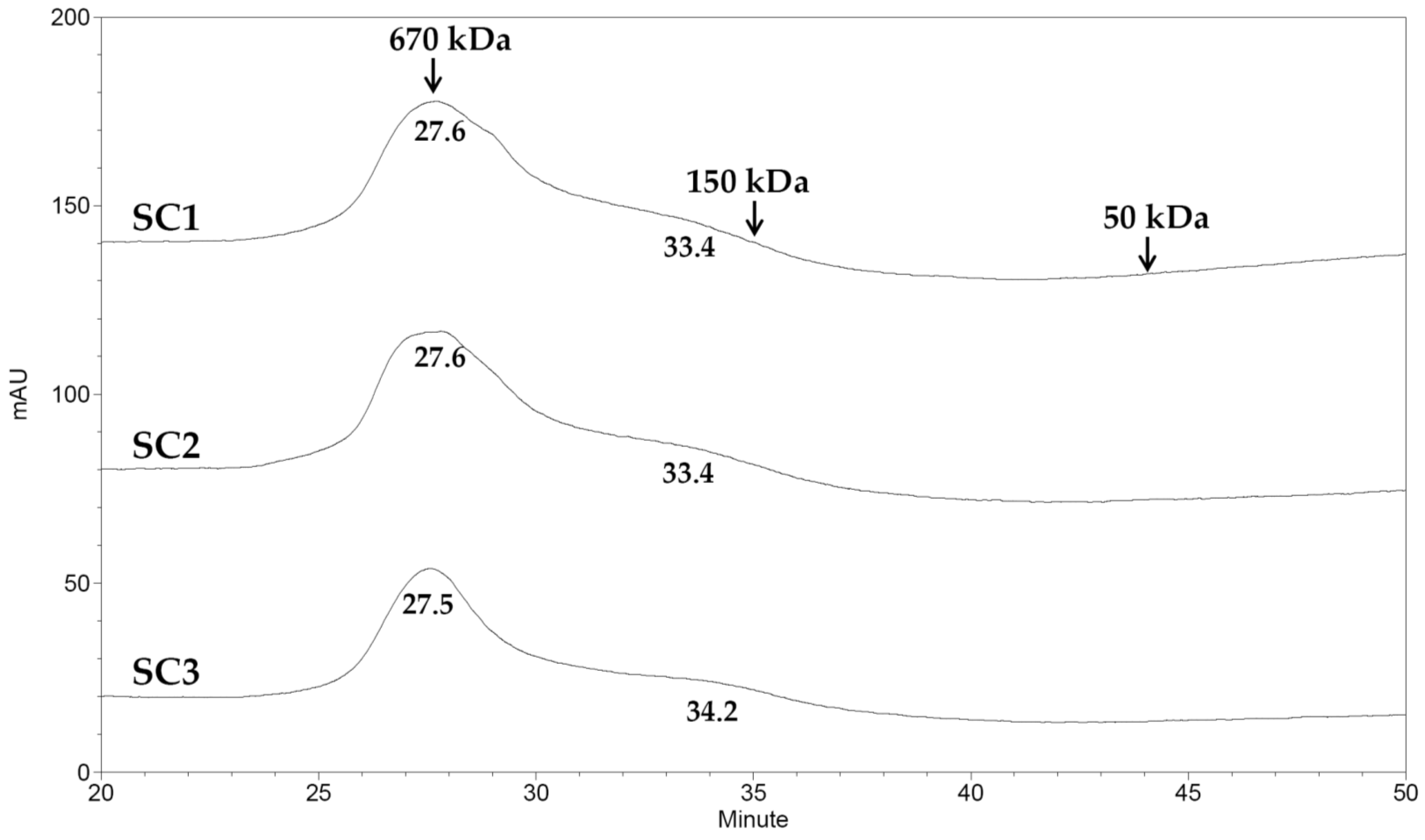
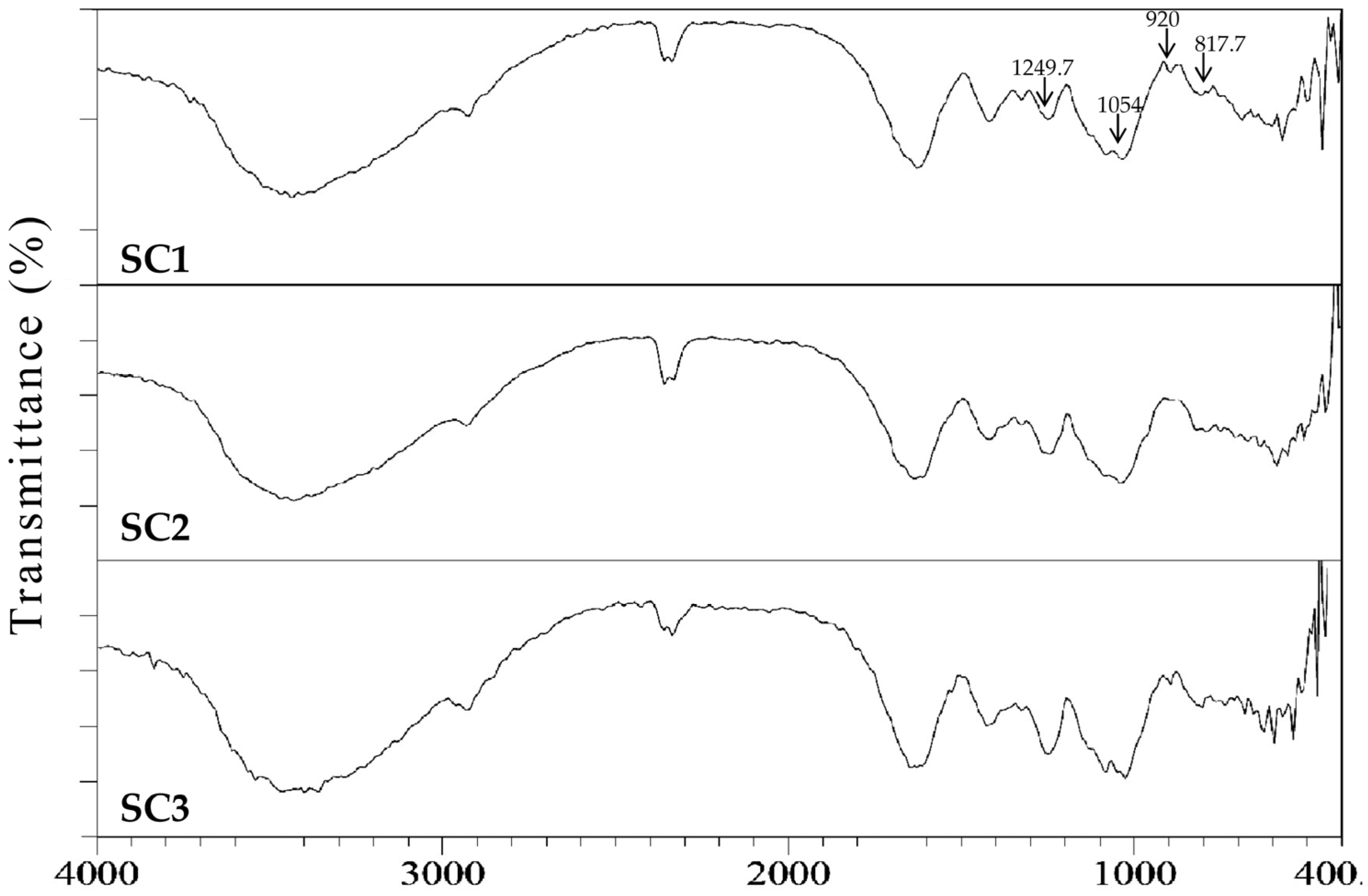
2.2. Physicochemical and Compositional Analyses of Fucoidans for SC1, SC2, and SC3
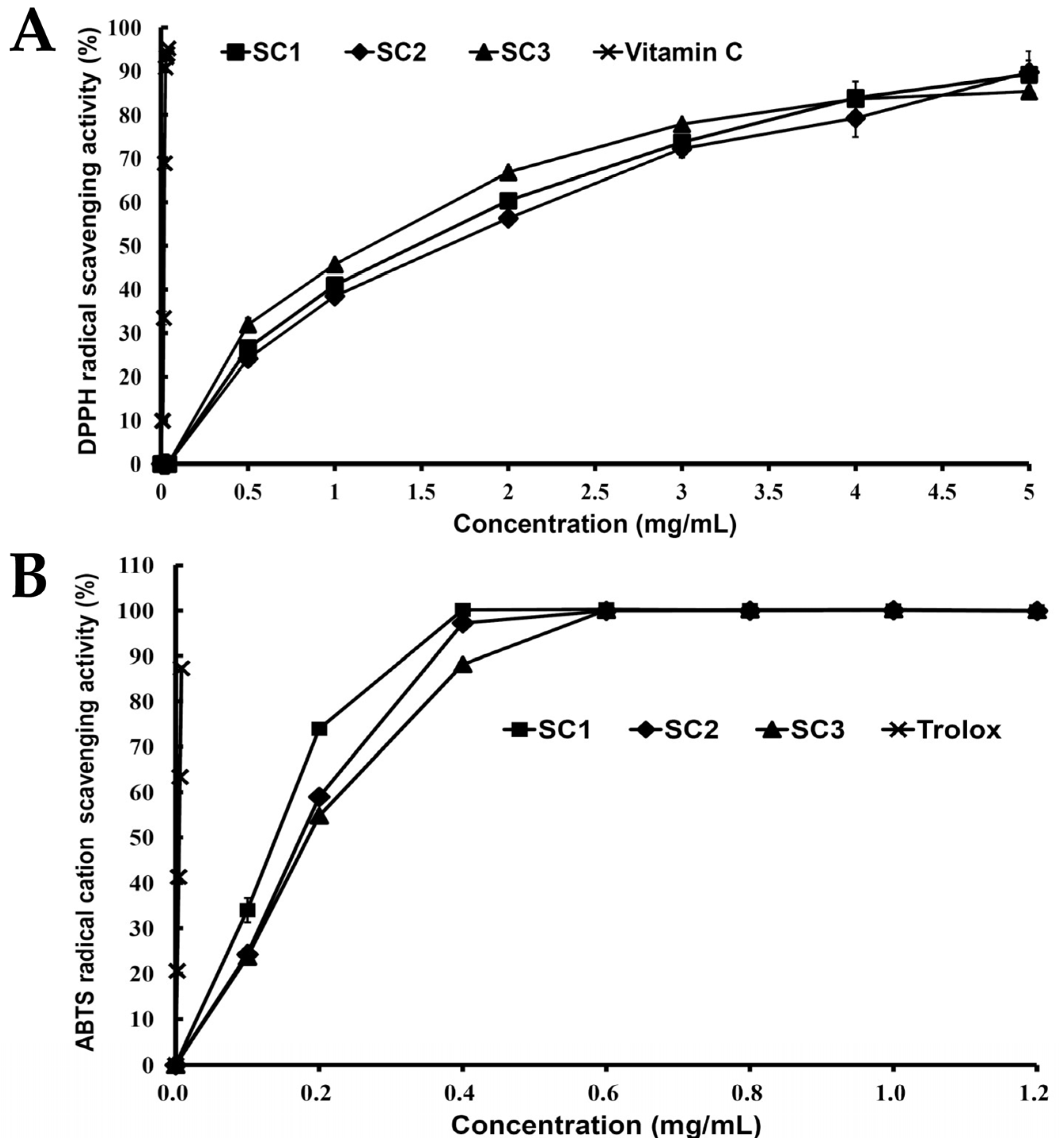
2.3. Antioxidant Activities of SC1, SC2, and SC3
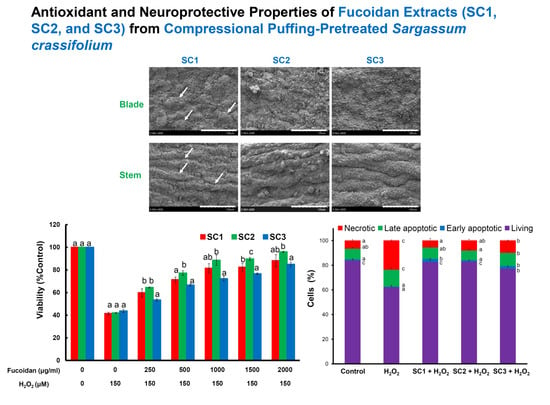
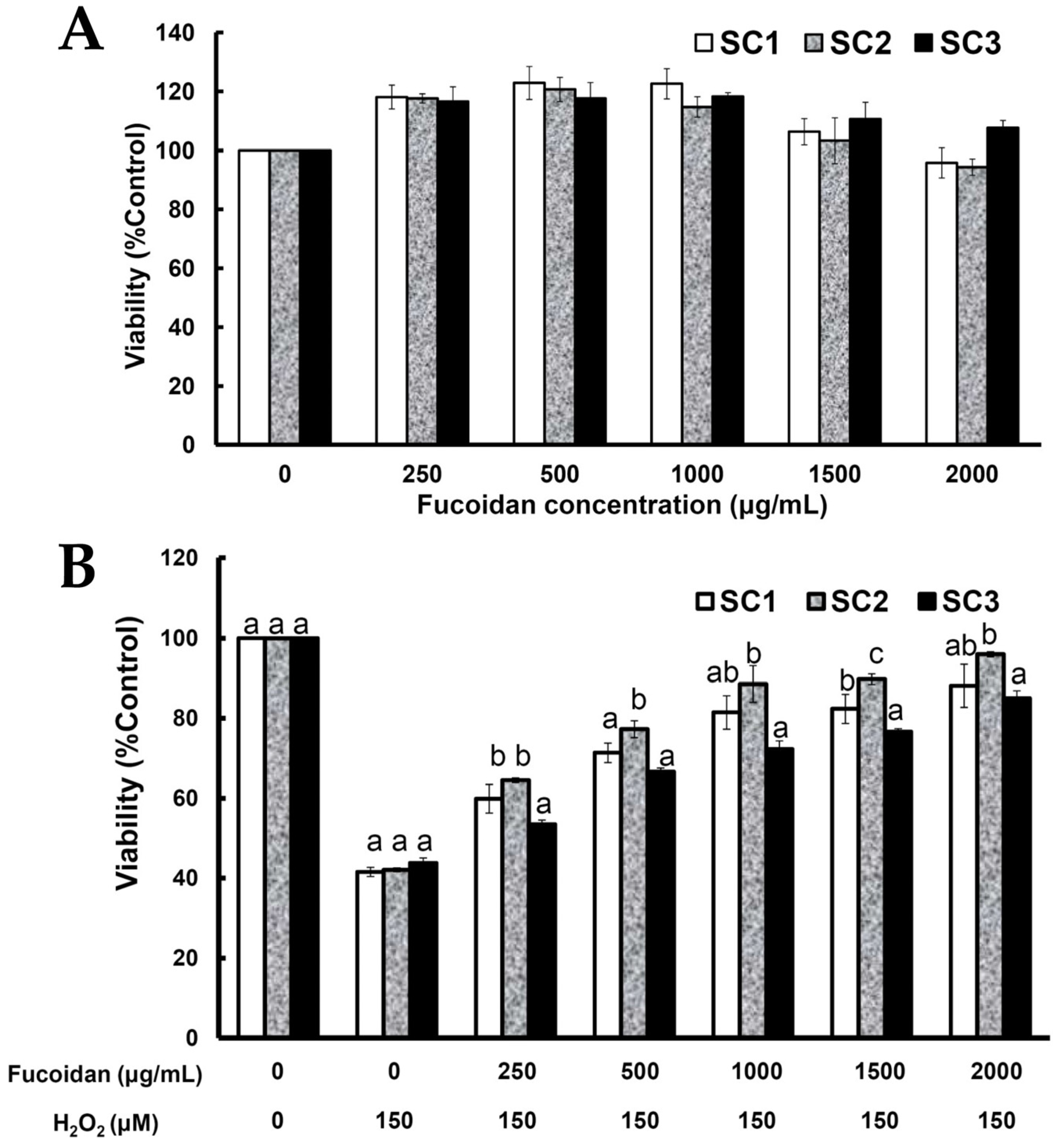
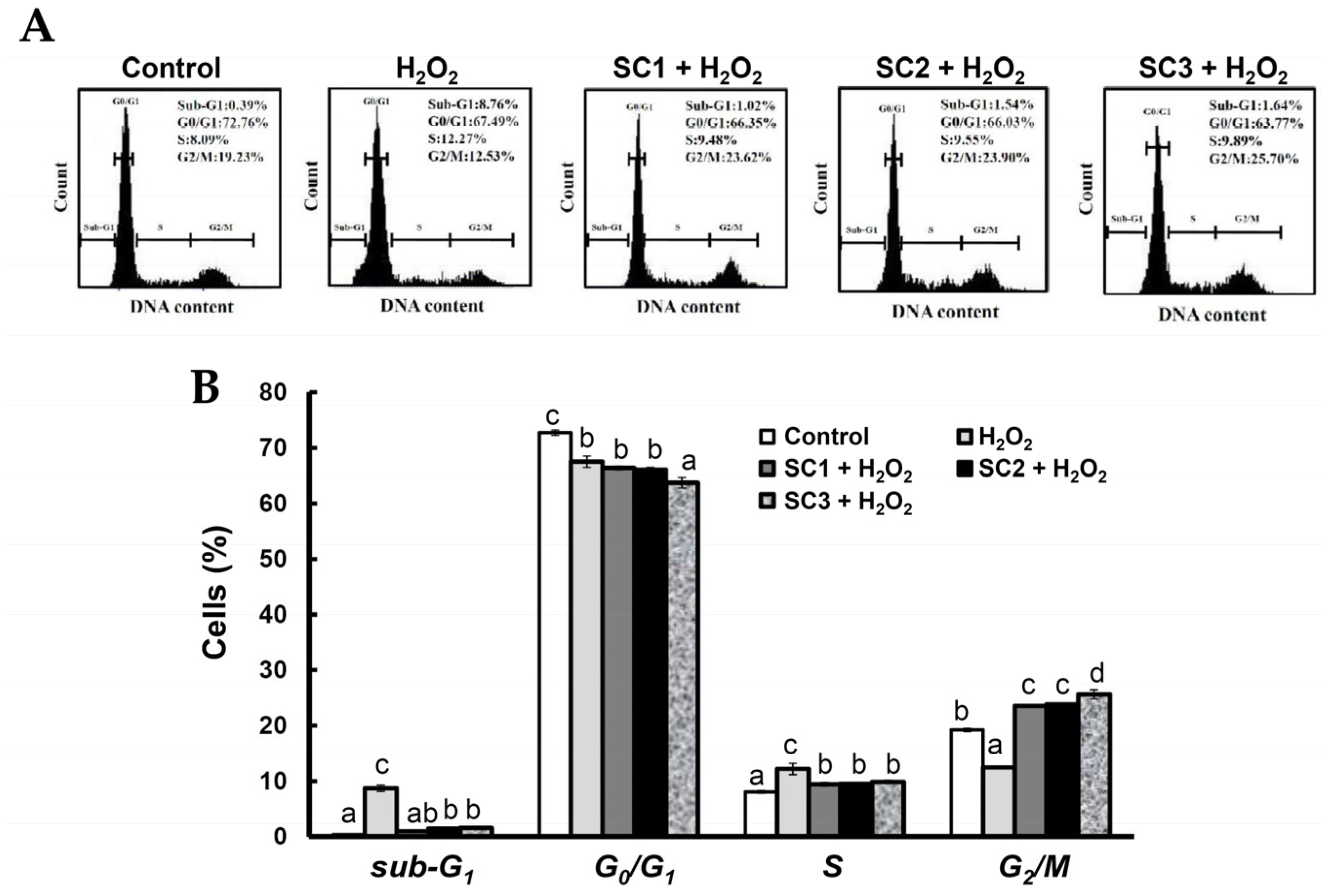
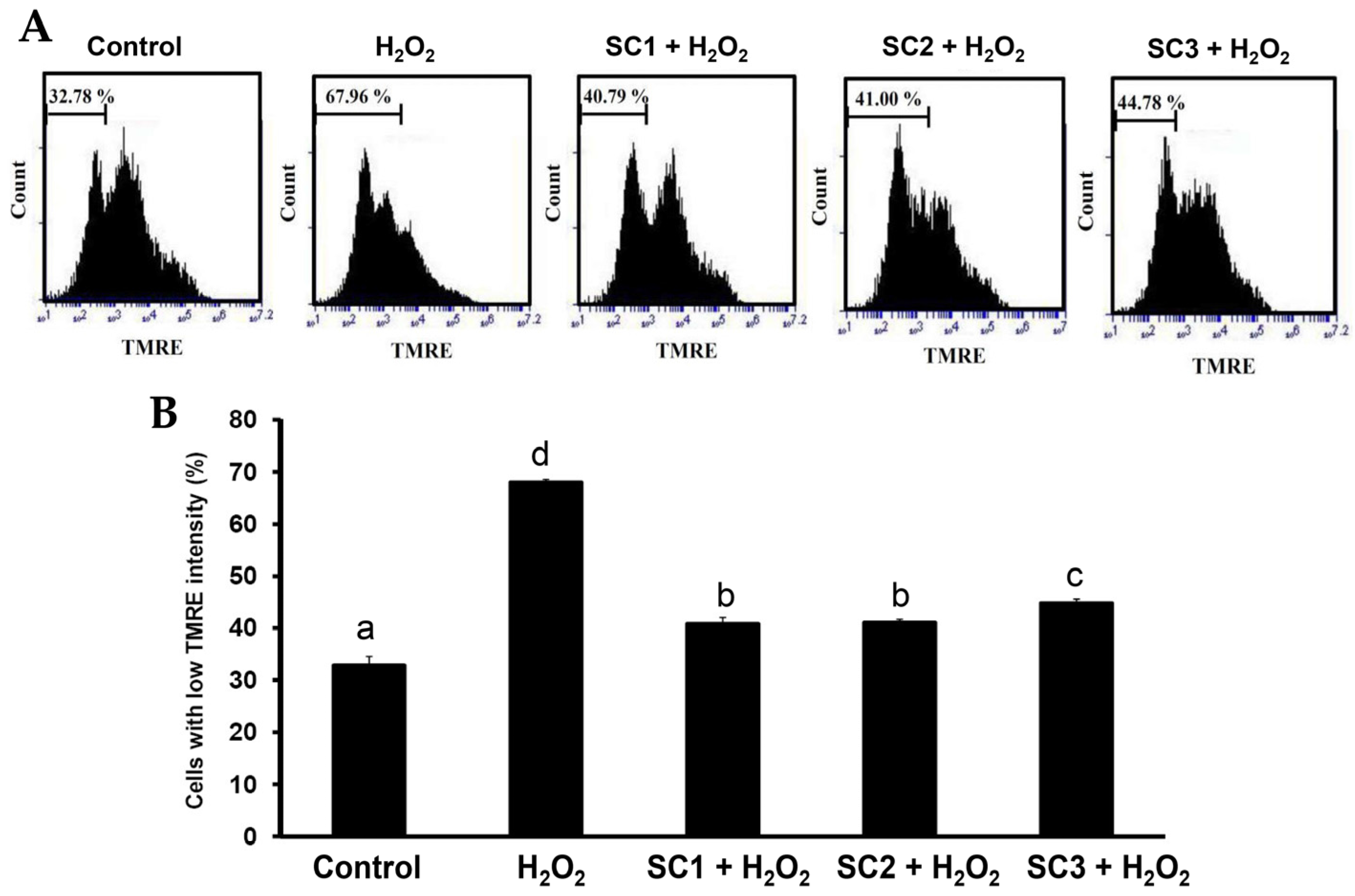
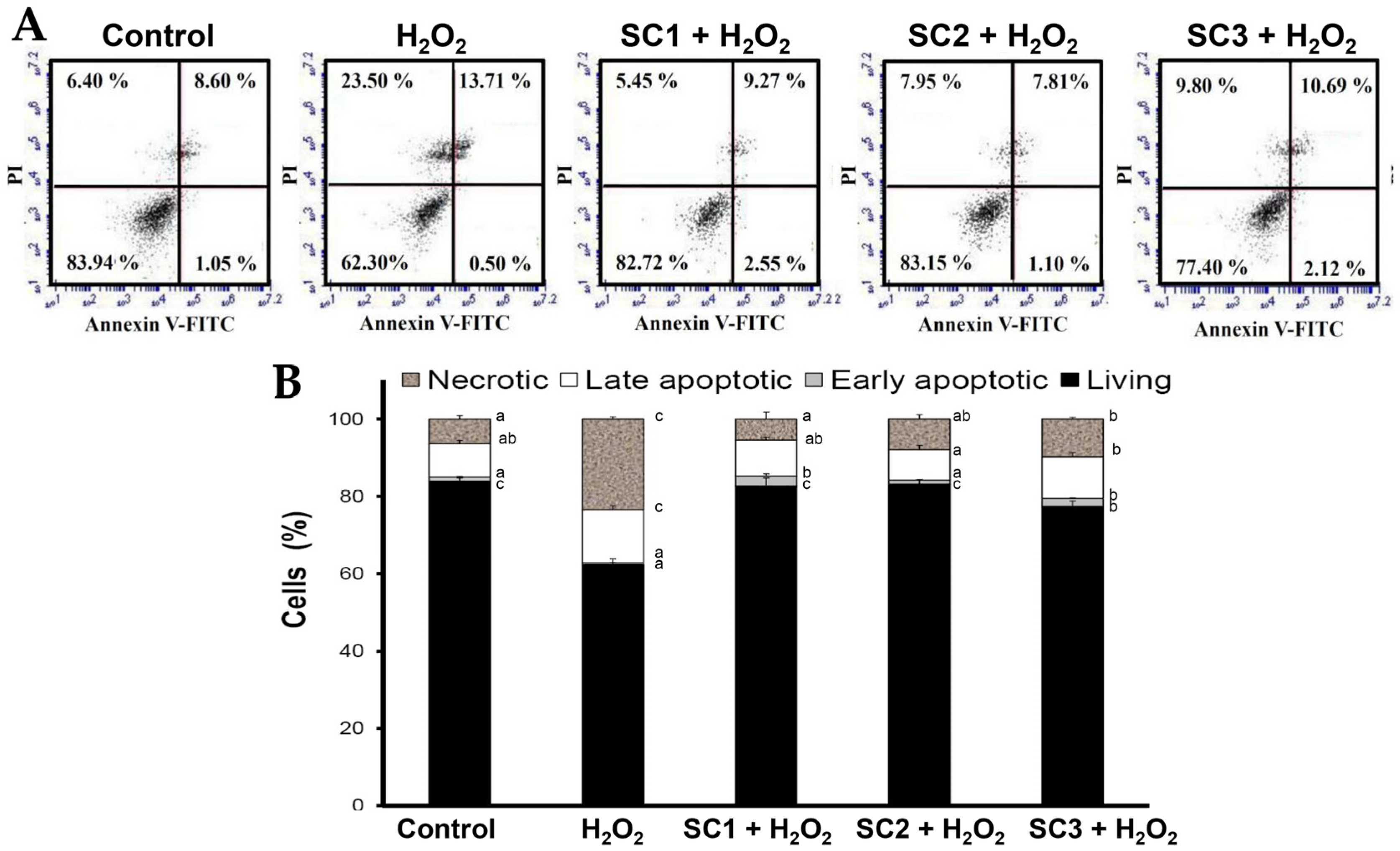
2.4. Neuroprotective Activities of SC1, SC2, and SC3
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Compressional-Puffing Procedure
3.3. Warm Water Extraction Procedure
3.4. Analytical Methods
3.5. Color Analysis
3.6. Sensory Evaluation
3.7. Bulk Density
3.8. Analysis of Monosaccharide Composition
3.9. Molecular Weight Analysis
3.10. Scanning Electron Microscopy (SEM) Examination
3.11. Fourier Transform Infrared (FTIR) Spectroscopy
3.12. DPPH Radical Scavenging Activity
3.13. ABTS Radical Cation Scavenging Activity
3.14. Cell Culture
3.15. Cell Viability Test
3.16. Cell Cycle Analysis
3.17. Mitochondrial Membrane Potential (MMP) Analysis
3.18. Annexin V-Fluorescein Isothiocyanate (FITC) Staining Analysis
3.19. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nordberg, J.; Arnér, E.S. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Bio. Med. 2001, 31, 1287–1312. [Google Scholar] [CrossRef]
- Vijayabaskar, P.; Vaseela, N.; Thirumaran, G. Potential antibacterial and antioxidant properties of a sulfated polysaccharide from the brown marine algae Sargassum swartzii. Chin. J. Nat. Med. 2012, 10, 421–428. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Drake, J.; Pocernich, C.; Castegna, A. Evidence of oxidative damage in Alzheimer’s disease brain: Central role for amyloid β-peptide. Trends Mol. Med. 2001, 7, 548–554. [Google Scholar] [CrossRef]
