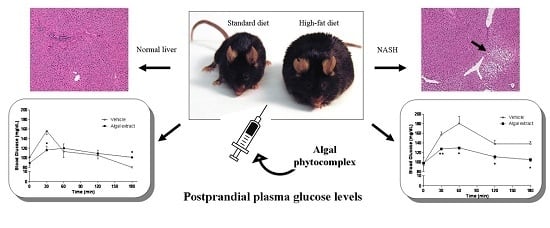
The Phytocomplex from Fucus vesiculosus and Ascophyllum nodosum Controls Postprandial Plasma Glucose Levels: An In Vitro and In Vivo Study in a Mouse Model of NASH
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. NMR, HPLC-DAD, and GC-MS Analysis
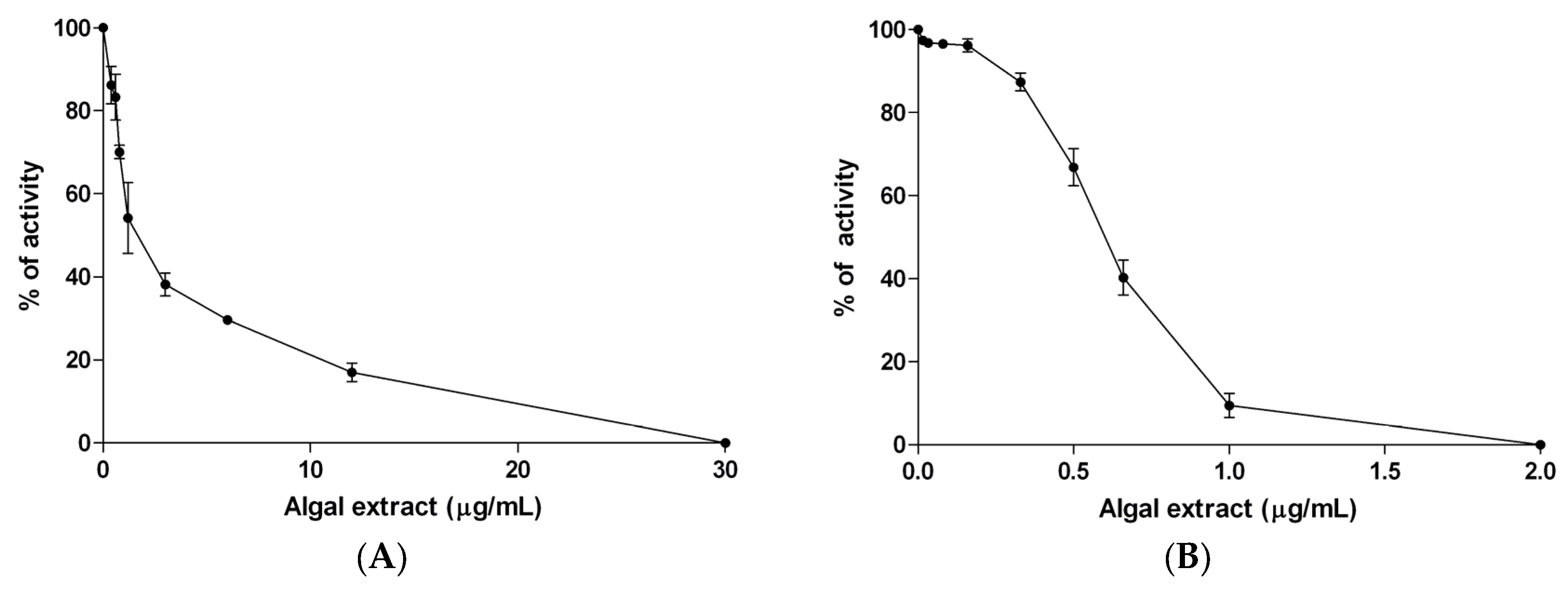
2.3. α-Amylase Activity Assay
2.4. α-Glucosidase Activity Assay
2.5. Animals and Treatments
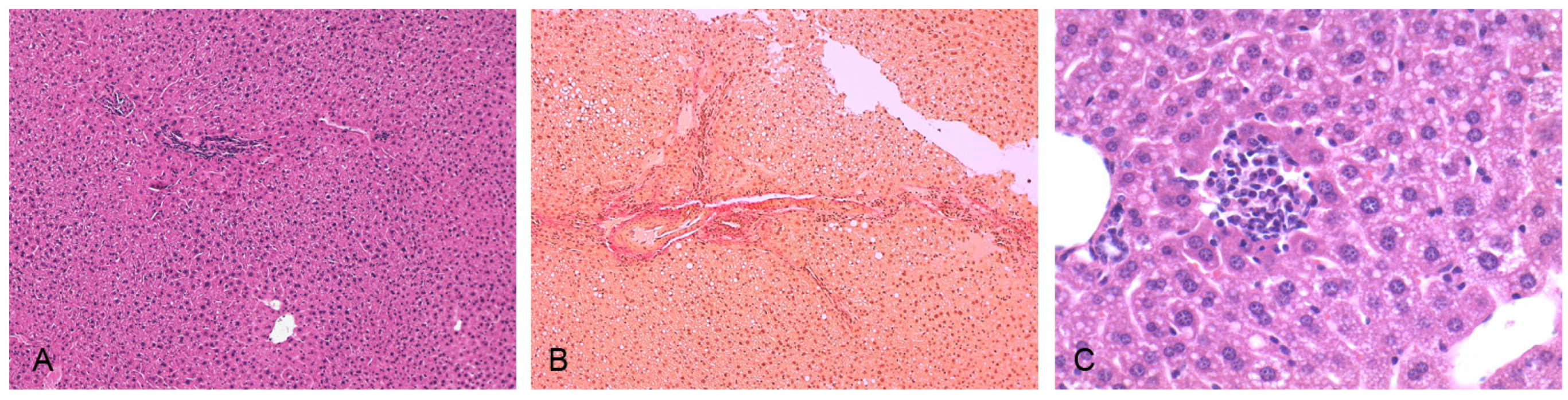
2.6. Histological Analysis
2.7. Statistical Analysis
3. Results
3.1. Phytochemical Fingerprint of the Algal Extract
3.2. In Vitro Studies
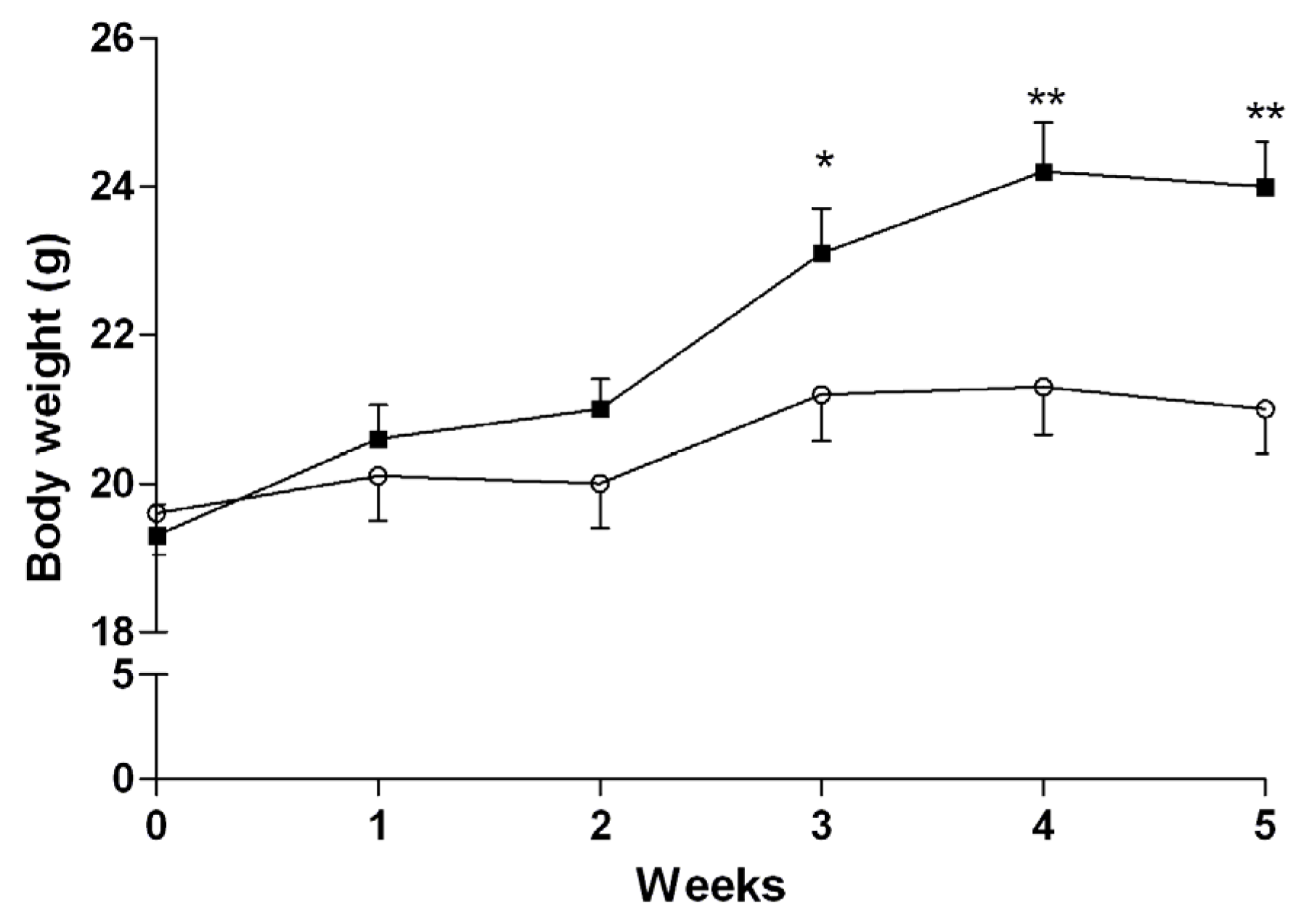
3.3. Induction of NASH
3.4. In Vivo Studies
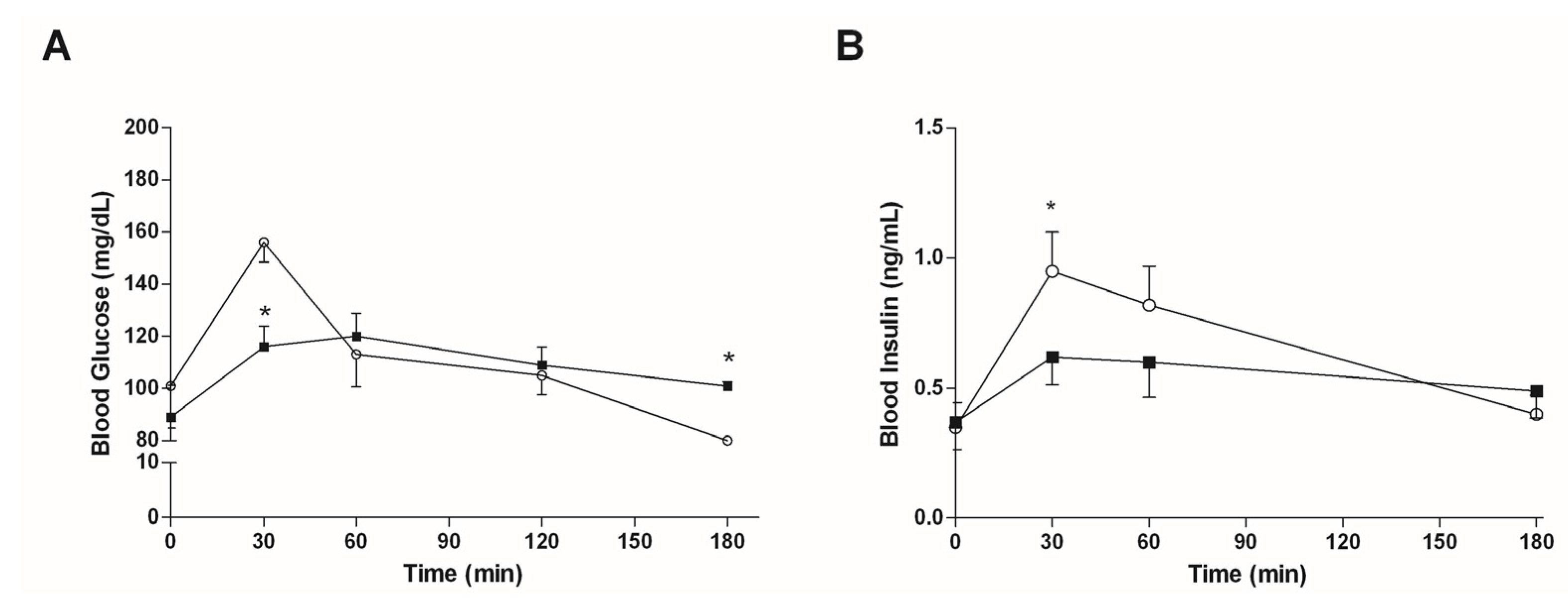
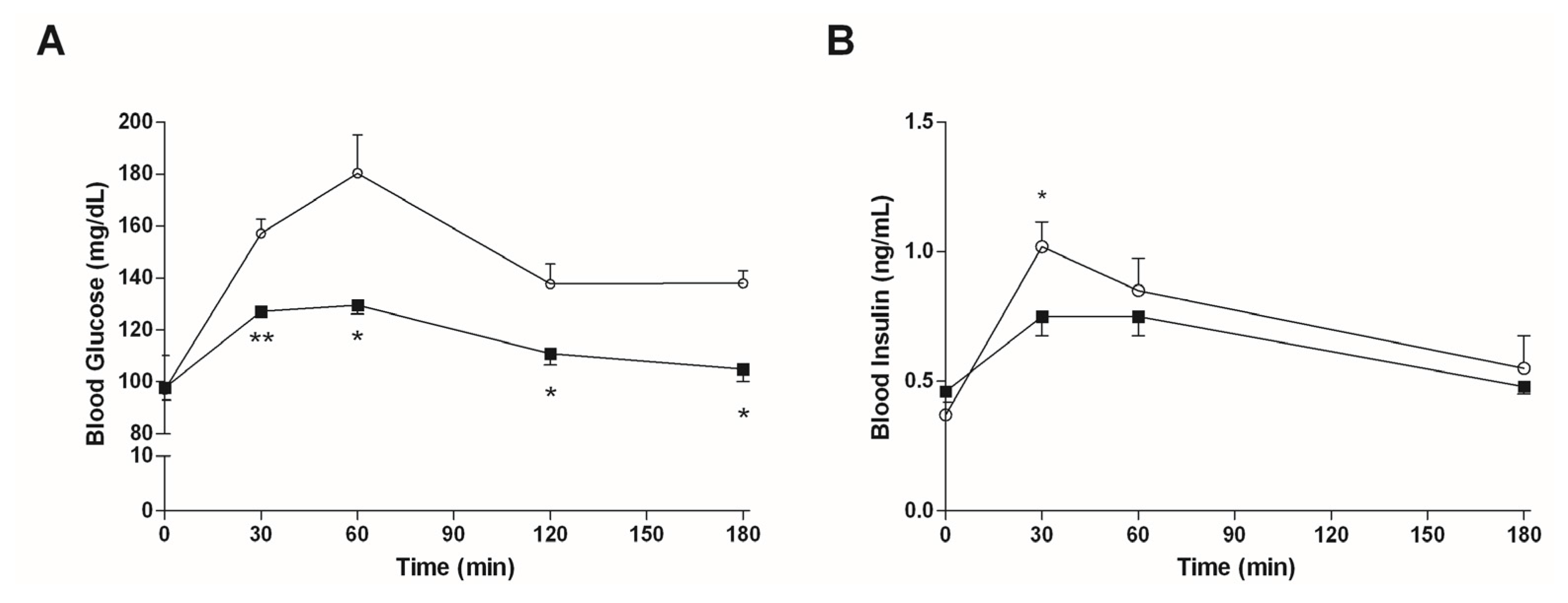
3.4.1. Mice Fed with a Normal Diet
3.4.2. Mice Fed with HFD
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ruperez, P.; Ahrazem, O.; Leal, J.A. Potential antioxidant capacity of sulfated polysaccharides from the edible marine brown seaweed Fucus vesiculosus. J. Agric. Food Chem. 2002, 50, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Rindi, F.; Soler-Vila, A.; Guiry, M.D. Taxonomy of Marine Macroalgae Used as Sources of Bioactive Compounds. In Marine Bioactive Compounds: Sources, Characterization and Applications; Hayes, M., Ed.; Springer: Boston, MA, USA, 2012; pp. 1–53. [Google Scholar]
- Apostolidis, E.; Lee, C.M. In vitro potential of Ascophyllum nodosum phenolic antioxidant-mediated alpha-glucosidase and alpha-amylase inhibition. J. Food Sci. 2010, 75, H97–H102. [Google Scholar] [CrossRef] [PubMed]
