The Effect of Phloroglucinol, A Component of Ecklonia cava Extract, on Hepatic Glucose Production
Abstract
:1. Introduction
2. Results
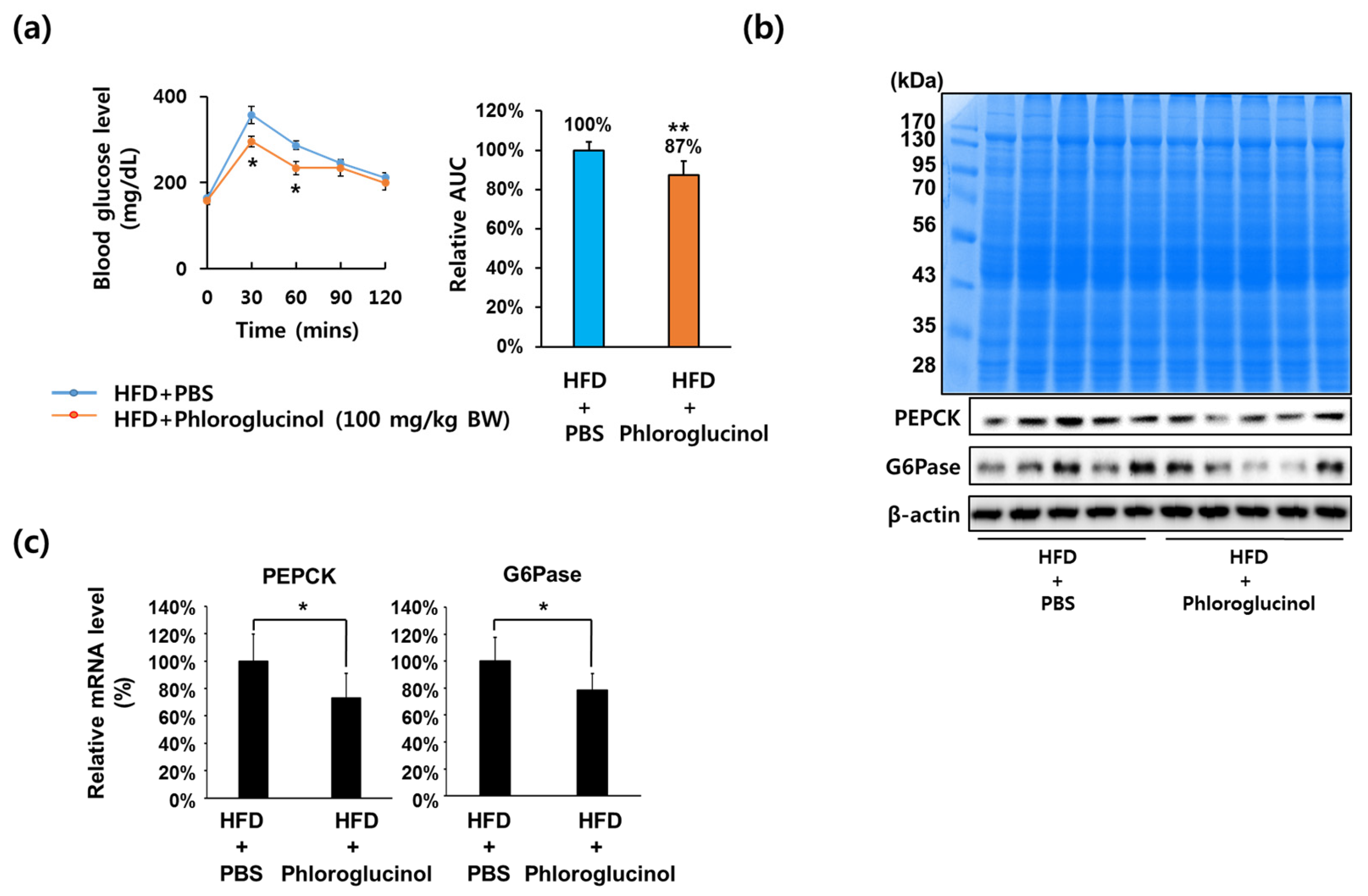
2.1. Administration of Phloroglucinol Improved Glucose Tolerance and Reduced PEPCK and G6Pase Expression Levels in HFD-Induced Obese Mice
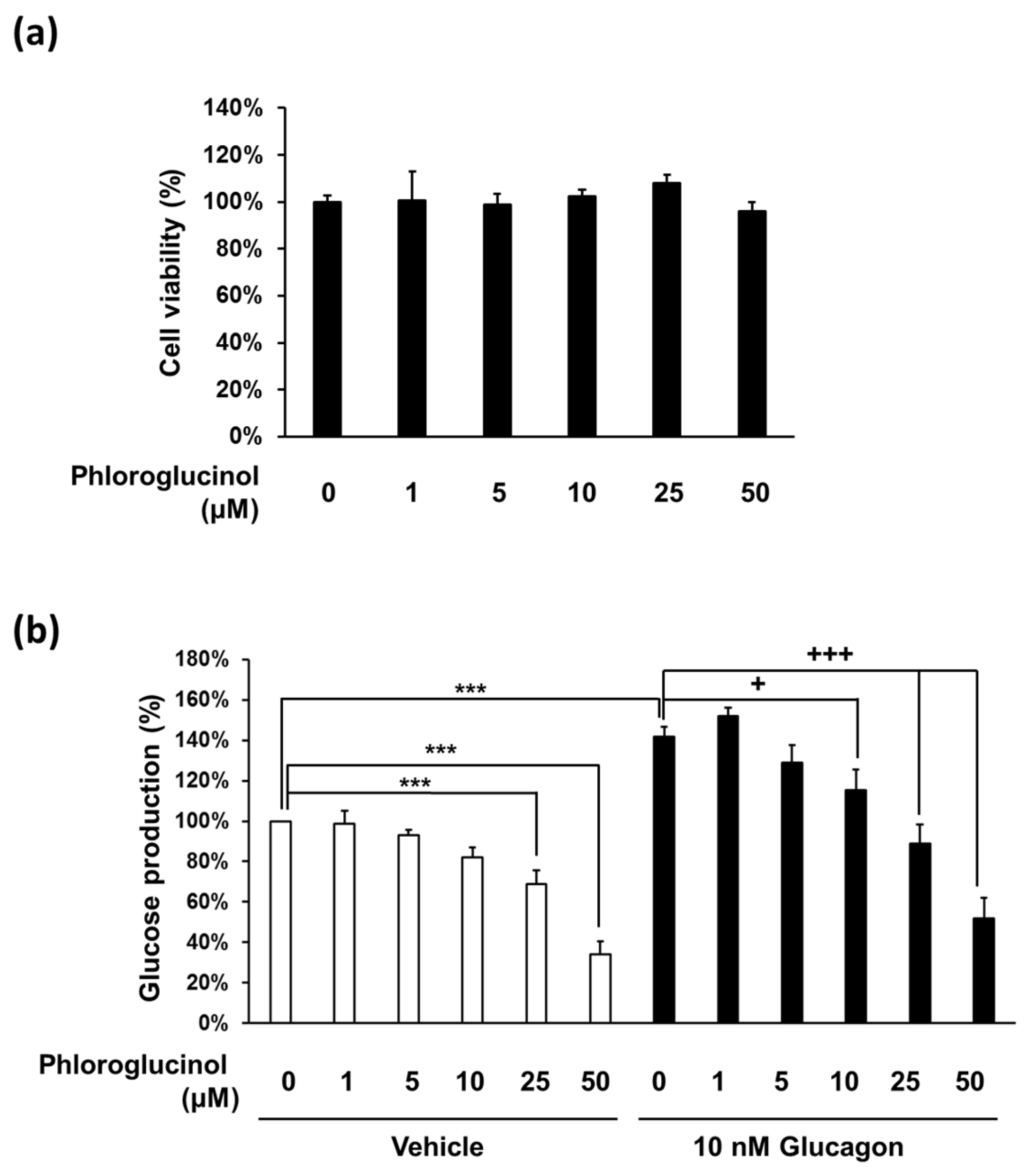
2.2. Phloroglucinol Reduced the Glucose Production in Mouse Primary Hepatocyte
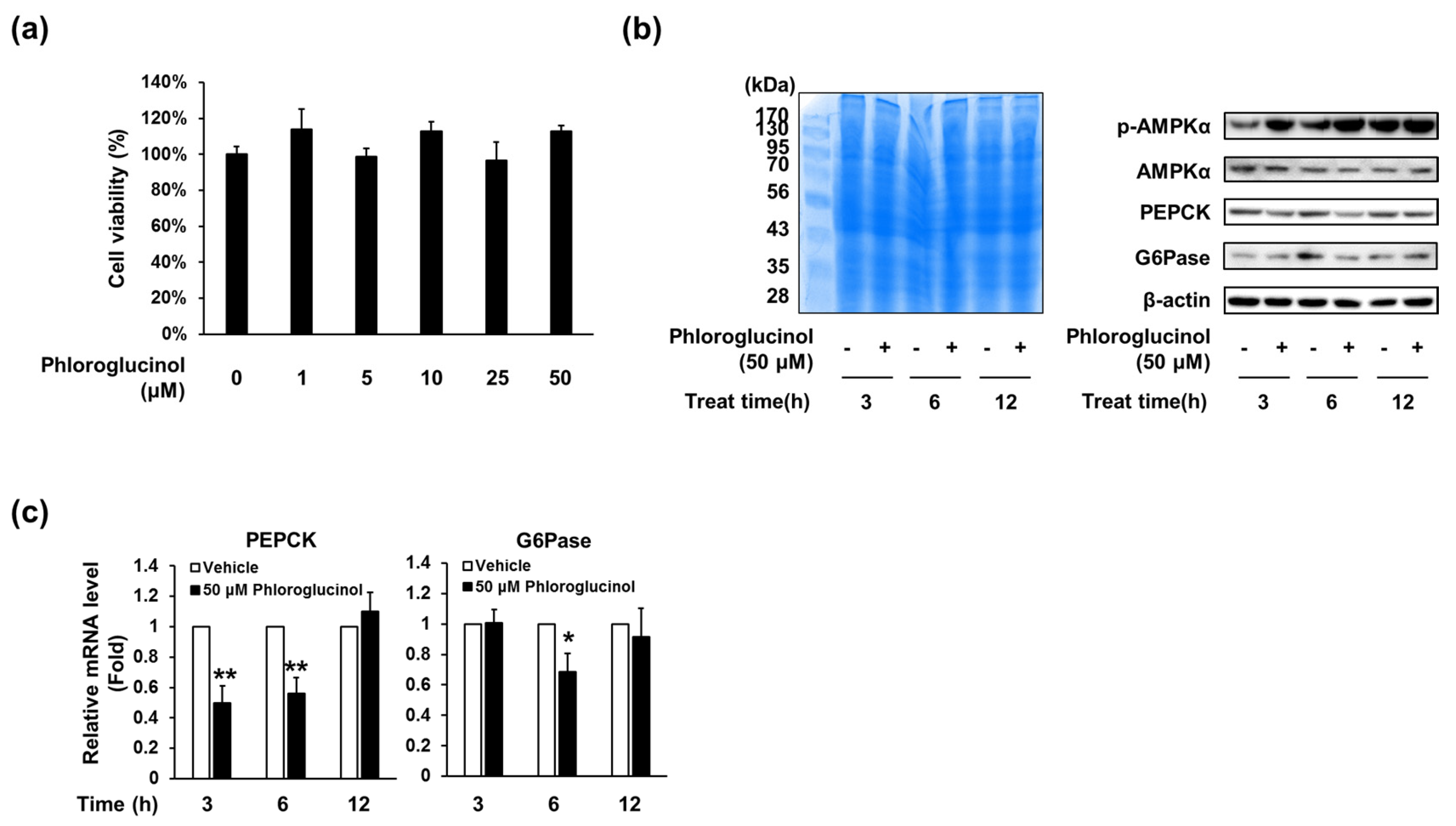
2.3. Phloroglucinol Decreased PEPCK and G6Pase Gene and Protein Expression in HepG2 Cells
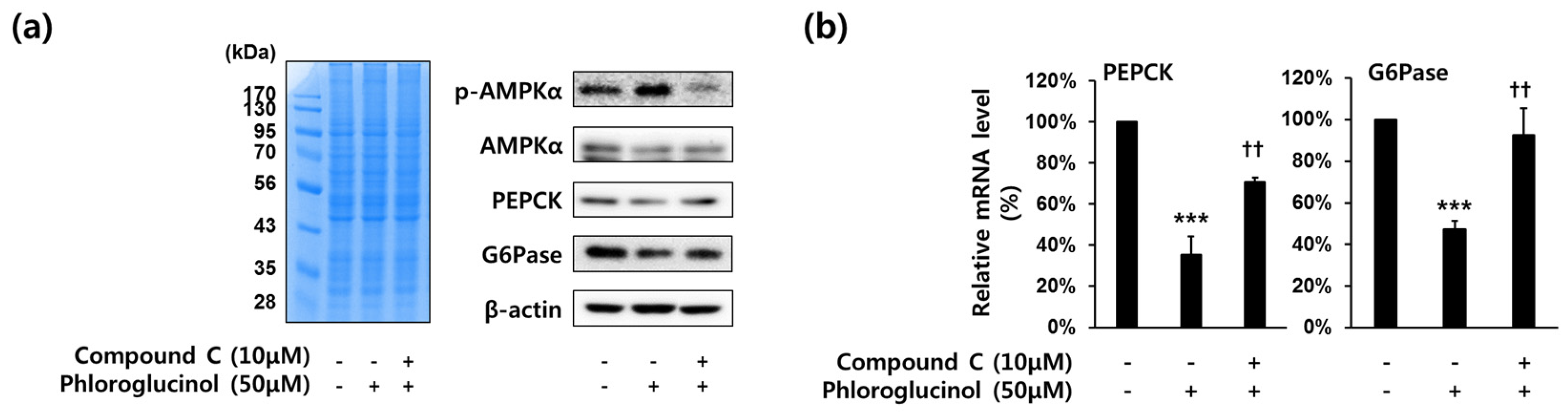
2.4. Decrease of PEPCK and G6Pase Gene Expressions by Phloroglucinol in HepG2 Cells Was Mediated by AMPKα Activation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals and Administration of Phloroglucinol to HFD-Induced Obese Mice
4.3. Glucose Tolerance Tests
4.4. Isolation of Mouse Primary Hepatocytes
4.5. Measurement of Hepatic Glucose Production
4.6. Cell Culture and Viability Assay
4.7. RT-qPCR Analysis
4.8. Western Blot Analysis
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bogardus, C.; Lillioja, S.; Howard, B.V.; Reaven, G.; Mott, D. Relationships between insulin secretion, insulin action, and fasting plasma glucose concentration in nondiabetic and noninsulin-dependent diabetic subjects. J. Clin. Investig. 1984, 74, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Stumvoll, M.; Nurjhan, N.; Perriello, G.; Dailey, G.; Gerich, J.E. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N. Engl. J. Med. 1995, 333, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Nordlie, R.C.; Foster, J.D.; Lange, A.J. Regulation of glucose production by the liver. Annu. Rev. Nutr. 1999, 19, 379–406. [Google Scholar] [CrossRef] [PubMed]
