Antimicrobial Compounds from Eukaryotic Microalgae against Human Pathogens and Diseases in Aquaculture
Abstract
:1. Introduction
2. Antibacterial Activity from Microalgae
2.1. Antibacterial Activity from Microalgae against Human Pathogenic Bacteria
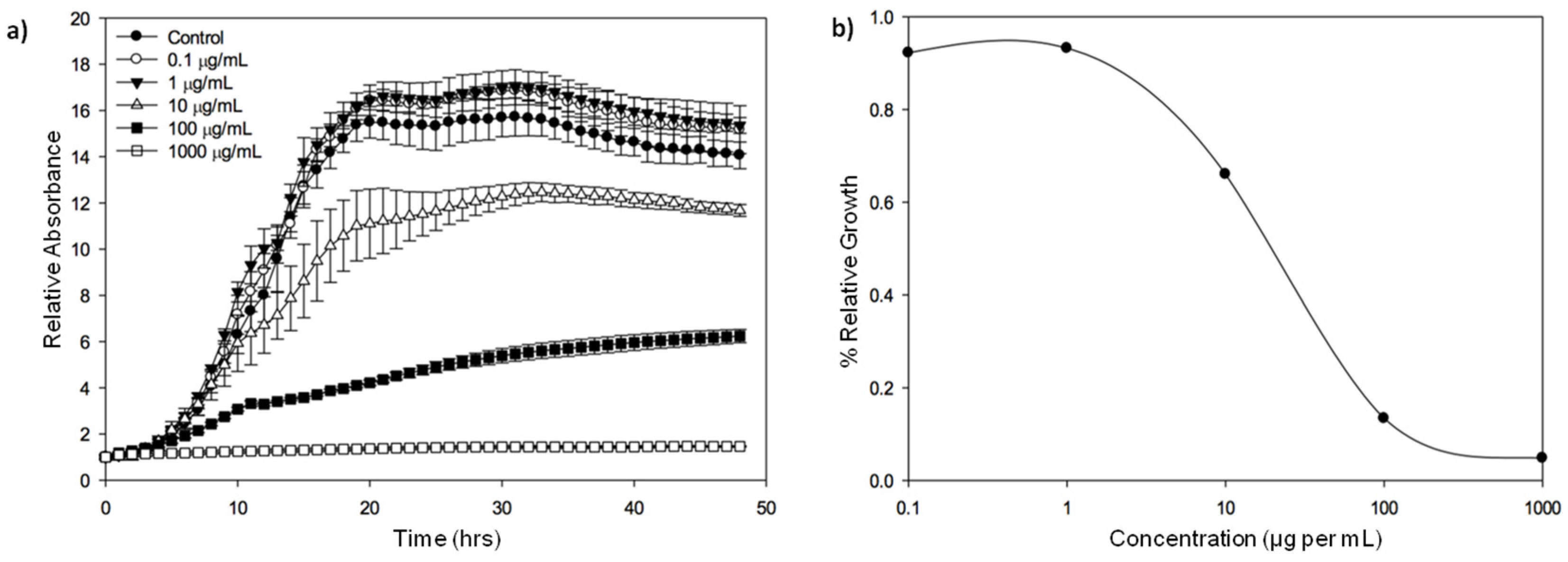
2.1.1. Toward Improving Extraction Techniques
2.1.2. Diversity of Antibacterial Compounds Extracted from Microalgae
2.2. Use of Microalgae against Pathogenic Bacteria in Aquaculture
2.2.1. Benefits of the “Green-Water” Technique against Bacterial Diseases in Aquaculture
2.2.2. Microalgae to Improve the Live-Food Quality
2.2.3. In Vitro Efficiency of Microalgal Compounds against Marine Bacteria
2.2.4. Interactions between Microalgae and Marine Bacteria
3. Antifungal Activity from Microalgae
3.1. Antifungal Activity from Microalgae against Human Pathogens
3.2. Potential Use of Microalgae against Fungal Diseases in Aquaculture
4. Antiviral Activity from Microalgae
4.1. Antiviral Activity from Microalgae against Pathogenic Human Viruses
4.2. Potential Use of Microalgae against Viruses in Aquaculture
5. Conclusions and Final Remarks
Acknowledgments
Conflicts of Interest
References
- Borowitzka, M.A. Microalgae as sources of pharmaceuticals and other biologically active compounds. J. Appl. Phycol. 1995, 7, 3–15. [Google Scholar] [CrossRef]
- Bhatnagar, I.; Kim, S.-K. Immense essence of excellence: Marine microbial bioactive compounds. Mar. Drugs 2010, 8, 2673–2701. [Google Scholar] [CrossRef] [PubMed]
- Dyshlovoy, S.A.; Honecker, F. Marine compounds and cancer: Where do we stand? Mar. Drugs 2015, 13, 5657–5665. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.K.; Zi-rong, Xu. Biomedical compounds from marine organisms. Mar. Drugs 2004, 2, 123–146. [Google Scholar]
- Kang, H.K.; Seo, C.H.; Park, Y. Marine peptides and their anti-infective activities. Mar. Drugs 2015, 13, 618–654. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.S.; Rodríguez, A.D.; Taglialatela-Scafati, O.; Fusetani, N. Marine Pharmacology in 2009–2011: Marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous systems, and other miscellaneous mechanisms of action. Mar. Drugs 2013, 11, 2510–2573. [Google Scholar] [PubMed]
- Suleria, H.A.R.; Osborne, S.; Masci, P.; Gobe, G. Marine-based nutraceuticals: An innovative trend in the food and supplement industries. Mar. Drugs 2015, 13, 6336–6351. [Google Scholar] [CrossRef] [PubMed]
- Encarnacao, T.; Pais, A.A.; Campos, M.G.; Burrows, H.D. Cyanobacteria and microalgae: A renewable source of bioactive compounds and other chemicals. Sci. Prog. 2015, 98, 145–168. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kate, B.N.; Banerjee, U.C. Bioactive compounds from cyanobacteria and microalgae: An overview. Crit. Rev. Biotechnol. 2005, 25, 73–95. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y. Microalgal metabolites. Curr. Opin. Microbiol. 2003, 6, 236–243. [Google Scholar] [CrossRef]
- Talero, E.; García-Mauriño, S.; Ávila-Román, J.; Rodríguez-Luna, A.; Alcaide, A.; Motilva, V. Bioactive compounds isolated from microalgae in chronic inflammation and cancer. Mar. Drugs 2015, 13, 6152–6209. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.L.; Yasin, N.H.M.; Derek, C.J.C.; Lim, J.K. Microalgae as a sustainable energy source for biodiesel production: A review. Renew. Sustain. Energy Rev. 2011, 15, 584–593. [Google Scholar] [CrossRef]
- Ghasemi, Y.; Rasoul-Amini, S.; Naseri, A.T.; Montazeri-Najafabady, N.; Mobasher, M.A.; Dabbagh, F. Microalgae biofuel potentials (Review). Appl. Biochem. Microbiol. 2012, 48, 126–144. [Google Scholar] [CrossRef]
- Halim, R.; Danquah, M.K.; Webley, P.A. Extraction of oil from microalgae for biodiesel production: A review. Biotechnol. Adv. 2012, 30, 709–732. [Google Scholar] [CrossRef] [PubMed]
- Barra, L.; Chandrasekaran, R.; Corato, F.; Brunet, C. The challenge of ecophysiological biodiversity for biotechnological applications of marine microalgae. Mar. Drugs 2014, 12, 1641–1675. [Google Scholar] [CrossRef] [PubMed]
- Cadoret, J.-P.; Garnier, M.; Saint-Jean, B. Microalgae, functional genomics and biotechnology. Adv. Bot. Res. 2012, 64, 285–341. [Google Scholar]
- Priyadarshani, I.; Biswajit, R. Commercial and industrial applications of micro algae—A review. J. Algal Biomass Utln. 2012, 3, 89–100. [Google Scholar]
- De Jesus Raposo, M.F.; de Morais, R.M.S.C.; de Morais, A.M.M.B. Health applications of bioactive compounds from marine microalgae. Life Sci. 2013, 93, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Yaakob, Z.; Ali, E.; Zainal, A.; Mohamad, M.; Takriff, M.S. An overview: Biomolecules from microalgae for animal feed and aquaculture. J. Biol. Res. 2014, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- De Jesus Raposo, M.F.; de Morais, R.M.S.C.; Bernardo de Morais, A.M.M. Bioactivity and applications of sulphated polysaccharides from marine microalgae. Mar. Drugs 2013, 11, 233–252. [Google Scholar] [CrossRef] [PubMed]
- De Jesus Raposo, M.F.; de Morais, A.M.B.; de Morais, R.M.S.C. Marine polysaccharides from algae with potential biomedical applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef] [PubMed]
- Amaro, H.M.; Guedes, A.C.; Malcata, F.X. Chapter: Antimicrobial activities of microalgae: An invited review. In Science against Microbial Pathogens: Communicating Current Research and Technological Advances; Formatex Microbiology Book Series; Mendez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2011; Volume 2. [Google Scholar]
- De Jesus Raposo, M.F.; de Morais, A.M.M.B.; de Morais, R.M.S.C. Emergent sources of prebiotics: Seaweeds and microalgae. Mar. Drugs 2016, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- De Jesus Raposo, M.F.; de Morais, A.M.M.B.; de Morais, R.M.S.C. Carotenoids from marine microalgae: A valuable natural source for the prevention of chronic diseases. Mar. Drugs 2015, 13, 5128–5155. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.J.; Desbois, A.P.; Dyrynda, E.A. Conventional and unconventional antimicrobials from fish, marine invertebrates and micro-algae. Mar. Drugs 2010, 8, 1213–1262. [Google Scholar] [CrossRef] [PubMed]
- Muller-Feuga, A. The role of microalgae in aquaculture: Situation and trends. J. Appl. Phycol. 2000, 12, 527–534. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Microalgae for aquaculture: Opportunities and constraints. J. Appl. Phycol. 1997, 9, 393–401. [Google Scholar] [CrossRef]
- Lorenz, R.T.; Cysewski, G.R. Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends Biotechnol. 2000, 18, 160–167. [Google Scholar] [CrossRef]
- Gastineau, R.; Turcotte, F.; Pouvreau, J.-B.; Morançais, M.; Fleurence, J.; Windarto, E.; Prasetiya, F.S.; Arsad, S.; Jaouen, P.; Babin, M.; et al. Marennine, promising blue pigments from a widespread Haslea diatom species complex. Mar. Drugs 2014, 12, 3161–3189. [Google Scholar] [CrossRef] [PubMed]
- Neori, A. “Green water” microalgae: The leading sector in world aquaculture. J. Appl. Phycol. 2010, 23, 143–149. [Google Scholar] [CrossRef]
- Papandroulakis, N.; Divanach, P.; Anastasiadis, P.; Kentouri, M. The pseudo-green water technique for intensive rearing of sea bream (Sparus aurata) larvae. Aquac. Int. 2001, 9, 205–216. [Google Scholar] [CrossRef]
- Naas, K.E.; N˦ss, T.; Harboe, T. Enhanced first feeding of halibut larvae (Hippoglossus hippoglossus L.) in green water. Aquaculture 1992, 105, 143–156. [Google Scholar] [CrossRef]
- Reitan, K.I.; Rainuzzo, J.R.; Øie, G.; Olsen, Y. A review of the nutritional effects of algae in marine fish larvae. Aquaculture 1997, 155, 207–221. [Google Scholar] [CrossRef]
- Supamattaya, K.; Kiriratnikom, S.; Boonyaratpalin, M.; Borowitzka, L. Effect of a Dunaliella extract on growth performance, health condition, immune response and disease resistance in black tiger shrimp (Penaeus monodon). Aquaculture 2005, 248, 207–216. [Google Scholar] [CrossRef]
- Van der Meeren, T.; Mangor-Jensen, A.; Pickova, J. The effect of green water and light intensity on survival, growth and lipid composition in Atlantic cod (Gadus morhua) during intensive larval rearing. Aquaculture 2007, 265, 206–217. [Google Scholar] [CrossRef]
- Cahu, C.L.; Zambonino Infante, J.L.; Péres, A.; Quazuguel, P.; Le Gall, M.M. Algal addition in sea bass (Dicentrarchus labrax) larvae rearing: Effect on digestive enzymes. Aquaculture 1998, 161, 479–489. [Google Scholar] [CrossRef]
- Palmer, P.J.; Burke, M.J.; Palmer, C.J.; Burke, J.B. Developments in controlled green-water larval culture technologies for estuarine fishes in Queensland, Australia and elsewhere. Aquaculture 2007, 272, 1–21. [Google Scholar] [CrossRef]