- Kandra, L.; Gyemant, G.; Zajacz, A.; Batta, G. Inhibitory effects of tannin on human salivary alpha-amylase. Biochem. Biophys. Res. Commun. 2004, 319, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Nwosu, F.; Morris, J.; Lund, V.A.; Stewart, D.; Ross, H.A.; McDougall, G.J. Anti-proliferative and potential anti-diabetic effects of phenolic-rich extracts from edible marine algae. Food Chem. 2011, 126, 1006–1012. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N. Bioactive potential and possible health effects of edible brown seaweeds. Trends Food Sci. Technol. 2011, 22, 315–326. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed]
- Lahaye, M. Marine algae as sources of fibres: Determination of soluble and insoluble dietary fibre contents in some? Sea vegetables? J. Sci. Food Agric. 1991, 54, 587–594. [Google Scholar] [CrossRef]
- Mabeau, S.; Fleurence, J. Seaweed in food products: Biochemical and nutritional aspects. Trends Food Sci. Technol. 1993, 4, 103–107. [Google Scholar] [CrossRef]
- Landin, K.; Holm, G.; Tengborn, L.; Smith, U. Guar gum improves insulin sensitivity, blood lipids, blood pressure, and fibrinolysis in healthy men. Am. J. Clin. Nutr. 1992, 56, 1061–1065. [Google Scholar] [PubMed]
- Ou, S.; Kwok, K.; Li, Y.; Fu, L. In vitro study of possible role of dietary fiber in lowering postprandial serum glucose. J. Agric. Food Chem. 2001, 49, 1026–1029. [Google Scholar] [CrossRef] [PubMed]
- Atashrazm, F.; Lowenthal, R.M.; Woods, G.M.; Holloway, A.F.; Dickinson, J.L. Fucoidan and cancer: A multifunctional molecule with anti-tumor potential. Mar. Drugs 2015, 13, 2327–2346. [Google Scholar] [CrossRef] [PubMed]