- Pilkis, S.J.; Granner, D.K. Molecular physiology of the regulation of hepatic gluconeogenesis and glycolysis. Annu. Rev. Physiol. 1992, 54, 885–909. [Google Scholar] [CrossRef] [PubMed]
- Gastaldelli, A.; Baldi, S.; Pettiti, M.; Toschi, E.; Camastra, S.; Natali, A.; Landau, B.R.; Ferrannini, E. Influence of obesity and type 2 diabetes on gluconeogenesis and glucose output in humans: A quantitative study. Diabetes 2000, 49, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, W.A.; Jeon, Y.J. Exploiting biological activities of brown seaweed ecklonia cava for potential industrial applications: A review. Int. J. Food Sci. Nutr. 2012, 63, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Jung, H.J.; Jeong, H.Y.; Kwon, H.J.; Ali, M.Y.; Choi, J.S. Phlorotannins isolated from the edible brown alga ecklonia stolonifera exert anti-adipogenic activity on 3t3-l1 adipocytes by downregulating c/ebp alpha and ppar gamma. Fitoterapia 2014, 92, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Han, J.S.; Heo, S.J.; Hwang, J.Y.; Jeon, Y.J. Protective effects of dieckol isolated from ecklonia cava against high glucose-induced oxidative stress in human umbilical vein endothelial cells. Toxicol. In Vitro 2010, 24, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Satake, N.; Yamashita, H.; Tamura, A.; Sasaki, M.; Matsui-Yuasa, I.; Tabuchi, M.; Akahoshi, Y.; Terada, M.; Kojima-Yuasa, A. Ecklonia cava polyphenol protects the liver against ethanol-induced injury in rats. Biochim. Biophys. Acta 