Borrelidins C–E: New Antibacterial Macrolides from a Saltern-Derived Halophilic Nocardiopsis sp.
Abstract
:1. Introduction
2. Results
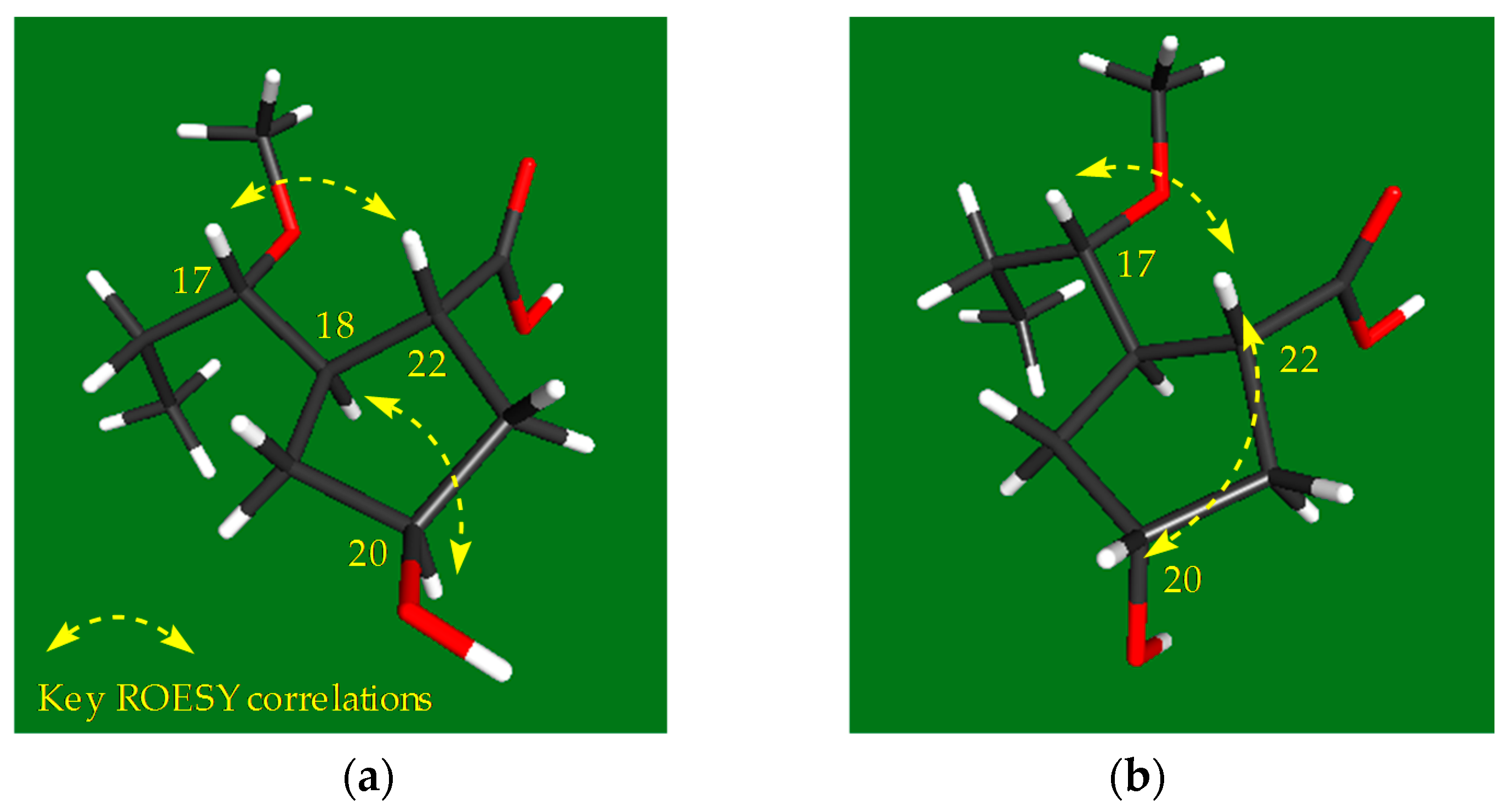
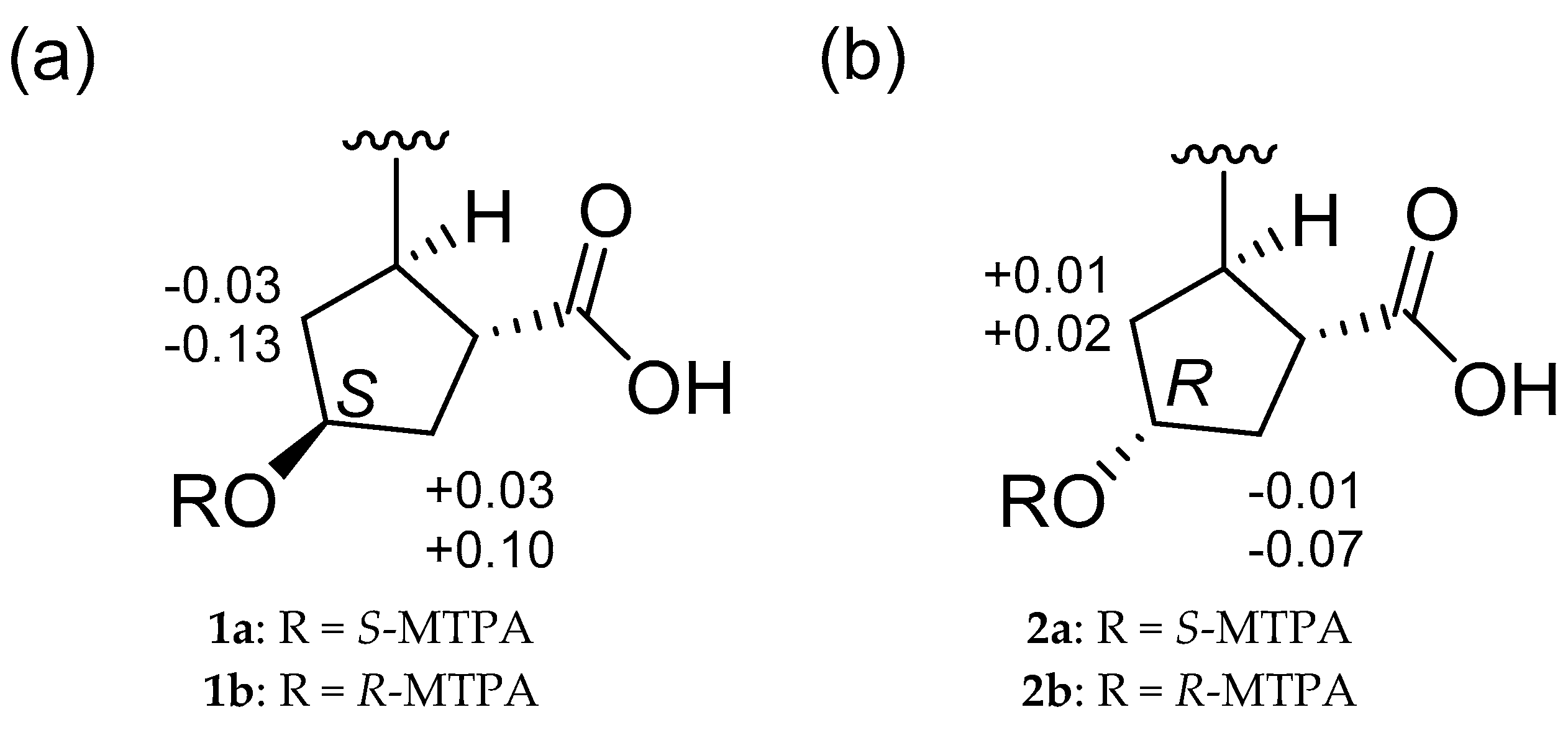
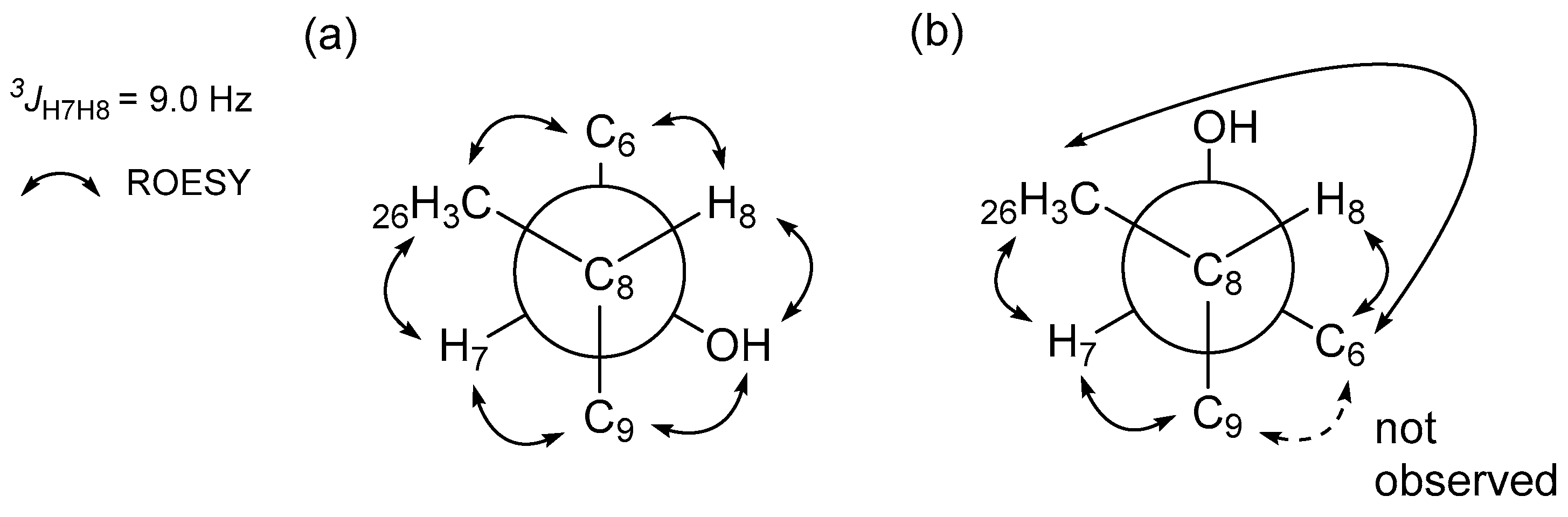
2.1. Structural Elucidation
2.2. Bioactivities of the Borrelidins
3. Experimental Section
3.1. General Experimental Procedures
3.2. Isolation, Cultivation, and Extraction of the Halophilic Actinomycete Strain
3.3. Isolation of the Borrelidins
3.3.1. Borrelidin C (1)
3.3.2. Borrelidin D (2)
3.3.3. Borrelidin E (3)
3.4. MTPA Esterification of Borrelidins C–D (1–2)
3.4.1. Bis-S-MTPA Ester (1a) of Borrelidin C (1)
3.4.2. Bis-R-MTPA Ester (1b) of Borrelidin C (1)
3.4.3. Bis-S-MTPA Ester (2a) of Borrelidin D (2)
3.4.4. Bis-R-MTPA Ester (2b) of Borrelidin D (2)
3.5. Antibacterial Activity Assay
3.6. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jensen, P.R.; Moore, B.S.; Fenical, W. The marine actinomycete genus Salinispora: A model organism for secondary metabolite discovery. Nat. Prod. Rep. 2015, 32, 738–751. [Google Scholar] [CrossRef] [PubMed]
- Fenical, W.; Jensen, P.R. Developing a new resource for drug discovery: Marine actinomycete bacteria. Nat. Chem. Biol. 2006, 2, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.R.; Gontang, E.; Mafnas, C.; Mincer, T.J.; Fenical, W. Culturable marine actinomycete diversity from tropical Pacific Ocean sediments. Environ. Microbiol. 2005, 7, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Duangmal, K.; Suksaard, P.; Pathom-aree, W.; Mingma, R.; Matsumoto, A.; Takahashi, Y. Actinopolyspora salinaria sp. nov., a halophilic actinomycete isolated from solar saltern soil. Int. J. Syst. Evol. Microbiol. 2016, 66, 1660–1665. [Google Scholar] [PubMed]
