Molecular Weight-Dependent Immunostimulative Activity of Low Molecular Weight Chitosan via Regulating NF-κB and AP-1 Signaling Pathways in RAW264.7 Macrophages
Abstract
:1. Introduction
2. Results
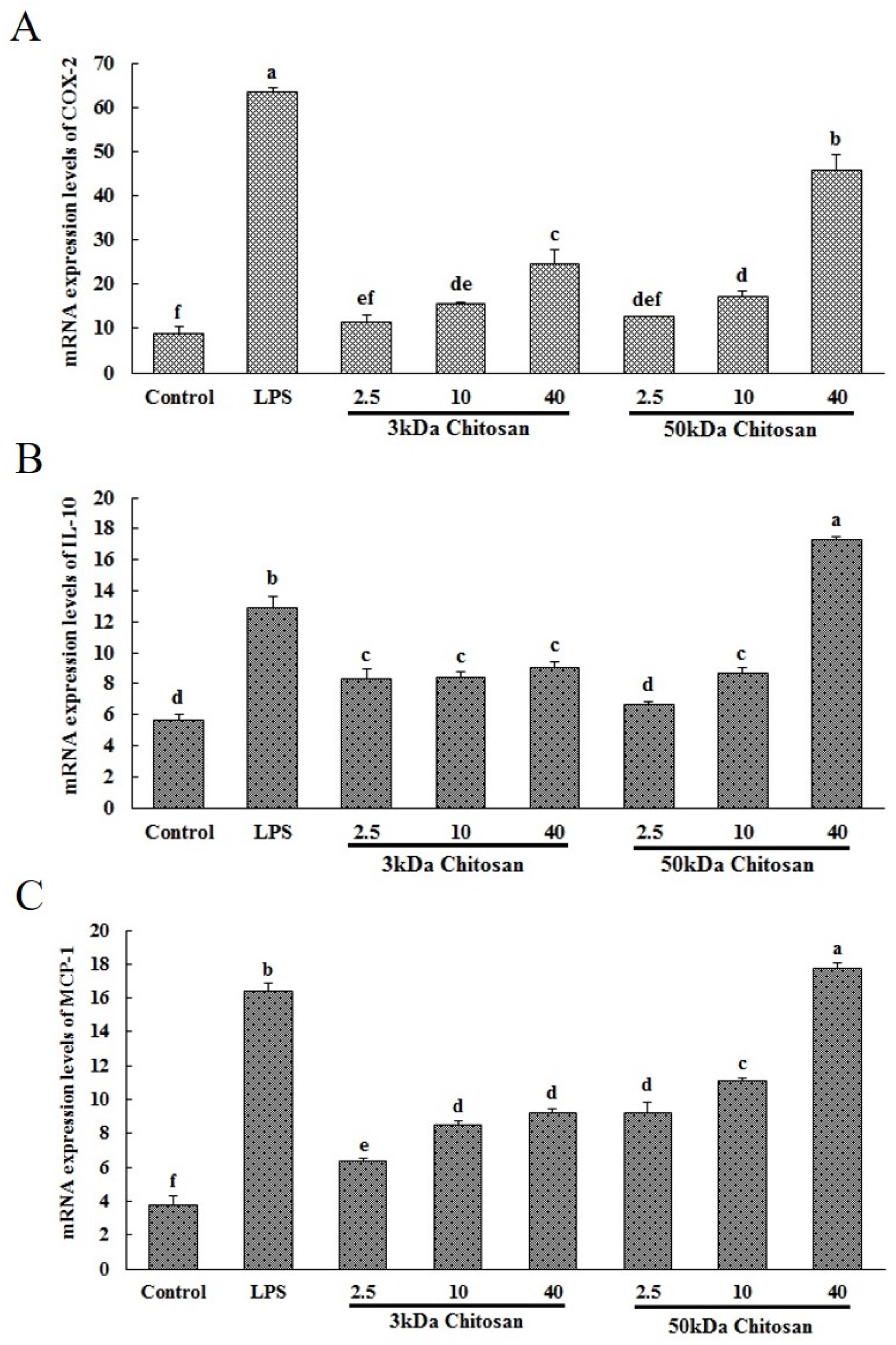
2.1. Effects of LMWCs on the mRNA Expression Levels of COX-2, IL-10 and MCP-1 in RAW264.7 Macrophages
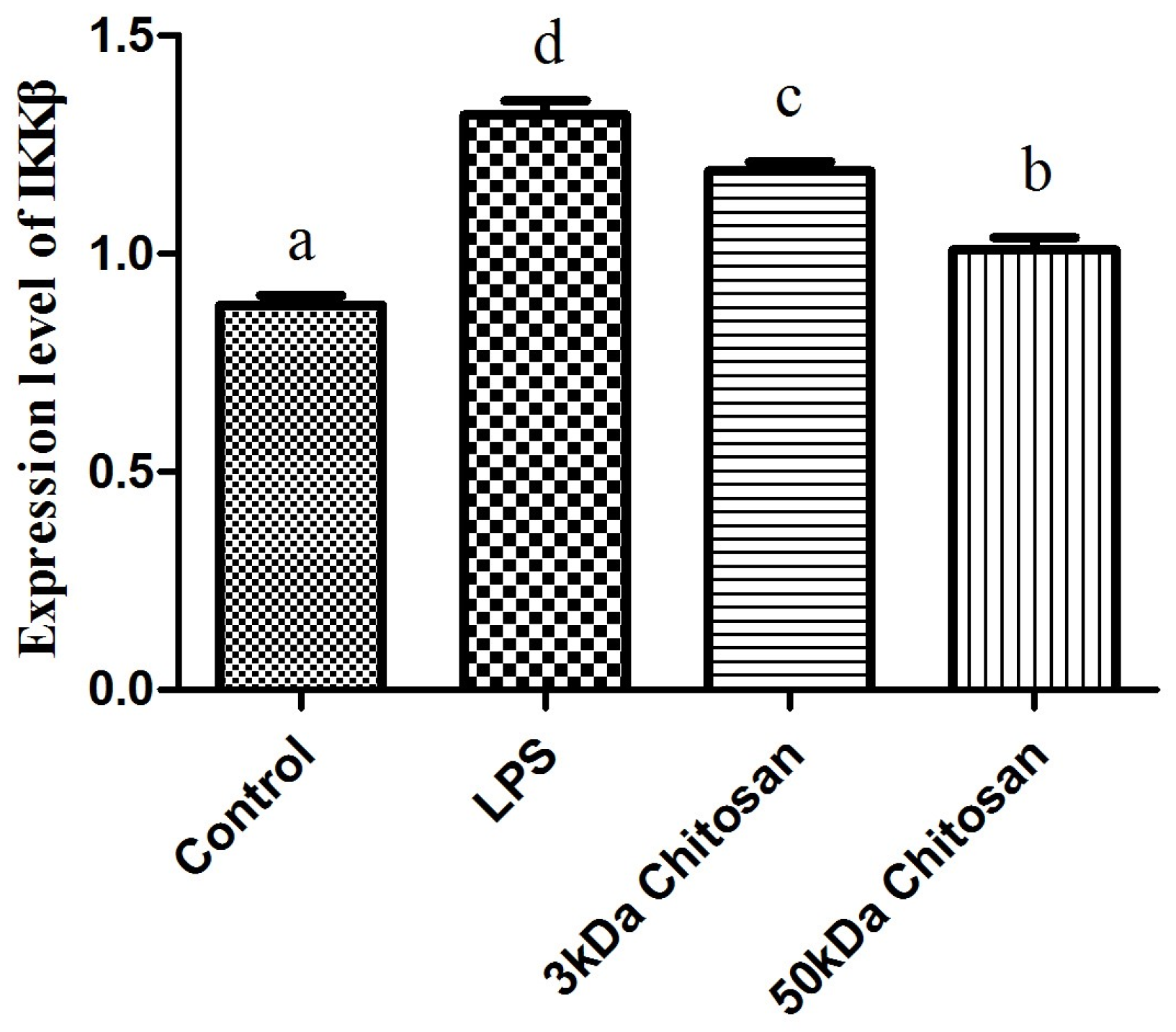
2.2. Effects of LMWCs on the mRNA Expression Levels of IKKβ in RAW264.7 Macrophages
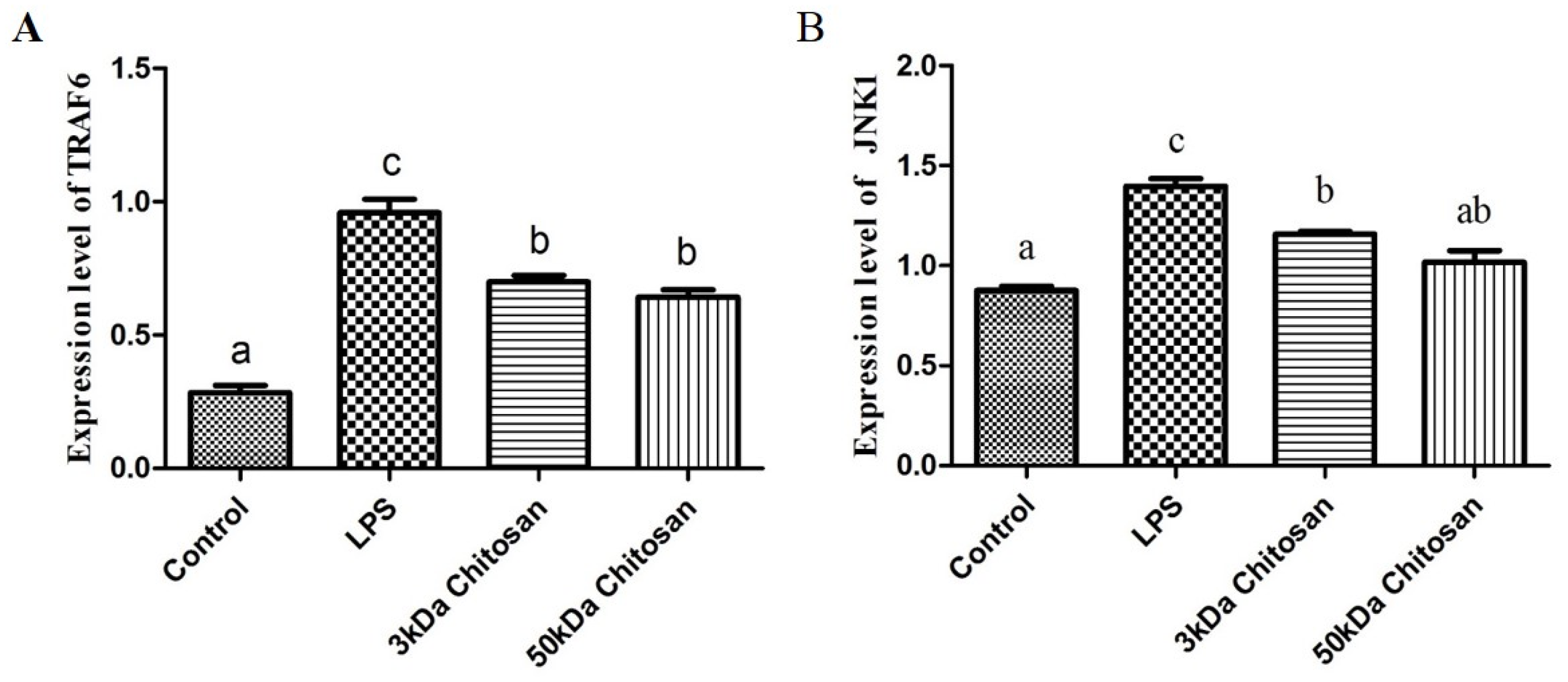
2.3. Effect of LMWCs on the mRNA Expression Levels of Key Molecules (TRAF6, JNK1) in RAW264.7 Macrophages
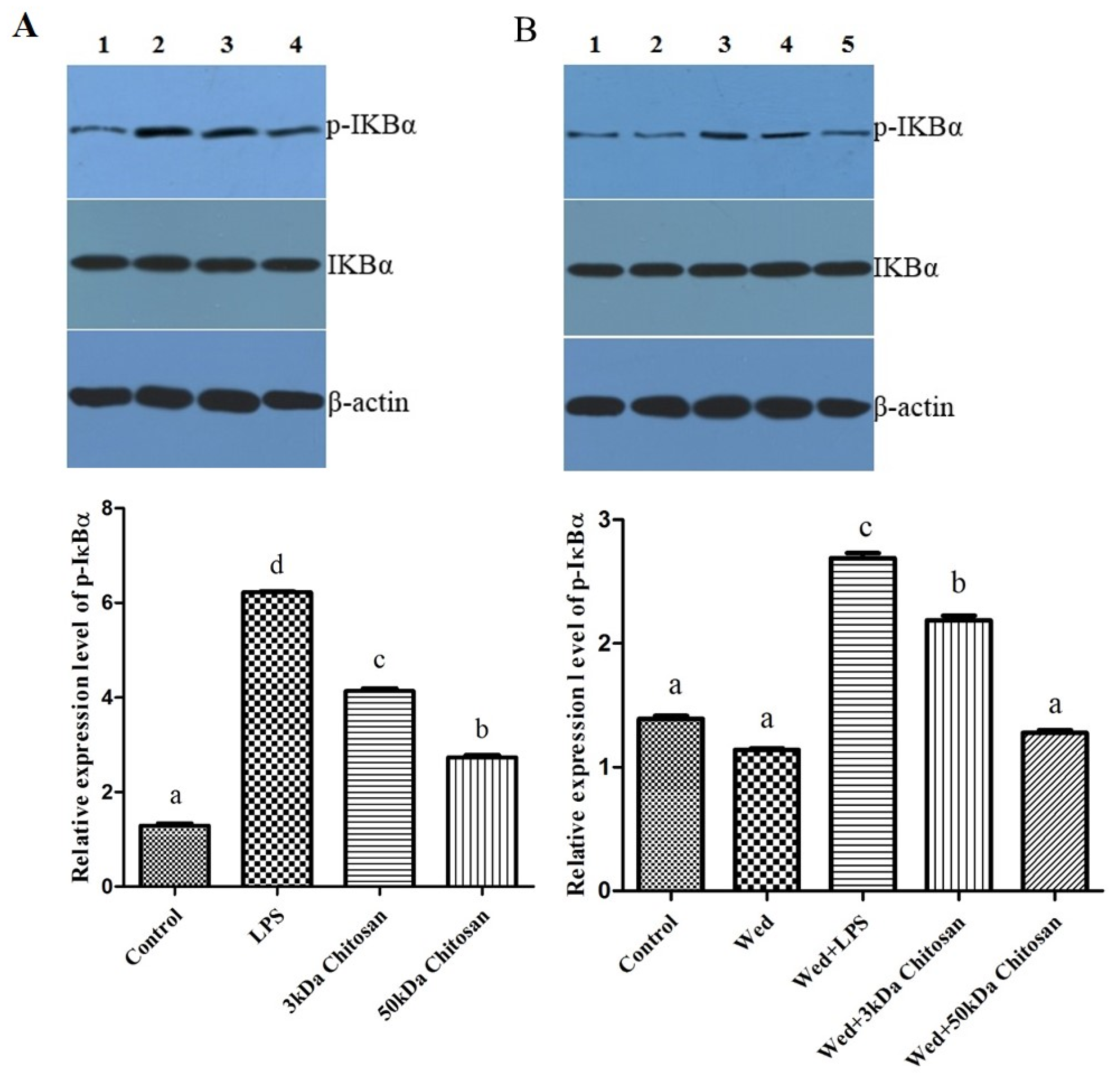
2.4. Effects of LMWCs on the Phosphorylation of IKBα in the RAW264.7 Cells
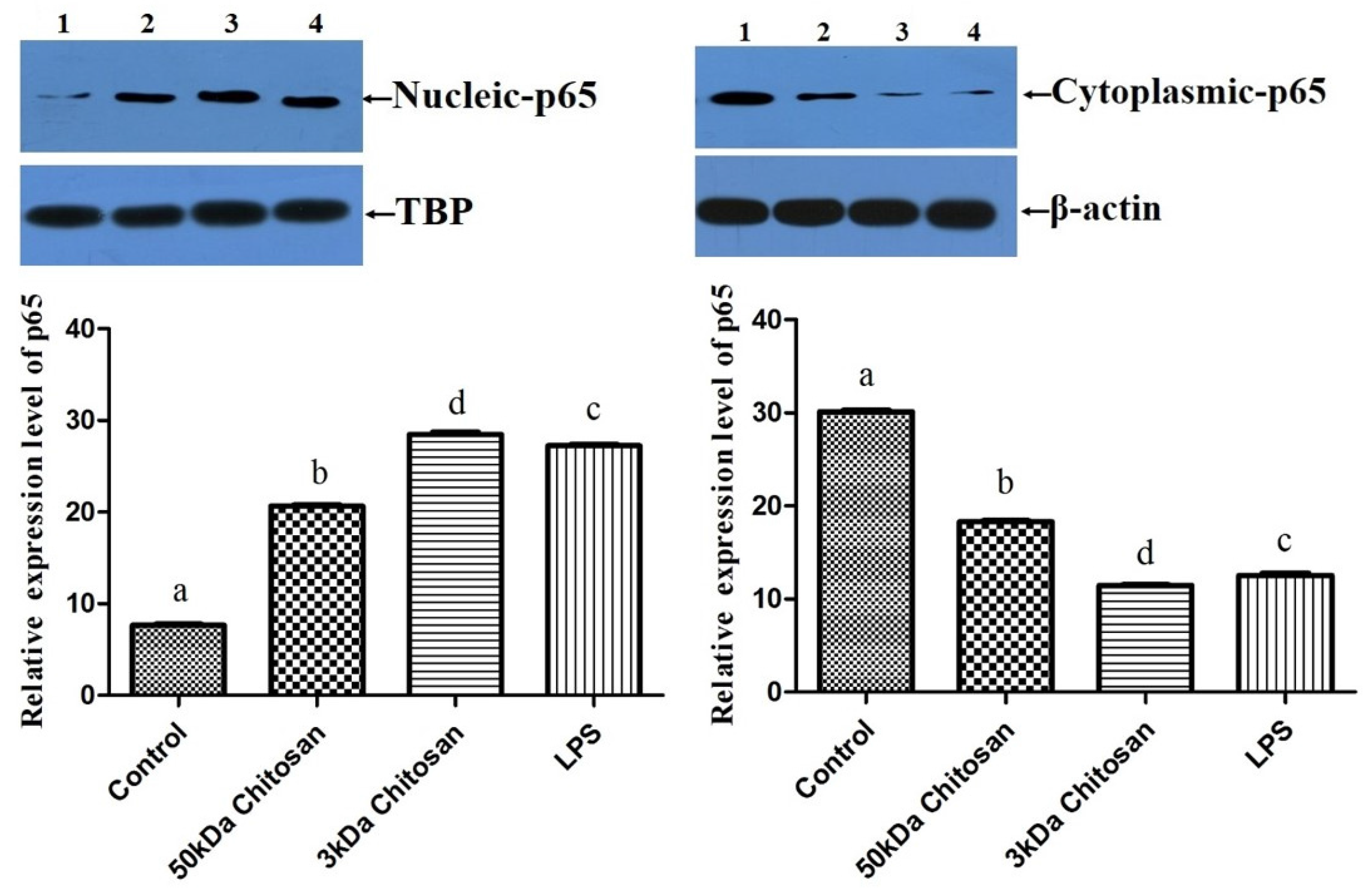
2.5. Effects of LMWCs on the Protein Expression of p65 in RAW264.7 Macrophages
2.6. Effects of LMWCs on the Expression of AP-1 (C-Jun and C-Fos) in RAW264.7 Macrophages
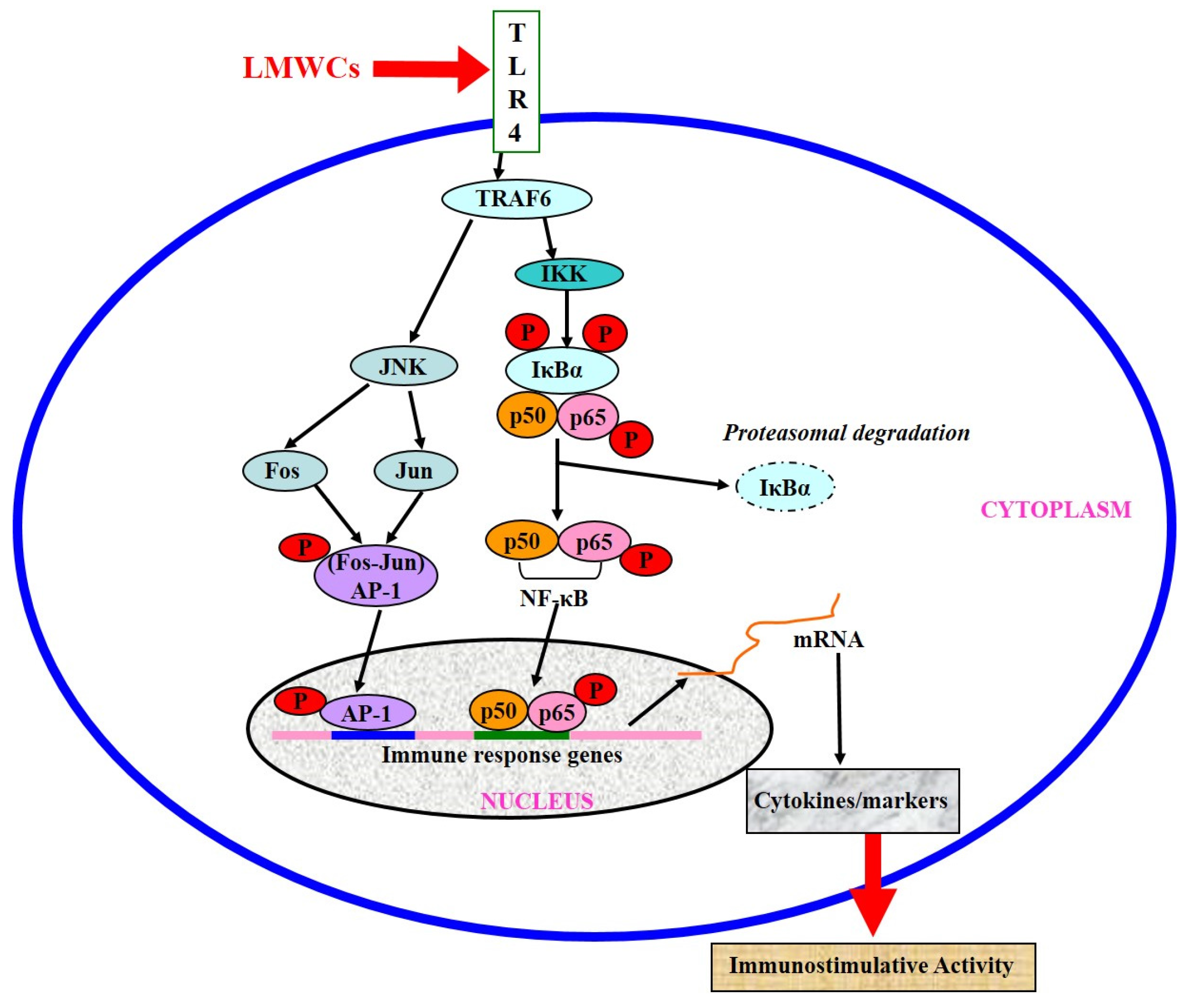
3. Discussion
4. Experimental Section
4.1. Chemicals and Reagents
4.2. Cell Culture and Treatment
4.3. Real-Time Fluorescent Quantitative Reverse Transcription-Polymerase Chain Reaction (RT-PCR) Analysis
4.4. Western Blot Analysis
4.5. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zaharoff, D.A.; Rogers, C.J.; Hance, K.W.; Schlom, J.; Greiner, J.W. Chitosan solution enhances both humoral and cell-mediated immune responses to subcutaneous vaccination. Vaccine 2007, 25, 2085–2094. [Google Scholar] [CrossRef] [PubMed]
