Fluorescent Property of Chitosan Oligomer and Its Application as a Metal Ion Sensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
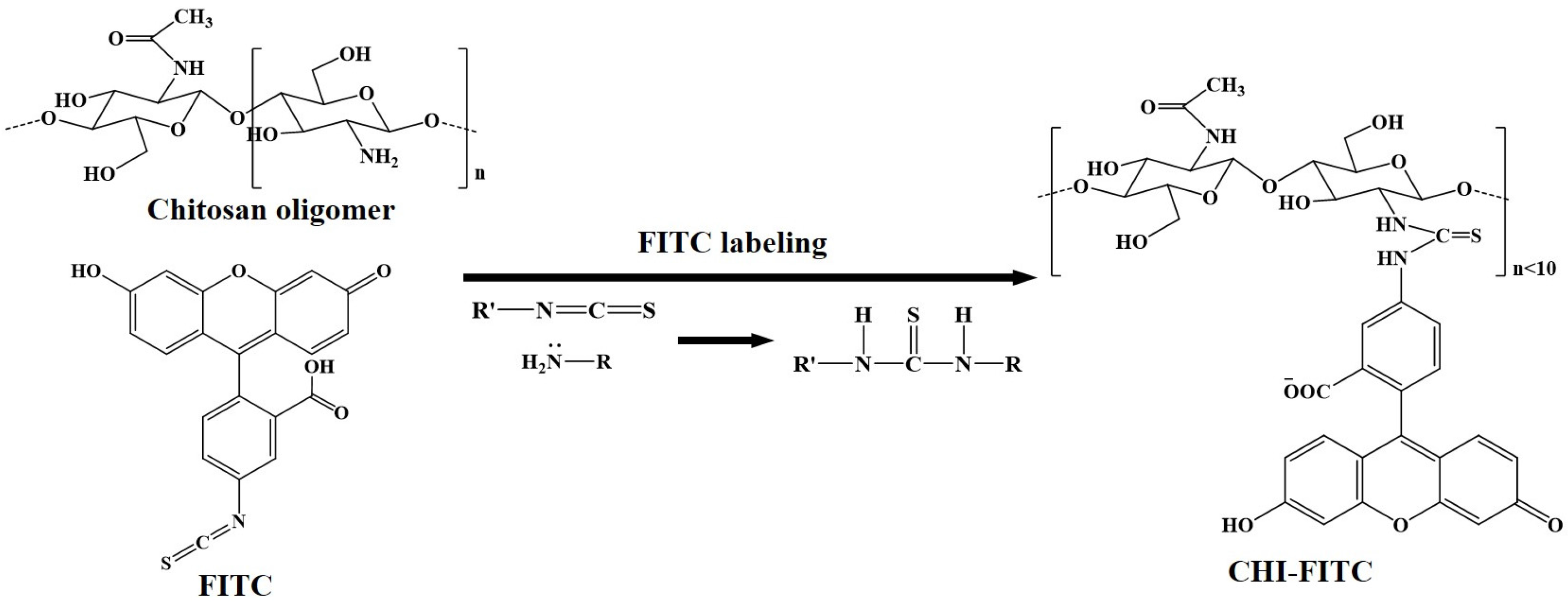
2.2. Preparation of CHI Oligomer-FITC Complexes
2.3. Structural Analyses of the CHI Oligomer and CHI Oligomer-FITC Complexes
2.4. Fluorescent Analyses of the CHI Oligomer and CHI Oligomer-FITC Complexes
2.5. Adsorption Behaviors of Metal Ions onto the CHI Oligomer-FITC Complex
3. Results and Discussion
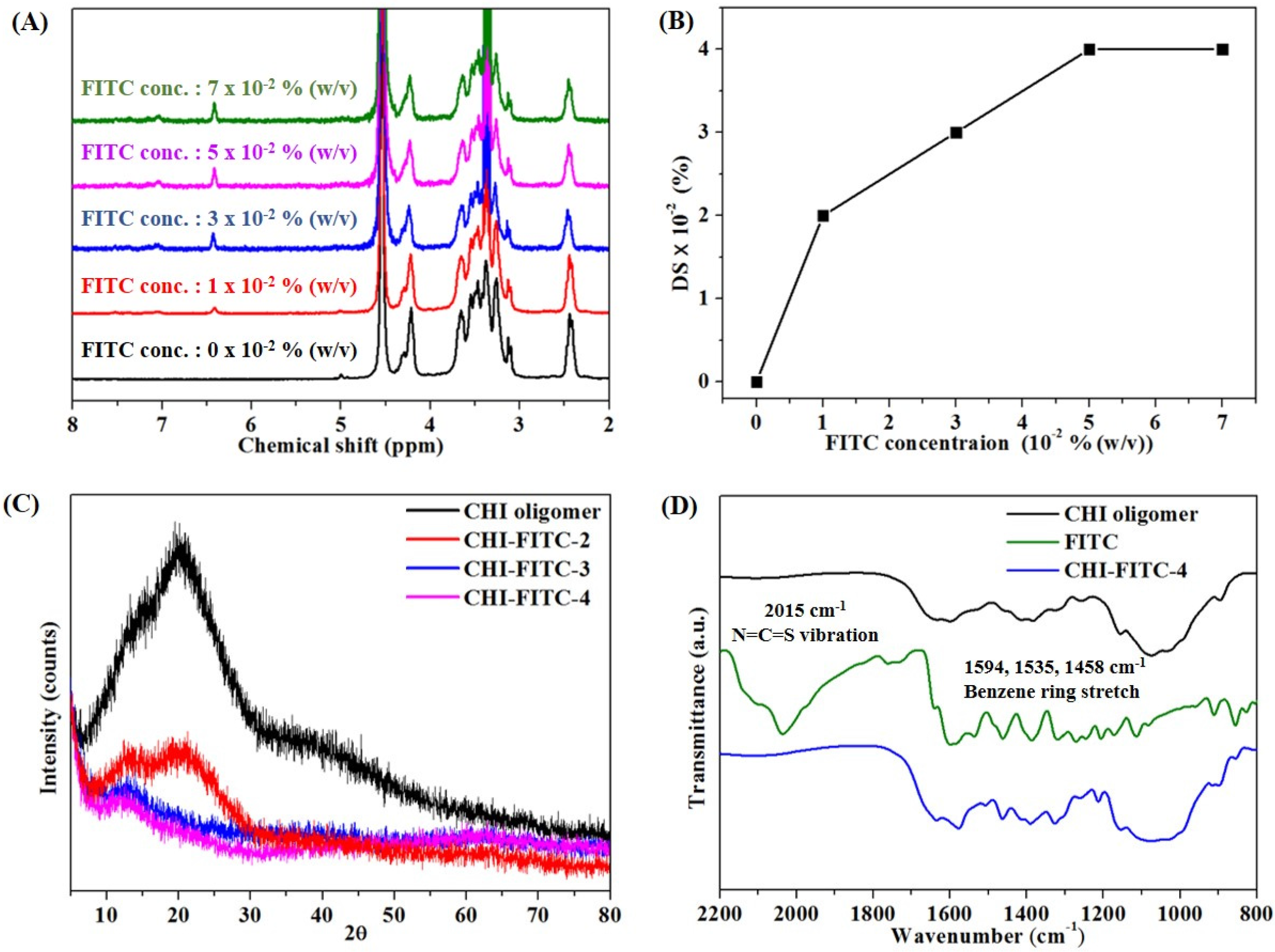
3.1. Structural and Fluorescent Analyses of the CHI Oligomer
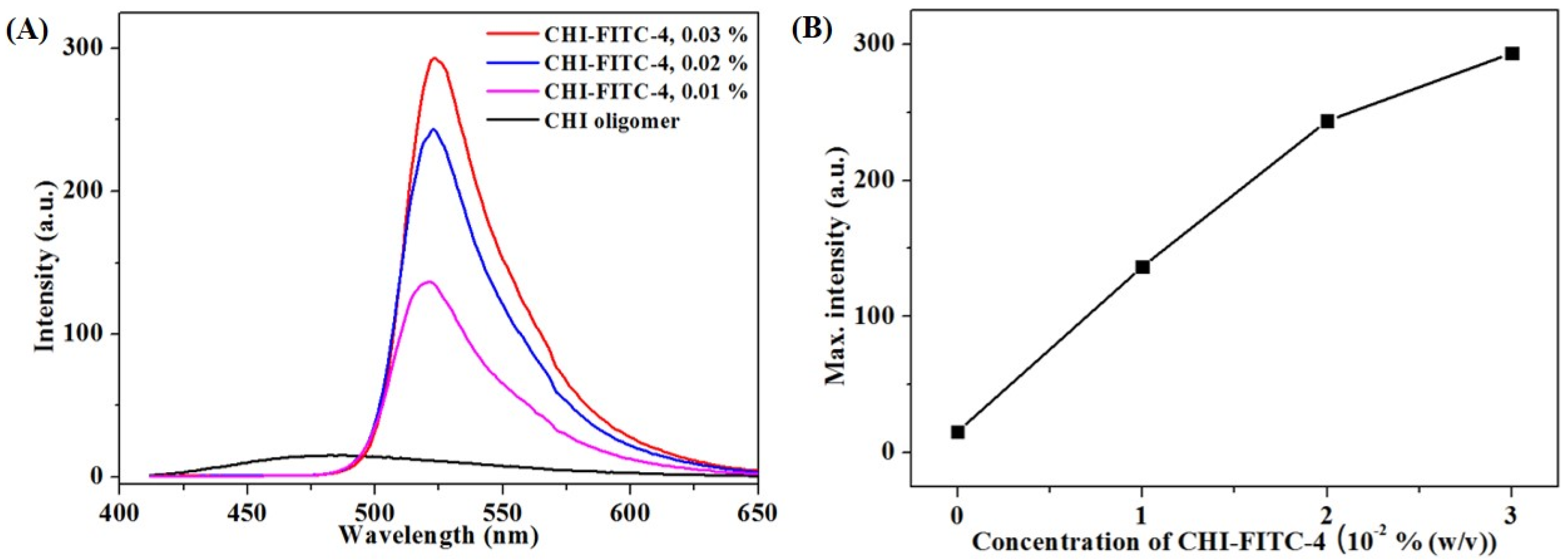
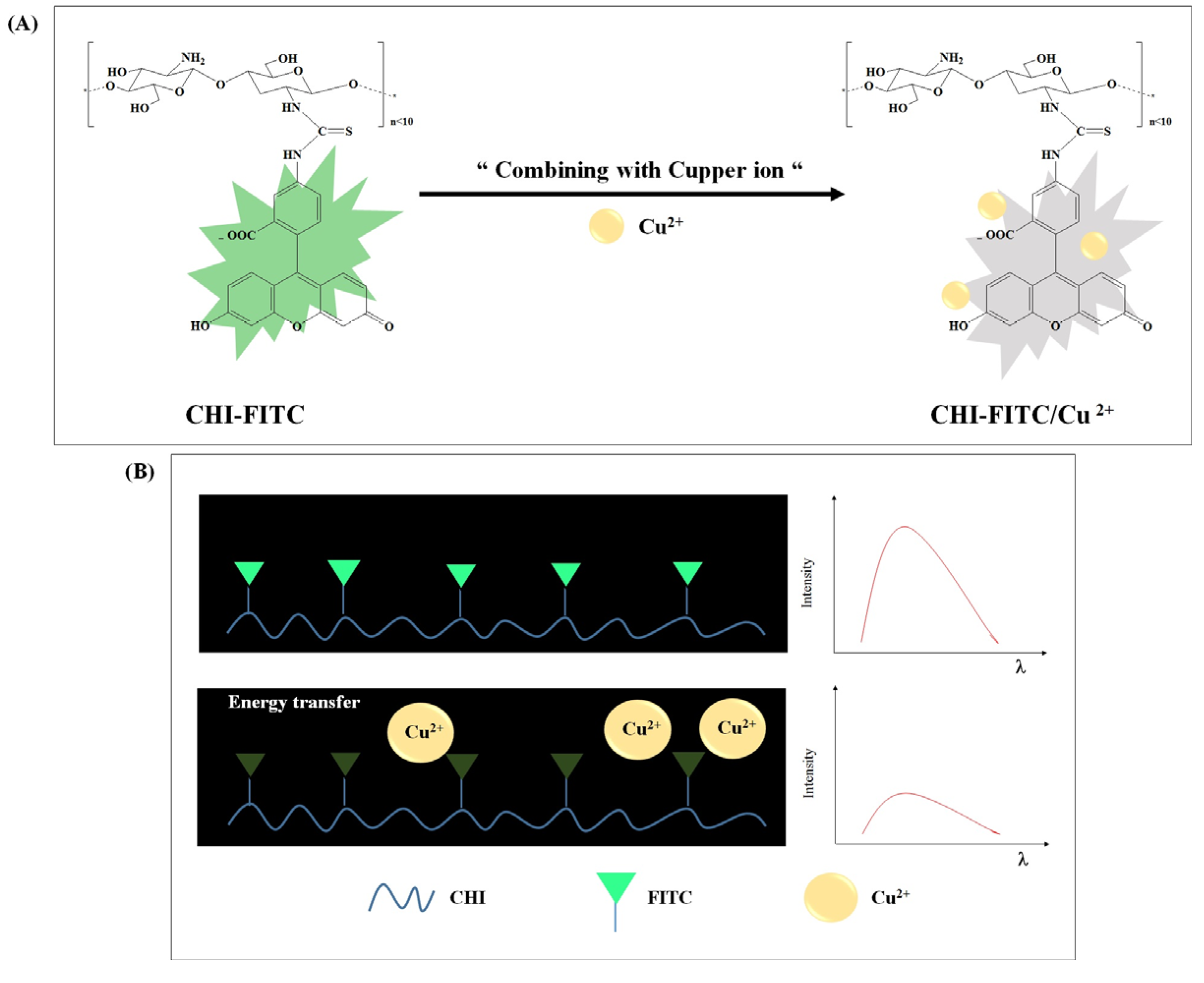
3.2. Structural and Fluorescent Analyses of the CHI Oligomer-FITC Complexes
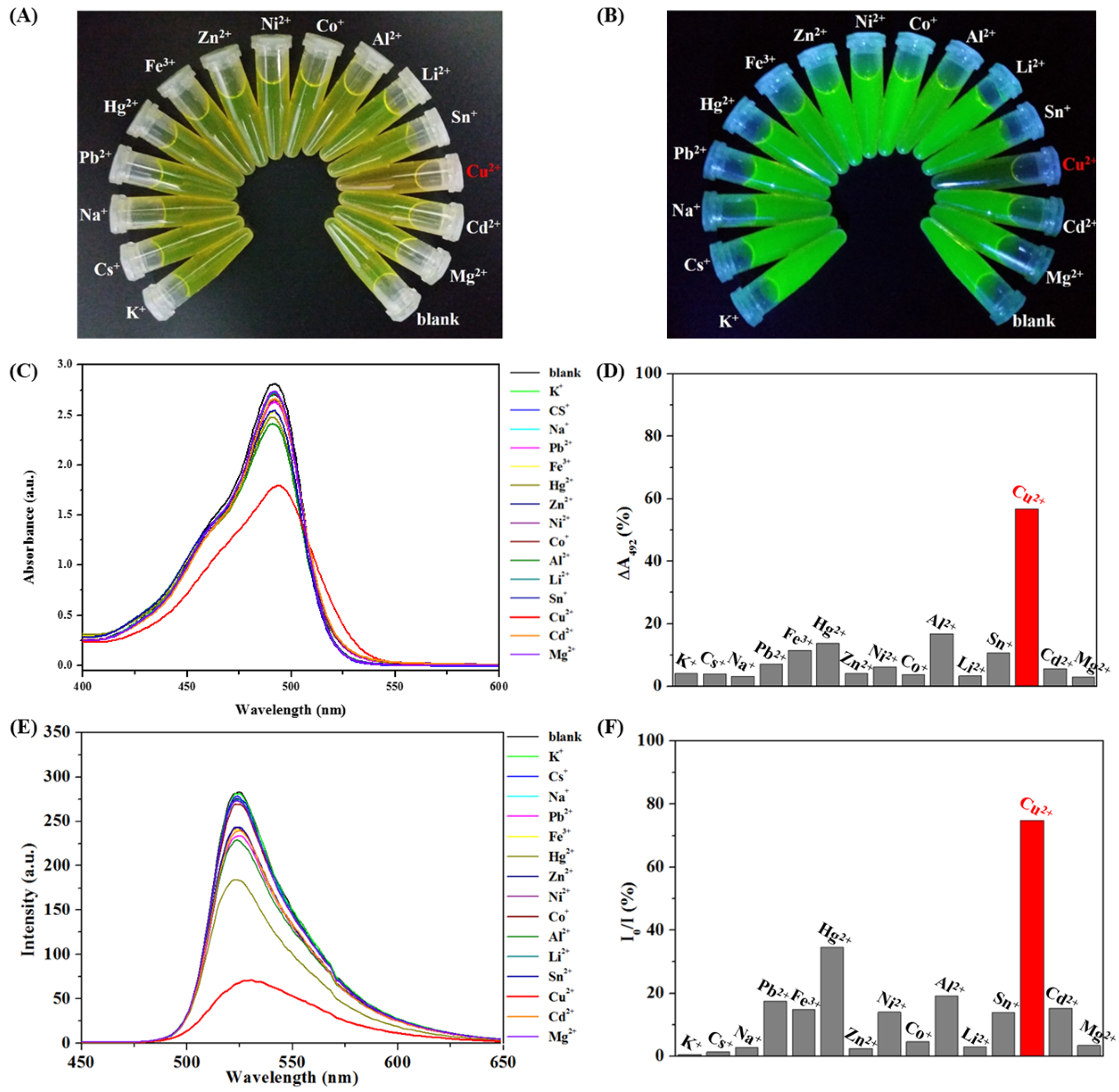
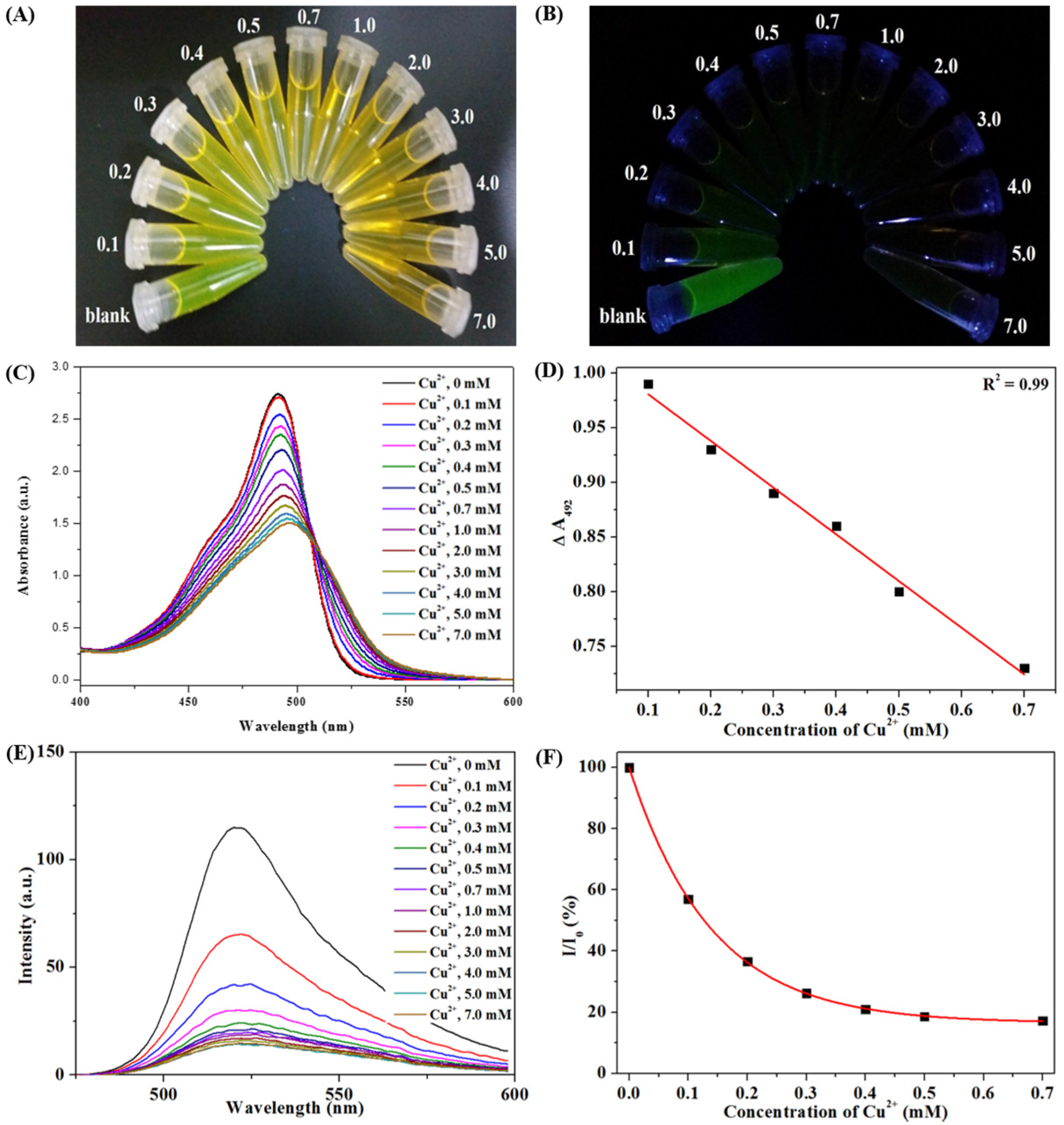
3.3. Colorimetric Sensing of CHI-FITC-4 against Metal Ions