- Marques, A.; Thanh, T.H.; Sorgeloos, P.; Bossier, P. Use of microalgae and bacteria to enhance protection of gnotobiotic Artemia against different pathogens. Aquaculture 2006, 258, 116–126. [Google Scholar] [CrossRef]
- Tendencia, E.A.; dela Peña, M. Investigation of some components of the greenwater system which makes it effective in the initial control of luminous bacteria. Aquaculture 2003, 218, 115–119. [Google Scholar] [CrossRef]
- Burja, A.M.; Banaigs, B.; Abou-Mansour, E.; Grant Burgess, J.; Wright, P.C. Marine cyanobacteria—A prolific source of natural products. Tetrahedron 2001, 57, 9347–9377. [Google Scholar] [CrossRef]
- Costa, M.; Costa-Rodrigues, J.; Fernandes, M.H.; Barros, P.; Vasconcelos, V.; Martins, R. Marine cyanobacteria compounds with anticancer properties: A review on the implication of apoptosis. Mar. Drugs 2012, 10, 2181–2207. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Ratha, S.K.; Sood, A.; Chaudhary, V.; Prasanna, R. New insights into the biodiversity and applications of cyanobacteria (blue-green algae)—Prospects and challenges. Algal Res. 2013, 2, 79–97. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Menakha, M. Pharmaceutical applications of cyanobacteria—A review. J. Acute Med. 2015, 5, 15–23. [Google Scholar] [CrossRef]
- Deschênes, J.-S.; Boudreau, A.; Tremblay, R. Mixotrophic production of microalgae in pilot-scale photobioreactors: Practicability and process considerations. Algal Res. 2015, 10, 80–86. [Google Scholar] [CrossRef]
- Pradhan, J.; Das, S.; Das, B.K. Antibacterial activity of freshwater microalgae: A review. Afr. J. Pharm. Pharmacol. 2014, 8, 809–818. [Google Scholar]
- Jin, L.; Quan, C.; Hou, X.; Fan, S. Potential Pharmacological Resources: Natural Bioactive Compounds from Marine-Derived Fungi. Mar. Drugs 2016, 14, 76. [Google Scholar] [CrossRef] [PubMed]
- Shannon, E.; Abu-Ghannam, N. Antibacterial Derivatives of Marine Algae: An Overview of Pharmacological Mechanisms and Applications. Mar. Drugs 2016, 14, 81. [Google Scholar] [CrossRef] [PubMed]
- Pratt, R.; Daniels, T.C.; Eiler, J.J.; Gunnison, J.B.; Kumler, W.D.; Oneto, J.F.; Strait, L.A.; Spoehr, H.A.; Hardin, G.J.; Milner, H.W.; et al. Chlorellin, an antibacterial substance from Chlorella. Science 1944, 99, 351–352. [Google Scholar] [CrossRef] [PubMed]
- Mudimu, O.; Rybalka, N.; Bauersachs, T.; Born, J.; Friedl, T.; Schulz, R. Biotechnological screening of microalgal and cyanobacterial strains for biogas production and antibacterial and antifungal effects. Metabolites 2014, 4, 373–393. [Google Scholar] [CrossRef] [PubMed]
- Ördög, V.; Stirk, W.A.; Lenobel, R.; Bancířová, M.; Strnad, M.; van Staden, J.; Szigeti, J.; Németh, L. Screening microalgae for some potentially useful agricultural and pharmaceutical secondary metabolites. J. Appl. Phycol. 2004, 16, 309–314. [Google Scholar] [CrossRef]
- Pane, G.; Cacciola, G.; Giacco, E.; Mariottini, G.L.; Coppo, E. Assessment of the antimicrobial activity of algae extracts on bacteria responsible of external otitis. Mar. Drugs 2015, 13, 6440–6452. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.; Ohta, S.; Ikegami, N.; Miyata, H.; Kashimoto, T.; Kondo, M. Antibiotic substances produced by a marine green alga, Dunaliella primolecta. Bioresour. Technol. 1993, 44, 149–153. [Google Scholar] [CrossRef]
- Duff, D.C.B.; Bruce, D.L.; Antia, N.J. The Antibacterial Activity of Marine Planktonic Algae. Can. J. Microbiol. 1966, 12, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Kellam, S.J.; Walker, J.M. Antibacterial activity from marine microalgae in laboratory culture. Br. Phycol. J. 1989, 24, 191–194. [Google Scholar] [CrossRef]
- Ohta, S.; Chang, T.; Ikegami, N.; Kondo, M.; Miyata, H. Antibiotic substance produced by a newly isolated marine microalga, Chlorococcum HS-101. Bull. Environ. Contam. Toxicol. 1993, 50, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Srinivasakumar, K.P.; Rajashekhar, M. In vitro studies on bactericidal activity and sensitivity pattern of isolated marine microalgae against selective human bacterial pathogens. Indian J. Sci. Technol. 2009, 2, 16–23. [Google Scholar]
- Viso, A.C.; Pesando, D.; Baby, C. Antibacterial and antifungal properties of some marine diatoms in culture. Bot. Mar. 1987, 30, 41–46. [Google Scholar] [CrossRef]
- Cannell, R.J.P.; Owsianka, A.M.; Walker, J.M. Results of a large-scale screening programme to detect antibacterial activity from freshwater algae. Br. Phycol. J. 1988, 23, 41–44. [Google Scholar] [CrossRef]
- Katircioglu, H.; Beyatli, Y.; Aslim, B.; Yüksekdag, Z.; Atici, T. Screening for antimicrobial agent production of some microalgae in freshwater. Internet J. Microbiol. 2005, 2, 1–5. [Google Scholar]
- Safonova, E.; Reisser, W. Growth promoting and inhibiting effects of extracellular substances of soil microalgae and cyanobacteria on Escherichia coli and Micrococcus luteus. Phycol. Res. 2005, 53, 189–193. [Google Scholar] [CrossRef]
- Ghasemi, Y.; Moradian, A.; Mohagheghzadeh, A.; Shokravi, S.; Morowvat, M.H. Antifungal and antibacterial activity of the microalgae collected from paddy fields of Iran: Characterization of antimicrobial activity of Chroococcus dispersus. J. Biol. Sci. 2007, 7, 904–910. [Google Scholar]
- Abedin, R.; Taha, H. Antibacterial and antifungal activity of cyanobacteria and green microalgae. Evaluation of medium components by Plackett-Burman design for antimicrobial activity of Spirulina platensis. Glob. J. Biotechnol. Biochem. 2008, 3, 22–31. [Google Scholar]
- Ohta, S.; Shiomi, Y.; Kawashima, A.; Aozasa, O.; Nakao, T.; Nagate, T.; Kitamura, K.; Miyata, H. Antibiotic effect of linolenic acid from Chlorococcum strain HS-101 and Dunaliella primolecta on methicillin-resistant Staphylococcus aureus. J. Appl. Phycol. 1995, 7, 121–127. [Google Scholar] [CrossRef]
- Bhagavathy, S.; Sumathi, P.; Jancy Sherene Bell, I. Green algae Chlorococcum humicola—A new source of bioactive compounds with antimicrobial activity. Asian Pac. J. Trop. Biomed. 2011, 1, S1–S7. [Google Scholar] [CrossRef]
- Herrero, M.; Ibáñez, E.; Cifuentes, A.; Reglero, G.; Santoyo, S. Dunaliella salina microalga pressurized liquid extracts as potential antimicrobials. J. Food Prot. 2006, 69, 2471–2477. [Google Scholar] [PubMed]
- Mendiola, J.A.; Santoyo, S.; Cifuentes, A.; Reglero, G.; Ibáñez, E.; Señoráns, F.J. Antimicrobial activity of sub- and supercritical CO2 extracts of the green alga Dunaliella salina. J. Food Prot. 2008, 71, 2138–2143. [Google Scholar] [PubMed]
- Lustigman, B. Comparison of antibiotic production from four ecotypes of the marine alga, Dunaliella. Bull. Environ. Contam. Toxicol. 1988, 40, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Meizoso, I.; Jaime, L.; Santoyo, S.; Señoráns, F.J.; Cifuentes, A.; Ibáñez, E. Subcritical water extraction and characterization of bioactive compounds from Haematococcus pluvialis microalga. J. Pharm. Biomed. Anal. 2010, 51, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, S.; Rodríguez-Meizoso, I.; Cifuentes, A.; Jaime, L.; García-Blairsy Reina, G.; Señorans, F.J.; Ibáñez, E. Green processes based on the extraction with pressurized fluids to obtain potent antimicrobials from Haematococcus pluvialis microalgae. LWT—Food Sci. Technol. 2009, 42, 1213–1218. [Google Scholar] [CrossRef]
- Guedes, A.C.; Barbosa, C.R.; Amaro, H.M.; Pereira, C.I.; Malcata, F.X. Microalgal and cyanobacterial cell extracts for use as natural antibacterial additives against food pathogens. Int. J. Food Sci. Technol. 2011, 46, 862–870. [Google Scholar] [CrossRef]
- Najdenski, H.M.; Gigova, L.G.; Iliev, I.I.; Pilarski, P.S.; Lukavský, J.; Tsvetkova, I.V.; Ninova, M.S.; Kussovski, V.K. Antibacterial and antifungal activities of selected microalgae and cyanobacteria. Int. J. Food Sci. Technol. 2013, 48, 1533–1540. [Google Scholar] [CrossRef]
- Ingebrigtsen, R.A.; Hansen, E.; Andersen, J.H.; Eilertsen, H.C. Light and temperature effects on bioactivity in diatoms. J. Appl. Phycol. 2015, 28, 939–950. [Google Scholar] [CrossRef] [PubMed]
- Del Pilar Sánchez-Saavedra, M.; Licea-Navarro, A.; Bernáldez-Sarabia, J. Evaluation of the antibacterial activity of different species of phytoplankton. Rev. Biol. Mar. Oceanogr. 2010, 45, 531–536. [Google Scholar] [CrossRef]
- Mendiola, J.A.; Torres, C.F.; Toré, A.; Martín-Álvarez, P.J.; Santoyo, S.; Arredondo, B.O.; Señoráns, F.J.; Cifuentes, A.; Ibáñez, E. Use of supercritical CO2 to obtain extracts with antimicrobial activity from Chaetoceros muelleri microalga. A correlation with their lipidic content. Eur. Food Res. Technol. 2007, 224, 505–510. [Google Scholar] [CrossRef]