- Reed, T.T. Lipid peroxidation and neurodegenerative disease. Free Radic. Biol. Med. 2011, 51, 1302–1319. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Abu-Ghannam, N. Bioactive potential and possible health effects of edible brown seaweeds. Trends Food Sci. Technol. 2011, 22, 315–326. [Google Scholar] [CrossRef]
- Kim, M.M.; Rajapakse, N.; Kim, S.K. Anti-inflammatory effect of Ishige okamurae ethanolic extract via inhibition of NF-κB transcription factor in RAW 264.7 cells. Phytother. Res. 2009, 23, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Kim, S.K. Neuroprotective effects of marine algae. Mar. Drugs 2011, 9, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Kang, H. Neuroprotective effect of Sargassum thunbergii (Mertens ex Roth) Kuntze in activated murine microglial cells. Trop. J. Pharm. Res. 2015, 14, 235–240. [Google Scholar] [CrossRef]
- Jin, W.H.; Zhang, W.J.; Wang, J.; Yao, J.T.; Xie, E.; Liu, D.C.; Duan, D.; Zhang, Q.B. A study of neuroprotective and antioxidant activities of heteropolysaccharides from six Sargassum species. Int. J. Biol. Macromol. 2014, 67, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Ale, M.T.; Maruyama, H.; Tamauchi, H.; Mikkelsen, J.; Meyer, A.S. Fucoidan from Sargassum sp. and Fucus vesiculosus reduces cell viability of lung carcinoma and melanoma cells in vitro and activates natural killer cells in mice in vivo. Int. J. Biol. Macromol. 2011, 49, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.L.; Dong, C.H.; Yin, J.G.; Shen, J.Y.; Tian, J.W.; Li, C.M. Neuroprotective effect of fucoidan on H2O2-induced apoptosis in PC12 cells via activation of PI3K/Akt pathway. Cell. Mol. Neurobiol. 