Depolymerization of Fucosylated Chondroitin Sulfate with a Modified Fenton-System and Anticoagulant Activity of the Resulting Fragments
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Reaction Conditions on the Molecular Weights of Oxidative Depolymerized Products
2.2. Free Radical Degradation of fCS-Ib in a Controllable Fenton System
2.2.1. GPC, PAGE and Chemical Compositional Analysis
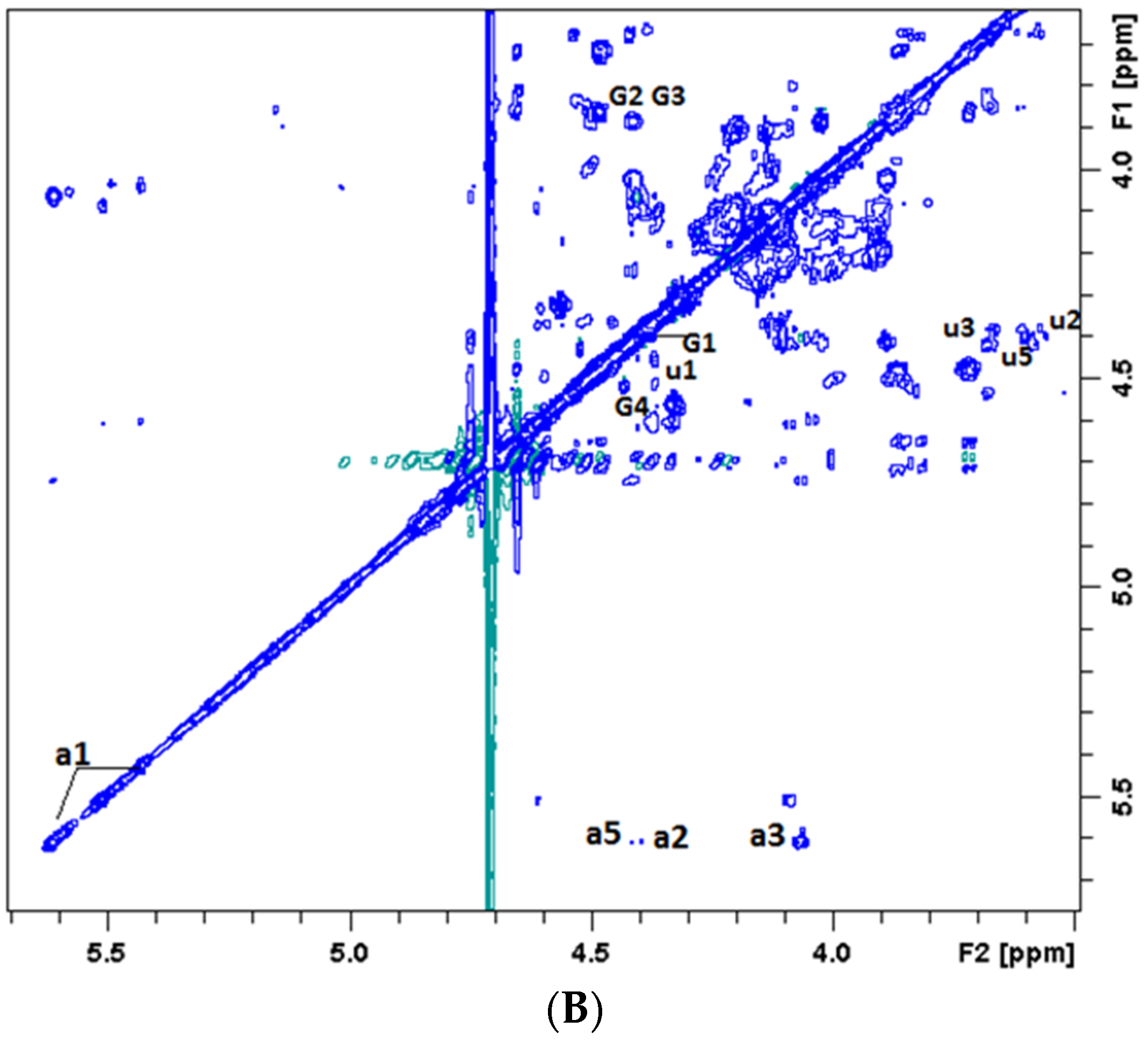
2.2.2. NMR Analysis of the Degradation Products
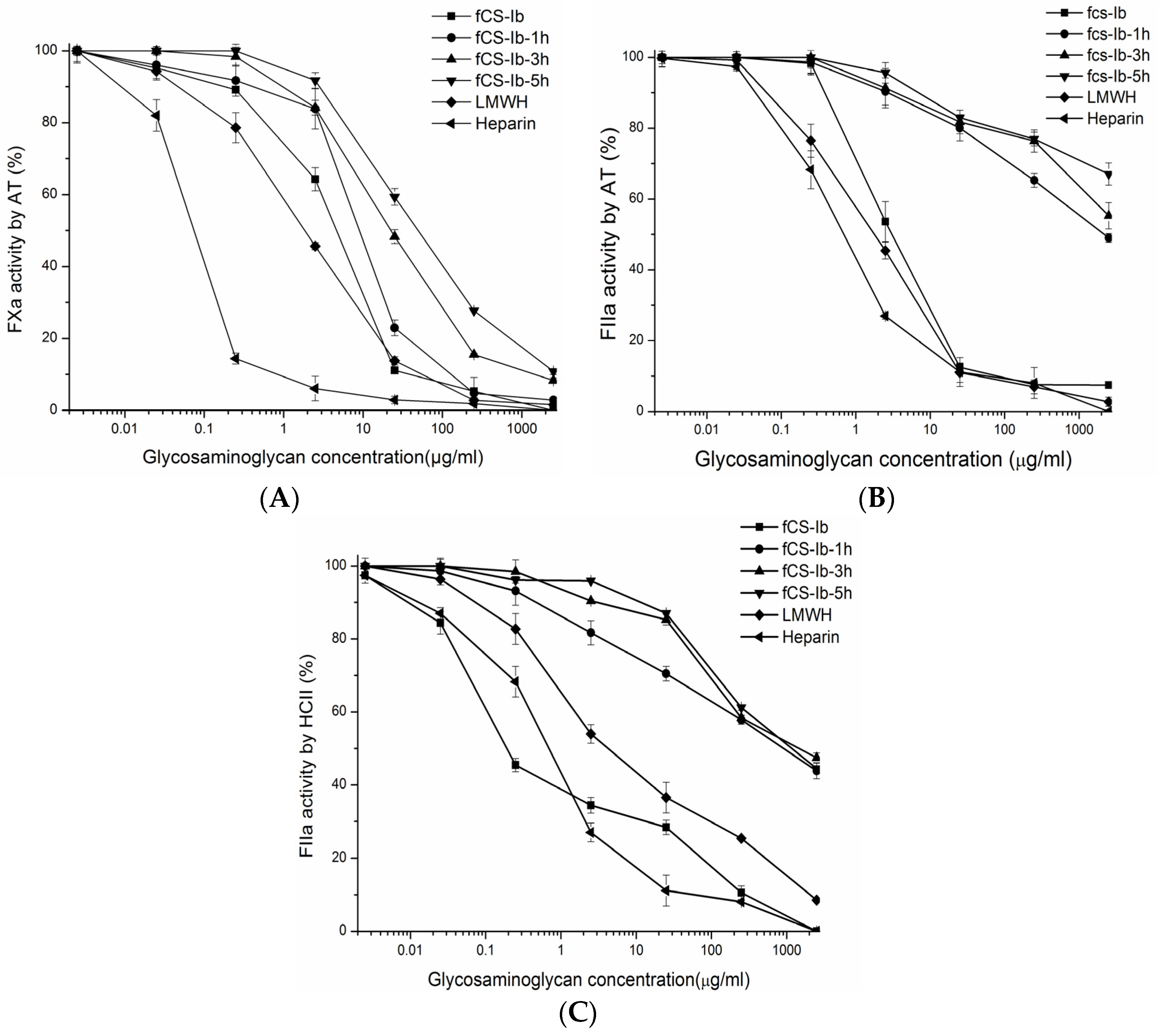
2.3. In Vitro Anticoagulant Activity Analysis of Oxidative Degradation Products
3. Experimental Section
3.1. Isolation and Purification of fCS-Ib
3.2. Free Radical Degradation of fCS-Ib in a Modified Fenton System
3.3. Chemical Composition Analysis of Oligosaccharide Fragments
3.4. NMR Analysis of Oligosaccharide Fragments
3.5. Anticoagulant Assays
3.6. Inhibition of Thrombin or FXa by AT III and HCII in the Presence of fCS-Ib and Its Depolymerized Products
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gao, N.; Wu, M.Y.; Liu, S.; Lian, W.; Li, Z.; Zhao, J.H. Preparation and characterization of O-acylatedfucosylated chondroitin sulfate from sea cucumber. Mar. Drgus 2012, 10, 1647–1661. [Google Scholar] [CrossRef] [PubMed]
- Melo-Filho, N.M.; Belmir, C.L.; Gonçalves, R.G.; Takiya, C.M., Jr.; Leite, M.; Pavão, M.S.G.; Mourão, P.A.S. Fucosylated chondroitin sulfate attenuates renal fibrosis in animals submitted to unilateral ureteral obstruction: A P-selectin-mediated event. Am. J. Physiol. Ren. Physiol. 2010, 299, F1299–F1307. [Google Scholar] [CrossRef] [PubMed]