- Findlay, J.A.; Patil, A.D. Antibacterial constituents of the diatom Navicula delognei. J. Nat. Prod. 1984, 47, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Desbois, A.P.; Lebl, T.; Yan, L.; Smith, V. Isolation and structure characterization of two antibacterial free fatty acids from marine diatom, Phaeodactylum tricornutum. Appl. Microbiol. Biotechnol. 2008, 81, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Desbois, A.P.; Mearns-Spragg, A.; Smith, V.J. A fatty acid from the diatom Phaeodactylum tricornutum is antibacterial against diverse bacteria including multi-resistant Staphylococcus aureus (MRSA). Mar. Biotechnol. 2008, 11, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, R.; Karthikayen, R.; Periyanayagi, R.; Sasikala, V.; Balasubramanian, T. Antibacterial activity of the marine diatom, Rhizosolenia alata (Brightwell, 1858) against human pathogens. Res. J. Microbiol. 2007, 2, 98–100. [Google Scholar]
- Walter, C.S.; Mahesh, R. Antibacterial and antifungal activities of some marine diatoms in culture. Indian J. Mar. Sci. 2000, 29, 238–242. [Google Scholar]
- Cooper, S.; Battat, A.; Marsot, P.; Sylvestre, M. Production of antibacterial activities by two Bacillariophyceae grown in dialysis culture. Can. J. Microbiol. 1983, 29, 338–341. [Google Scholar] [CrossRef]
- Bruce, D.L.; Duff, D.C.; Antia, N.J. The identification of two antibacterial products of the marine planktonic alga Isochrysis galbana. Microbiology 1967, 48, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Pawlik-Skowrońska, B. Resistance, accumulation and allocation of zinc in two ecotypes of the green alga Stigeoclonium tenue Kütz. coming from habitats of different heavy metal concentrations. Aquat. Bot. 2003, 75, 189–198. [Google Scholar] [CrossRef]
- Herrero, M.; Mendiola, J.A.; Plaza, M.; Ibañez, E. Screening for Bioactive Compounds from Algae. In Advanced Biofuels and Bioproducts; Lee, J.W., Ed.; Springer: New York, NY, USA, 2013; pp. 833–872. [Google Scholar]
- Jørgensen, E.G. Antibiotic substances from cells and culture solutions of unicellular algae with special reference to some chlorophyll derivatives. Physiol. Plant. 1962, 15, 530–545. [Google Scholar] [CrossRef]
- Desbois, A.P.; Walton, M.; Smith, V.J. Differential antibacterial activities of fusiform and oval morphotypes of Phaeodactylum tricornutum (Bacillariophyceae). J. Mar. Biol. Assoc. UK 2010, 90, 769–774. [Google Scholar] [CrossRef]
- Jüttner, F. Liberation of 5,8,11,14,17-Eicosapentaenoic Acid and Other Polyunsaturated Fatty Acids from Lipids as a Grazer Defense Reaction in Epilithic Diatom Biofilms. J. Phycol. 2001, 37, 744–755. [Google Scholar] [CrossRef]
- Kabara, J.J.; Swieczkowski, D.M.; Conley, A.J.; Truant, J.P. Fatty acids and derivatives as antimicrobial agents. Antimicrob. Agents Chemother. 1972, 2, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Galbraith, H.; Miller, T.B. Physicochemical effects of long chain fatty acids on bacterial cells and their protoplasts. J. Appl. Bacteriol. 1973, 36, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.Q.; O’Connor, C.J.; Roberton, A.M. Antibacterial actions of fatty acids and monoglycerides against Helicobacter pylori. FEMS Immunol. Med. Microbiol. 2003, 36, 9–17. [Google Scholar] [CrossRef]
- Petschow, B.W.; Batema, R.P.; Ford, L.L. Susceptibility of Helicobacter pylori to bactericidal properties of medium-chain monoglycerides and free fatty acids. Antimicrob. Agents Chemother. 1996, 40, 302–306. [Google Scholar] [PubMed]
- Austin, B. Infectious Disease in Aquaculture: Prevention and Control; Woodhead Publishing: Cambridge, UK, 2012. [Google Scholar]
- Austin, B.; Austin, D.A. Bacterial Fish Pathogens: Disease of Farmed and Wild Fish; Springer Science & Business Media: Berlin, Germany, 2007. [Google Scholar]
- Vandenberghe, J.; Thompson, F.L.; Gomez-Gil, B.; Swings, J. Phenotypic diversity amongst Vibrio isolates from marine aquaculture systems. Aquaculture 2003, 219, 9–20. [Google Scholar] [CrossRef]
- Aguirre-Guzmán, G.; Mejia Ruíz, H.; Ascencio, F. A review of extracellular virulence product of Vibrio species important in diseases of cultivated shrimp. Aquac. Res. 2004, 35, 1395–1404. [Google Scholar] [CrossRef]
- Austin, B.; Zhang, X.-H. Vibrio harveyi: A significant pathogen of marine vertebrates and invertebrates. Lett. Appl. Microbiol. 2006, 43, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Beaz-Hidalgo, R.; Diéguez, A.L.; Cleenwerck, I.; Balboa, S.; Doce, A.; de Vos, P.; Romalde, J.L. Vibrio celticus sp. nov., a new Vibrio species belonging to the Splendidus clade with pathogenic potential for clams. Syst. Appl. Microbiol. 2010, 33, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Kesarcodi-Watson, A.; Kaspar, H.; Lategan, M.J.; Gibson, L. Two pathogens of Greenshell mussel larvae, Perna canaliculus: Vibrio splendidus and a V. coralliilyticus/neptunius-like isolate. J. Fish Dis. 2009, 32, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Lambert, C.; Nicolas, J.L.; Cilia, V.; Corre, S. Vibrio pectenicida sp. nov., a pathogen of scallop (Pecten maximus) larvae. Int. J. Syst. Bacteriol. 1998, 48, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Prado, S.; Romalde, J.L.; Montes, J.; Barja, J.L. Pathogenic bacteria isolated from disease outbreaks in shellfish hatcheries. First description of Vibrio neptunius as an oyster pathogen. Dis. Aquat. Org. 2005, 67, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Travers, M.-A.; Boettcher Miller, K.; Roque, A.; Friedman, C.S. Bacterial diseases in marine bivalves. J. Invertebr. Pathol. 2015, 131, 11–31. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.M.; Hargrave, B.T.; Haya, K. Antibiotic Use in Finfish Aquaculture: Modes of Action, Environmental Fate, and Microbial Resistance. In Environmental Effects of Marine Finfish Aquaculture; Hargrave, B.T., Ed.; Handbook of Environmental Chemistry; Springer: Berlin, Germany, 2005; pp. 341–357. [Google Scholar]
- Cabello, F.C. Heavy use of prophylactic antibiotics in aquaculture: A growing problem for human and animal health and for the environment. Environ. Microbiol. 2006, 8, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Bermdez-Almada, M.C.; Espinosa-Plascenci, A. The Use of Antibiotics in Shrimp Farming. In Health and Environment in Aquaculture; Carvalho, E., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Gräslund, S.; Bengtsson, B.E. Chemicals and biological products used in south-east Asian shrimp farming, and their potential impact on the environment—A review. Sci. Total Environ. 2001, 280, 93–131. [Google Scholar] [CrossRef]
- Primavera, J.H.; Lavilla-Pitogo, C.R.; Ladja, J.M.; Dela Peña, M.R. A survey of chemical and biological products used in intensive prawn farms in the Philippines. Mar. Pollut. Bull. 1993, 26, 35–40. [Google Scholar] [CrossRef]
- Silva, E.F.; Soares, M.A.; Calazans, N.F.; Vogeley, J.L.; do Valle, B.C.; Soares, R.; Peixoto, S. Effect of probiotic (Bacillus spp.) addition during larvae and postlarvae culture of the white shrimp Litopenaeus vannamei. Aquac. Res. 2012, 44, 13–21. [Google Scholar] [CrossRef]
- Holbach, M.; Robert, R.; Boudry, P.; Petton, B.; Archambault, P.; Tremblay, R. Scallop larval survival from erythromycin treated broodstock after conditioning without sediment. Aquaculture 2015, 437, 312–317. [Google Scholar] [CrossRef]
- Prado, S.; Romalde, J.L.; Barja, J.L. Review of probiotics for use in bivalve hatcheries. Vet. Microbiol. 2010, 145, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque Costa, R.; Araújo, R.L.; Souza, O.V.; Vieira, R.H.S.D.F. Antibiotic-Resistant Vibrios in Farmed Shrimp. BioMed Res. Int. 2015, 2015, 505914. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Alarcón, C.; Miranda, C.D.; Singer, R.S.; López, Y.; Rojas, R.; Bello, H.; Domínguez, M.; González-Rocha, G. Detection of the floR gene in a diversity of florfenicol resistant Gram-negative bacilli from freshwater salmon farms in Chile. Zoonoses Public Health 2010, 57, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.D.; Zemelman, R. Bacterial resistance to oxytetracycline in Chilean salmon farming. Aquaculture 2002, 212, 31–47. [Google Scholar] [CrossRef]
- Sapkota, A.; Sapkota, A.R.; Kucharski, M.; Burke, J.; McKenzie, S.; Walker, P.; Lawrence, R. Aquaculture practices and potential human health risks: Current knowledge and future priorities. Environ. Int. 2008, 34, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Citarasu, T. Natural antimicrobial compounds for use in aquaculture. In Infectious Disease in Aquaculture: Prevention and Control; Austin, B., Ed.; Woodhead Publishing: Cambridge, UK, 2012; pp. 419–457. [Google Scholar]