2012, 1820, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Jin, Y.B.; Lee, H.; Cha, M.; Sohn, E.T.; Moon, J.; Park, C.; Chun, S.; Jung, E.S.; Hong, J.S.; et al. Brown alga ecklonia cava attenuates type 1 diabetes by activating ampk and akt signaling pathways. Food Chem. Toxicol. 2010, 48, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Min, K.H.; Han, J.S.; Lee, D.H.; Park, D.B.; Jung, W.K.; Park, P.J.; Jeon, B.T.; Kim, S.K.; Jeon, Y.J. Effects of brown alga, ecklonia cava on glucose and lipid metabolism in C57BL/KsJ-db/db mice, a model of type 2 diabetes mellitus. Food Chem. Toxicol. 2012, 50, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Park, E.Y.; Kim, E.H.; Kim, M.H.; Seo, Y.W.; Lee, J.I.; Jun, H.S. Polyphenol-rich fraction of brown alga ecklonia cava collected from gijang, korea, reduces obesity and glucose levels in high-fat diet-induced obese mice. Evid.-Based Complement. Altern. Med. 2012, 2012, 418912. [Google Scholar] [CrossRef] [PubMed]
- Park, E.Y.; Choi, H.; Yoon, J.Y.; Lee, I.Y.; Seo, Y.; Moon, H.S.; Hwang, J.H.; Jun, H.S. Polyphenol-rich fraction of ecklonia cava improves nonalcoholic fatty liver disease in high fat diet-fed mice. Mar. Drugs 2015, 13, 6866–6883. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C.; Chang, H.H.; Chan, C.P.; Chou, H.Y.; Chang, B.E.; Yeung, S.Y.; Wang, T.M.; Jeng, J.H. Antiplatelet effect of phloroglucinol is related to inhibition of cyclooxygenase, reactive oxygen species, erk/p38 signaling and thromboxane a(2) production. Toxicol. Appl. Pharm. 