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Ilium, L. Chitosan and its use as a pharmaceutical excipient. Pharm. Res. 1998, 15, 1326–1331. [Google Scholar] [CrossRef]
- Agrawal, P.; Strijkers, G.J.; Nicolay, K. Chitosan-based systems for molecular imaging. Adv. Drug Deliv. Rev. 2010, 62, 42–58. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Pan, X.; Ren, W.; Gu, L.; Wang, G.; Liu, Y. Photoluminescence from chitosan for bio-imaging. Aust. J. Chem. 2014, 67, 1422–1426. [Google Scholar] [CrossRef]
- Cormode, D.P.; Skajaa, T.; Fayad, Z.A.; Mulder, J.M. Nanotechnology in medical imaging probe design and applications. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Gonil, P.; Sajomsang, W.; Ruktanonchai, U.R.; Ubol, P.N.; Treetong, A.; Opanasopit, P.; Puttipipatkhachorn, S. Synthesis and fluorescence properties of N‑substituted 1‑cyanobenz[f]isoindole chitosan polymers and nanoparticles for live cell Imaging. Biomacromolecules 2014, 15, 2879–2888. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, F.; Chen, S.; Zhao, Q.; Liao, B.; Long, Y.; Zeng, Y.; Xia, X. Enhanced fluorescence of chitosan based on size change of micelles and application to directly selective detecting Fe3+ in humanserum. Biosens. Bioelectron. 2013, 42, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Qaqish, R.; Amiji, M. Synthesis of a fluorescent chitosan derivative and its application for the study of chitosan-mucin interactions. Carbohydr. Polym. 1999, 38, 99–107. [Google Scholar] [CrossRef]
- Keana, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I.; Halliwell, B.; Gajewski, E.; Dizdaroglu, M. Copper-ion-dependent damage to the bases in DNA in the presence of hydrogen