- González-Davis, O.; Ponce-Rivas, E.; Sánchez-Saavedra, M.D.P.; Muñoz-Márquez, M.-E.; Gerwick, W.H. Bioprospection of microalgae and cyanobacteria as biocontrol agents against Vibrio campbellii and their use in white shrimp Litopenaeus vannamei culture. J. World Aquac. Soc. 2012, 43, 387–399. [Google Scholar] [CrossRef]
- Das, B.K.; Pradhan, J.; Pattnaik, P.; Samantaray, B.R.; Samal, S.K. Production of antibacterials from the freshwater alga Euglena viridis (Ehren). World J. Microbiol. Biotechnol. 2005, 21, 45–50. [Google Scholar] [CrossRef]
- Gastineau, R.; Hardivillier, Y.; Leignel, V.; Tekaya, N.; Morançais, M.; Fleurence, J.; Davidovich, N.; Jacquette, B.; Gaudin, P.; Hellio, C.; et al. Greening effect on oysters and biological activities of the blue pigments produced by the diatom Haslea karadagensis (Naviculaceae). Aquaculture 2012, 368, 61–67. [Google Scholar] [CrossRef]
- Gastineau, R.; Pouvreau, J.-B.; Hellio, C.; Morançais, M.; Fleurence, J.; Gaudin, P.; Bourgougnon, N.; Mouget, J.-L. Biological activities of purified marennine, the blue pigment responsible for the greening of oysters. J. Agric. Food Chem. 2012, 60, 3599–3605. [Google Scholar] [CrossRef] [PubMed]
- Pouvreau, J.-B. Purification et Caractérisation du Pigment Bleu-Vert “Marennine” Synthétisé par la Diatomée Marine Haslea ostrearia (Gaillon/Bory) Simonsen: Propriétés Physico-Chimiques et Activités Biologiques. Ph.D. Thesis, Université de Nantes, Nantes, France, 2006. [Google Scholar]
- Naviner, M.; Bergé, J.-P.; Durand, P.; Le Bris, H. Antibacterial activity of the marine diatom Skeletonema costatum against aquacultural pathogens. Aquaculture 1999, 174, 15–24. [Google Scholar] [CrossRef]
- Berland, B.R.; Bonin, D.J.; Cornu, A.L.; Maestrini, S.Y.; Marino, J.-P. The antibacterial substances of the marine alga Stichochrysis immobilis (chrysophyta). J. Phycol. 1972, 8, 383–392. [Google Scholar] [CrossRef]
- Austin, B.; Day, J.G. Inhibition of prawn pathogenic Vibrio spp. by a commercial spray-dried preparation of Tetraselmis suecica. Aquaculture 1990, 90, 389–392. [Google Scholar] [CrossRef]
- Austin, B.; Baudet, E.; Stobie, M. Inhibition of bacterial fish pathogens by Tetraselmis suecica. J. Fish Dis. 1992, 15, 55–61. [Google Scholar] [CrossRef]
- Lio-Po, G.D.; Leaño, E.M.; Peñaranda, M.M.D.; Villa-Franco, A.U.; Sombito, C.D.; Guanzon, N.G., Jr. Anti-luminous Vibrio factors associated with the “green water” grow-out culture of the tiger shrimp Penaeus monodon. Aquaculture 2005, 250, 1–7. [Google Scholar] [CrossRef]
- Kokou, F.; Makridis, P.; Kentouri, M.; Divanach, P. Antibacterial activity in microalgae cultures. Aquac. Res. 2012, 43, 1520–1527. [Google Scholar] [CrossRef]
- Molina-Cárdenas, C.A.; del Pilar Sánchez-Saavedra, M.; Lizárraga-Partida, M.L. Inhibition of pathogenic Vibrio by the microalgae Isochrysis galbana. J. Appl. Phycol. 2014, 26, 2347–2355. [Google Scholar] [CrossRef]
- Terekhova, V.E.; Aizdaicher, N.A.; Buzoleva, L.S.; Somov, G.P. Influence of extrametabolites of marine microalgae on the reproduction of the bacterium Listeria monocytogenes. Russ. J. Mar. Biol. 2009, 35, 355–358. [Google Scholar] [CrossRef]
- Kogure, K.; Simidu, U.; Taga, N. Effect of Skeletonema costatum (Grev.) Cleve on the growth of marine bacteria. J. Exp. Mar. Biol. Ecol. 1979, 36, 201–215. [Google Scholar] [CrossRef]
- Makridis, P.; Costa, R.A.; Dinis, M.T. Microbial conditions and antimicrobial activity in cultures of two microalgae species, Tetraselmis chuii and Chlorella minutissima, and effect on bacterial load of enriched Artemia metanauplii. Aquaculture 2006, 255, 76–81. [Google Scholar] [CrossRef]
- Turcotte, F. Effet Protecteur d’un Pigment (Marennine) de Diatomée Bleue Contre la Bactérie Pathogène Vibrio splendidus chez les Larves de Moule Bleue Mytilus edulis: Utilisation Potentielle en Ecloseries de Vivalves. Master’s Thesis, Université du Québec à Rimouski, Rimouski, QC, Canada, 2014. [Google Scholar]
- Olsen, A.I.; Olsen, Y.; Attramadal, Y.; Christie, K.; Birkbeck, T.H.; Skjermo, J.; Vadstein, O. Effects of short term feeding of microalgae on the bacterial flora associated with juvenile Artemia franciscana. Aquaculture 2000, 190, 11–25. [Google Scholar] [CrossRef]
- Regunathan, C.; Wesley, S.G. Control of Vibrio spp. in shrimp hatcheries using the green algae Tetraselmis suecica. Asian Fish. Sci. 2004, 17, 147–158. [Google Scholar]
- Salvesen, I.; Skjermo, J.; Vadstein, O. Growth of turbot (Scophthalmus maximus L.) during first feeding in relation to the proportion of r/K-strategists in the bacterial community of the rearing water. Aquaculture 1999, 175, 337–350. [Google Scholar] [CrossRef]
- Lavilla-Pitogo, C.R.; Albright, L.J.; Paner, M.G. Will microbial manipulation sustain the ecological balance in shrimp (Penaeus monodon) hatcheries? In Advances in Shrimp Biotechnology, Proceedings of the 5th Asian Fisheries Forum on Special Session on Shrimp Biotechnology, Chiengmai, Thailand, 11–14 November 1998; Flegel, T.W., Ed.; National Center for Genetic Engineering and Biotechnology: Bangkok, Thailand, 1998; pp. 185–192. [Google Scholar]
- Kusumaningrum, H.P.; Zainuri, M. Detection of bacteria and fungi associated with Penaeus monodon postlarvae mortality. Procedia Environ. Sci. 2015, 23, 329–337. [Google Scholar] [CrossRef]
- Davey, M.R.; Anthony, P.; Power, J.B.; Lowe, K.C. Plant protoplasts: Status and biotechnological perspectives. Biotechnol. Adv. 2005, 23, 131–171. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-S.; Tsai, H.-J. Transgenic microalgae as a non-antibiotic bactericide producer to defend against bacterial pathogen infection in the fish digestive tract. Fish Shellfish Immunol. 2009, 26, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Øie, G.; Reitan, K.I.; Olsen, Y. Comparison of rotifer culture quality with yeast plus oil and algal-based cultivation diets. Aquac. Int. 1994, 2, 225–238. [Google Scholar]
- Pouvreau, J.-B.; Morançais, M.; Massé, G.; Rosa, P.; Robert, J.-M.; Fleurence, J.; Pondaven, P. Purification of the blue-green pigment “marennine” from the marine tychopelagic diatom Haslea ostrearia (Gaillon/Bory) Simonsen. J. Appl. Phycol. 2006, 18, 769–781. [Google Scholar] [CrossRef]
- Garnier, M.; Labreuche, Y.; Garcia, C.; Robert, M.; Nicolas, J.-L. Evidence for the Involvement of Pathogenic Bacteria in Summer Mortalities of the Pacific Oyster Crassostrea gigas. Microb. Ecol. 2007, 53, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Garnier, M.; Labreuche, Y.; Nicolas, J.-L. Molecular and phenotypic characterization of Vibrio aestuarianus subsp. francensis subsp. nov., a pathogen of the oyster Crassostrea gigas. Syst. Appl. Microbiol. 2008, 31, 358–365. [Google Scholar] [PubMed]
- Gay, M.; Renault, T.; Pons, A.-M.; Le Roux, F. Two Vibrio splendidus related strains collaborate to kill Crassostrea gigas: Taxonomy and host alterations. Dis. Aquat. Org. 2004, 62, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Goudenège, D.; Travers, M.A.; Lemire, A.; Petton, B.; Haffner, P.; Labreuche, Y.; Tourbiez, D.; Mangenot, S.; Calteau, A.; Mazel, D.; et al. A single regulatory gene is sufficient to alter Vibrio aestuarianus pathogenicity in oysters. Environ. Microbiol. 2015, 17, 4189–4199. [Google Scholar] [CrossRef] [PubMed]
- Genard, B.; Miner, P.; Nicolas, J.-L.; Moraga, D.; Boudry, P.; Pernet, F.; Tremblay, R. Integrative Study of Physiological Changes Associated with Bacterial Infection in Pacific Oyster Larvae. PLoS ONE 2013, 8, e64534. [Google Scholar] [CrossRef] [PubMed]
- Travers, M.-A.; Mersni Achour, R.; Haffner, P.; Tourbiez, D.; Cassone, A.-L.; Morga, B.; Doghri, I.; Garcia, C.; Renault, T.; Fruitier-Arnaudin, I.; et al. First description of French V. tubiashii strains pathogenic to mollusk: I. Characterization of isolates and detection during mortality events. J. Invertebr. Pathol. 2014, 123, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Anaissie, E.; Bodey, G.P. Nosocomial fungal infections. Old problems and new challenges. Infect. Dis. Clin. N. Am. 1989, 3, 867–882. [Google Scholar]
- Alangaden, G.J. Nosocomial fungal infections: Epidemiology, infection control, and prevention. Infect. Dis. Clin. N. Am. 2011, 25, 201–225. [Google Scholar] [CrossRef] [PubMed]
- Manuel, R.J.; Kibbler, C.C. The epidemiology and prevention of invasive aspergillosis. J. Hosp. Infect. 1998, 39, 95–109. [Google Scholar] [CrossRef]