- Kim, S.-H.; Shin, Y.; Lee, S.K.; Shin, J.; Oh, D.-C.; Lee, S.-H.; Oh, K.-B. Salternamides A-D from a halophilic Streptomyces sp. actinobacterium. J. Nat. Prod. 2015, 78, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Shin, Y.; Lee, S.K.; Shin, J.; Oh, D.-C. Salternamide E from a saltern-derived marine actinomycete Streptomyces sp. Nat. Prod. Sci. 2015, 21, 273–277. [Google Scholar] [CrossRef]
- Bach, D.-H.; Kim, S.-H.; Hong, J.-Y.; Park, H.J.; Oh, D.-C.; Lee, S.K. Salternamide A suppresses hypoxia-induced accumulation of HIF-1α and induces apoptosis in human colorectal cancer cells. Mar. Drugs 2015, 13, 6962–6976. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Ha, T.-K.-Q.; Oh, W.K.; Shin, J.; Oh, D.-C. Antiviral indolosesquiterpenoid xiamycins C-E from a halophilic actinomycete. J. Nat. Prod. 2016, 79, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Jampolsky, L.M.; Goldberg, M.W. Borrelidin, a new antibiotic with antiborrelia activity and penicillin-enhancement properties. Arch. Biochem. 1949, 22, 476–478. [Google Scholar] [PubMed]
- Pretsch, E.; Bȕhlmann, P.; Affolter, C. Structure Determination of Organic Compounds—Tables of Spectral Data; Springer: New York, NY, USA, 2000. [Google Scholar]
- Freire, F.; Seco, J.M.; Quiñoá, E.; Riguera, R. Determining the absolute stereochemistry of secondary/secondary diols by 1H NMR: Basis and applications. J. Org. Chem. 2005, 70, 3778–3790. [Google Scholar] [CrossRef] [PubMed]