- Borsig, L.; Wang, L.; Cavalcante, M.C.M.; Cardilo-Reis, L.; Ferreira, P.L.; Mourao, P.A.S.; Esko, J.D.; Pavao, M.S.G. Selectin blocking activity of a fucosylated chondroitin sulfate glycosaminoglycan from sea cucumber: Effect on tumor metastasis and neutrophil recruitment. J. Biol. Chem. 2007, 282, 14984–14991. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, Z.L.; Zhang, M.S.; Meng, X.M.; Xia, X.K.; Yuan, W.P.; Xue, F.; Liu, C.H. Antioxidant and antihyperlipidemic activities of polysaccharides from sea cucumber Apostichopusjaponicus. Carbohydr. Polym. 2012, 90, 1664–1670. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, R.J.; Oliveira, S.N.; Pomin, V.H.; Mecawi, A.S.; Araujo, I.G.; Mourao, P.A. Effects of oversulfated and fucosylated chondroitin sulfates on coagulation. Thromb. Haemost. 2010, 103, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Mourao, P.A.S.; Catherine, B.; Jacqueline, T.; Bruno, D.; Andreae, B.; Fischer, A.A. Inactivation of thrombin by a fucosylated chondroitin sulfate from Echinoderm. Thromb. Res. 2001, 102, 167–176. [Google Scholar] [CrossRef]