- Wey, S.B.; Mori, M.; Pfaller, M.A.; Woolson, R.F.; Wenzel, R.P. Hospital-acquired candidemia: The attributable mortality and excess length of stay. Arch. Intern. Med. 1988, 148, 2642–2645. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.A.; Rice, L.B. Antifungal agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 1999, 12, 501–517. [Google Scholar] [PubMed]
- Accoceberry, I.; Noël, T. Antifongiques: Cibles cellulaires et mécanismes de résistance. Thérapie 2006, 61, 195–199. [Google Scholar] [CrossRef] [PubMed]
- El Amraoui, B.; El Amraoui, M.; Cohen, N.; Fassouane, A. Anti-Candida and anti-Cryptococcus antifungal produced by marine microorganisms. J. Med. Mycol. 2014, 24, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.C.F.; Wong, J.H.; Pan, W.L.; Chan, Y.S.; Yin, C.M.; Dan, X.L.; Wang, H.X.; Fang, E.F.; Lam, S.K.; Ngai, P.H.K.; et al. Antifungal and antiviral products of marine organisms. Appl. Microbiol. Biotechnol. 2014, 98, 3475–3494. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.-H.; Jang, S.; Heo, Y.M.; Min, M.; Lee, H.; Lee, Y.M.; Lee, H.; Kim, J.-J. Investigation of marine-derived fungal diversity and their exploitable biological activities. Mar. Drugs 2015, 13, 4137–4155. [Google Scholar] [CrossRef] [PubMed]
- Shishido, T.K.; Humisto, A.; Jokela, J.; Liu, L.; Wahlsten, M.; Tamrakar, A.; Fewer, D.P.; Permi, P.; Andreote, A.P.D.; Fiore, M.F.; et al. Antifungal compounds from cyanobacteria. Mar. Drugs 2015, 13, 2124–2140. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-T.; Xue, Y.-R.; Liu, C.-H. A brief review of bioactive metabolites derived from deep-sea fungi. Mar. Drugs 2015, 13, 4594–4616. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Meng, W.; Cao, C.; Wang, J.; Shan, W.; Wang, Q. Antibacterial and antifungal compounds from marine fungi. Mar. Drugs 2015, 13, 3479–3513. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, M.J. Activité antibactérienne d’une Diatomée marine: Asterionella notata (Grun). Rev. Intern. Oceanogr. Med. 1969, 25, 103–165. [Google Scholar]
- Nagai, H.; Satake, M.; Yasumoto, T. Antimicrobial activities of polyether compounds of dinoflagellate origins. J. Appl. Phycol. 1990, 2, 305–308. [Google Scholar] [CrossRef]
- Pesando, D.; Gnassia-Barelli, M.; Gueho, E. Antifungal properties of some marine algae. In Marine Algae in Pharmaceutical Science; Walter de Gruyter: Berlin, Germany; New York, NY, USA, 1979; pp. 461–472. [Google Scholar]
- Kellam, S.J.; Cannell, R.J.P.; Owsianka, A.M.; Walker, J.M. Results of a large-scale screening programme to detect antifungal activity from marine and freshwater microalgae in laboratory culture. Br. Phycol. J. 1988, 23, 45–47. [Google Scholar] [CrossRef]
- Gueho, E.; Pesando, D.; Barelli, M. Propriétés antifongiques d’une diatomeé Chaetoceros lauderi ralfs C C. Mycopathologia 1977, 60, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Pesando, D.; Gnassia-Barelli, M.; Gueho, E. Partial characterization of a specific antibiotic, antifungal substance isolated from the marine diatom Chaetoceros lauderi Ralfs. In Marine Algae in Pharmaceutical Science; Walter de Gruyter: Berlin, Germany; New York, NY, USA, 1979; Volume 1, pp. 447–459. [Google Scholar]
- Washida, K.; Koyama, T.; Yamada, K.; Kita, M.; Uemura, D. Karatungiols A and B, two novel antimicrobial polyol compounds, from the symbiotic marine dinoflagellate Amphidinium sp. Tetrahedron Lett. 2006, 47, 2521–2525. [Google Scholar] [CrossRef]
- Nagai, H.; Mikami, Y.; Yazawa, K.; Gonoi, T.; Yasumoto, T. Biological activities of novel polyether antifungals, gambieric acids A and B from a marine dinoflagellate Gambierdiscus toxicus. J. Antibiot. 1993, 46, 520–522. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.M.; Michaels, L.; Burkholder, P.R. Goniodomin, a new antibiotic from a dinoflagellate. J. Antibiot. 1968, 21, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Makabe, K.; Yamaguchi, K.; Konosu, S.; Wälchli, M.R. Goniodomin a, a novel polyether macrolide from the dinoflagellate Goniodoma pseudogoniaulax. Tetrahedron Lett. 1988, 29, 1149–1152. [Google Scholar] [CrossRef]
- Houdai, T.; Matsuoka, S.; Matsumori, N.; Murata, M. Membrane-permeabilizing activities of amphidinol 3, polyene-polyhydroxy antifungal from a marine dinoflagellate. Biochim. Biophys. Acta 2004, 1667, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Satake, M.; Murata, M.; Yasumoto, T.; Fujita, T.; Naoki, H. Amphidinol, a polyhydroxy-polyene antifungal agent with an unprecedented structure, from a marine dinoflagellate, Amphidinium klebsii. J. Am. Chem. Soc. 1991, 113, 9859–9861. [Google Scholar] [CrossRef]
- Yanong, R.P.E. Fungal diseases of fish. Vet. Clin. N. Am. Exot. Anim. Pract. 2003, 6, 377–400. [Google Scholar] [CrossRef]
- Noga, E.J. Fish Disease: Diagnosis and Treatment; John Wiley & Sons: New York, NY, USA, 2011. [Google Scholar]
- Anater, A.; Manyes, L.; Meca, G.; Ferrer, E.; Luciano, F.B.; Pimpão, C.T.; Font, G. Mycotoxins and their consequences in aquaculture: A review. Aquaculture 2016, 451, 1–10. [Google Scholar] [CrossRef]
- Trakranrungsie, N. Plant derived antifungals-trends and potential applications in veterinary medicine: A mini-review. In Science against Microbial Pathogens: Communicating Current Research and Technological Advances; Mendez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2011. [Google Scholar]
- Hussein, M.M.A.; El-Feki, M.A.; Hatai, K.; Yamamoto, A. Inhibitory effects of thymoquinone from Nigella sativa on pathogenic Saprolegnia in fish. Biocontrol Sci. 2002, 7, 31–35. [Google Scholar] [CrossRef]
- Mori, T.; Hirose, H.; Hanjavanit, C.; Hatai, K. Antifungal activities of plant extracts against some aquatic fungi. Biocontrol Sci. 2002, 7, 187–191. [Google Scholar] [CrossRef]
- Udomkusonsri, P.; Trongvanichnam, K.; Limpoka, M.; Klangkaew, N.; Kusucharit, N. In vitro efficacy of the antifungal activity of some Thai medicinal-plants on the pathogenic fungus, Saprolegnia parasitica H2, in fish. Kasetsart J. Nat. Sci. 2007, 41, 56–61. [Google Scholar]
- Hussein, M.M.A.; Wada, S.; Hatai, K.; Yamamoto, A. Antimycotic activity of eugenol against selected water molds. J. Aquat. Anim. Health 2000, 12, 224–229. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Mirzargar, S.S.; Ebrahim Zadeh Mousavi, H.; Omid Baigi, R.; Khosravi, A.; Bahonar, A.; Ahmadi, M. Evaluation of antifungal activity of new combined essential oils in comparaison with malachite green on hatching rate in rainbow trout (Oncorhynchus mykiss) eggs. J. Fish. Aquat. Sci. 2009, 4, 103–110. [Google Scholar]
- Rhoobunjongde, W.; Hatai, K.; Wada, S.; Kubota, S.S. Fusarium moniliforme (Sheldon) isolated from gills of kuruma prawn Penaeus japonicus (Bate) with black gill disease. Nippon Suisan Gakkaishi 1991, 57, 629–635. [Google Scholar] [CrossRef]
- Tolosa, J.; Font, G.; Mañes, J.; Ferrer, E. Natural occurrence of emerging Fusarium mycotoxins in feed and fish from aquaculture. J. Agric. Food Chem. 2014, 62, 12462–12470. [Google Scholar] [CrossRef] [PubMed]
- Grovel, O.; Pouchus, Y.F.; Verbist, J.-F. Accumulation of gliotoxin, a cytotoxic mycotoxin from Aspergillus fumigatus, in blue mussel (Mytilus edulis). Toxicon 2003, 42, 297–300. [Google Scholar] [CrossRef]
- Matallah-Boutiba, A.; Ruiz, N.; Sallenave-Namont, C.; Grovel, O.; Amiard, J.-C.; Pouchus, Y.F.; Boutiba, Z. Screening for toxigenic marine-derived fungi in Algerian mussels and their immediate environment. Aquaculture 2012, 342–343, 75–79. [Google Scholar] [CrossRef]
- Karthikeyan, V.; Gopalakrishnan, A. A novel report of phytopathogenic fungi Gilbertella persicaria infection on Penaeus monodon. Aquaculture 2014, 430, 224–229. [Google Scholar] [CrossRef]
- Karthikeyan, V.; Selvakumar, P.; Gopalakrishnan, A. A novel report of fungal pathogen Aspergillus awamori causing black gill infection on Litopenaeus vannamei (pacific white shrimp). Aquaculture 2015, 444, 36–40. [Google Scholar] [CrossRef]
- Kitazato, K.; Wang, Y.; Kobayashi, N. Viral infectious disease and natural products with antiviral activity. Drug Discov. Ther. 2007, 1, 14–22. [Google Scholar] [PubMed]
- Yasuhara-Bell, J.; Lu, Y. Marine compounds and their antiviral activities. Antivir. Res. 2010, 86, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Husseiny, S.; Ibrahem, A.B.B.; El Sayed, A.F.M.; Mohammed, F.A. In vitro evaluation of anti-microbial activities of marine streptomyces against viral models, bacterial and fungal Strains. Int. J. Virol. 2015, 11, 20–31. [Google Scholar]
- Vo, T.-S.; Ngo, D.-H.; Ta, Q.V.; Kim, S.-K. Marine organisms as a therapeutic source against herpes simplex virus infection. Eur. J. Pharm. Sci. 2011, 44, 11–20. [Google Scholar] [CrossRef]