- Matsumori, N.; Kaneno, D.; Murata, M.; Nakamura, H.; Tachibana, K. Stereochemical determination of acyclic structures based on carbon-proton spin-coupling constants. A method of configuration analysis for natural products. J. Org. Chem. 1999, 64, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Hurley, D.; McCusker, M.P.; Fanning, S.; Martins, M. Salmonella-host interactions—Modulation of the host innate immune system. Front. Immunol. 2014, 5, 481. [Google Scholar] [CrossRef] [PubMed]
- Schulze, C.J.; Bray, W.M.; Loganzo, F.; Lam, M.-H.; Szal, T.; Villalobos, A.; Koehn, F.E.; Linington, R.G. Borrelidin B: Isolation, biological activity, and implications for nitrile biosynthesis. J. Nat. Prod. 2014, 77, 2570–2574. [Google Scholar] [CrossRef] [PubMed]


| C/H | 1 | 2 | 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| δH a | Mult (J in Hz) | δC b | δH a | Mult (J in Hz) | δC b | δH a | Mult (J in Hz) | δC b | |
| 1 | – | – | 172.4 s | – | – | 172.7 s | – | – | 172.7 s |
| 2 | 2.79 | m | 39.3 t | 2.82 | m | 41.1 t | 2.92 | m | 41.3 t |
| 2.76 | m | 2.80 | m | 2.79 | m | ||||
| 3 | 4.37 | m | 70.8 d | 4.34 | m | 71.2 d | 4.44 | m | 69.8 d |
| 4 | 1.98 | m | 36.5 d | 1.92 | m | 36.9 d | 1.96 | m | 36.8 d |
| 5 | 1.34 | m | 43.9 t | 1.26 | m | 44.9 t | 1.72 | m | 41.5 t |
| 0.95 | m | 0.94 | m | 1.66 | m | ||||
| 6 | 2.10 | m | 27.6 d | 2.00 | m | 27.9 d | 2.37 | m | 32.2 d |
| 7 | 1.07 | m | 48.1 t | 1.05 | m | 48.6 t | 3.26 | br d (9.5) | 81.5 d |
| 1.00 | m | 0.95 | m | ||||||
| 8 | 1.73 | m | 26.8 d | 1.72 | m | 27.1 d | 2.01 | m | 34.4 d |
| 9 | 1.41 | ddd (13.0, 13.0, 2.5) | 37.9 t | 1.38 | ddd (13.0, 13.0, 2.5) | 38.1 t | 1.37 | m | 36.3 t |
| 0.98 | m | 0.99 | m | 1.15 | m | ||||
| 10 | 2.28 | m | 35.6 d | 2.30 | m | 36.0 d | 2.36 | m | 36.1 d |
| 11 | 4.57 | d (9.5) | 72.2 d | 4.58 | d (9.5) | 72.6 d | 4.62 | d (9.5) | 72.7 d |
| 12 | – | – | 120.1 s | – | – | 120.6 s | – | – | 120.6 s |