- Read, R.C.; Naylor, S.C.; Potter, C.W.; Bond, J.; Jabbal-Gill, I.; Fisher, A.; Illum, L.; Jennings, R. Effective nasal influenza vaccine delivery using chitosan. Vaccine 2005, 23, 4367–4374. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.H.; Cosgrove, C.; McNeela, E.A.; Sexton, A.; Giemza, R.; Jabbal-Gill, I.; Church, A.; Lin, W.; Illum, L.; Podda, A.; et al. Protective levels of diphtheria-neutralizing antibody induced in healthy volunteers by unilateral priming-boosting intranasal immunization associated with restricted ipsilateral mucosal secretory immunoglobulin a. Infect. Immun. 2003, 71, 726–732. [Google Scholar] [CrossRef] [PubMed]
- McNeela, E.A.; Jabbal-Gill, I.; Illum, L.; Pizza, M.; Rappuoli, R.; Podda, A.; Lewis, D.J.; Mills, K.H. Intranasal immunization with genetically detoxified diphtheria toxin induces T cell responses in humans: Enhancement of Th2 responses and toxin-neutralizing antibodies by formulation with chitosan. Vaccine 2004, 22, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.-S.; Xu, Y.-L.; Zou, X.-T.; Xu, Z.-R. Chitosan Nanoparticles Act as an Adjuvant to Promote both Th1 and Th2 Immune Responses Induced by Ovalbumin in Mice. Mar. Drugs 2011, 9, 1038–1055. [Google Scholar] [CrossRef] [PubMed]
- Eun, J.J.; Dal, K.Y.; Shin, H.L.; Hong, K.N.; Jong, G.H.; Prinyawiwatkul, W. Antibacterial activity of chitosans with different degrees of deacetylation and viscosities. Int. J. Food Sci. Technol. 2010, 45, 676–682. [Google Scholar]
- Artan, M.; Karadeniz, F.; Karagozlu, M.Z.; Kim, M.M.; Kim, S.K. Anti-HIV-1 activity of low molecular weight sulfated chitooligosaccharides. Carbohydr. Res. 2010, 345, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.C.; Spindola, H.; de Sousa, V.; Santos-Silva, A.; Pintado, M.E.; Malcata, F.X.; Carvalho, J.E. Anti-inflammatory activity of chitooligosaccharides in vivo. Mar. Drugs 2010, 8, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.N.; Lee, S.H.; Kim, M.; Kim, S.K. Production of chitin-oligosaccharides with different molecular weights and their antioxidant effect in RAW264.7 cells. J. Funct. Foods 2009, 1, 188–198. [Google Scholar] [CrossRef]