2012, 32, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Wu, S.J.; Yang, W.N.; Kuan, A.W.; Chen, C.Y. Antioxidant activities of crude extracts of fucoidan extracted from Sargassum glaucescens by a compressional-puffing-hydrothermal extraction process. Food Chem. 2016, 197, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Yamaguchi, Y.; Noda, K.; Fujii, T.; Shinohara, N.; Ushirokawa, T.; Sugawa-Katayama, Y.; Katayama, M. Seasonal variation in the chemical composition of a marine brown alga, Sargassum horneri (Turner) C. Agardh. J. Food Compost. Anal. 2011, 24, 231–236. [Google Scholar] [CrossRef]
- Chiang, P.S.; Lee, D.J.; Whiteley, C.G.; Huang, C.Y. Antioxidant phenolic compounds from Pinus morrisconicola using compressional-puffing pretreatment and water-ethanol extraction: Optimization of extraction parameters. J. Taiwan Inst. Chem. Eng. 2017, 70, 7–14. [Google Scholar] [CrossRef]
- An, Y.E.; Ahn, S.C.; Yang, D.C.; Park, S.J.; Kim, B.Y.; Baik, M.Y. Chemical conversion of ginsenosides in puffed red ginseng. LWT Food Sci. Technol. 2011, 44, 370–374. [Google Scholar] [CrossRef]
- Varnalis, A.I.; Brennan, J.G.; MacDougall, D.B. A proposed mechanism of high-temperature puffing of potato. Part I. The influence of blanching and drying conditions on the volume of puffed cubes. J. Food Eng. 2001, 48, 361–367. [Google Scholar] [CrossRef]
- Moore, R.E. Volatile compounds from marine algae. Acc. Chem. Res 1977, 10, 40–47. [Google Scholar] [CrossRef]
- Cho, E.H.; Park, K.H.; Kim, S.Y.; Oh, C.S.; Bang, S.I.; Chae, H.J. Process development for deodorization of fucoidan using a combined method of solvent extraction and spray drying. KSBB J. 2011, 26, 49–56. [Google Scholar] [CrossRef]
- Khalafu, S.H.S.; Mustapha, W.A.W.; Lim, S.J.; Maskat, M.Y. The effect of deodorization on volatile compositions of fucoidan extracted from brown seaweed (Sargassum sp.). AIP Conf. Proc. 2016, 1784, 030043. [Google Scholar]