- Mak, W.; Wang, S.K.; Liu, T.; Hamid, N.; Li, Y.; Lu, J.; White, W.L. Anti-Proliferation Potential and Content of Fucoidan Extracted from Sporophyll of New Zealand Undaria pinnatifida. Front. Nutr. 2014, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, H.; Mitani, T.; Kawabata, J.; Takahashi, K. Two new bromophenols from the red alga Odonthalia corymbifera. J. Nat. Prod. 1999, 62, 882–884. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Hansen, P.E.; Lin, X. Bromophenols in marine algae and their bioactivities. Mar. Drugs 2011, 9, 1273–1292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tiller, C.; Shen, J.; Wang, C.; Girouard, G.S.; Dennis, D.; Barrow, C.J.; Miao, M.; Ewart, H.S. Antidiabetic properties of polysaccharide- and polyphenolic-enriched fractions from the brown seaweed Ascophyllum nodosum. Can. J. Physiol. Pharmacol. 2007, 85, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Sales, P.M.; Souza, P.M.; Simeoni, L.A.; Silveira, D. alpha-Amylase inhibitors: A review of raw material and isolated compounds from plant source. J. Pharm. Pharm. Sci. 2012, 15, 141–183. [Google Scholar] [CrossRef] [PubMed]
- Etxeberria, U.; de la Garza, A.L.; Campion, J.; Martinez, J.A.; Milagro, F.I. Antidiabetic effects of natural plant extracts via inhibition of carbohydrate hydrolysis enzymes with emphasis on pancreatic alpha amylase. Expert Opin. Ther. Targets 2012, 16, 269–297. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Nguyen, T.H.; Kurihara, H.; Kim, S.M. Alpha-glucosidase inhibitory activity of bromophenol purified from the red alga Polyopes lancifolia. J. Food Sci. 2010, 75, H145–H150. [Google Scholar] [PubMed]
- Rabasa-Lhoret, R.; Chiasson, J. Alpha-Glucosidase Inhibitors. In International Textbook of Diabetes Mellitus; John Wiley & Sons, Ltd.: London, UK, 2004. [Google Scholar]