- Wu, M.Y.; Wen, D.D.; Gao, N.; Xiao, C.; Yang, L.; Xu, L.; Lian, W.; Peng, W.L.; Jiang, J.M.; Zhao, J.H. Anticoagulant and antithrombotic evaluation of native fucosylated chondroitin sulfates and their derivatives as selective inhibitors of intrinsic factor Xase. Eur. J. Med. Chem. 2015, 92, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Kitazato, K.; Kitazato, K.T.; Sasaki, E.; Minamiguchi, K.; Nagase, H. Prolonged bleeding time induced by anticoagulant glycosaminoglycans in dogs is associated with the inhibition of thrombin-induced platelet aggregation. Thromb. Res. 2003, 112, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Buyue, Y.; Sheehan, J.P. Fucosylated chondroitin sulfate inhibits plasma thrombin generation via targeting of the factor IXa heparin-binding exosite. Blood 2009, 114, 3092–3100. [Google Scholar] [CrossRef] [PubMed]
- Kenji, K.; Keiko, T.K.; Eiji, S.; Kazuhisa, M.; Hideki, N. DHG, a new depolymerized holothurian glycosaminoglycan, exerts an antithrombotic effect with less bleeding than unfractionated or low molecular weight heparin, in rats. Thromb. Res. 1996, 84, 111–120. [Google Scholar]
- Zhao, L.Y.; Lai, S.S.; Huang, R.; Wu, M.Y.; Gao, N.; Xu, L.; Qin, H.B.; Peng, W.L.; Zhao, J.H. Structure and anticoagulant activity of fucosylated glycosaminoglycan degraded by deaminative cleavage. Carbohydr. Polym. 2013, 98, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Ye, X.Q.; Guo, X.; Liao, N.B.; Yin, X.Z.; Hu, Y.J.; Sun, Y.J.; Liu, D.H.; Chen, S.G. Depolymerization of fucosylated chondroitin sulfate from sea cucumber, Pearsonothuriagraeffei, via 60Co irradiation. Carbohydr. Polym. 