- Ju, C.X.; Yue, W.; Yang, Z.H.; Zhang, Q.; Yang, X.; Liu, Z.; Zhang, F. Antidiabetic effect and mechanism of chitooligosaccharides. Biol. Pharm. Bull. 2010, 33, 1511–1516. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Gong, L.; Gu, X.; Ding, F. Chitooligosaccharides promote peripheral nerve regeneration in a rabbit common peronial nerve crush injury model. Microsurgery 2009, 29, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Zhu, F.; Han, X.; Xu, Z.; Zhao, Y.; Miao, Z. Mechanism of anti-angiogenic activities of chitooligosaccharides may be through inhibiting heparanase activity. Med. Hypothesis 2009, 73, 205–206. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Da Silva, C.A.; Lee, J.Y.; Hartl, D.; Elias, J.A. Chitin regulation of immune responses: An old molecule with new roles. Curr. Opin. Immunol. 2008, 20, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Min, M.; Du, N.; Gu, Y.; Hode, T.; Naylor, M.; Chen, D.; Nordquist, R.E.; Chen, W.R. Chitin, Chitosan, and Glycated Chitosan Regulate Immune Responses: The Novel Adjuvants for Cancer Vaccine. Clin. Dev. Immunol. 2013, 2013, 387023. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Mikami, T.; Okawa, Y.; Tokoro, A.; Suzuki, S.; Suzuki, M. Antitumor effect of hexa-N-acetylchitohexaose and chitohexaose. Carbohydr. Res. 1986, 151, 403–408. [Google Scholar] [CrossRef]
- Okamoto, Y.; Inoue, A.; Miyatake, K.; Ogihara, K.; Shigemasa, Y.; Minami, S. Effects of chitin/chitosan and their oligomers/monomers on migrations of macrophages. Macromol. Biosci. 2003, 3, 587–590. [Google Scholar] [CrossRef]