- Porte, D. Clinical importance of insulin secretion and its interaction with insulin resistance in the treatment of type 2 diabetes mellitus and its complications. Diabetes Metab. Res. 2001, 17, 181–188. [Google Scholar] [CrossRef]
- Miele, L.; Targher, G. Understanding the association between developing a fatty liver and subsequent cardio-metabolic complications. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 1243–1245. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Targher, G.; Day, C.P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Nadalin, S.; Malago, M.; Valentin-Gamazo, C.; Testa, G.; Baba, H.A.; Liu, C.; Fruhauf, N.R.; Schaffer, R.; Gerken, G.; Frilling, A.; et al. Preoperative donor liver biopsy for adult living donor liver transplantation: Risks and benefits. Liver Transplant. 2005, 11, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Cobbold, J.F.; Anstee, Q.M.; Goldin, R.D.; Williams, H.R.; Matthews, H.C.; North, B.V.; Absalom, N.; Thomas, H.C.; Thursz, M.R.; Cox, R.D.; et al. Phenotyping murine models of non-alcoholic fatty liver disease through metabolic profiling of intact liver tissue. Clin. Sci. 2009, 116, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Gambino, R.; Cassader, M.; Pagano, G. Meta-analysis: Natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann. Med. 2011, 43, 617–649. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.F.; Pories, W.J.; Caro, J.F. Liver pathology in diabetes mellitus and morbid obesity. Clinical, pathological, and biochemical considerations. Pathol. Annu. 1989, 24 Pt 1, 275–302. [Google Scholar] [PubMed]