2013, 93, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Mourão, P.A.; Pereira, M.S.; Pavão, M.S.; Mulloy, B.; Tollefsen, D.M.; Mowinckel, M.C.; Abildgaard, U. Structure and anticoagulant activity of a fucosylated chondroitin sulfate from echinoderm sulfated fucose branches on the polysaccharide account for its high anticoagulant action. J. Biol. Chem. 1996, 271, 23973–23984. [Google Scholar] [PubMed]
- Dong, X.D.; Pan, R.J.; Zou, S.M.; He, M.L.; Wang, C.H. Oxidative degradation of the sulfated polysaccharide isolated from sea cucumber Holothuri nobilis. Process Biochem. 2015, 50, 294–301. [Google Scholar] [CrossRef]
- Achour, O.; Bridiau, N.; Godhbani, A.; Le Joubioux, F.; BordenaveJuchereau, S.; Sannier, F.; Piot, J.; Fruitier Arnaudin, I.; Maugard, T. Ultrasonic-assisted preparation of a low molecular weight heparin (LMWH) with anticoagulant activity. Carbohydr. Polym. 2013, 97, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Petit, A.C.; Noiret, N.; Sinquin, C.; Ratiskol, J.; Guézennec, J.; Colliec-Jouault, S. Free-radical depolymerization with metallic catalysts of an exopolysaccharide produced by a bacterium isolated from a deep-sea hydrothermal vent polychaete annelid. Carbohydr. Polym. 2006, 64, 597–602. [Google Scholar] [CrossRef]
- Wu, M.Y.; Xu, S.M.; Zhao, J.H.; Kang, H.; Ding, H. Free-radical depolymerization of glycosaminoglycan from sea cucumber Thelenataananas by hydrogen peroxide and copper ions. Carbohydr. Polym. 2010, 80, 1116–1124. [Google Scholar] [CrossRef]