- Skriptsova, A.V.; Shevchenko, N.M.; Tarbeeva, D.V.; Zvyagintseva, T.N. Comparative study of polysaccharides from reproductive and sterile tissues of five brown seaweeds. Mar. Biotechnol. 2012, 14, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Peranginangin, R.; Saepudin, E. Purification and characterization of fucoidan from the brown seaweed Sargassum binderi Sonder. Squalen Bull. Mar. Fish. Postharvest Biotech. 2015, 10, 79–87. [Google Scholar]
- Wang, C.Y.; Wu, T.C.; Hsieh, S.L.; Tsai, Y.H.; Yeh, C.W.; Huang, C.Y. Antioxidant activity and growth inhibition of human colon cancer cells by crude and purified fucoidan preparations extracted from Sargassum cristaefolium. J. Food Drug Anal. 2015, 23, 766–777. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Wang, X.M.; Zhao, M.X.; Yu, S.C.; Qi, H.M. The immunological and antioxidant activities of polysaccharides extracted from Enteromorpha linza. Int. J. Biol. Macromol. 2013, 57, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.F.; Liu, M.; Fang, Z.X.; Wu, J.L.; Zhang, Q.Q. Composition and cytotoxicity of a novel polysaccharide from brown alga (Laminaria japonica). Carbohydr. Polym. 2012, 89, 1022–1026. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Astani, A.; Ghosh, T.; Schnitzler, P.; Ray, B. Polysaccharides from Sargassum tenerrimum: Structural features, chemical modification and anti-viral activity. Phytochemistry 2010, 71, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Hu, N.; Wu, Y.L.; Pan, Y.J.; Sun, C.R. Preliminary studies on the chemical characterization and antioxidant properties of acidic polysaccharides from Sargassum fusiforme. J. Zhejiang Univ. Sci. B 2008, 9, 721. [Google Scholar] [CrossRef] [PubMed]