- Gerber, P.; Dutcher, J.D.; Adams, E.V.; Sherman, J.H. Protective Effect of Seaweed Extracts for Chicken Embryos Infected with Influenza B or Mumps Virus. Exp. Biol. Med. 1958, 99, 590–593. [Google Scholar] [CrossRef]
- Ehresmann, D.W.; Deig, E.F.; Hatch, M.T.; DiSalvo, L.H.; Vedros, N.A. Antiviral Substances from California Marine Algae1. J. Phycol. 1977, 13, 37–40. [Google Scholar] [CrossRef]
- Bouhlal Rhimou, R.H. Antiviral activity of the extracts of Rhodophyceae from Morocco. Afr. J. Biotechnol. 2010, 9, 7968–7975. [Google Scholar] [CrossRef]
- Luescher-Mattli, M. Algae, a possible source for new drugs in the treatment of HIV and other viral diseases. Curr. Med. Chem.—Anti-Infect. Agents 2003, 2, 219–225. [Google Scholar] [CrossRef]
- Schaeffer, D.J.; Krylov, V.S. Anti-HIV activity of extracts and compounds from algae and cyanobacteria. Ecotoxicol. Environ. Saf. 2000, 45, 208–227. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.R.; Robaina, M.C.S.; Mendes, G.S.; Silva, T.S.L.; Gestinari, L.M.S.; Pamplona, O.S.; Yoneshigue-Valentin, Y.; Kaiser, C.R.; Romanos, M.T.V. Antiviral activity of extracts from Brazilian seaweeds against herpes simplex virus. Rev. Bras. Farmacogn. 2012, 22, 714–723. [Google Scholar] [CrossRef]
- Wijesekara, I.; Pangestuti, R.; Kim, S.-K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2011, 84, 14–21. [Google Scholar] [CrossRef]
- Arif, J.M.; Farooqui, A.; Siddiqui, M.H.; Al-Karrawi, M.; Al-Hazmi, A.; Al-Sagair, O.A. Chapter 5: Novel bioactive peptides from cyanobacteria: Functional, biochemical, and biomedical significance. In Studies in Natural Products Chemistry; Bioactive Natural Products; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 36, pp. 111–161. [Google Scholar]
- Hayashi, T.; Hayashi, K.; Maeda, M.; Kojima, I. Calcium spirulan, an inhibitor of enveloped virus replication, from a blue-green alga Spirulina platensis. J. Nat. Prod. 1996, 59, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Rechter, S.; König, T.; Auerochs, S.; Thulke, S.; Walter, H.; Dörnenburg, H.; Walter, C.; Marschall, M. Antiviral activity of Arthrospira-derived spirulan-like substances. Antivir. Res. 2006, 72, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Zorofchian Moghadamtousi, S.; Abubakar, S.; Zandi, K. Antiviral potential of algae polysaccharides isolated from marine sources: A review. BioMed Res. Int. 2015, 2015, 825203. [Google Scholar] [CrossRef] [PubMed]
- Huleihel, M.; Ishanu, V.; Tal, J.; Arad, S. Antiviral effect of red microalgal polysaccharides on Herpes simplex and Varicella zoster viruses. J. Appl. Phycol. 2001, 13, 127–134. [Google Scholar] [CrossRef]
- Santoyo, S.; Plaza, M.; Jaime, L.; Ibañez, E.; Reglero, G.; Señorans, F.J. Pressurized liquid extraction as an alternative process to obtain antiviral agents from the edible microalga Chlorella vulgaris. J. Agric. Food Chem. 2010, 58, 8522–8527. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S.; Ono, F.; Shiomi, Y.; Nakao, T.; Aozasa, O.; Nagate, T.; Kitamura, K.; Yamaguchi, S.; Nishi, M.; Miyata, H. Anti-Herpes Simplex Virus substances produced by the marine green alga, Dunaliella primolecta. J. Appl. Phycol. 1998, 10, 349–356. [Google Scholar] [CrossRef]
- Santoyo, S.; Jaime, L.; Plaza, M.; Herrero, M.; Rodriguez-Meizoso, I.; Ibañez, E.; Reglero, G. Antiviral compounds obtained from microalgae commonly used as carotenoid sources. J. Appl. Phycol. 2011, 24, 731–741. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.F.; de Morais, A.M.M.B.; de Morais, R.M.S.C. Influence of sulphate on the composition and antibacterial and antiviral properties of the exopolysaccharide from Porphyridium cruentum. Life Sci. 2014, 101, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Radonić, A.; Thulke, S.; Achenbach, J.; Kurth, A.; Vreemann, A.; König, T.; Walter, C.; Possinger, K.; Nitsche, A. Anionic polysaccharides from phototrophic microorganisms exhibit antiviral activities to Vaccinia virus. J. Antivir. Antiretrovir. 2010, 2, 51–55. [Google Scholar] [CrossRef]
- Huleihel, M.; Ishanu, V.; Tal, J.; Arad, S. Activity of Porphyridium sp. polysaccharide against Herpes simplex viruses in vitro and in vivo. J. Biochem. Biophys. Methods 2002, 50, 189–200. [Google Scholar] [CrossRef]
- Talyshinsky, M.M.; Souprun, Y.Y.; Huleihel, M.M. Anti-viral activity of red microalgal polysaccharides against retroviruses. Cancer Cell Int. 2002, 2. [Google Scholar] [CrossRef]
- Bergé, J.P.; Bourgougnon, N.; Alban, S.; Pojer, F.; Billaudel, S.; Chermann, J.C.; Robert, J.M.; Franz, G. Antiviral and anticoagulant activities of a water-soluble fraction of the marine diatom Haslea ostrearia. Planta Med. 1999, 65, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-B.; Hayashi, K.; Hirata, M.; Kuroda, E.; Suzuki, E.; Kubo, Y.; Hayashi, T. Antiviral sulfated polysaccharide from Navicula directa, a diatom collected from deep-sea water in Toyama bay. Biol. Pharm. Bull. 2006, 29, 2135–2139. [Google Scholar] [CrossRef] [PubMed]
- Hasui, M.; Matsuda, M.; Okutani, K.; Shigeta, S. In vitro antiviral activities of sulfated polysaccharides from a marine microalga (Cochlodinium polykrikoides) against human immunodeficiency virus and other enveloped viruses. Int. J. Biol. Macromol. 1995, 17, 293–297. [Google Scholar] [CrossRef]
- Yim, J.H.; Kim, S.J.; Ahn, S.H.; Lee, C.K.; Rhie, K.T.; Lee, H.K. Antiviral effects of sulfated exopolysaccharide from the marine microalga Gyrodinium impudicum strain KG03. Mar. Biotechnol. 2004, 6, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Yim, J.H.; Kim, S.-Y.; Kim, H.S.; Lee, W.G.; Kim, S.J.; Kang, P.-S.; Lee, C.-K. In vitro inhibition of influenza A virus infection by marine microalga-derived sulfated polysaccharide p-KG03. Antivir. Res. 2012, 93, 253–259. [Google Scholar] [CrossRef]
- Ahne, W. Viral infections of aquatic animals with special reference to Asian aquaculture. Annu. Rev. Fish Dis. 1994, 4, 375–388. [Google Scholar] [CrossRef]
- Arzul, I.; Corbeil, S.; Morga, B.; Renault, T. Viruses infecting marine molluscs. J. Invertebr. Pathol. 2016. under review. [Google Scholar]
- Crane, M.; Hyatt, A. Viruses of Fish: An Overview of Significant Pathogens. Viruses 2011, 3, 2025–2046. [Google Scholar] [CrossRef] [PubMed]
- Segarra, A.; Pépin, J.F.; Arzul, I.; Morga, B.; Faury, N.; Renault, T. Detection and description of a particular Ostreid herpesvirus 1 genotype associated with massive mortality outbreaks of Pacific oysters, Crassostrea gigas, in France in 2008. Virus Res. 2010, 153, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Arzul, I.; Renault, T.; Lipart, C.; Davison, A.J. Evidence for interspecies transmission of oyster herpesvirus in marine bivalves. J. Gen. Virol. 2001, 82, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Casado, E.; Estepa, A.; Coll, J.M. A comparative review on European-farmed finfish RNA viruses and their vaccines. Vaccine 2011, 29, 2657–2671. [Google Scholar] [CrossRef] [PubMed]
- Sommerset, I.; Krossøy, B.; Biering, E.; Frost, P. Vaccines for fish in aquaculture. Expert Rev. Vaccines 2005, 4, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Loker, E.S.; Adema, C.M.; Zhang, S.-M.; Kepler, T.B. Invertebrate immune systems—Not homogeneous, not simple, not well understood. Immunol. Rev. 2004, 198, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Rowley, A.F.; Pope, E.C. Vaccines and crustacean aquaculture—A mechanistic exploration. Aquaculture 2012, 334–337, 1–11. [Google Scholar] [CrossRef]
- Johnson, K.N.; van Hulten, M.C.W.; Barnes, A.C. “Vaccination” of shrimp against viral pathogens: Phenomenology and underlying mechanisms. Vaccine 2008, 26, 4885–4892. [Google Scholar] [CrossRef] [PubMed]
- Venegas, C.A.; Nonaka, L.; Mushiake, K.; Nishizawa, T.; Murog, K. Quasi-immune response of Penaeus japonicus to penaeid rod-shaped DNA virus (PRDV). Dis. Aquat. Org. 2000, 42, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Green, T.J.; Montagnani, C. Poly I: C induces a protective antiviral immune response in the Pacific oyster (Crassostrea gigas) against subsequent challenge with Ostreid herpesvirus (OsHV-1 μvar). Fish Shellfish Immunol. 2013, 35, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Jemaà, M.; Morin, N.; Cavelier, P.; Cau, J.; Strub, J.M.; Delsert, C. Adult somatic progenitor cells and hematopoiesis in oysters. J. Exp. Biol. 2014, 217, 3067–3077. [Google Scholar] [CrossRef] [PubMed]
- Sivasankar, P.; Anix Vivek Santhiya, A.; Kanaga, V. A review on plants and herbal extracts against viral diseases in aquaculture. J. Med. Plants Stud. 2015, 3, 75–79. [Google Scholar]
