Enzyme-Assisted Discovery of Antioxidant Peptides from Edible Marine Invertebrates: A Review
Abstract
:1. Introduction
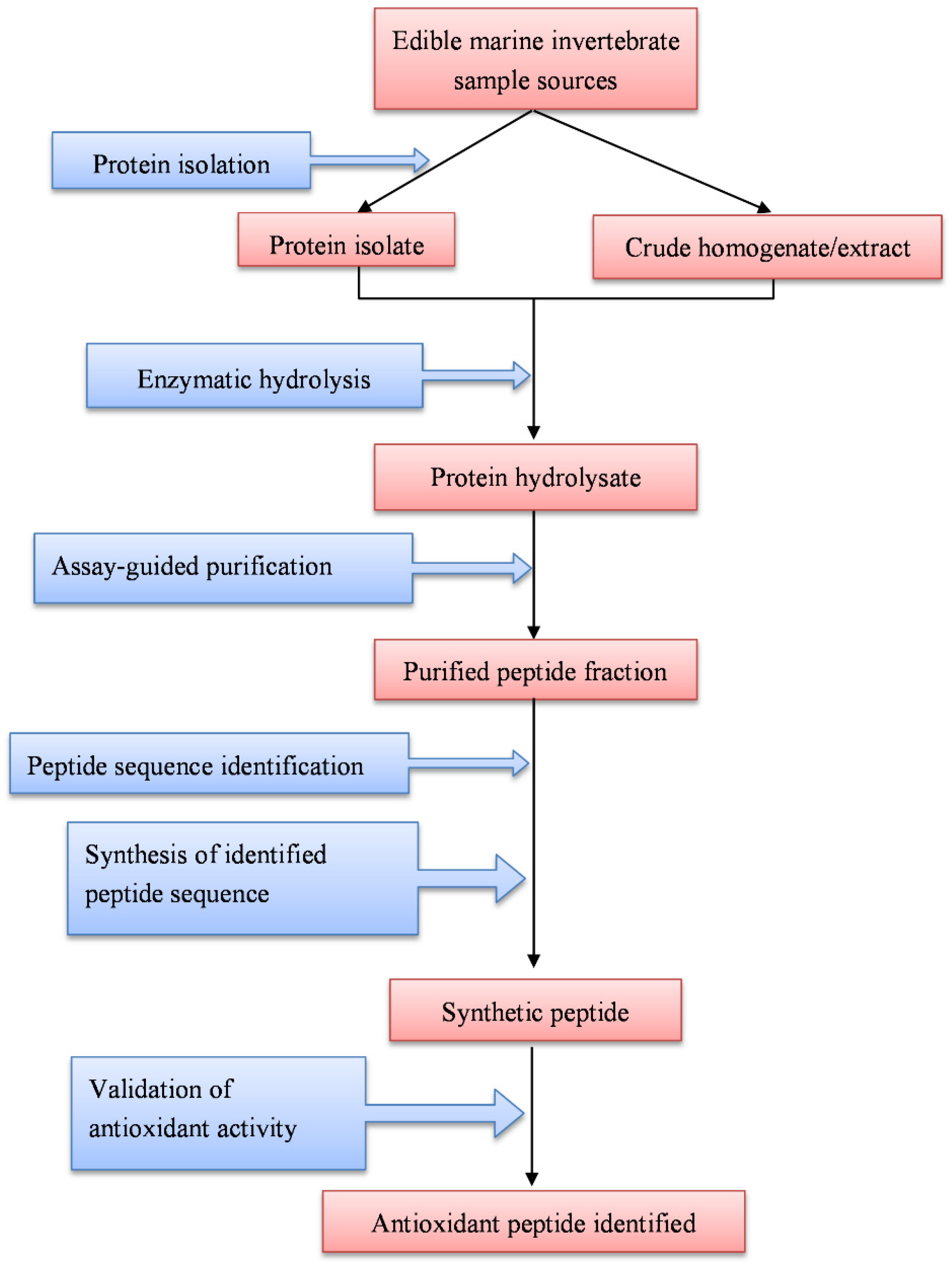
2. Enzyme-Assisted Production, Purification, and Identification of Antioxidant Peptides
2.1. Production of Antioxidant Peptides
2.2. Purification of Antioxidant Peptides
2.3. Identification of Antioxidant Peptides
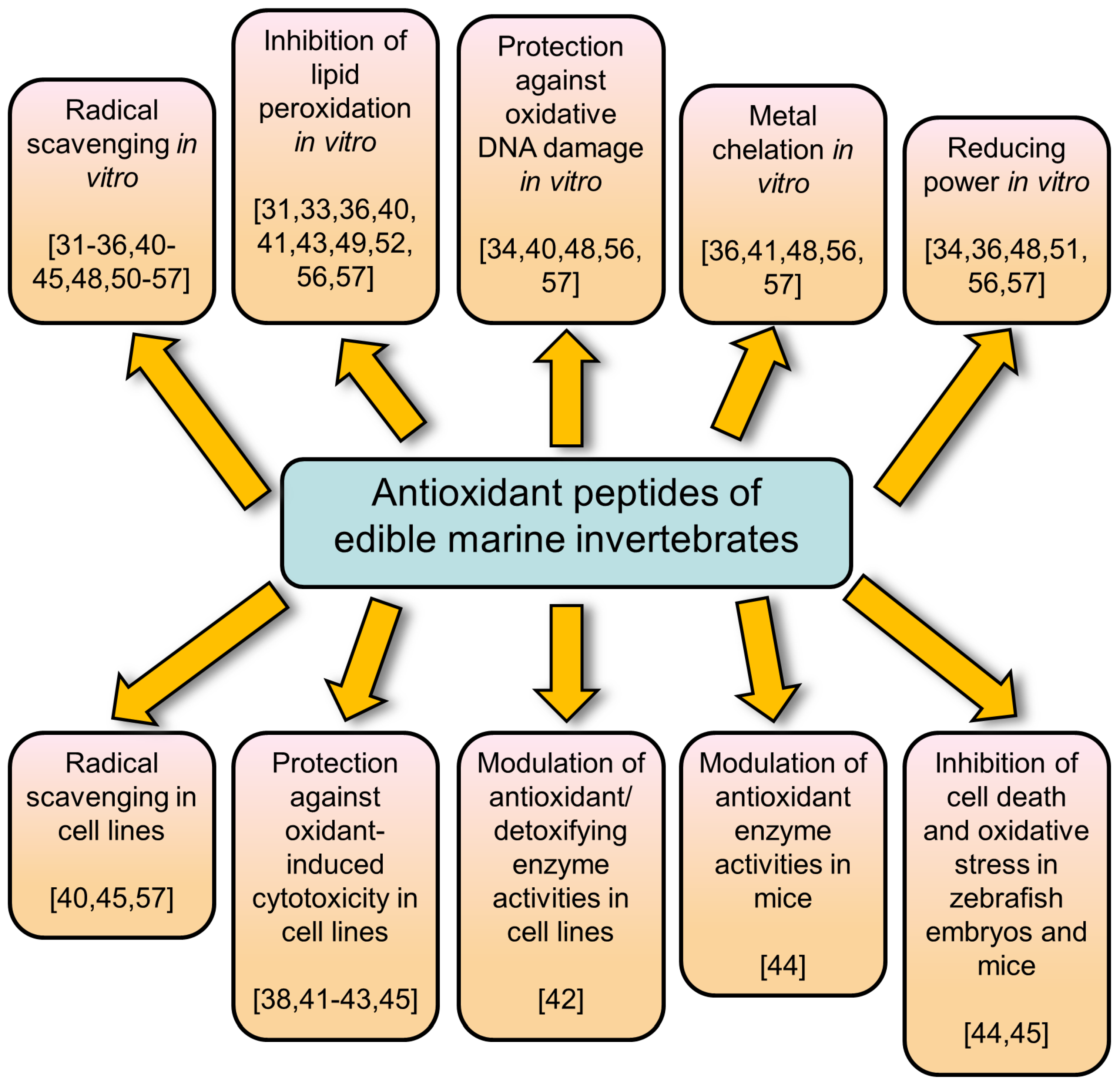
3. Evaluation of Antioxidant Activities
4. Molecular Characteristics and Structure–Activity Relationship
5. Potential Applications in Food, Therapy and Cosmetics
6. Current Gaps in Knowledge and Future Perspectives
6.1. Biological Significance
6.2. Stability
6.3. Application of In Silico Tools
6.4. Multifunctionality
6.5. Safety
6.6. Need for More Intensive Research on Processing Wastes
7. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Weidinger, A.; Kozlov, A. Biological activities of reactive oxygen and nitrogen species: Oxidative stress versus signal transduction. Biomolecules 2015, 5, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.-W.; Zhang, J.; Townsend, D.M.; Tew, K.D. Oxidative stress, redox regulation and diseases of cellular differentiation. BBA-Gen. Subj. 2015, 1850, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Luca, M.; Luca, A.; Calandra, C. The role of oxidative damage in the pathogenesis and progression of Alzheimer’s disease and vascular dementia. Oxid. Med. Cell. Longev. 2015, 2015, 504678. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Jahandideh, F.; Wu, J. Food-Derived bioactive peptides on inflammation and oxidative stress. Biomed. Res. Int. 2014, 2014, 608979. [Google Scholar] [CrossRef] [PubMed]
- Reeg, S.; Grune, T. Protein oxidation in aging: Does it play a role in aging progression? Antioxid. Redox Signal. 2015, 23, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Fukushima, W.; Tanaka, K.; Sasaki, S.; Kiyohara, C.; Tsuboi, Y.; Yamada, T.; Oeda, T.; Miki, T.; Kawamura, N.; et al. Dietary intake of antioxidant vitamins and risk of Parkinson’s disease: A case–control study in Japan. Eur. J. Neurol. 2011, 18, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Bo, Y.; Lu, Y.; Zhao, Y.; Zhao, E.; Yuan, L.; Lu, W.; Cui, L.; Lu, Q. Association between dietary vitamin C intake and risk of esophageal cancer: A dose-response meta-analysis. Int. J. Cancer 2016, 138, 1843–1850. [Google Scholar] [CrossRef] [PubMed]
- Aboonabi, A.; Singh, I. The effectiveness of antioxidant therapy in aspirin resistance, diabetes population for prevention of thrombosis. Biomed. Pharmacother. 2016, 83, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Tamay-Cach, F.; Quintana-Pérez, J.C.; Trujillo-Ferrara, J.G.; Cuevas-Hernández, R.I.; Del Valle-Mondragón, L.; García-Trejo, E.M.; Arellano-Mendoza, M.G. A review of the impact of oxidative stress and some antioxidant therapies on renal damage. Ren. Fail. 2016, 38, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Bielli, A.; Scioli, M.G.; Mazzaglia, D.; Doldo, E.; Orlandi, A. Antioxidants and vascular health. Life Sci. 2015, 143, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Shahidi, F. Methods for the assessment of antioxidant activity in foods. In Handbook of Antioxidants for Food Preservation; Shahidi, F., Ed.; Woodhead Publishing: Oxford, UK, 2015; pp. 287–333. [Google Scholar]