- Tissot, B.; Salpin, J.Y.; Martinez, M.; Gaigeot, M.P.; Daniel, R. Differentiation of the fucoidan sulfated L-fucose isomers constituents by CE-ESIMS and molecular modeling. Carbohydr. Res. 2006, 341, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lu, F.; Wei, X.J.; Zhao, R.X. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Cui, N.; Bo, Z.X.; Xiang, F.X. Structural determinant and its underlying molecular mechanism of STPC2 related to anti-angiogenic activity. Mar. Drugs 2017, 15, 48. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Q.B.; Zhang, Z.S.; Song, H.F.; Li, P.C. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2010, 46, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Ananthi, S.; Raghavendran, H.R.B.; Sunil, A.G.; Gayathri, V.; Ramakrishnan, G.; Vasanthi, H.R. In vitro antioxidant and in vivo anti-inflammatory potential of crude polysaccharide from Turbinaria ornata (Marine Brown Alga). Food Chem. Toxicol. 2010, 48, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Imbs, T.I.; Skriptsova, A.V.; Zvyagintseva, T.N. Antioxidant activity of fucose-containing sulfated polysaccharides obtained from Fucus evanescens by different extraction methods. J. Appl. Phycol. 2015, 27, 545–553. [Google Scholar] [CrossRef]
- Bilan, M.I.; Grachev, A.A.; Ustuzhanina, N.E.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. Structure of a fucoidan from the brown seaweed Fucus evanescens C. Ag. Carbohyd. Res. 2002, 337, 719–730. [Google Scholar] [CrossRef]
- Leong, L.P.; Shui, G. An investigation of antioxidant capacity of fruits in Singapore markets. Food Chem. 2002, 76, 69–75. [Google Scholar] [CrossRef]
- Magliaro, B.C.; Saldanha, C.J. Clozapine protects PC-12 cells from death due to oxidative stress induced by hydrogen peroxide via a cell-type specific mechanism involving inhibition of extracellular signal-regulated kinase phosphorylation. Brain Res. 2009, 