2012, 263, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.P.; Sidana, J.; Bansal, P.; Foley, W.J. Phloroglucinol compounds of therapeutic interest: Global patent and technology status. Expert Opin. Ther. Pat. 2009, 19, 847–866. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.P.; Bharate, S.B. Phloroglucinol compounds of natural origin. Nat. Prod. Rep. 2006, 23, 558–591. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.; Bing, S.J.; Cho, J.; Ahn, G.; Kim, D.S.; Al-Amin, M.; Park, S.J.; Jee, Y. Phloroglucinol protects small intestines of mice from ionizing radiation by regulating apoptosis-related molecules: A comparative immunohistochemical study. J. Histochem. Cytochem. 2013, 61, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.A.; Lee, K.H.; Chae, S.; Zhang, R.; Jung, M.S.; Ham, Y.M.; Baik, J.S.; Lee, N.H.; Hyun, J.W. Cytoprotective effect of phloroglucinol on oxidative stress induced cell damage via catalase activation. J. Cell Biochem. 2006, 97, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Chapman, E.E.; Langler, R.F.; Crandall, I. Phloroglucinol sulfonic acid esters as antimalarial/anticancer agents. J. Sulfur Chem. 2008, 29, 607–618. [Google Scholar] [CrossRef]
- Jeong, K.J.; Kim do, Y.; Quan, H.Y.; Jo, H.K.; Kim, G.W.; Chung, S.H. Effects of eugenol on hepatic glucose production and ampk signaling pathway in hepatocytes and c57bl/6j mice. Fitoterapia 2014, 93, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jeon, Y.J. Anti-diabetic effects of brown algae derived phlorotannins, marine polyphenols through diverse mechanisms. Fitoterapia 2013, 86, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Hou, M.F.; Huang, H.W.; Chang, F.R.; Yeh, C.C.; Tang, J.Y.; Chang, H.W. Marine