- Agyei, D.; Danquah, M.K.; Sarethy, I.P.; Pan, S. Antioxidative peptides derived from food proteins. In Free Radicals in Human Health and Disease; Rani, V., Yadav, S.U.C., Eds.; Springer: New Delhi, India, 2015; pp. 417–430. [Google Scholar]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Sampath Kumar, N.S.; Nazeer, R.A.; Jaiganesh, R. Purification and identification of antioxidant peptides from the skin protein hydrolysate of two marine fishes, horse mackerel (Magalaspis cordyla) and croaker (Otolithes ruber). Amino Acids 2011, 42, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H. Marine peptides: Bioactivities and applications. Mar. Drugs 2015, 13, 4006–4043. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S. Peptide therapeutics market: Forecast and analysis 2015–2025. Chim. Oggi Chem. Today 2016, 34, 5–7. [Google Scholar]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Sila, A.; Bougatef, A. Antioxidant peptides from marine by-products: Isolation, identification and application in food systems. A review. J. Funct. Foods 2016, 21, 10–26. [Google Scholar] [CrossRef]
- Lemes, A.C.; Sala, L.; Ores, J.d.C.; Braga, A.R.C.; Egea, M.B.; Fernandes, K.F. A review of the latest advances in encrypted bioactive peptides from protein-rich waste. Int. J. Mol. Sci. 2016, 17, 950. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.-H.; Vo, T.-S.; Ngo, D.-N.; Wijesekara, I.; Kim, S.-K. Biological activities and potential health benefits of bioactive peptides derived from marine organisms. Int. J. Biol. Macromol. 2012, 51, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Samaranayaka, A.G.P.; Li-Chan, E.C.Y. Food-derived peptidic antioxidants: A review of their production, assessment, and potential applications. J. Funct. Foods 2011, 3, 229–254. [Google Scholar] [CrossRef]
- Xiong, Y.L. Antioxidant peptides. In Bioactive Proteins and Peptides as Functional Foods and Nutraceuticals; Mine, Y., Li-Chan, E., Jiang, B., Eds.; Wiley-Blackwell: Oxford, UK, 2010; pp. 29–42. [Google Scholar]
- Kim, S.-K.; Wijesekara, I. Marine-Derived Peptides: Development and Health Prospects. In Marine Proteins and Peptides: Biological Activities and Applications; Kim, S.-K., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2013; pp. 1–3. [Google Scholar]
- Kim, S.-K.; Wijesekara, I. Development and biological activities of marine-derived bioactive peptides: A review. J. Funct. Foods 2010, 2, 1–9. [Google Scholar] [CrossRef]
- Li, Y.; Yu, J. Research progress in structure-activity relationship of bioactive peptides. J. Med. Food 2015, 18, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.-B.; He, T.-P.; Li, H.-B.; Tang, H.-W.; Xia, E.-Q. The structure-activity relationship of the antioxidant peptides from natural proteins. Molecules 2016, 21, 72. [Google Scholar] [CrossRef] [PubMed]
- Scopus. Available online: http://www.scopus.com (accessed on 29 November 2016).