- Yang, E.J.; Kim, J.G.; Kim, J.Y.; Kim, S.C.; Lee, N.H.; Hyun, C. Anti-inflammatory effect of chitosan oligosaccharides in RAW264.7 cells. Cent. Eur. J. Biol. 2009, 5, 95–102. [Google Scholar]
- Wu, N.; Wen, Z.; Xiang, X.; Huang, Y.; Gao, Y.; Qu, Y. Immunostimulative activity of low molecular weight chitosans in RAW264.7 macrophages. Mar. Drugs 2015, 13, 6210–6225. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Wang, C.Y.; Yang, F.Y.; Wang, B.S.; Chen, J.Y.; Lin, L.T.; Leu, J.D.; Chiu, S.J.; Chen, F.D.; Lee, Y.J.; et al. Synergistic effects of glycated chitosan with high-intensity focused ultrasound on suppression of metastases in a syngeneic breast tumor model. Cell Death Dis. 2014, 5, e1178. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.A.; Chalouni, C.; Williams, A.; Hartl, D.; Lee, C.G.; Elias, J.A. Chitin is a size-dependent regulator of macrophage TNF and IL-10 production. J. Immunol. 2009, 182, 3573–3582. [Google Scholar] [CrossRef] [PubMed]
- Bueter, C.L.; Lee, C.K.; Rathinam, V.A.; Healy, G.J.; Taron, C.H.; Specht, C.A.; Levitz, S.M. Chitosan but not chitin activates the inflammasome by a mechanism dependent upon phagocytosis. J. Biol. Chem. 2011, 286, 35447–35455. [Google Scholar] [CrossRef] [PubMed]
- Farias-Eisner, R.; Sherman, M.P.; Aeberhard, E.; Chaudhuri, G. Nitric oxide is an important mediator for tumoricidal activity in vivo. Proc. Natl. Acad. Sci. USA 1994, 91, 9407–9411. [Google Scholar] [CrossRef] [PubMed]
- Rauh, M.J.; Ho, V.; Pereira, C.; Sham, A.; Sly, L.M.; Lam, V.; Huxham, L.; Minchinton, A.I.; Mui, A.; Krystal, G. SHIP represses the generation of alternatively activated macrophages. Immunity 2005, 23, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002, 23, 549–555. [Google Scholar] [CrossRef]