- Chen, S.G.; Xue, C.H.; Tang, Q.J.; Yu, G.L.; Chai, W.G. Comparison of structures and anticoagulant activities of fucosylated chondroitin sulfates from different sea cucumbers. Carbohydr. Polym. 2011, 83, 688–696. [Google Scholar] [CrossRef]
- Chen, S.G.; Li, G.Y.; Wu, N.; Guo, X.; Liao, N.B.; Ye, X.Q.; Liu, D.H.; Xue, C.H.; Chai, W.G. Sulfation pattern of the fucose branch is important for the anticoagulant and antithrombotic activities of fucosylated chondroitin sulfates. Biochim. Biophys. Acta 2013, 1830, 3054–3066. [Google Scholar] [CrossRef] [PubMed]
- Rota, C.; Liverani, L.; Spelta, F.; Mascellani, G.; Tomasi, A.; Iannone, A.; Vismara, E. Free radical generation during chemical depolymerization of heparin. Anal. Biochem. 2005, 344, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Ofman, D.; Slim, G.C.; Watt, D.K.; Yorke, S.C. Free radical induced oxidative depolymerisation of chondroitin sulphate and dermatan sulphate. Carbohydr. Polym. 1997, 33, 47–56. [Google Scholar] [CrossRef]
- Yue, W.; Yao, P.J.; Wei, Y.N.; Li, S.Q.; Lai, F.; Liu, X.M. An innovative method for preparation of acid-free-water-soluble low-molecular-weight chitosan (AFWSLMWC). Food Chem. 2008, 108, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wang, J.; Jin, W.H.; Zhang, H.; Zhang, Q.B. Degradation of Laminaria japonica fucoidan by hydrogen peroxide and antioxidant activities of the degradation products of different molecular weights. Carbohydr. Polym. 2012, 87, 153–159. [Google Scholar] [CrossRef]