- Smith, B.W.; Adams, L.A. Nonalcoholic fatty liver disease and diabetes mellitus: Pathogenesis and treatment. Nat. Rev. Endocrinol. 2011, 7, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Vonghia, L.; Ruyssers, N.; Schrijvers, D.; Pelckmans, P.; Michielsen, P.; De Clerck, L.; Ramon, A.; Jirillo, E.; Ebo, D.; De Winter, B.; et al. CD4+ROR gamma t++ and Tregs in a Mouse Model of Diet-Induced Nonalcoholic Steatohepatitis. Mediat. Inflamm. 2015, 2015, 239623. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Terauchi, Y. Lessons from mouse models of high-fat diet-induced NAFLD. Int. J. Mol. Sci. 2013, 14, 21240–21257. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Anguenot, R.; Fillion, C.; Beaulieu, M.; Bérubé, J.; Richard, D. Effect of a commercially-available algal phlorotannins extract on digestive enzymes and carbohydrate absorption in vivo. Food Res. Int. 2011, 44, 3026–3029. [Google Scholar] [CrossRef]
- Ghosh, S.; Ahire, M.; Patil, S.; Jabgunde, A.; Bhat Dusane, M.; Joshi, B.N.; Pardesi, K.; Jachak, S.; Dhavale, D.D.; Chopade, B.A. Antidiabetic Activity of Gnidia glauca and Dioscorea bulbifera: Potent Amylase and Glucosidase Inhibitors. Evid. Based Complement. Altern. Med. 2012, 2012, 929051. [Google Scholar] [CrossRef] [PubMed]
- Jegou, C.; Kervarec, N.; Cerantola, S.; Bihannic, I.; Stiger-Pouvreau, V. NMR use to quantify phlorotannins: The case of Cystoseira tamariscifolia, a phloroglucinol-producing brown macroalga in Brittany (France). Talanta 2015, 135, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Paradis, M.E.; Couture, P.; Lamarche, B. A randomised crossover placebo-controlled trial investigating the effect of brown seaweed (Ascophyllum nodosum and Fucus vesiculosus) on postchallenge plasma glucose and insulin levels in men and women. Appl. Physiol. Nutr. Metab. 