- Minkiewicz, P.; Dziuba, J.; Iwaniak, A.; Dziuba, M.; Darewicz, M. BIOPEP database and other programs for processing bioactive peptide sequences. J. AOAC Int. 2008, 91, 965–980. [Google Scholar] [PubMed]
- Halim, N.R.A.; Yusof, H.M.; Sarbon, N.M. Functional and bioactive properties of fish protein hydolysates and peptides: A comprehensive review. Trends Food Sci. Technol. 2016, 51, 24–33. [Google Scholar] [CrossRef]
- Senevirathne, M.; Kim, S.-K. Development of bioactive peptides from fish proteins and their health promoting ability. Adv. Food Nutr. Res. 2012, 65, 235–248. [Google Scholar] [PubMed]
- Wang, B.; Li, L.; Chi, C.F.; Ma, J.H.; Luo, H.Y.; Xu, Y.F. Purification and characterisation of a novel antioxidant peptide derived from blue mussel (Mytilus edulis) protein hydrolysate. Food Chem. 2013, 138, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, Y.S.; Ahn, C.B.; Je, J.Y. Partial purification and identification of three antioxidant peptides with hepatoprotective effects from blue mussel (Mytilus edulis) hydrolysate by peptic hydrolysis. J. Funct. Foods 2016, 20, 88–95. [Google Scholar] [CrossRef]
- Chi, C.F.; Hu, F.Y.; Wang, B.; Li, T.; Ding, G.F. Antioxidant and anticancer peptides from the protein hydrolysate of blood clam (Tegillarca granosa) muscle. J. Funct. Foods 2015, 15, 301–313. [Google Scholar] [CrossRef]
- Li, R.; Yang, Z.S.; Sun, Y.; Li, L.; Wang, J.B.; Ding, G. Purification and antioxidant property of antioxidative oligopeptide from short-necked clam (Ruditapes philippinarum) hydrolysate in vitro. J. Aquat. Food Prod. Technol. 2015, 24, 556–565. [Google Scholar] [CrossRef]
- Wang, Q.; Li, W.; He, Y.; Ren, D.; Kow, F.; Song, L.; Yu, X. Novel antioxidative peptides from the protein hydrolysate of oysters (Crassostrea talienwhanensis). Food Chem. 2014, 145, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Asha, K.K.; Remya Kumari, K.R.; Ashok Kumar, K.; Chatterjee, N.S.; Anandan, R.; Mathew, S. Sequence determination of an antioxidant peptide obtained by enzymatic hydrolysis of oyster Crassostrea madrasensis (Preston). Int. J. Pept. Res. Ther. 2016, 1–13. [Google Scholar] [CrossRef]
- Jung, W.K.; Rajapakse, N.; Kim, S.K. Antioxidative activity of a low molecular weight peptide derived from the sauce of fermented blue mussel, Mytilus edulis. Eur. Food Res. Technol. 2005, 220, 535–539. [Google Scholar] [CrossRef]
- Rajapakse, N.; Mendis, E.; Jung, W.K.; Je, J.Y.; Kim, S.K. Purification of a radical scavenging peptide from fermented mussel sauce and its antioxidant properties. Food Res. Int. 2005, 38, 175–182. [Google Scholar] [CrossRef]
- Kleekayai, T.; Harnedy, P.A.; O’Keeffe, M.B.; Poyarkov, A.A.; CunhaNeves, A.; Suntornsuk, W.; FitzGerald, R.J. Extraction of antioxidant and ACE inhibitory peptides from Thai traditional fermented shrimp pastes. Food Chem. 2015, 176, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.-J.; Jung, W.-K.; Byun, H.-G.; Kim, S.-K. Protective effect of an antioxidative peptide purified from gastrointestinal digests of oyster, Crassostrea gigas against free radical induced DNA damage. Bioresour. Technol. 2008, 99, 3365–3371. [Google Scholar] [CrossRef] [PubMed]
- Mendis, E.; Rajapakse, N.; Byun, H.-G.; Kim, S.-K. Investigation of jumbo squid (Dosidicus gigas) skin gelatin peptides for their in vitro antioxidant effects. Life Sci. 2005, 77, 2166–2178. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Q.; Li, J.; Zhou, B. Peptides derived from Rhopilema esculentum hydrolysate exhibit angiotensin converting enzyme (ACE) inhibitory and antioxidant abilities. Molecules 2014, 19, 13587–13602. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, N.; Mendis, E.; Byun, H.-G.; Kim, S.-K. Purification and in vitro antioxidative effects of giant squid muscle peptides on free radical-mediated oxidative systems. J. Nutr. Biochem. 2005, 16, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-K.; Oh, H.-J.; Kim, Y.-S.; Hwang, J.-W.; Ahn, C.-B.; Lee, J.S.; Jeon, Y.-J.; Moon, S.-H.; Sung, S.H.; Jeon, B.-T.; et al. Purification of a novel peptide derived from Mytilus coruscus and in vitro/in vivo evaluation of its bioactive properties. Fish Shellfish Immunol. 2013, 34, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.C.; Kim, E.A.; Jung, W.K.; Kim, W.S.; Lee, S.C.; Son, K.T.; Kim, J.I.; Jeon, Y.J. A hexameric peptide purified from Styela plicata protects against free radical-induced oxidative stress in cells and zebrafish model. RSC Adv. 2016, 6, 54169–54178. [Google Scholar] [CrossRef]