1283, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Wang, H.; Li, Q.; Qian, Y.F.; Yao, W.B. Salidroside inhibits H2O2-induced apoptosis in PC 12 cells by preventing cytochrome c release and inactivating of caspase cascade. Acta Biochim. Biophys. Sin. (Shanghai) 2008, 40, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Bao, Y.M.; Jiang, B.; An, L.J. Protective effect of protocatechuic acid from Alpinia oxyphylla on hydrogen peroxide-induced oxidative PC12 cell death. Eur. J. Pharmacol. 2006, 538, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Crompton, M. The mitochondrial permeability transition pore and its role in cell death. Biochem. J. 1999, 341, 233–249. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.Q.; Feng, J.Q.; Chen, J.; Chen, P.X.; Zhi, J.L.; Cui, Y.; Guo, R.X.; Yu, H.M. Protection of oxidative preconditioning against apoptosis induced by H2O2 in PC12 cells: Mechanisms via MMP, ROS, and Bcl-2. Brain Res. 2005, 1057, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Kroemer, G. The pathophysiology of mitochondrial cell death. Science 2004, 305, 626–629. [Google Scholar] [CrossRef] [PubMed]
- Van Engeland, M.; Nieland, L.J.; Ramaekers, F.C.; Schutte, B.; Reutelingsperger, C.P. Annexin V-affinity assay: A review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry 1998, 31, 1–9. [Google Scholar] [CrossRef]
- Gibbons, M.N. The determination of methylpentoses. Analyst 1955, 80, 268–276. [Google Scholar] [CrossRef]
- Filisetti-Cozzi, T.M.; Carpita, N.C. Measurement of uronic acids without interference from neutral sugars. Anal. Biochem. 1991, 197, 157–162. [Google Scholar] [CrossRef]
- Honya, M.; Kinoshita, T.; Ishikawa, M.; Mori, H.; Nisizawa, K. Monthly determination of alginate, M/G ratio, mannitol, and minerals in cultivated Laminaria japonica. Bull. Jpn. Soc. Sci. Fish. 1993, 59, 295–299. [Google Scholar] [CrossRef]