2011, 36, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Murakami, T.; Shimada, H.; Matsuda, H.; Yamahara, J.; Tanabe, G.; Muraoka, O. Salacinol, potent antidiabetic principle with unique thiosugar sulfonium sulfate structure from the Ayurvedic traditional medicine Salacia reticulata in Sri Lanka and India. Tetrahedron Lett. 1997, 38, 8367–8370. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Morikawa, T.; Matsuda, H.; Tanabe, G.; Muraoka, O. Absolute stereostructure of potent alpha-glucosidase inhibitor, Salacinol, with unique thiosugar sulfonium sulfate inner salt structure from Salacia reticulata. Bioorg. Med. Chem. 2002, 10, 1547–1554. [Google Scholar] [CrossRef]
- McDougall, G.J.; Shpiro, F.; Dobson, P.; Smith, P.; Blake, A.; Stewart, D. Different Polyphenolic Components of Soft Fruits Inhibit alpha-Amylase and alpha-Glucosidase. J. Agric. Food Chem. 2005, 53, 2760–2766. [Google Scholar] [CrossRef] [PubMed]
- Koh, L.W.; Wong, L.L.; Loo, Y.Y.; Kasapis, S.; Huang, D. Evaluation of different teas against starch digestibility by mammalian glycosidases. J. Agric. Food Chem. 2010, 58, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Lo Piparo, E.; Scheib, H.; Frei, N.; Williamson, G.; Grigorov, M.; Chou, C.J. Flavonoids for controlling starch digestion: Structural requirements for inhibiting human alpha-amylase. J. Med. Chem. 2008, 51, 3555–3561. [Google Scholar] [CrossRef] [PubMed]
- O'Sullivan, L.; Murphy, B.; McLoughlin, P.; Duggan, P.; Lawlor, P.G.; Hughes, H.; Gardiner, G.E. Prebiotics from marine macroalgae for human and animal health applications. Mar. Drugs 2010, 8, 2038–2064. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Rioux, L.; Turgeon, S.L. Alpha-amylase and alpha-glucosidase inhibition is differentially modulated by fucoidan obtained from Fucus vesiculosus and Ascophyllum nodosum. Phytochemistry 2014, 98, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Kongstad, K.T.; Wiese, S.; Jager, A.K.; Staerk, D. Edible seaweed as future functional food: Identification of alpha-glucosidase inhibitors by combined use of high-resolution alpha-glucosidase inhibition profiling and HPLC-HRMS-SPE-NMR. Food Chem. 2016, 203, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, A.L.; Kacinik, V.; Lyon, M.R.; Wolever, T.M. Reduction of postprandial glycemia by the novel viscous polysaccharide PGX, in a dose-dependent manner, independent of food form. J. Am. Coll. Nutr. 2010, 29, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, A.L.; Kacinik, V.; Lyon, M.; Wolever, T.M. Effect of adding the novel fiber, PGX®, to commonly consumed foods on glycemic response, glycemic index and GRIP: A simple and effective strategy for reducing post prandial blood glucose levels—A randomized, controlled trial. Nutr. J. 2010, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Zuberi, A.R.; Zhang, X.H.; Macgowan, J.; Qin, J.; Ye, X.; Son, L.; Wu, Q.; Lian, K.; Cefalu, W.T. Effects of dietary fibers on weight gain, carbohydrate metabolism, and gastric ghrelin gene expression in mice fed a high-fat diet. Metabolism 2007, 56, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
| Retention Time (min) | Methyl Ester of Fatty Acid |
|---|---|
| 13.75 | Methyl myristate |
| 15.88 | Methyl hexadecanoate |
| 16.15 | Methyl palmitate |
| 18.58 | Methyl linoleate |
| 18.68 | Methyl oleate |
| 19.09 | Methyl stearate |
| 21.22 | Methyl arachidonate |
| IC50 Value (μg/mL) | ||
|---|---|---|
| α-Amylase | α-Glucosidase | |