- Wu, R.B.; Wu, C.L.; Liu, D.; Yang, X.H.; Huang, J.F.; Zhang, J.; Liao, B.; He, H.L.; Li, H. Overview of antioxidant peptides derived from marine resources: The sources, characteristic, purification, and evaluation methods. Appl. Biochem. Biotechnol. 2015, 176, 1815–1833. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.-H.; Kim, S.-K. Marine bioactive peptides as potential antioxidants. Curr. Protein Pept. Sci. 2013, 14, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Suetsuna, K. Antioxidant peptides from the protease digest of prawn (Penaeus japonicus) muscle. Mar. Biotechnol. 2000, 2, 5–10. [Google Scholar] [PubMed]
- Wu, H.T.; Jin, W.G.; Sun, S.G.; Li, X.S.; Duan, X.H.; Li, Y.; Yang, Y.T.; Han, J.R.; Zhu, B.W. Identification of antioxidant peptides from protein hydrolysates of scallop (Patinopecten yessoensis) female gonads. Eur. Food Res. Technol. 2016, 242, 713–722. [Google Scholar] [CrossRef]
- Kang, N.; Ko, S.C.; Samarakoon, K.; Kim, E.A.; Kang, M.C.; Lee, S.C.; Kim, J.; Kim, Y.T.; Kim, J.S.; Kim, H.; et al. Purification of antioxidative peptide from peptic hydrolysates of Mideodeok (Styela clava) flesh tissue. Food Sci. Biotechnol. 2013, 22, 541–547. [Google Scholar] [CrossRef]
- Alemán, A.; Giménez, B.; Pérez-Santin, E.; Gómez-Guillén, M.C.; Montero, P. Contribution of Leu and Hyp residues to antioxidant and ACE-inhibitory activities of peptide sequences isolated from squid gelatin hydrolysate. Food Chem. 2011, 125, 334–341. [Google Scholar] [CrossRef]
- Jung, W.-K.; Qian, Z.-J.; Lee, S.-H.; Choi, S.Y.; Sung, N.J.; Byun, H.-G.; Kim, S.-K. Free radical scavenging activity of a novel antioxidative peptide isolated from in vitro gastrointestinal digests of Mytilus coruscus. J. Med. Food 2007, 10, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Hwang, J.W.; Kim, Y.S.; Ahn, C.B.; Jeon, Y.J.; Kweon, H.J.; Bahk, Y.Y.; Moon, S.H.; Jeon, B.T.; Park, P.J. A novel bioactive peptide derived from enzymatic hydrolysis of Ruditapes philippinarum: Purification and investigation of its free-radical quenching potential. Process Biochem. 2013, 48, 325–330. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, G.; Jiang, J. Purification and characterization of a new DPPH radical scavenging peptide from shrimp processing by-products hydrolysate. J. Aquat. Food Prod. Technol. 2013, 22, 281–289. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, C.; Jiang, A. Antioxidant peptides isolated from sea cucumber Stichopus Japonicus. Eur. Food Res. Technol. 2012, 234, 441–447. [Google Scholar] [CrossRef]
- Sudhakar, S.; Nazeer, R.A. Preparation of potent antioxidant peptide from edible part of shortclub cuttlefish against radical mediated lipid and DNA damage. LWT-Food Sci. Technol. 2015, 64, 593–601. [Google Scholar] [CrossRef]
- Sudhakar, S.; Nazeer, R.A. Structural characterization of an Indian squid antioxidant peptide and its protective effect against cellular reactive oxygen species. J. Funct. Foods 2015, 14, 502–512. [Google Scholar] [CrossRef]
- Grienke, U.; Silke, J.; Tasdemir, D. Bioactive compounds from marine mussels and their effects on human health. Food Chem. 2014, 142, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Vega, J.A.; Olivera-Castillo, L.; Gómez-Ruiz, J.T.; Hernández-Ledesma, B. Release of multifunctional peptides by gastrointestinal digestion of sea cucumber (Isostichopus badionotus). J. Funct. Foods 2013, 5, 869–877. [Google Scholar] [CrossRef]
- Amarowicz, R.; Shahidi, F. Antioxidant activity of peptide fractions of capelin protein hydrolysates. Food Chem. 1997, 58, 355–359. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Wijesekara, I.; Vo, T.-S.; Van Ta, Q.; Kim, S.-K. Marine food-derived functional ingredients as potential antioxidants in the food industry: An overview. Food Res. Int. 2011, 44, 523–529. [Google Scholar] [CrossRef]
- Zhuang, H.; Tang, N.; Yuan, Y. Purification and identification of antioxidant peptides from corn gluten meal. J. Funct. Foods 2013, 5, 1810–1821. [Google Scholar] [CrossRef]
- PepDraw. Available online: http://www.tulane.edu/~biochem/WW/PepDraw/ (accessed on 30 July 2016).
- Harnedy, P.A.; FitzGerald, R.J. Bioactive peptides from marine processing waste and shellfish: A review. J. Funct. Foods 2012, 4, 6–24. [Google Scholar] [CrossRef]
- IARC TP53 Database. Available online: http://p53.iarc.fr/AAProperties.aspx (accessed on 30 July 2016).