- Balasubramanian, G.; Sarathi, M.; Venkatesan, C.; Thomas, J.; Hameed, A.S.S. Studies on the immunomodulatory effect of extract of Cyanodon dactylon in shrimp, Penaeus monodon, and its efficacy to protect the shrimp from white spot syndrome virus (WSSV). Fish Shellfish Immunol. 2008, 25, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Fabregas, J.; García, D.; Fernandez-Alonso, M.; Rocha, A.I.; Gómez-Puertas, P.; Escribano, J.M.; Otero, A.; Coll, J.M. In vitro inhibition of the replication of haemorrhagic septicaemia virus (VHSV) and African swine fever virus (ASFV) by extracts from marine microalgae. Antivir. Res. 1999, 44, 67–73. [Google Scholar] [CrossRef]
- Katharios, P.; Papadakis, I.; Prapas, A.; Dermon, C.; Ampatzis, K.; Divanach, P. Mortality control of viral encephalopathy and retinopathy in 0+ grouper Epinephelus marginatus after prolonged bath in dense Chlorella minutissima culture. Bull. Eur. Assoc. Fish Pathol. 2005, 25, 28–31. [Google Scholar]

| Microalgae Species | Antibacterial Compound/Fraction | (G+) Bacteria Growth Inhibition | (G−) Bacteria Growth Inhibition | References |
|---|---|---|---|---|
| Green microalgae | ||||
| Chlamydomonas reinhardtii | Aqueous or methanolic and exanolic extracts | Bacillus subtilis, Staphylococcus aureus, Staphylococcus epidermidis | Escherichia coli, Pseudomonas aeruginosa, Salmonella typhi | [62] |
| Chlorella minutissima | Ethanolic extracts | S. aureus | E. coli, P. aeruginosa | [51] |
| Chlorella pyrenoidosa | Various organic solvent extracts | B. subtilis, S. aureus | E. coli, P. aeruginosa | [63] |
| Chlorella vulgaris | Chlorellin | B. subtilis, S. aureus, Streptococcus pyogenes | E. coli, P. aeruginosa | [49] |
| Chlorella vulgaris | Aqueous or methanolic and hexanolic extracts | B. subtilis, S. aureus, S. epidermidis | E. coli, P. aeruginosa, S. typhi | [62] |
| Chlorococcum HS-101 | alpha-linolenic acid | B. subtilis, Bacillus cereus, S. aureus, MRSA | Enterobacter aerogenes | [53,56,64] |
| Chlorococcum humicola | Various organic solvent extracts and purified pigments (carotenoid, chlorophyll) | B. subtilis, S. aureus | E. coli, P. aeruginosa, Salmonella typhimurium, Klebsiella pneumoniae, Vibrio cholerae | [65] |
| Desmococcus olivaceus | Ethanolic extracts | S. aureus | E. coli, P. aeruginosa | [51] |
| Dunaliella primolecta | Polyunsatured fatty acids: alpha-linolenic acid | B. cereus, B. subtilis, S. aureus, MRSA | E. aerogenes | [53,64] |
| Dunaliella salina | Indolic derivative, polyunsaturated fatty acids, beta-ionone and neophytadiene | S. aureus | E. coli, P. aeruginosa | [52,66,67] |
| Dunaliella sp. | Lysed cells | S. epidermidis, Micrococcus luteus | Proteus vulgaris | [68] |
| Haematococcus pluvialis | Short-chain fatty acids | S. aureus | E. coli | [69,70] |
| Klebsormidium sp. | Pellet | B. subtilis | Ne | [50] |
| Pseudokirchneriella subcapitata | Methanolic extracts | S. aureus | P. aeruginosa | [52] |
| Scenedesmus obliquus | Long chain fatty acids | S. aureus | E. coli, P. aeruginosa, Salmonella sp. | [71] |
| Scenedesmus quadricauda | Various organic solvent extracts | B. subtilis, S. aureus | E. coli, P. aeruginosa | [63] |
| Scenedesmus sp. | Ethanolic extracts | S. aureus | E. coli, P. aeruginosa | [51] |
| Red microalgae | ||||
| Porphyridium aerugineum | Phycobiliproteins | S. aureus, S. pyogenes | Nt | [72] |
| Porphyridium purpureum | Methanolic extracts | B. subtilis | E. coli | [50] |
| Porphyridium sordidum | Pellet | B. subtilis | E. coli, Pseudomonas fluorescens | [50] |
| Rhodella reticulata | Exopolysaccharides | S. aureus, B. cereus, S. pyogenes | Ne | [72] |
| Diatoms | ||||
| Asterionella glacialis | Whole cell | S. aureus, S. epidermidis, M. luteus, Sarcina sp. | E. coli | [58] |
| Attheya longicornis | Methanolic extracts | S. aureus, MRSA | Ne | [73] |
| Chaetoceros muelleri | Unsaturated fatty acid-containing lipidic fractions (triglycerides and docosa-pentaenoic acid (DPA)) | B. subtilis, S. aureus | E. coli | [74,75] |
| Navicula delognei (Parlibellus delognei) | transphytol ester, hexadecatetraenoic and octadecatetraenoic acids | S. aureus, S. epidermidis | S. typhimurium, P. vulgaris | [76] |
| Phaeodactylum tricornutum | eicosapentaenoic acid (EPA), palmitoleic and hexadecatrienoic acids (HTA) | B. cereus, Bacillus weihenstephanensis, S. aureus, S. epidermidis, MRSA | Ne | [77,78] |
| Rhizosolenia alata | Various organic solvent extracts | B. subtilis, S. aureus | E. coli, P. aeruginosa, P. vulgaris, S. typhi, V. cholerae | [79] |
| Thalassiothrix frauenfeldii | Non-axenic culture and organic solvent extracts | B. subtilis, S. aureus | E. coli, P. aeruginosa, Salmonella paratyphi, S. typhi, V. cholerae | [80] |
| Skeletonema costatum | Aqueous and organic extracts | B. subtilis, S. aureus | P. aeruginosa | [81] |
| Skeletonema costatum | Various organic solvents extracts | S. aureus, Staphylococcus peoria, S. fecalis, S. pyogenes | Ne | [54] |
| Haptophytes | ||||
| Isochrysis galbana | Chlorophyll a derivatives: pheophytin a and chlorophyllide a | S. aureus, Streptococcus faecalis, S. pyogenes, Micrococcus sp. | Nt | [54,82] |
| Microalgae Species | Compound/Fraction Tested | Target Bacteria/Antibacterial Effect | References |
|---|---|---|---|
| In vitro experiments | |||
| Chaetoceros lauderi | Whole cell | Vibrio anguillarum, Aeromonas salmonicida | [58] |
| Dunaliella tertiolecta | Aqueous extract | Vibrio campbellii | [115] |
| Euglena viridis | Organic solvent extracts | Aeromonas hydrophila, Edwardsiella tarda, Pseudomonas aeruginosa, Pseudomonas fluorescens, Pseudomonas putida Vibrio alginolyticus, V. anguillarum, Vibrio fluvialis, Vibrio harveyi, Vibrio parahaemolyticus | [116] |
| Haslea karadagensis | Purified pigment (intra- and extracellular forms) | Polaribacter irgensii, Pseudoalteromonas elyakowii, Vibrio aestuarianus | [117] |
| Haslea ostrearia | Purified marennine (intra- and extracellular forms) | P. irgensii, P. elyakowii, V. aestuarianus | [118] |
| Purified marennine (intracellular form) | V. anguillarum | [119] | |
| Purified marennine (extracellular form) | Vibrio splendidus-related | [30] | |
| Phaeodactylum tricornutum | Aqueous and organic extracts | Alcaligenes cupidus, Alteromonas communis, Alteromonas haloplanktis, Vibrio fischeri, V. parahaemolyticus | [81] |
| Polyunsaturated free fatty acid | Vibrio anguillarum, M. luteus, Photobacterium sp. | [78] | |
| Skeletonema costatum | Aqueous and Organic extracts | A. cupidus, A. communis, Pseudomonas marina, V. fischeri, V. parahaemolyticus | [81] |
| Organic and purified extracts | V. anguillarum, Vibrio mytili T, Vibrio spp. S322, Vibrio spp. VRP | [120] | |
| Aqueous extracts | Vibrio campbellii | [115] | |
| Stichochrysis immobilis | Microalgal homogenates | Xanthomonas sp. 1, Flavobacterium sp. 1, Pseudomonas sp. Strain 101 | [121] |
| Tetraselmis suecica | Microalgal supernatant and microalgal homogenates of a commercial spray-dried preparation | A. hydrophila, A. salmonicida, Serratia liquefaciens, V. alginolyticus, V. anguillarum, V. parahaemolyticus, Vibrio salmonicida, Vibrio vulnificus, Yersinia ruckeri | [122,123] |
| Co-culture experiments | |||
| Chaetoceros calcitrans | Axenic culture | V. harveyi | [124] |
| Chlorella minutissima | Axenic culture | V. alginolyticus, V. anguillarum, Vibrio lentus, V. parahaemolyticus, Vibrio scophthalmi, V. splendidus | [125] |
| Chlorella sp. | Axenic culture | V. harveyi | [40] |
| Isochrysis galbana | Non-axenic culture | V. alginolyticus, V. campbellii, V. harveyi | [126] |
| Isochrysis sp. | Axenic culture | V. alginolyticus, V. lentus, V. splendidus, V. scophthalmi, V. parahaemolyticus, V. anguillarum | [125] |
| Nannochloropsis sp. | Axenic culture | V. alginolyticus, V. anguillarum, V. lentus, V. parahaemolyticus, V. scophthalmi, V. splendidus | [125] |
| Nitzchia sp. | Axenic culture | V. harveyi | [124] |
| S. costatum | Exometabolites in the culture fluid | Listeria monocytogenes | [127] |
| Axenic culture | Pseudomonas sp., Vibrio sp. | [128] | |
| Tetraselmis chui | Axenic culture | V. alginolyticus, V. anguillarum, V. lentus, V. parahaemolyticus, V. scophthalmi, V. splendidus | [125] |
| In vivo experiments | |||
| C. minutissima | 30 min incubation of enriched Artemia metanauplii | Decrease of the bacterial load in Artemia and diminution of presumptive Vibrio | [129] |
| D. tertiolecta | Daily diet of Artemia franciscana | Protection against V. campbellii and V. proteolyticus | [39] |
| H. ostrearia | Incubation of Mytilus edulis larvae with supernatant containing extracellular pigments | Higher survival and physiological conditions of larvae challenged with V. splendidus-related | [130] |