peroxide. Biochem. J. 1991, 273, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Kobal, A.B.; Horvat, M.; Prezelj, M.; Briski, A.S.; Krsnik, M.; Dizdarevic, T.; Mazej, D.; Falnoga, I.; Stibilj, V.; Arneric, N.; et al. The impact of long-term past exposure to elemental mercury on antioxidative capacity and lipid peroxidation in mercury miners. J. Trace Elem. Med. Biol. 2004, 17, 261–274. [Google Scholar] [CrossRef]
- Gumpu, M.B.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. A review on detection of heavy metal ions in water-an electrochemical approach. Sens. Actuators B Chem. 2015, 213, 515–533. [Google Scholar] [CrossRef]
- Jameson, D.M.; Croney, J.C.; Moens, P.D. Fluorescence: Basic concepts, practical aspects, and some anecdotes. Methods Enzymol. 2003, 360, 1–43. [Google Scholar] [PubMed]
- Pan, X.; Wang, G.; Lay, C.L.; Tan, B.H.; He, C.; Liu, Y. Photoluminescence from amino-containing polymer in the presence of CO2: Carbamato anion formed as a fluorophore. Sci. Rep. 2013, 3, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lee, S.; Liu, Y.; Lee, M.; Yin, J.; Sessler, J.L.; Yoon, J. Anion-activated, thermoreversible gelation system for the capture, release, and visual monitoring of CO2. Sci. Rep. 2014, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dulkeith, E.; Morteani, A.C.; Niedereichholz, T.; Klar, T.A.; Feldmann, J. Fluorescence quenching of dye molecules near gold nanoparticles: Radiative and nonradiative effects. Phys. Rev. Lett. 2002, 89, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Serdyuk, O.V.; Heckel, C.M.; Tsogoeva, S.B. Bifunctional primary amine-thioureas in asymmetric organocatalysis. Org. Biomol. Chem. 2013, 11, 7051–7071. [Google Scholar] [CrossRef] [PubMed]