- Chan, K.M.; Decker, E.A.; Feustman, C. Endogenous skeletal muscle antioxidants. Crit. Rev. Food Sci. Nutr. 1994, 34, 403–426. [Google Scholar] [CrossRef] [PubMed]
- Dhaval, A.; Yadav, N.; Purwar, S. Potential applications of food derived bioactive peptides in management of health. Int. J. Pept. Res. Ther. 2016, 1–22. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.-M.; Chi, C.-F.; Luo, H.-Y.; Deng, S.-G.; Ma, J.-Y. Isolation and characterization of collagen and antioxidant collagen peptides from scales of croceine croaker (Pseudosciaena crocea). Mar. Drugs 2013, 11, 4641–4661. [Google Scholar] [CrossRef] [PubMed]
- Thorkelsson, G.; Kristinsson, H.G. Bioactive Peptides from Marine Sources. State of Art. Report to the NORA Fund; Matis Food Research, Innovation and Technology: Reykjavik, Iceland, 2009; pp. 1–19. [Google Scholar]
- Hartmann, R.; Meisel, H. Food-Derived peptides with biological activity: From research to food applications. Curr. Opin. Biotechnol. 2007, 18, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Dziuba, B.; Dziuba, M. Milk proteins-derived bioactive peptides in dairy products: Molecular, biological and methodological aspects. Acta Sci. Pol. Technol. Aliment. 2014, 13, 5–26. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.C.; Andrade, J.C.; Silva, F.M.; Rocha-Santos, T.A.P.; Duarte, A.C.; Gomes, A.M. Antioxidative peptides: Trends and perspectives for future research. Curr. Med. Chem. 2013, 20, 4575–4594. [Google Scholar] [CrossRef] [PubMed]
- Sivaraman, B.; Shakila, R.J.; Jeyasekaran, G.; Sukumar, D.; Manimaran, U.; Sumathi, G. Antioxidant activities of squid protein hydrolysates prepared with papain using response surface methodology. Food Sci. Biotechnol. 2016, 25, 665–672. [Google Scholar] [CrossRef]
- Jridi, M.; Lassoued, I.; Nasri, R.; Ayadi, M.A.; Nasri, M.; Souissi, N. Characterization and potential use of cuttlefish skin gelatin hydrolysates prepared by different microbial proteases. Biomed. Res. Int. 2014, 2014, 461728:1–461728:14. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.S.; Dora, K.C. Antioxidative activity of protein hydrolysate produced by alcalase hydrolysis from shrimp waste (Penaeus monodon and Penaeus indicus). J. Food Sci. Technol. (Mysore) 2014, 51, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Nikoo, M.; Benjakul, S.; Ehsani, A.; Li, J.; Wu, F.; Yang, N.; Xu, B.; Jin, Z.; Xu, X. Antioxidant and cryoprotective effects of a tetrapeptide isolated from Amur sturgeon skin gelatin. J. Funct. Foods 2014, 7, 609–620. [Google Scholar] [CrossRef]
- Nikoo, M.; Regenstein, J.M.; Ghomi, M.R.; Benjakul, S.; Yang, N.; Xu, X. Study of the combined effects of a gelatin-derived cryoprotective peptide and a non-peptide antioxidant in a fish mince model system. LWT-Food Sci. Technol. 2015, 60, 358–364. [Google Scholar] [CrossRef]
- Aluko, R.E. Amino acids, peptides, and proteins as antioxidants for food preservation. In Handbook of Antioxidants for Food Preservation; Shahidi, F., Ed.; Woodhead Publishing: Oxford, UK, 2015; pp. 105–140. [Google Scholar]
- Cho, J.; Won, K.; Wu, D.; Soong, Y.; Liu, S.; Szeto, H.H.; Hong, M.K. Potent mitochondria-targeted peptides reduce myocardial infarction in rats. Coron. Artery Dis. 2007, 18, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Li, S.; Wu, M.; Wei, J.; Ren, Y.; Du, C.; Wu, H.; Han, C.; Duan, H.; Shi, Y. Mitochondria-Targeted peptide SS-31 attenuates renal injury via an antioxidant effect in diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 2016, 310, F547–F559. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, X.; Li, M.; Li, J.; Xiao, W.; Ma, W.; Chen, X.; Liang, X.; Tang, S.; Luo, Y. Mitochondria-targeted antioxidant peptide SS31 protects the retinas of diabetic rats. Curr. Mol. Med. 2013, 13, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Righi, V.; Constantinou, C.; Mintzopoulos, D.; Khan, N.; Mupparaju, S.P.; Rahme, L.G.; Swartz, H.M.; Szeto, H.H.; Tompkins, R.G.; Tzika, A.A. Mitochondria-targeted antioxidant promotes recovery of skeletal muscle mitochondrial function after burn trauma assessed by in vivo 31P nuclear magnetic resonance and electron paramagnetic resonance spectroscopy. FASEB J. 2013, 27, 2521–2530. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Kaneki, M.; Andreas, J.; Tompkins, R.G.; Martyn, J.A.J. Novel mitochondria-targeted antioxidant peptide ameliorates burn-induced apoptosis and endoplasmic reticulum stress in the skeletal muscle of mice. Shock 2011, 36, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Homayouni-Tabrizi, M.; Asoodeh, A.; Abbaszadegan, M.R.; Shahrokhabadi, K.; Nakhaie Moghaddam, M. An identified antioxidant peptide obtained from ostrich (Struthio camelus) egg white protein hydrolysate shows wound healing properties. Pharm. Biol. 2015, 53, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Lintner, K. Peptides and proteins. In Cosmetic Dermatology: Products and Procedures; Draelos, Z.D., Ed.; John Wiley & Sons, Ltd.: Oxford, UK, 2015; pp. 308–317. [Google Scholar]
- Reddy, B.; Jow, T.; Hantash, B.M. Bioactive oligopeptides in dermatology: Part I. Exp. Dermatol. 2012, 21, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.Y.; Jow, T.; Hantash, B.M. Bioactive oligopeptides in dermatology: Part II. Exp. Dermatol. 2012, 21, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Falla, T.J. Cosmeceuticals and peptides. Clin. Dermatol. 2009, 27, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Pickart, L.; Vasquez-Soltero, J.; Margolina, A. GHK-Cu may prevent oxidative stress in skin by regulating copper and modifying expression of numerous antioxidant genes. Cosmetics 2015, 2, 236–247. [Google Scholar] [CrossRef]