- Uchiyama, H.; Dobashi, Y.; Ohkouchi, K.; Nagasawa, K. Chemical change involved in the oxidative reductive depolymerization of hyaluronic acid. J. Biol. Chem. 1990, 265, 7753–7759. [Google Scholar] [PubMed]
- Lin, C.Z.; Guan, H.S.; Li, H.H.; Yu, G.L.; Gu, C.X.; Li, G.Q. The influence of molecular mass of sulfated propylene glycol ester of low-molecular-weight alginate on anticoagulant activities. Eur. Polym. J. 2007, 43, 3009–3015. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Wu, M.Y.; Xiao, C.; Yang, L.; Zhou, L.T.; Gao, N.; Li, Z.; Chen, J.C.; Chen, J.; Liu, J.K.; et al. Discovery of an intrinsic tenase complex inhibitor: Pure nonasaccharide from fucosylated glycosaminoglycan. Proc. Natl. Acad. Sci. USA 2015, 112, 8284–8289. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Y.; Masuko, S.; Green, D.E.; Xu, Y.; Li, L.; Zhang, F.; Linhardt, R.J. N-sulfotestosteronan, a novel substrate for heparan sulfate 6-O-sulfotransferases and its analysis by oxidative degradation. Biopolymers 2013, 99, 675–685. [Google Scholar] [CrossRef] [PubMed]
- John, P.S.; Erik, N.W. Depolymerized holothurian glycosaminoglycan and heparin inhibit the intrinsic tenase complex by a common antithrombin-independent mechanism. Blood 2006, 107, 3876–3882. [Google Scholar]