- Bishop, G.A. The multifaceted roles of TRAFs in the regulation of B-cell function. Nat. Rev. Immunol. 2004, 4, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Zimmermann, A.G.; Roberts, R.A.; Zhang, L.; Swanson, K.V.; Wen, H.; Davis, B.K.; Allen, I.C.; Holl, E.K.; Ye, Z.; et al. The innate immune sensor NLRC3 attenuates Toll-like receptor signaling via modification of the signaling adaptor TRAF6 and transcription factor NF-κB. Nat. Immunol. 2012, 13, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Lin, S.C.; Lamothe, B.; Lu, M.; Lo, Y.C.; Hura, G.; Zheng, L.; Rich, R.L.; Campos, A.D.; Myszka, D.G.; et al. E2 interaction and dimerization in the crystal structure of TRAF6. Nat. Struct. Mol. Biol. 2009, 16, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Fujimoto, Y.; Lucas, P.C.; Nakano, H.; Fukase, K.; Núñez, G.; Inohara, N. A critical role of RICK/RIP2 polyubiquitination in Nod-induced NF-κB activation. EMBO J. 2008, 27, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Delhase, M. The IκB kinase (IKK) and NF-κB: Key elements of proinflammatory signaling. Semin. Immunol. 2000, 12, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Beutler, B. Tlr4: Central component of the sole mammalian LPS sensor. Curr. Opin. Immunol. 2000, 12, 20–26. [Google Scholar] [CrossRef]
- Janeway, C.A., Jr.; Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, W.; Peng, Y.; Han, B.; Yang, Y. Toll like receptor 4 (TLR4) mediates the stimulating activities of chitosan oligosaccharide on macrophages. Int. Immunopharmacol. 2014, 23, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Li, S.; Wang, W.; Wang, S.; Zou, M.; Guo, Y.; Fan, J.; Du, Y.; Zhang, J. The effects of chitosan oligosaccharide on the activation of murine spleen CD11c+ dendritic cells via Toll-like receptor 4. Carbohydr. Polym. 2011, 83, 1075–1081. [Google Scholar] [CrossRef]