| Tetraselmis sp. | 4 h incubation of Artemia franciscana | Diminution of associated bacteria, better bacterial diversity and the flora less dominated by V. alginolyticus | [131] |
| T. suecica | Food supplement for the Atlantic salmon Solmo salar | Reduction of A. hydrophila, A. salmonicida, Serratia liquefaciens, V. anguillarum, V. salmonicida, Y. ruckeri infections, reduction of bacterial populations in water tanks and increase of the microbial communities in the digestive tract | [123] |
| Food supplement for the broodstock and partial live larvae feed for the white prawn Fenneropenaeus indicus | Reduction of Vibrio numbers in the water tank, better egg hatching rate and survival rate of the larvae | [132] |
| Microalgae Species | Antifungal Compounds/Fraction | Target Microorganims | References | |
|---|---|---|---|---|
| Green microalgae | ||||
| Chlorella vulgaris | Microalgal supernatant | Yeast: Candida kefyr Mold: Aspergillus fumigatus, Aspergillus niger | [62] | |
| Chlorococcum humicola | Organic solvent extracts and pigments: beta carotene, Chlorophyll a and Chlorophyll b | Yeast: C. albicans Mold: A. flavus, A. niger | [65] | |
| Heterochlorella luteoviridis | Microalgal supernatant | Yeast: C. albicans | [50] | |
| Haematococcus pluvialis | Short-chain fatty acids | Yeast: C. albicans | [70] | |
| Scenedesmus quadricauda | Organic solvent extracts | Yeast: C. albicans, S. cerevisiae Mold: A. flavus, A. niger, P. herquei Other: A. brassicae, F. moniliforme, Helminthosporium sp. | [63] | |
| Red microalgae | ||||
| Porphyridium aerugineum | Phycobiliproteins | Yeast: C. albicans | [72] | |
| Porphyridium purpureum | Microalgal supernatant | Yeast: C. albicans | [50] | |
| Rhodella reticulata | Exopolysaccharides | Yeast: C. albicans | [72] | |
| Diatoms | ||||
| Chaetoceros lauderi | Polysaccharides | Mold: A. fumigatus, Blastomyces dermatitidis, Emmonsia parva, Madurella mycetomi, Sporothrix schenckii Dermatophyte: Epidermophyton floccosum, Microsporum audouini, Microsporum canis, Microsporum ferrugineum, Microsporum gypseum, Microsporum nanum, Microsporum persicolor, Trichophyton spp. | [58,162,163] | |
| Chaetoceros muelleri | Lipidic fractions: triglycerides, docosapentaenoic acid (DPA) | Yeast: C. albicans | [75] | |
| Haslea karadagensis | Purified pigment (intra- and extracellular forms) | Corollospora maritima, Lulworthia sp., Dendryphiella salina | [117] | |
| Thalassiothrix frauenfeldii | culture filtrates and organic solvent extracts | Yeast: C. albicans, Candida glabrata, Candida krusei, Candida tropicalis, Cryptococcus neoformans Mold: A. niger | [80] | |
| Dinoflagellates | ||||
| Amphidinium sp. | Polyols: karatungiols A(1) | Mold: A. niger | [164] | |
| Gambierdiscus toxicus | Gambieric acids A and B forms | Mold: A. fumigatus, A. niger, Aspergillus oryzae, Penicillium citrinum, Penicillium chrysogenum, Paecilomyces variotii Dermatophyte: E. floccosum, T. mentagrophytes | [165] | |
| Goniodoma pseudogonyaulax | Goniodomin A (polyether macrolide) | Yeast: C. albicans, C. neoformans, S. cerevisiae Mold: Penicillium sp. Dermatophyte: T. mentagrophytes | [166,167] | |
| Prorocentrum lima | Polyethers | Yeast: Candida rugosa Mold: A. niger, Penicillium funiculosum | [159] | |
| Microalgae Species | Antiviral Compound and Cytotoxicity (μg/mL) | Target Virus | Mechanism of Action and Efficiency (μg/mL) | References |
|---|---|---|---|---|
| Green microalgae | ||||
| Chlorella vulgaris | Polysaccharide-rich fraction CC50 > 1600 (Vero cells) | HSV-1 | Inhibits attachment, replication | [201] |
| IC50 = 61 | ||||
| Dunaliella primolecta | Pheophorbide-like compound Not cytotoxic (Vero cells) | HSV-1 | Inhibits adsorption, invasion | [202] |
| MIC = 5 (totally inhibit the CPE) | ||||
| Dunaliella salina | Short chain fatty acids, β-ionone, neophytadiene, phytol, palmitic and α-linolenic acids CC50 = 1711 (Vero cells) | HSV-1 | Inhibits infectivity | [203] |
| IC50 = 85 | ||||
| Haematococcus pluvialis | Polysaccharide-rich fraction CC50 = 1867 (Vero cells) | HSV-1 | Inhibits attachment, penetration, replication | [203] |
| IC50 = 99 | ||||
| Red microalgae | ||||
| Porphyridium cruentum | Sulphated exopolysaccharide Not cytotoxic at 100 (HeL cells) | Inhibits penetration, replication | [204] | |
| HSV-1 | EC50 (HSV-1) = 34 | |||
| HSV-2 | EC50 (HSV-2) = 12 | |||
| Vaccina | EC50 (Vaccina) = 12 | |||
| Porphyridium purpureum | Exopolysaccharide Not cytotoxic at 500 (HEp-2 cells) | Vaccina | Interaction with free viral particles | [205] |
| IC50 = 0.65 | ||||
| Porphyridium sp. | Sulphated polysaccharide Not cytotoxic at 250 (Vero cells) and 2000 (in vivo in rats) | HSV-1 | In vitro: inhibits adsorption, replication | [200,206] |
| CPE50 = 1 | ||||
| In vivo: prevents the development of symptoms at 100 | ||||
| Inhibits adsorption, replication | ||||
| HSV-2 | CPE50 (HSV-2) = 5 | |||
| VZV | CPE50 (VZV) = 0.7 | |||
| Porphyridium sp. | Purified polysaccharide Not cytotoxic at 1000 (NIH/3T3 cells) | MuSV/MuLV | Inhibits the production of retroviruses in the cells | [207] |
| RT50 reduction = 5 | ||||
| MuSV-124 | Inhibits cell transformation | |||
| ffu50 protection = 10 | ||||
| Diatoms | ||||
| Haslea karadagensis | Purified pigment: intra- and extracellular forms | HSV-1 | Inhibits infection, cell destruction | [117] |
| CC50 (Int) = 87 | EC50 (Int) = 62 | |||
| CC50(Ext) > 200 (Vero cells) | EC50 (Ext) = 23 | |||
| Haslea ostrearia | Purified pigment: intra- and extracellular forms | HSV-1 | Inhibits infection, cell destruction | [117] |
| CC50 (Int) > 200 (Vero cells) | EC50 (Int) = 24 | |||
| CC50 (Ext) = 107 (Vero cells) | EC50 (Ext) = 27 | |||
| Water soluble extract CC50 > 200 (Vero and MT-4 cells) | HSV-1 | Inhibits replication | [208] | |
| EC50 = 14 | ||||
| Navicula directa | Sulphated polysaccharide: Naviculan | Inhibits adhesion, penetration | [209] | |
| CC50 (HSV-1) = 3800 (Vero cells) | HSV-1 | IC50 (HSV-1) = 14 | ||
| CC50 (HSV-2) = 3800 (Vero cells) | HSV-2 | IC50 (HSV-2) = 7.4 | ||
| CC50 (IFV-A)= 5400 (MDCK cells) | IFV-A | IC50 (IFV-A) = 170 | ||
| CC50 (HIV-1) = 4000 (HeLA cells) | HIV-1 | IC50 (HIV-1) = 53 | ||
| Dinoflagellates | ||||
| Cochlodinium polykrikoides | Extracellular sulphated polysaccharides: A1 and A2 | Inhibits replication and the CPE | [210] | |
| CC50 (HIV-1) > 100 (MT-4 cells) | HIV-1 | IC50 (HIV-1) = 1.7 | ||
| CC50 (IFV-A) > 100 (MDCK cells) | IFV-A | IC50 (IFV-A) = 0.45–1 | ||
| CC50 (IFV-B) > 100 (MDCK cells) | IFV-B | IC50 (IFV-B) = 7.1–8.3 | ||
| CC50 (RSV-A) > 100 (Hep-2 cells) | RSV-A | IC50 (RSV-A) = 2–3 | ||
| CC50 (RSV-B) > 100 (Hep-2 cells) | RSV-B | IC50 (RSV-B) = 0.8 | ||
| A1 | HSV-1 | IC50 = 4.5 | ||
| CC50 > 100 (HMV-2 cells) | ||||
| A2 | PFluV-2 | IC50 = 0.8 | ||
| CC50 > 100 (HMV-2 cells) | ||||
| Gyrodinium impudicum | Purified sulphated exopolysaccharide: p-KG03 | EMCV | Inhibits the development of the CPE, suppress tumor cell growth EC50 = 27 | [211] |
| CC50 = 3.4 (MT-4 cells) | ||||
| CC50 = 59.9 (Vero cells) | ||||
| CC50 > 1000 (HeLa cells) | ||||
| Not in MDCK cells CC50 > 100 | Inhibits adsorption | [212] | ||
| IFV-A | EC50 (IFV-A) = 0.19–0.48 | |||
| IFV-B | EC50 (IFV-B) = 0.26 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falaise, C.; François, C.; Travers, M.-A.; Morga, B.; Haure, J.; Tremblay, R.; Turcotte, F.; Pasetto, P.; Gastineau, R.; Hardivillier, Y.; et al. Antimicrobial Compounds from Eukaryotic Microalgae against Human Pathogens and Diseases in Aquaculture. Mar. Drugs 2016, 14, 159. https://doi.org/10.3390/md14090159
Falaise C, François C, Travers M-A, Morga B, Haure J, Tremblay R, Turcotte F, Pasetto P, Gastineau R, Hardivillier Y, et al. Antimicrobial Compounds from Eukaryotic Microalgae against Human Pathogens and Diseases in Aquaculture. Marine Drugs. 2016; 14(9):159. https://doi.org/10.3390/md14090159
Chicago/Turabian StyleFalaise, Charlotte, Cyrille François, Marie-Agnès Travers, Benjamin Morga, Joël Haure, Réjean Tremblay, François Turcotte, Pamela Pasetto, Romain Gastineau, Yann Hardivillier, and et al. 2016. "Antimicrobial Compounds from Eukaryotic Microalgae against Human Pathogens and Diseases in Aquaculture" Marine Drugs 14, no. 9: 159. https://doi.org/10.3390/md14090159
APA StyleFalaise, C., François, C., Travers, M.-A., Morga, B., Haure, J., Tremblay, R., Turcotte, F., Pasetto, P., Gastineau, R., Hardivillier, Y., Leignel, V., & Mouget, J.-L. (2016). Antimicrobial Compounds from Eukaryotic Microalgae against Human Pathogens and Diseases in Aquaculture. Marine Drugs, 14(9), 159. https://doi.org/10.3390/md14090159