- Panagos, C.G.; August, D.P.; Jesson, C.; Uhrín, D. Photochemical depolymerisation of dermatan sulfate and analysis of the generated oligosaccharides. Carbohydr. Polym. 2016, 140, 13–19. [Google Scholar] [CrossRef] [PubMed]
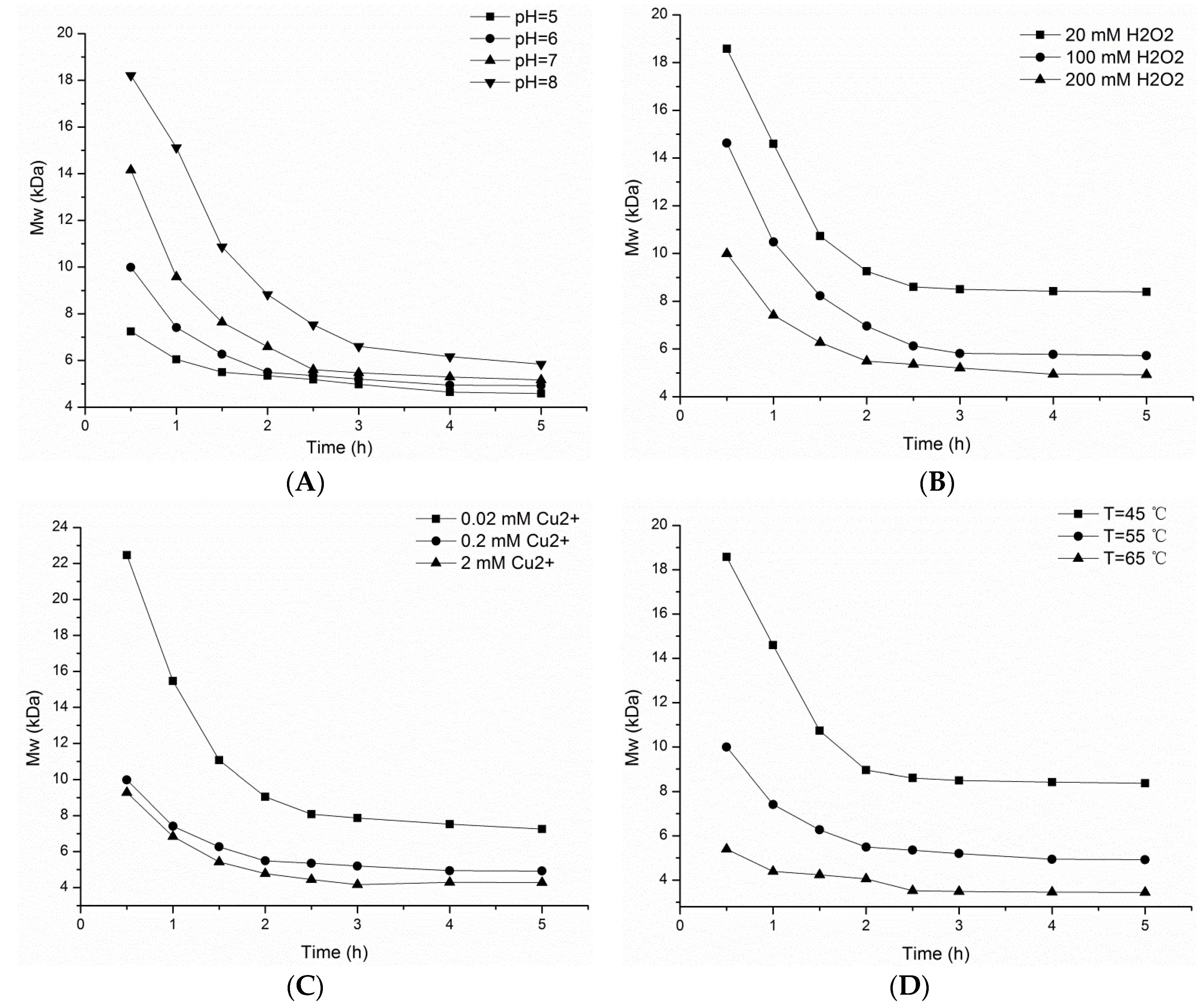
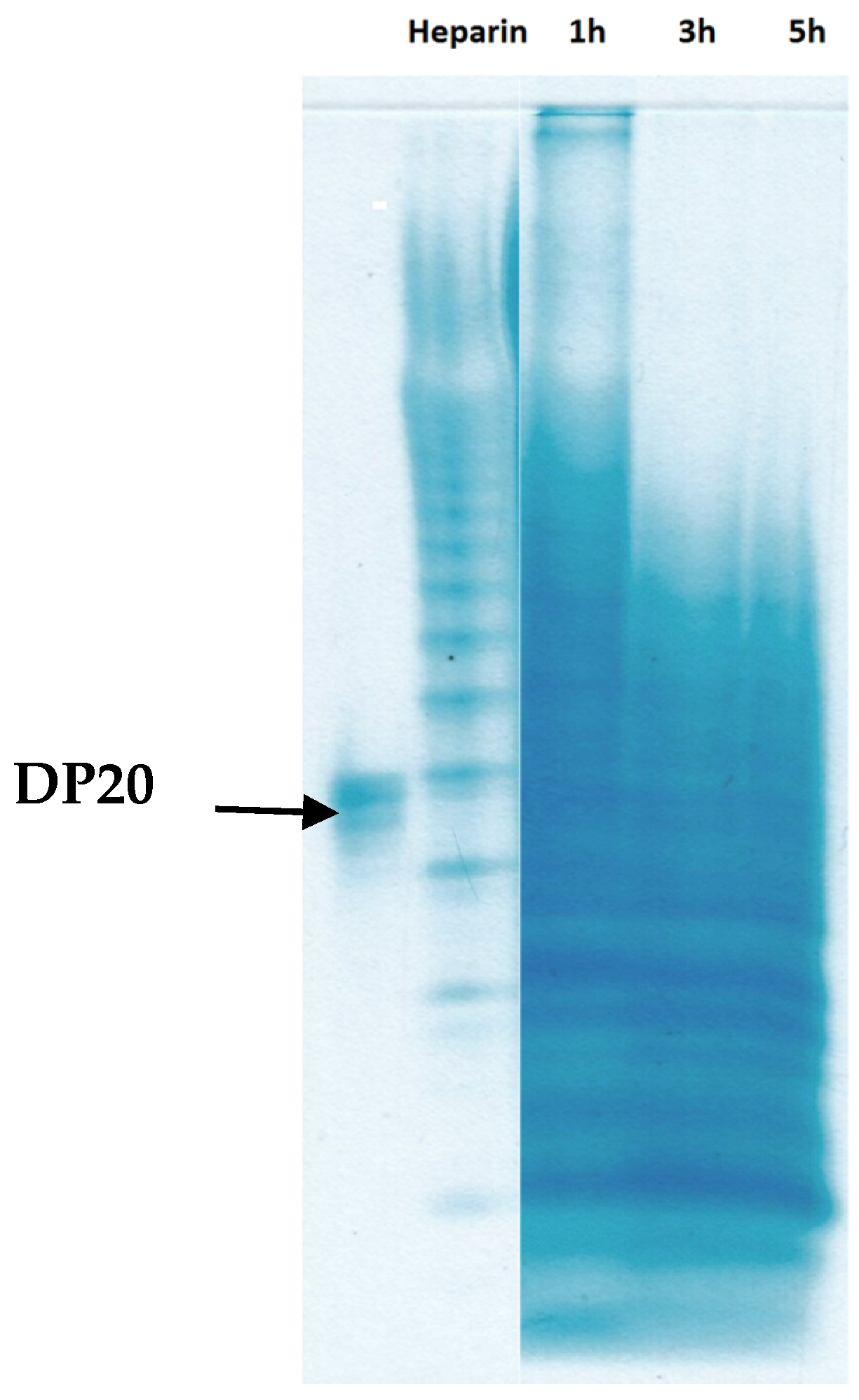
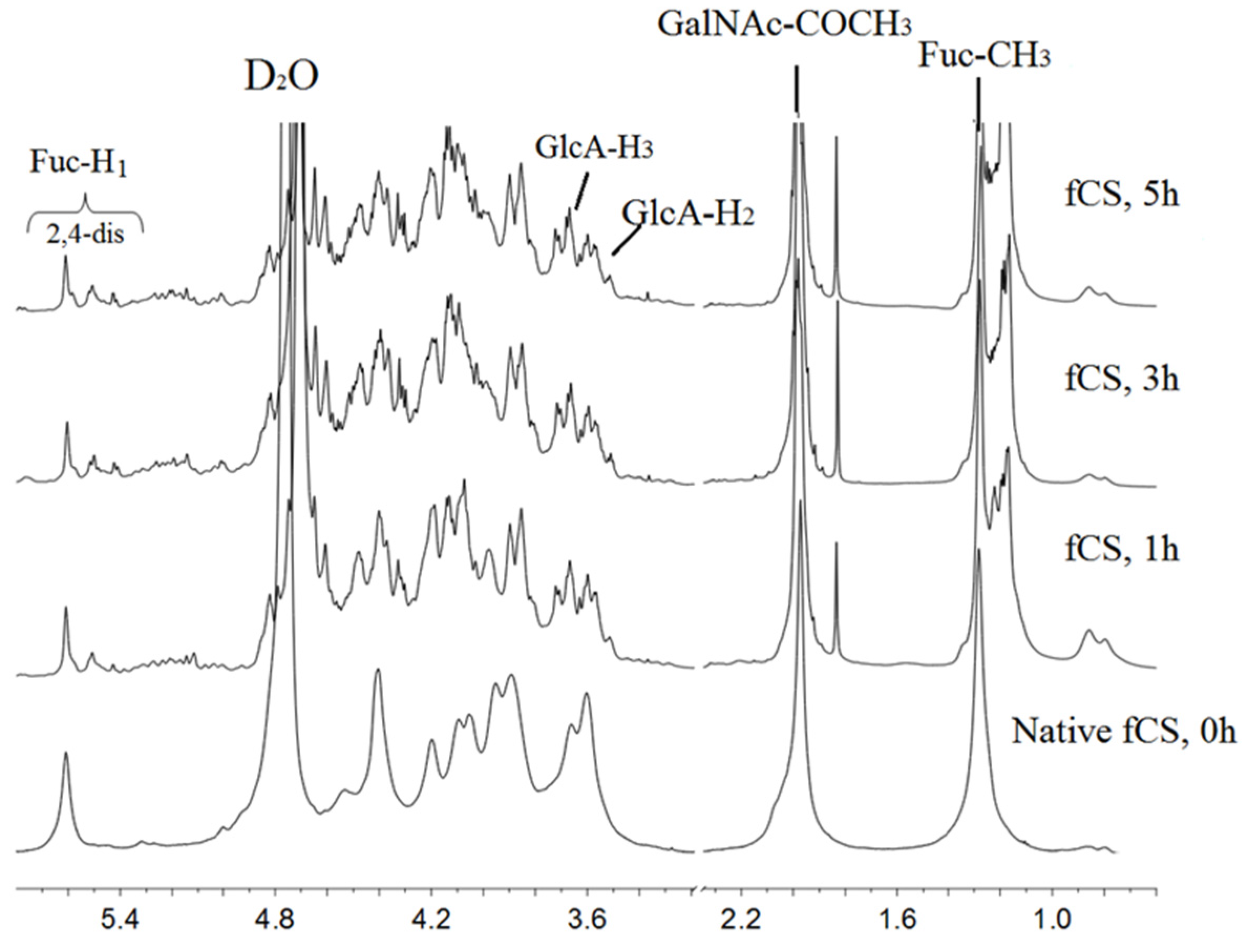
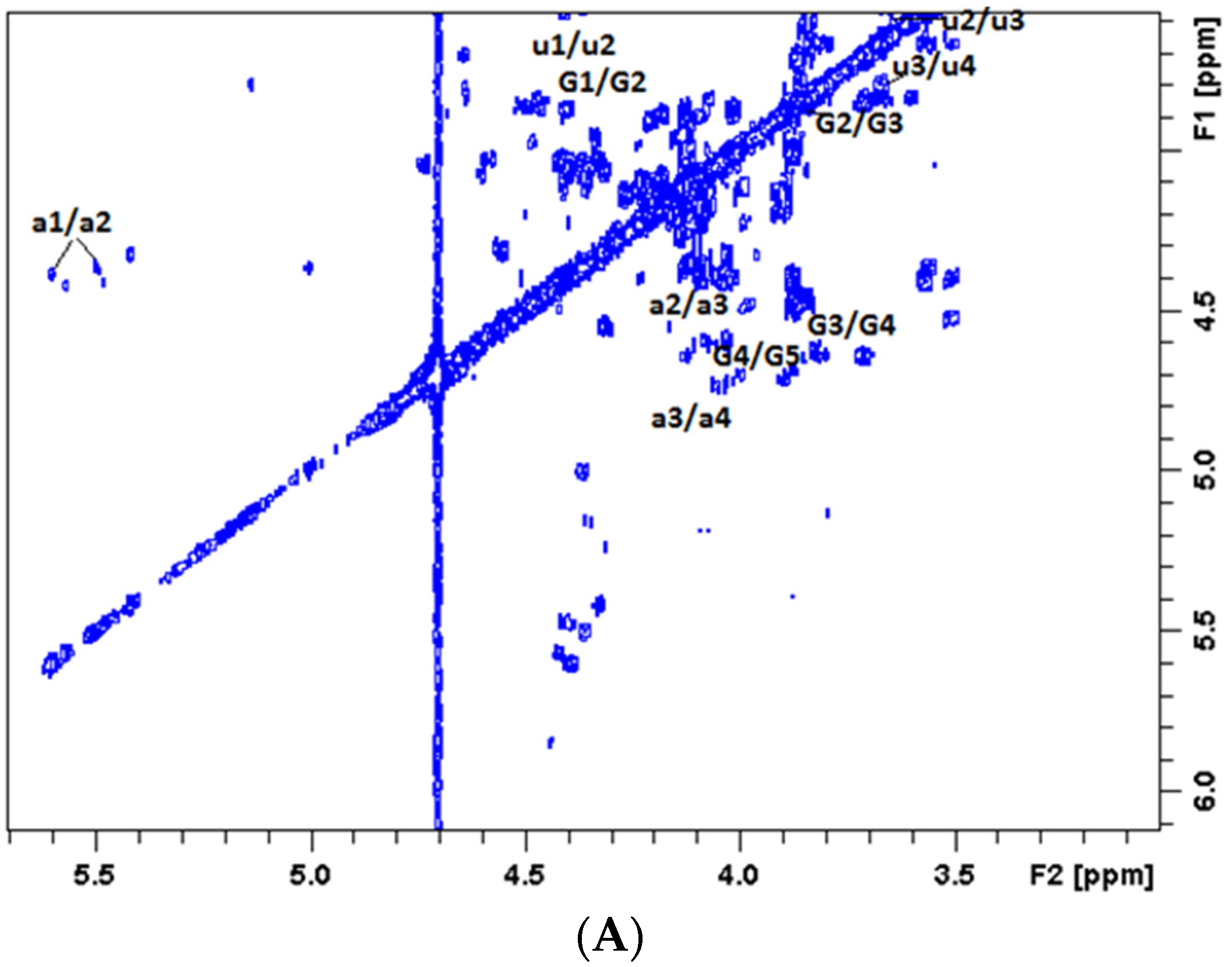
| Samples | Average of Molecular Weight (n = 3) | Polydispersity (Mw/Mn) | |
|---|---|---|---|
| Weight Average (Mw) (kDa) | Number-Average (Mn) (kDa) | ||
| fCS-Ib | 109 ± 6.13 | 94.78 ± 3.97 | 1.15 ± 0.04 |
| DfCS-1 | 7.4 ± 0.486 | 4.60 ± 0.36 | 1.61 ± 0.02 |
| DfCS-3 | 5.2 ± 0.140 | 2.89 ± 0.41 | 1.80 ± 0.18 |
| DfCS-5 | 4.3 ± 0.126 | 2.38 ± 0.40 | 1.80 ± 0.21 |
| LMWH | 6.4 ± 0.538 | 5.0 ± 0.55 | 1.28 ± 0.03 |
| UFH | 18.6 ± 0.224 | 13.47 ± 0.58 | 1.38 ± 0.04 |
| Samples | Mw (kDa) | Molar Ratios a | ||
|---|---|---|---|---|
| GlcA | GalNAc | Fuc | ||
| fCS-Ib | 109 | 1.43 | 1 | 1.71 |
| DfCS-1 | 7.4 | 1.35 | 1 | 1.70 |
| DfCS-3 | 5.2 | 1.32 | 1 | 1.72 |
| DfCS-5 | 4.3 | 1.30 | 1 | 1.71 |
| Samples | Mw (kDa) | APTT */TT * (IU/mg) | EC50 (μg/mL) (Anti-FIIa/AT) ** | EC50 (μg/mL) (Anti-FIIa/HCII) ** | EC50 (μg/mL) (Anti-FXa/AT) ** | Anti-Xa/Anti-IIa |
|---|---|---|---|---|---|---|
| fCS-Ib | 109 | 187 157 | 3.2 | 0.2 | 4.7 | 0.2 |
| DfCS-1 | 7.4 | 103.8 34.3 | > 1500 | 857.5 | 8.9 | 88 |
| DfCS-3 | 5.2 | 60.5 < 1 | > 1500 | 1490 | 22.8 | 42 |