- Sonthalia, S.; Daulatabad, D.; Sarkar, R. Glutathione as a skin whitening agent: Facts, myths, evidence and controversies. Indian J. Dermatol. Venereol. Leprol. 2016, 82, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Chahal, B.; Majumder, K.; You, S.-J.; Wu, J. Identification of novel antioxidative peptides derived from a thermolytic hydrolysate of ovotransferrin by LC-MS/MS. J. Agric. Food. Chem. 2010, 58, 7664–7672. [Google Scholar] [CrossRef] [PubMed]
- Jahandideh, F.; Chakrabarti, S.; Davidge, S.T.; Wu, J. Antioxidant peptides identified from ovotransferrin by the ORAC method did not show anti-inflammatory and antioxidant activities in endothelial cells. J. Agric. Food. Chem. 2016, 64, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Tenore, G.C.; Ritieni, A.; Campiglia, P.; Stiuso, P.; Di Maro, S.; Sommella, E.; Pepe, G.; D’Urso, E.; Novellino, E. Antioxidant peptides from “Mozzarella di Bufala Campana DOP” after simulated gastrointestinal digestion: In vitro intestinal protection, bioavailability, and anti-haemolytic capacity. J. Funct. Foods 2015, 15, 365–375. [Google Scholar] [CrossRef]
- Fernández-Musoles, R.; Salom, J.B.; Castelló-Ruiz, M.; Contreras, M.d.M.; Recio, I.; Manzanares, P. Bioavailability of antihypertensive lactoferricin B-derived peptides: Transepithelial transport and resistance to intestinal and plasma peptidases. Int. Dairy J. 2013, 32, 169–174. [Google Scholar] [CrossRef]
- Renukuntla, J.; Vadlapudi, A.D.; Patel, A.; Boddu, S.H.S.; Mitra, A.K. Approaches for enhancing oral bioavailability of peptides and proteins. Int. J. Pharm. 2013, 447, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Bruno, B.J.; Miller, G.D.; Lim, C.S. Basics and recent advances in peptide and protein drug delivery. Ther. Deliv. 2013, 4, 1443–1467. [Google Scholar] [CrossRef] [PubMed]
- Vermeirssen, V.; Camp, J.V.; Verstraete, W. Bioavailability of angiotensin I converting enzyme inhibitory peptides. Br. J. Nutr. 2004, 92, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [PubMed]
- Darewicz, M.; Borawska, J.; Pliszka, M. Carp proteins as a source of bioactive peptides—An in silico approach. Czech J. Food Sci. 2016, 34, 111–117. [Google Scholar] [CrossRef]
- Huang, B.B.; Lin, H.C.; Chang, Y.W. Analysis of proteins and potential bioactive peptides from tilapia (Oreochromis spp.) processing co-products using proteomic techniques coupled with BIOPEP database. J. Funct. Foods 2015, 19, 629–640. [Google Scholar] [CrossRef]
- Garcia-Mora, P.; Peñas, E.; Frias, J.; Zieliński, H.; Wiczkowski, W.; Zielińska, D.; Martínez-Villaluenga, C. High-pressure-assisted enzymatic release of peptides and phenolics increases angiotensin converting enzyme I inhibitory and antioxidant activities of pinto bean hydrolysates. J. Agric. Food. Chem. 2016, 64, 1730–1740. [Google Scholar] [CrossRef] [PubMed]
- Zenezini Chiozzi, R.; Capriotti, A.L.; Cavaliere, C.; La Barbera, G.; Piovesana, S.; Samperi, R.; Laganà, A. Purification and identification of endogenous antioxidant and ACE-inhibitory peptides from donkey milk by multidimensional liquid chromatography and nanoHPLC-high resolution mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 5657–5666. [Google Scholar] [CrossRef] [PubMed]
- Hikida, A.; Ito, K.; Motoyama, T.; Kato, R.; Kawarasaki, Y. Systematic analysis of a dipeptide library for inhibitor development using human dipeptidyl peptidase IV produced by a Saccharomyces cerevisiae expression system. Biochem. Biophys. Res. Commun. 2013, 430, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Chaudhary, K.; Dhanda, S.K.; Bhalla, S.; Usmani, S.S.; Gautam, A.; Tuknait, A.; Agrawal, P.; Mathur, D.; Raghava, G.P.S. SATPdb: A database of structurally annotated therapeutic peptides. Nucleic Acids Res. 2015, 44, D1119–D1126. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Su, Z.-D.; Wei, H.-H.; Chen, W.; Lin, H. Prediction of cell-penetrating peptides with feature selection techniques. Biochem. Biophys. Res. Commun. 2016, 477, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Kapoor, P.; Kumar, R.; Chaudhary, K.; Gautam, A.; Raghava, G.P.S. In silico models for designing and discovering novel anticancer peptides. Sci. Rep. 2013, 3, 2984. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, R.; Wal, J.M.; Bernard, H.; Pentzien, A.K. Cytotoxic and allergenic potential of bioactive proteins and peptides. Curr. Pharm. Des. 2007, 13, 897–920. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, G. Safety of protein hydrolysates, fractions thereof and bioactive peptides in human nutrition. Eur. J. Clin. Nutr. 2009, 63, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Christer, H.; Andersson, S.; Arpaia, D.; Casacuberta, J.; Davies, H.; Jardin, P.; Flachowsky, G. Scientific opinion on the assessment of allergenicity of GM plants and microorganisms and derived food and feed. EFSA J. 2010, 8, 1700. [Google Scholar]
- Lafarga, T.; Wilm, M.; Wynne, K.; Hayes, M. Bioactive hydrolysates from bovine blood globulins: Generation, characterisation, and in silico prediction of toxicity and allergenicity. J. Funct. Foods 2016, 24, 142–155. [Google Scholar] [CrossRef]