| Algal extract | 1.49 ± 0.32 | 0.604 ± 0.004 |
| Acarbose | 130.2 ± 2.5 | 207.2 ± 5.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabbia, D.; Dall’Acqua, S.; Di Gangi, I.M.; Bogialli, S.; Caputi, V.; Albertoni, L.; Marsilio, I.; Paccagnella, N.; Carrara, M.; Giron, M.C.; et al. The Phytocomplex from Fucus vesiculosus and Ascophyllum nodosum Controls Postprandial Plasma Glucose Levels: An In Vitro and In Vivo Study in a Mouse Model of NASH. Mar. Drugs 2017, 15, 41. https://doi.org/10.3390/md15020041
Gabbia D, Dall’Acqua S, Di Gangi IM, Bogialli S, Caputi V, Albertoni L, Marsilio I, Paccagnella N, Carrara M, Giron MC, et al. The Phytocomplex from Fucus vesiculosus and Ascophyllum nodosum Controls Postprandial Plasma Glucose Levels: An In Vitro and In Vivo Study in a Mouse Model of NASH. Marine Drugs. 2017; 15(2):41. https://doi.org/10.3390/md15020041
Chicago/Turabian StyleGabbia, Daniela, Stefano Dall’Acqua, Iole Maria Di Gangi, Sara Bogialli, Valentina Caputi, Laura Albertoni, Ilaria Marsilio, Nicola Paccagnella, Maria Carrara, Maria Cecilia Giron, and et al. 2017. "The Phytocomplex from Fucus vesiculosus and Ascophyllum nodosum Controls Postprandial Plasma Glucose Levels: An In Vitro and In Vivo Study in a Mouse Model of NASH" Marine Drugs 15, no. 2: 41. https://doi.org/10.3390/md15020041
APA StyleGabbia, D., Dall’Acqua, S., Di Gangi, I. M., Bogialli, S., Caputi, V., Albertoni, L., Marsilio, I., Paccagnella, N., Carrara, M., Giron, M. C., & De Martin, S. (2017). The Phytocomplex from Fucus vesiculosus and Ascophyllum nodosum Controls Postprandial Plasma Glucose Levels: An In Vitro and In Vivo Study in a Mouse Model of NASH. Marine Drugs, 15(2), 41. https://doi.org/10.3390/md15020041