| 13 | 6.83 | d (11.0) | 143.7 d | 6.76 | d (11.5) | 143.2 d | 6.83 | d (11.0) | 143.4 d |
| 14 | 6.66 | dd (14.0, 11.0) | 127.7 d | 6.68 | dd (14.0, 11.5) | 128.1 d | 6.71 | dd (14.0, 11.0) | 127.9 d |
| 15 | 6.33 | m | 139.1 d | 6.27 | ddd (14.0, 11.0, 4.0) | 139.5 d | 6.33 | ddd (14.0, 11.0, 4.0) | 139.9 d |
| 16 | 2.60 | m | 36.2 t | 2.57 | m | 36.6 t | 2.57 | m | 37.0 t |
| 2.57 | m | 2.49 | m | 2.5 | m | ||||
| 17 | 5.65 | m | 76.7 d | 5.37 | m | 76.8 d | 5.38 | m | 76.8 d |
| 18 | 3.22 | m | 44.7 d | 3.49 | m | 45.1 d | 3.09 | m | 46.8 d |
| 19 | 2.42 | m | 39.1 t | 2.26 | m | 40.8 t | 1.90 | m | 30.3 t |
| 1.80 | m | 1.60 | m | 1.27 | m | ||||
| 20 | 4.79 | m | 72.4 d | 4.59 | m | 73.3 d | 1.79 | m | 26.2 t |
| 1.62 | m | ||||||||
| 21 | 2.46 | m | 41.6 t | 2.38 | m | 40.8 t | 2.14 | m | 32.3 t |
| 2.41 | m | 2.20 | m | 1.98 | m | ||||
| 22 | 3.44 | ddd (8.5, 8.5, 8.5) | 48.3 d | 3.07 | ddd (8.0, 8.0, 8.0) | 49.7 d | 2.89 | ddd (7.5, 7.5, 7.5) | 50.8 d |
| 23 | – | – | 179.4 s | – | – | 181.9 s | – | – | 181.2 s |
| 24 | 0.85 | d (7.0) | 18.3 q | 0.89 | d (7.0) | 18.9 q | 0.92 | d (7.0) | 17.9 q |
| 25 | 0.97 | d (6.5) | 18.6 q | 0.92 | d (6.5) | 18.7 q | 1.18 | d (6.5) | 11.6 q |
| 26 | 0.91 | d (6.5) | 20.6 q | 0.90 | d (6.5) | 21.0 q | 1.31 | d (6.5) | 16.7 q |
| 27 | 1.28 | d (6.5) | 15.4 q | 1.30 | d (6.5) | 15.7 q | 1.37 | d (6.5) | 15.8 q |
| 28 | – | – | 118.3 s | – | – | 118.6 s | – | – | 118.6 s |
| MIC in µM | Gram-Positive | Gram-Negative | |||||
|---|---|---|---|---|---|---|---|
| S. aureus | E. faecalis | E. faecium | P. hauseri | K. pneumoniae | S. enterica | E. coli | |
| Borrelidin C (1) | >250 | 250 | >250 | >250 | >250 | 16 | >250 |
| Borrelidin D (2) | >250 | >250 | >250 | >250 | >250 | 63 | >250 |