- Gupta, S.; Kapoor, P.; Chaudhary, K.; Gautam, A.; Kumar, R.; Raghava, G.P.S. In silico approach for predicting toxicity of peptides and proteins. PLoS ONE 2013, 8, e0073957. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Raghava, G.P.S. AlgPred: Prediction of allergenic proteins and mapping of IgE epitopes. Nucleic Acids Res. 2006, 34, W202–W209. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, I.; Bangov, I.; Flower, D.R.; Doytchinova, I. AllerTOP v.2—A server for in silico prediction of allergens. J. Mol. Model. 2014, 20, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kapoor, P.; Chaudhary, K.; Gautam, A.; Kumar, R.; Raghava, G.P.S. Peptide toxicity prediction. In Computational Peptidology; Zhou, P., Huang, J., Eds.; Springer: New York, NY, USA, 2015; pp. 143–157. [Google Scholar]
- Gosslau, A. Assessment of food toxicology. Food Sci. Hum. Wellness 2016. [Google Scholar] [CrossRef]
- Nakchum, L.; Kim, S.M. Preparation of squid skin collagen hydrolysate as an antihyaluronidase, antityrosinase, and antioxidant agent. Prep. Biochem. Biotechnol. 2016, 46, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.A.; Oliveira, D.D.; Kurozawa, L.E. Production of peptides with radical scavenging activity and recovery of total carotenoids using enzymatic protein hydrolysis of shrimp waste. J. Food Biochem. 2016. [Google Scholar] [CrossRef]
- Tonon, R.V.; Dos Santos, B.A.; Couto, C.C.; Mellinger-Silva, C.; Brígida, A.I.S.; Cabral, L.M.C. Coupling of ultrafiltration and enzymatic hydrolysis aiming at valorizing shrimp wastewater. Food Chem. 2016, 198, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Mamelona, J.; Saint-Louis, R.; Pelletier, É. Nutritional composition and antioxidant properties of protein hydrolysates prepared from echinoderm byproducts. Int. J. Food Sci. Technol. 2010, 45, 147–154. [Google Scholar] [CrossRef]
- Amado, I.R.; Vázquez, J.A.; González, M.P.; Murado, M.A. Production of antihypertensive and antioxidant activities by enzymatic hydrolysis of protein concentrates recovered by ultrafiltration from cuttlefish processing wastewaters. Biochem. Eng. J. 2013, 76, 43–54. [Google Scholar] [CrossRef]
- Giménez, B.; López-Caballero, E.M.; Montero, P.M.; Gómez-Guillén, C.M. Antioxidant peptides from marine origin: Sources, properties and potential applications. In Antioxidant Polymers: Synthesis, Properties, and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 203–257. [Google Scholar]
- Lee, J.K.; Jeon, J.K.; Kim, S.K.; Byun, H.G. Characterization of bioactive peptides obtained from marine invertebrates. Adv. Food Nutr. Res. 2012, 65, 47–72. [Google Scholar] [PubMed]
- Yan, N.; Chen, X. Sustainability: Don’t waste seafood waste. Nature 2015, 524, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations (FAO). The State of World Fisheries and Aquaculture 2014. Opportunities and Challenges; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). The State of World Fisheries and Aquaculture 2016. Contributing to Food Security and Nutrition for All; Food and Agriculture Organization of the United Nations: Rome, Italy, 2016. [Google Scholar]
- Olsen, R.L.; Toppe, J.; Karunasagar, I. Challenges and realistic opportunities in the use of by-products from processing of fish and shellfish. Trends Food Sci. Technol. 2014, 36, 144–151. [Google Scholar] [CrossRef]
| Antioxidant Peptides | References |
|---|---|
| VKP, VKCFR | [42] |
| IKK, FKK, FIKK | [48] |
| HMSY, PEASY | [49] |
| LWHTH | [50] |
| LPHPSF | [45] |
| PIIVYWK, FSVVPSPK, TTANIEDRR | [32] |
| GPLGLLGFLGPLGLS | [51] |
| Species | Protease Used for Hydrolysis * | Antioxidant Parameters Used to Guide Purification and Characterize Purified Peptides | Purification Techniques | Peptide Sequence Identified | Validated with Synthetic Peptides | Reference |
|---|---|---|---|---|---|---|
| Oyster (Crassostrea madrasensis) | Papain | DPPH scavenging OH• scavenging # FRAP # Iron chelating # LPI # | UF SPE RP-HPLC | ISIGGQPAGRIVM | × | [36] |
| Oyster (Crassostrea gigas) | In vitro gastrointestinal digestion (Pepsin, Trypsin and α-Chymotrypsin | OH• scavenging O2•− scavenging Cellular radical scavenging Protection against OH•-induced DNA damage LPI | AEC RP-HPLC(×2) | LKQELEDLLEKQE | × | [40] |
| Oyster (Crassostrea talienwhanensis) | Subtilisin (Alcalase) | DPPH scavenging OH• scavenging | UF SEC RP-HPLC(×2) | PVMGA QHGV | × | [35] |
| Mussel (Mytilus coruscus) | Papain | DPPH scavenging OH• scavenging O2•− scavenging Alkyl radical scavenging In vivo antioxidant defense | UF AEC RP-HPLC(×2) GPC | SLPIGLMIAM | × | [44] |
| Mussel (Mytilus coruscus) | In vitro gastrointestinal digestion (Pepsin, Trypsin and α-Chymotrypsin) | LPI OH• scavenging O2•− scavenging Carbon-centered radical scavenging | AEC SEC RP-HPLC | LVGDEQAVPAVCVP | × | [52] |
| Blue mussel (Mytilus edulis) | Pepsin | DPPH scavenging ORAC Protection against H2O2-induced cytotoxicity | UF CEC RP-HPLC(×2) | PIIVYWK TTANIEDRR FSVVPSPK | √ | [32] |
| Blue mussel (Mytilus edulis) | Neutrase | DPPH scavenging OH• scavenging O2•− scavenging LPI | UF SEC RP-HPLC | YPPAK | × | [31] |
| Blood Clam (Tegillarca granosa) | Neutrase | DPPH scavenging ABTS scavenging OH• scavenging O2•− scavenging LPI | UF AEC SEC RP-HPLC | WPP QP | × | [33] |