- Huang, C.Y.; Kuo, J.M.; Wu, S.J.; Tsai, H.T. Isolation and characterization of fish scale collagen from tilapia (Oreochromis sp.) by a novel extrusion-hydro-extraction process. Food Chem. 2016, 190, 997–1006. [Google Scholar] [CrossRef] [PubMed]
| Variables of Compressional-Puffing | SC1 | SC2 | SC3 | |
| Mechanical compression | Pressure (kg/cm2) | 0 | 5 | 5 |
| Number of compression times | 0 | 3 | 3 | |
| Puffing | Temperature (°C) | 0 | 140 | 180 |
| Pressure (kg/cm2) | 0 | 1.7 | 6.3 | |
| Reaction time | Time (s) | 0 | 10 | 10 |
| Variables of Water Extraction | SC1 | SC2 | SC3 | |
| Extraction temperature | Temperature (°C) | 40 | 40 | 40 |
| Extraction time | Time (min) | 15 | 15 | 15 |
| Characteristics of Puffed Algal Samples | SC1 1 | SC2 1 | SC3 1 | |
| Degree of moisture loss (%) | 0.00 ± 0.00 a | 14.11 ± 2.36 b | 34.69 ± 6.02 c | |
| L | 41.73 ± 0.00 c | 41.66 ± 0.00 b | 41.38 ± 0.00 a | |
| a | 4.44 ± 0.00 c | 4.24 ± 0.01 b | 4.11 ± 0.00 a | |
| b | 3.55 ± 0.04 b | 3.36 ± 0.02 a | 3.35 ± 0.02 a | |
| Blackness | 58.54 ± 0.01 a | 58.59 ± 0.00 b | 58.86 ± 0.01 c | |
| Bulk density (g/mL) | 0.54 ± 0.00 c | 0.52 ± 0.00 b | 0.50 ± 0.00 a | |
| Hedonic scores of algal odor | 2.67 ± 0.79 a | 3.70 ± 0.97 b | 4.60 ± 0.84 c | |
| Extraction Yields of Fucoidan | SC1 1 | SC2 1 | SC3 1 | |
| Extraction yield (%) | 0.68 ± 0.07 a | 0.90 ± 0.04 b | 1.08 ± 0.04 c | |
| Chemical Composition | SC1 2 | SC2 2 | SC3 2 |
|---|---|---|---|
| Total sugar (%) | 46.43 ± 0.80 b | 41.74 ± 1.26 a | 56.41 ± 0.35 c |
| Uronic acid (%) | 12.68 ± 0.25 a | 15.83 ± 0.90 b | 23.55 ± 1.99 c |
| Fucose (%) | 28.77 ± 2.02 a b | 25.06 ± 1.75 a | 31.09 ± 1.00 b |
| Sulfate (%) | 23.84 ± 0.08 a | 23.59 ± 0.41 a | 22.08 ± 0.55 a |
| Protein (%) | 5.08 ± 0.32 b | 3.05 ± 0.48 a | 2.79 ± 0.17 a |