- Muzzarelli, R.A. Human enzymatic activities related to the therapeutic administration of chitin derivatives. Cell. Mol. Life Sci. 1997, 53, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Angel, P.; Karin, M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim. Biophys. Acta 1991, 1072, 129–157. [Google Scholar] [CrossRef]
- Shaulian, E.; Karin, M. AP-1 in cell proliferation and survival. Oncogene 2001, 20, 2390–2400. [Google Scholar] [CrossRef] [PubMed]
- Whitmarsh, A.J.; Davis, R.J. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J. Mol. Med. 1996, 74, 589–607. [Google Scholar] [CrossRef] [PubMed]
- Pearson, G.; Robinson, F.; Beers Gibson, T.; Xu, B.E.; Karandikar, M.; Berman, K.; Cobb, M.H. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr. Rev. 2001, 22, 153–183. [Google Scholar] [CrossRef] [PubMed]
- Giri, R.S.; Thaker, H.M.; Giordano, T.; Williams, J.; Rogers, D.; Sudersanam, V.; Vasu, K.K. Design, synthesis and characterization of novel 2-(2,4-disubstituted-thiazole-5-yl)-3-aryl-3Hquinazoline-4-one derivatives as inhibitors of NF-κB and AP-1 mediated transcription activation and as potential anti-inflammatory agents. Eur. J. Med. Chem. 2009, 44, 2184–2189. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Tilló, E.; Comalada, M.; Xaus, J.; Farrera, C.; Valledor, A.F.; Caelles, C.; Lloberas, J.; Celada, A. JNK1 Is required for the induction of Mkp1 expression in macrophages during proliferation and lipopolysaccharide-dependent activation. J. Biol. Chem. 2007, 282, 12566–12573. [Google Scholar] [CrossRef] [PubMed]