| Borrelidin E (3) | 250 | >250 | >250 | >250 | >250 | 250 | >250 |
| Borrelidin (4) | >260 | 33 | 65 | 16 | 65 | 0.51 | 260 |
| Ampicillin | 0.37 | 5.7 | 5.7 | 0.37 | >367 | 1.4 | 23 |
| IC50 in µM | A549 | HCT116 | SNU638 | SK-HEP1 | MDA-MB231 | K562 |
|---|---|---|---|---|---|---|
| Borrelidin C (1) | 9.1 | 10 | 5.5 | 63 | 96 | 5.7 |
| Borrelidin D (2) | 12 | 15 | 8.7 | 71 | 64 | 6.7 |
| Borrelidin E (3) | >100 | >100 | >100 | >100 | >100 | >100 |
| Borrelidin (4) | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 |
| Etoposide | 0.68 | 14 | 0.57 | 8.7 | 5.4 | 1.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Shin, D.; Kim, S.-H.; Park, W.; Shin, Y.; Kim, W.K.; Lee, S.K.; Oh, K.-B.; Shin, J.; Oh, D.-C. Borrelidins C–E: New Antibacterial Macrolides from a Saltern-Derived Halophilic Nocardiopsis sp. Mar. Drugs 2017, 15, 166. https://doi.org/10.3390/md15060166
Kim J, Shin D, Kim S-H, Park W, Shin Y, Kim WK, Lee SK, Oh K-B, Shin J, Oh D-C. Borrelidins C–E: New Antibacterial Macrolides from a Saltern-Derived Halophilic Nocardiopsis sp. Marine Drugs. 2017; 15(6):166. https://doi.org/10.3390/md15060166
Chicago/Turabian StyleKim, Jungwoo, Daniel Shin, Seong-Hwan Kim, Wanki Park, Yoonho Shin, Won Kyung Kim, Sang Kook Lee, Ki-Bong Oh, Jongheon Shin, and Dong-Chan Oh. 2017. "Borrelidins C–E: New Antibacterial Macrolides from a Saltern-Derived Halophilic Nocardiopsis sp." Marine Drugs 15, no. 6: 166. https://doi.org/10.3390/md15060166
APA StyleKim, J., Shin, D., Kim, S.-H., Park, W., Shin, Y., Kim, W. K., Lee, S. K., Oh, K.-B., Shin, J., & Oh, D.-C. (2017). Borrelidins C–E: New Antibacterial Macrolides from a Saltern-Derived Halophilic Nocardiopsis sp. Marine Drugs, 15(6), 166. https://doi.org/10.3390/md15060166