| Short-necked Clam (Ruditapes philippinarum) | α-Chymotrypsin | DPPH scavenging OH• scavenging Alkyl radicalscavenging O2•− scavenging | UF AEC RP-HPLC(×3) | SVEIQALCDM | × | [53] |
| Short-necked Clam (Ruditapes philippinarum) | Trypsin | DPPH scavenging Reducing power Protection against OH•-induced DNA damage | UF SEC RP-HPLC | GDQQK | × | [34] |
| Scallop (Patinopecten yessoensis) | Neutrase | DPPH scavenging # OH• scavenging Iron chelating # Reducing power # Protection against OH•-induced DNA damage | SEC | HMSY PEASY | √ | [49] |
| Jellyfish (Rhopilema esculentum) | Alcalase | OH• scavenging Protection against H2O2-induced cytotoxicity Cellular antioxidant enzyme activity | UF AEC RP-HPLC | VKP VKCFR | √ | [42] |
| Jumbo squid (Dosidicus gigas) | Trypsin | LPI OH• scavenging Carbon-centered radical scavenging Iron chelating # Protection against t-butyl hydroperoxide-induced cytotoxicity | UF CEC SEC RP-HPLC | FDSGPAGVL NGPLQAGQPGER | × | [41] |
| Giant squid (Dosidicus gigas) | Trypsin | LPI OH• scavenging O2•− scavenging Carbon-centered radical scavenging Protection against t-butyl hydroperoxide-induced cytotoxicity | UF CEC SEC RP-HPLC(×2) | NADFGLNGLEGLA NGLEGLK | × | [43] |
| Giant squid (Dosidicus gigas) (skin) | Alcalase | ABTS scavenging FRAP | UF SEC | GPLGLLGFLGPLGLS | √ | [51] |
| Shortclub cuttlefish (Sepia brevimana) | Trypsin | DPPH scavenging ABTS scavenging # O2•− scavenging # Total antioxidant capacity # Reducing power Iron chelating # LPI Protection against OH•-induced DNA damage | AEC SEC | I/L N I/L CCN | × | [56] |
| Indian squid (Loligo duvauceli) | α-chymotrypsin | DPPH scavenging OH• scavenging O2•− scavenging # Reducing power Iron chelating LPI Protection against OH•-induced DNA damage Cellular radical scavenging | AEC SEC | WCTSVS | × | [57] |
| Prawn (Penaeus japonicus) | Pepsin | LPI | SEC CEC RP-HPLC | IKK FKK FIKK | √ | [48] |
| Shrimp processing by-products | Alcalase | DPPH scavenging | Methanol extraction CEC SEC RP-HPLC(×2) | SVAMLFH | × | [54] |
| Sea cucumber (Stichopus japonicus) | Trypsin | OH• scavenging O2•− scavenging | SEC AEC SEC RP-HPLC | GPEPTGPT GAPQWLR | × | [55] |
| Sea squirt (Styela clava) | Pepsin | Peroxyl radical scavenging | SEC RP-HPLC | LWHTH | √ | [50] |
| Sea squirt (Styela plicata) | Trypsin | Peroxyl radical scavenging DPPH scavenging OH• scavenging Cellular radical scavenging Protection against APPH-induced cytotoxicity Protection against APPH-induced ROS generation and cell death in zebrafish embryos | AEC SEC RP-HPLC | LPHPSF | √ | [45] |
| Antioxidant Peptides | Molecular Mass (Da) | References |
|---|---|---|
| QP | 243.23 | [33] |
| VKP | 342 | [42] |
| IKK | 388 | [48] |
| WPP | 398.44 | [33] |
| FKK | 422 | [48] |
| QHGV | 440 | [35] |
| PVMGA | 518 | [35] |
| FIKK | 535 | [48] |
| HMSY | 536.16 | [49] |
| PEASY | 565.21 | [49] |
| YPPAK | 574 | [31] |
| GDQQK | 574.27 * | [34] |
| VKCFR | 651 | [42] |
| I/L N I/L CCN | 679.5 | [56] |
| WCTSVS | 682.5 | [57] |
| LWHTH | 692.2 | [50] |
| LPHPSF | 696.3 | [45] |
| NGLEGLK | 747 | [43] |
| SVAMLFH | 804.4 | [54] |
| FSVVPSPK | 860.09 | [32] |
| FDSGPAGVL | 880.18 | [41] |
| PIIVYWK | 1004.57 | [32] |
| SLPIGLMIAM | 1044.57 * | [44] |
| TTANIEDRR | 1074.54 | [32] |
| SVEIQALCDM | 1107.49 * | [53] |
| NGPLQAGQPGER | 1241.59 | [41] |
| ISIGGQPAGRIVM | 1297.72 | [36] |
| NADFGLNGLEGLA | 1307 | [43] |
| GPLGLLGFLGPLGLS | 1409.63 ** | [51] |
| GPEPTGPTGAPQWLR | 1563 | [55] |
| LVGDEQAVPAVCVP | 1590 | [52] |
| LKQELEDLLEKQE | 1600 | [40] |
| Antioxidant Peptides | Hydrophobic Amino Acid Residue (%) * | References |
|---|---|---|
| NGLEGLK | 28.57 | [43] |
| LKQELEDLLEKQE | 30.77 | [40] |
| NGPLQAGQPGER | 33.33 | [41] |
| IKK | 33.33 | [48] |
| FKK | 33.33 | [48] |
| WCTSVS | 33.33 | [57] |
| I/L N I/L CCN | 33.33 | [56] |
| FDSGPAGVL | 44.44 | [41] |
| NADFGLNGLEGLA | 46.15 | [43] |
| QP | 50 | [33] |
| FIKK | 50 | [48] |
| ISIGGQPAGRIVM | 53.85 | [36] |
| YPPAK | 60 | [31] |
| LVGDEQAVPAVCVP | 64.29 | [52] |
| WPP | 100 | [33] |
| Antioxidant Peptides | Hydrophobic Amino Acid Residue (%) * | References |
|---|---|---|
| TTANIEDRR | 22.22 | [32] |
| HMSY | 25 | [49] |
| IKK | 33.33 | [48] |
| FKK | 33.33 | [48] |
| PEASY | 40 | [49] |
| LWHTH | 40 | [50] |
| VKCFR | 40 | [42] |
| FIKK | 50 | [48] |
| GPLGLLGFLGPLGLS | 60 | [51] |
| FSVVPSPK | 62.5 | [32] |
| VKP | 66.67 | [42] |
| LPHPSF | 66.67 | [45] |
| PIIVYWK | 71.43 | [32] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chai, T.-T.; Law, Y.-C.; Wong, F.-C.; Kim, S.-K. Enzyme-Assisted Discovery of Antioxidant Peptides from Edible Marine Invertebrates: A Review. Mar. Drugs 2017, 15, 42. https://doi.org/10.3390/md15020042
Chai T-T, Law Y-C, Wong F-C, Kim S-K. Enzyme-Assisted Discovery of Antioxidant Peptides from Edible Marine Invertebrates: A Review. Marine Drugs. 2017; 15(2):42. https://doi.org/10.3390/md15020042
Chicago/Turabian StyleChai, Tsun-Thai, Yew-Chye Law, Fai-Chu Wong, and Se-Kwon Kim. 2017. "Enzyme-Assisted Discovery of Antioxidant Peptides from Edible Marine Invertebrates: A Review" Marine Drugs 15, no. 2: 42. https://doi.org/10.3390/md15020042
APA StyleChai, T.-T., Law, Y.-C., Wong, F.-C., & Kim, S.-K. (2017). Enzyme-Assisted Discovery of Antioxidant Peptides from Edible Marine Invertebrates: A Review. Marine Drugs, 15(2), 42. https://doi.org/10.3390/md15020042