- Guma, M.; Ronacher, L.M.; Firestein, G.S.; Karin, M.; Corr, M. JNK1 deficiency limits macrophage mediated antigen-induced arthritis. Arthritis Rheumatol. 2011, 63, 1603–1612. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Arron, J.R. TRAF6, a molecular bridge spanning adaptive immunity, innate immunity and osteoimmunology. Bioessays 2003, 25, 1096–1105. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.J.; Tsai, G.J. Chitooligosaccharides in Combination with Interferon-γ Increase Nitric Oxide Production via Nuclear Factor-κB Activation in Murine RAW264.7 Macrophages. Food Chem. Toxicol. 2007, 45, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, C.; Chen, X.; Wang, J.; Tian, J. Effects of Five Chitosan Oligosaccharides on Nuclear Factor-kappa B Signaling Pathway. J. Wuhan Univ. Technol.—Mater. Sci. Ed. 2012, 27, 276–279. [Google Scholar] [CrossRef]
- Bahar, B.; O’Doherty, J.V.; Maher, S.; McMorrow, J.; Sweeney, T. Chitooligosaccharide elicits acute inflammatory cytokine response through AP-1 pathway in human intestinal epithelial-like (Caco-2) cells. Mol. Immunol. 2012, 51, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Moynagh, P.N. The NF-kappaB Pathway. J. Cell Sci. 2005, 118, 4589–4592. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Shared Principles in NF-κB Signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔCt Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

| Gene | Genbank Accession | Primer Sequence | Product Size (bp) | Annealing (°C) |
|---|---|---|---|---|
| COX2 | NM_011198.3 | TCTGGCTTCGGGAGCACAAC | 144 | 64 |
| GGTGTTGCACGTAGTCTTCGATCA | ||||
| IL-10 | NM_010548.2 | CAGTCGGCCAGAGCCACAT | 144 | 64 |
| CTTGGCAACCCAAGTAACCCTT | ||||