algal natural products with anti-oxidative, anti-inflammatory, and anti-cancer properties. Cancer Cell Int. 2013, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A. An overview of the medical applications of marine skeletal matrix proteins. Mar. Drugs 2016, 14, 167. [Google Scholar] [CrossRef] [PubMed]
- Unnikrishnan, S.P.; Jayasri, A.M. Marine algae as a prospective source for antidiabetic compounds—A brief review. Curr. Diabetes Rev. 2016, in press. [Google Scholar]
- Nwosu, F.; Morris, J.; Lund, V.A.; Stewart, D.; Ross, H.A.; McDougall, G.J. Anti-proliferative and potential anti-diabetic effects of phenolic-rich extracts from edible marine algae. Food Chem. 2011, 126, 1006–1012. [Google Scholar] [CrossRef]
- Kang, K.A.; Bu, H.D.; Park, D.S.; Go, G.M.; Jee, Y.; Shin, T.; Hyun, J.W. Antioxidant activity of ethanol extract of callophyllis japonica. Phytother. Res. 2005, 19, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.I.; Yeh, C.C.; Lee, J.C.; Yi, S.C.; Huang, H.W.; Tseng, C.N.; Chang, H.W. Aqueous extracts of the edible gracilaria tenuistipitata are protective against h2o2-induced DNA damage, growth inhibition, and cell cycle arrest. Molecules 2012, 17, 7241–7254. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante-Silva, L.H.; da Matta, C.B.; de Araujo, M.V.; Barbosa-Filho, J.M.; de Lira, D.P.; de Oliveira Santos, B.V.; de Miranda, G.E.; Alexandre-Moreira, M.S. Antinociceptive and anti-inflammatory activities of crude methanolic extract of red alga bryothamnion triquetrum. Mar. Drugs 2012, 10, 1977–1992. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wang, F.; Wang, Z.; Lv, W.; Wang, W.; Wang, Y. Glucose uptake activities of bis (2, 3-dibromo-4, 5-dihydroxybenzyl) ether, a novel marine natural product from red alga odonthaliacorymbifera with protein tyrosine phosphatase 1b inhibition, in vitro and in vivo. PLoS ONE 2016, 11, e0147748. [Google Scholar] [CrossRef] [PubMed]
- Unnikrishnan, P.S.; Jayasri, M.A. Antidiabetic studies of chaetomorpha antennina extract using experimental models. J. Appl. Phycol. 2016, 1–10. [Google Scholar] [CrossRef]
- Lee, S.H.; Ko, S.C.; Kang, M.C.; Lee, D.H.; Jeon, Y.J. Octaphlorethol a, a marine algae product, exhibits antidiabetic effects in type 2 diabetic mice by activating amp-activated protein kinase and upregulating the expression of glucose transporter 4. Food Chem. Toxicol. 2016, 91, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Eo, H.; Jeon, Y.J.; Lee, M.; Lim, Y. Brown alga ecklonia cava polyphenol extract ameliorates hepatic lipogenesis, oxidative stress, and inflammation by activation of ampk and sirt1 in high-fat diet-induced obese mice. J. Agric. Food Chem. 2015, 63, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Han, J.S. Phloroglucinol protects ins-1 pancreatic beta-cells against glucotoxicity-induced apoptosis. Phytother. Res. 2015, 29, 1700–1706. [Google Scholar] [CrossRef] [PubMed]
- Srilatha, B.R.; Ananda, S. Antidiabetic effects of mukia maderaspatana and its phenolics: An in vitro study on gluconeogenesis and glucose uptake in rat tissues. Pharm. Biol. 2014, 52, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Viollet, B.; Foretz, M.; Guigas, B.; Horman, S.; Dentin, R.; Bertrand, L.; Hue, L.; Andreelli, F. Activation of amp-activated protein kinase in the liver: A new strategy for the management of metabolic hepatic disorders. J. Physiol. 