- Lavertu, M.; Xia, Z.; Serreqi, A.N.; Berrada, M.; Rodrigues, A.; Wang, D.; Buschmann, M.D.; Gupta, A. A validated 1H NMR method for the determination of the degree of deacetylation of chitosan. J. Pharm. Biomed. Anal. 2003, 32, 1149–1158. [Google Scholar] [CrossRef]
- Clark, G.L.; Smith, A.F. X-ray diffraction studies of chitin, chitosan, and derivatives. J. Phys. Chem. 1936, 40, 863–879. [Google Scholar] [CrossRef]
- Duarte, M.L.; Ferreira, M.C.; Marvão, M.R.; Rocha, J. An optimised method to determine the degree of acetylation of chitin and chitosan by FTIR spectroscopy. Int. J. Biol. Macromol. 2002, 31, 1–8. [Google Scholar] [CrossRef]
- Qiao, Y.; Zheng, X. Highly sensitive detection of copper ions by densely grafting fluorescein inside polyethyleneimine core-silica shell nanoparticles. Analyst 2015, 140, 8186–8193. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.M.; Kim, M.H.; Yoon, Y.I.; Park, W.H. Fluorescent Property of Chitosan Oligomer and Its Application as a Metal Ion Sensor. Mar. Drugs 2017, 15, 105. https://doi.org/10.3390/md15040105
Lee HM, Kim MH, Yoon YI, Park WH. Fluorescent Property of Chitosan Oligomer and Its Application as a Metal Ion Sensor. Marine Drugs. 2017; 15(4):105. https://doi.org/10.3390/md15040105
Chicago/Turabian StyleLee, Hun Min, Min Hee Kim, Young Il Yoon, and Won Ho Park. 2017. "Fluorescent Property of Chitosan Oligomer and Its Application as a Metal Ion Sensor" Marine Drugs 15, no. 4: 105. https://doi.org/10.3390/md15040105
APA StyleLee, H. M., Kim, M. H., Yoon, Y. I., & Park, W. H. (2017). Fluorescent Property of Chitosan Oligomer and Its Application as a Metal Ion Sensor. Marine Drugs, 15(4), 105. https://doi.org/10.3390/md15040105