| MCP-1 | NM_011333.3 | CTGCATCTGCCCTAAGGTCTTCA | 101 | 64 |
| AGTGCTTGAGGTGGTTGTGGAA | ||||
| TRAF6 | D84655.1 | GGAGGACAAGGTTGCCGAAAT | 73 | 63 |
| CCCAAACTTGCCAATCTTCCAA | ||||
| JNK1 | NM_001310454.1 | GCTCTCCAGCACCCATACATCA | 109 | 63 |
| CCTCTATTGTGTGCTCCCTCTCAT | ||||
| IKKβ | AF088910.1 | CAGAAGTACACCGTGACCGTTGA | 104 | 63 |
| CACTGCACAGGCTGCCAGTTA | ||||
| 18S | NR_003278 | CGGACACGGACAGGATTGACA | 94 | 64 |
| CCAGACAAATCGCTCCACCAACTA |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, B.; Wen, Z.-S.; Huang, Y.-J.; Xia, M.-S.; Xiang, X.-W.; Qu, Y.-L. Molecular Weight-Dependent Immunostimulative Activity of Low Molecular Weight Chitosan via Regulating NF-κB and AP-1 Signaling Pathways in RAW264.7 Macrophages. Mar. Drugs 2016, 14, 169. https://doi.org/10.3390/md14090169
Zheng B, Wen Z-S, Huang Y-J, Xia M-S, Xiang X-W, Qu Y-L. Molecular Weight-Dependent Immunostimulative Activity of Low Molecular Weight Chitosan via Regulating NF-κB and AP-1 Signaling Pathways in RAW264.7 Macrophages. Marine Drugs. 2016; 14(9):169. https://doi.org/10.3390/md14090169
Chicago/Turabian StyleZheng, Bin, Zheng-Shun Wen, Yun-Juan Huang, Mei-Sheng Xia, Xing-Wei Xiang, and You-Le Qu. 2016. "Molecular Weight-Dependent Immunostimulative Activity of Low Molecular Weight Chitosan via Regulating NF-κB and AP-1 Signaling Pathways in RAW264.7 Macrophages" Marine Drugs 14, no. 9: 169. https://doi.org/10.3390/md14090169
APA StyleZheng, B., Wen, Z.-S., Huang, Y.-J., Xia, M.-S., Xiang, X.-W., & Qu, Y.-L. (2016). Molecular Weight-Dependent Immunostimulative Activity of Low Molecular Weight Chitosan via Regulating NF-κB and AP-1 Signaling Pathways in RAW264.7 Macrophages. Marine Drugs, 14(9), 169. https://doi.org/10.3390/md14090169