| Polyphenols (%) | 3.52 ± 0.12 b | 2.63 ± 0.16 a | 2.77 ± 0.12 a |
| Alginate (%) | 9.54 ± 0.41 a | 9.60 ± 0.68 a | 8.84 ± 0.49 a |
| Sulfate/fucose (molar ratio) 1 | 1.42 ± 0.09 b | 1.62 ± 0.10 c | 1.22 ± 0.04 a |
| Monosaccharide Compositions (Molar Ratio) | SC1 | SC2 | SC3 |
| Fucose | 1 | 1 | 1 |
| Mannose | 0.13 | 0.17 | 0.29 |
| Rhamnose | 0.13 | 0.17 | 0.29 |
| Glucose | ND 3 | ND | ND |
| Glucuronic acid | 0.38 | 0.67 | 0.71 |
| Galactose | 0.75 | 1.16 | 1.00 |
| Xylose | 0.13 | 0.17 | 0.14 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.-N.; Chen, P.-W.; Huang, C.-Y. Compositional Characteristics and In Vitro Evaluations of Antioxidant and Neuroprotective Properties of Crude Extracts of Fucoidan Prepared from Compressional Puffing-Pretreated Sargassum crassifolium. Mar. Drugs 2017, 15, 183. https://doi.org/10.3390/md15060183
Yang W-N, Chen P-W, Huang C-Y. Compositional Characteristics and In Vitro Evaluations of Antioxidant and Neuroprotective Properties of Crude Extracts of Fucoidan Prepared from Compressional Puffing-Pretreated Sargassum crassifolium. Marine Drugs. 2017; 15(6):183. https://doi.org/10.3390/md15060183
Chicago/Turabian StyleYang, Wen-Ning, Po-Wei Chen, and Chun-Yung Huang. 2017. "Compositional Characteristics and In Vitro Evaluations of Antioxidant and Neuroprotective Properties of Crude Extracts of Fucoidan Prepared from Compressional Puffing-Pretreated Sargassum crassifolium" Marine Drugs 15, no. 6: 183. https://doi.org/10.3390/md15060183
APA StyleYang, W.-N., Chen, P.-W., & Huang, C.-Y. (2017). Compositional Characteristics and In Vitro Evaluations of Antioxidant and Neuroprotective Properties of Crude Extracts of Fucoidan Prepared from Compressional Puffing-Pretreated Sargassum crassifolium. Marine Drugs, 15(6), 183. https://doi.org/10.3390/md15060183