2006, 574, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Han, H.S.; Kang, G.; Kim, J.S.; Choi, B.H.; Koo, S.H. Regulation of glucose metabolism from a liver-centric perspective. Exp. Mol. Med 2016, 48, e218. [Google Scholar] [CrossRef] [PubMed]
| Gene Symbol | Sequences | |
|---|---|---|
| Human Cyclophilin B | Sense | 5′ TGCCATCGCCAAGGAGTAG 3′ |
| Anti-sense | 3′ TGCACAGACGGTCACTCAAA 5′ | |
| Human PEPCK | Sense | 5′ TGAAAGGCCTGGGGCACAT 3′ |
| Anti-sense | 3′ TTGCTTCAAGGCAAGGATCTCT 5′ | |
| Human G6Pase | Sense | 5′ TCATCTTGGTGTCCGTGATCG 3′ |
| Anti-sense | 3′ TTTATCAGGGGCACGGAAGTG 5′ | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, J.-Y.; Choi, H.; Jun, H.-S. The Effect of Phloroglucinol, A Component of Ecklonia cava Extract, on Hepatic Glucose Production. Mar. Drugs 2017, 15, 106. https://doi.org/10.3390/md15040106
Yoon J-Y, Choi H, Jun H-S. The Effect of Phloroglucinol, A Component of Ecklonia cava Extract, on Hepatic Glucose Production. Marine Drugs. 2017; 15(4):106. https://doi.org/10.3390/md15040106
Chicago/Turabian StyleYoon, Ji-Young, Hojung Choi, and Hee-Sook Jun. 2017. "The Effect of Phloroglucinol, A Component of Ecklonia cava Extract, on Hepatic Glucose Production" Marine Drugs 15, no. 4: 106. https://doi.org/10.3390/md15040106
APA StyleYoon, J.-Y., Choi, H., & Jun, H.-S. (2017). The Effect of Phloroglucinol, A Component of Ecklonia cava Extract, on Hepatic Glucose Production. Marine Drugs, 15(4), 106. https://doi.org/10.3390/md15040106