| D-fCS-5 | 4.3 | 34.8 < 1 | > 1500 | > 1500 | 52.9 | 38.6 |
| Heparin | 18.6 | 212 212 | 0.7 | 0.67 | 0.22 | 1 |
| LMWH | 6.4 | 69 64 | 1.82 | 5.45 | 2.35 | 4.1 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.-h.; Li, S.; Zhi, Z.-j.; Yan, L.-f.; Ye, X.-q.; Ding, T.; Yan, L.; Linhardt, R.J.; Chen, S.-g. Depolymerization of Fucosylated Chondroitin Sulfate with a Modified Fenton-System and Anticoagulant Activity of the Resulting Fragments. Mar. Drugs 2016, 14, 170. https://doi.org/10.3390/md14090170
Li J-h, Li S, Zhi Z-j, Yan L-f, Ye X-q, Ding T, Yan L, Linhardt RJ, Chen S-g. Depolymerization of Fucosylated Chondroitin Sulfate with a Modified Fenton-System and Anticoagulant Activity of the Resulting Fragments. Marine Drugs. 2016; 14(9):170. https://doi.org/10.3390/md14090170
Chicago/Turabian StyleLi, Jun-hui, Shan Li, Zi-jian Zhi, Lu-feng Yan, Xing-qian Ye, Tian Ding, Lei Yan, Robert John Linhardt, and Shi-guo Chen. 2016. "Depolymerization of Fucosylated Chondroitin Sulfate with a Modified Fenton-System and Anticoagulant Activity of the Resulting Fragments" Marine Drugs 14, no. 9: 170. https://doi.org/10.3390/md14090170
APA StyleLi, J.-h., Li, S., Zhi, Z.-j., Yan, L.-f., Ye, X.-q., Ding, T., Yan, L., Linhardt, R. J., & Chen, S.-g. (2016). Depolymerization of Fucosylated Chondroitin Sulfate with a Modified Fenton-System and Anticoagulant Activity of the Resulting Fragments. Marine Drugs, 14(9), 170. https://doi.org/10.3390/md14090170








