Recombinant Expression of a Modified Shrimp Anti-Lipopolysaccharide Factor Gene in Pichia pastoris GS115 and Its Characteristic Analysis
Abstract
:1. Introduction
2. Results
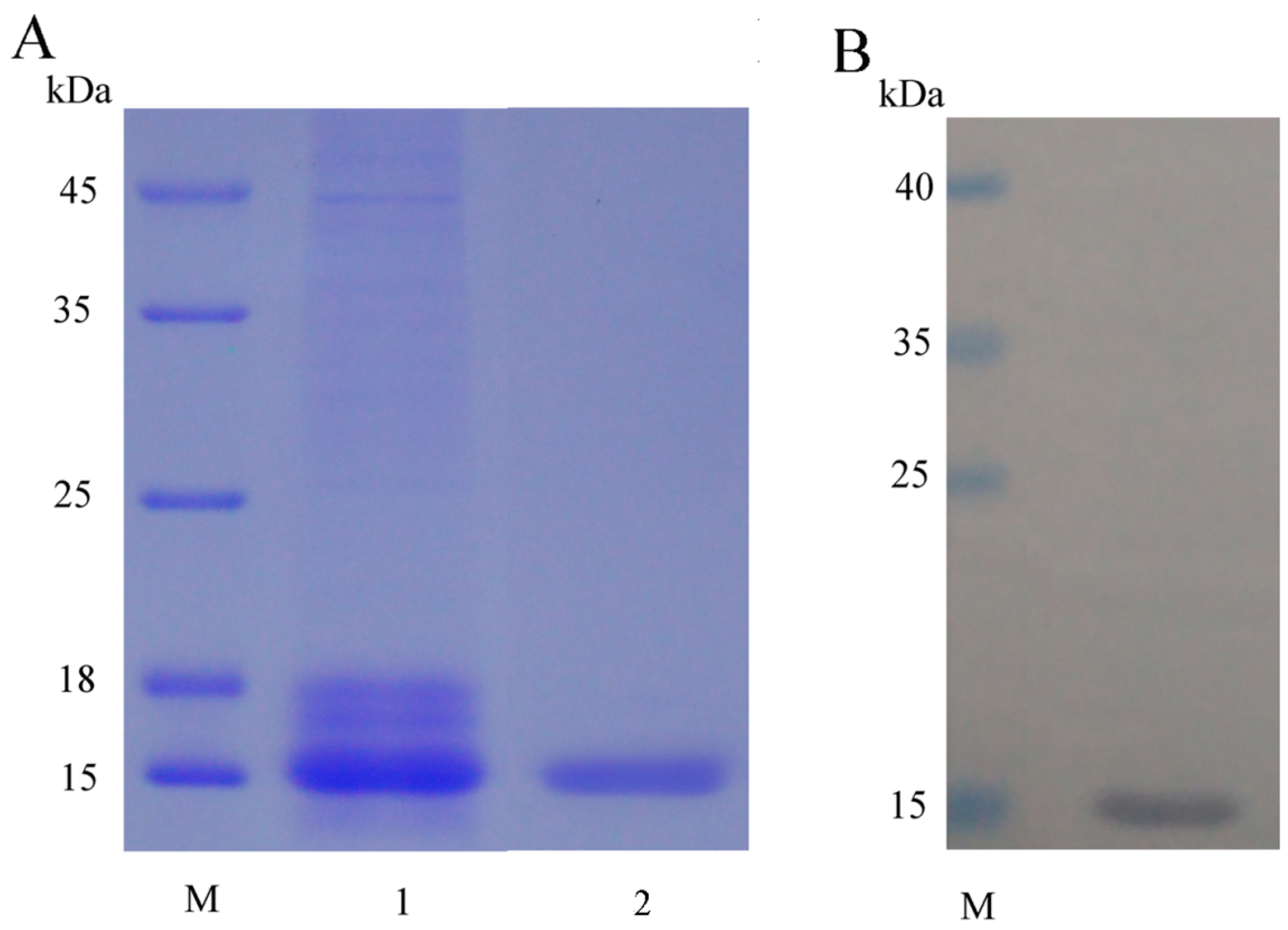
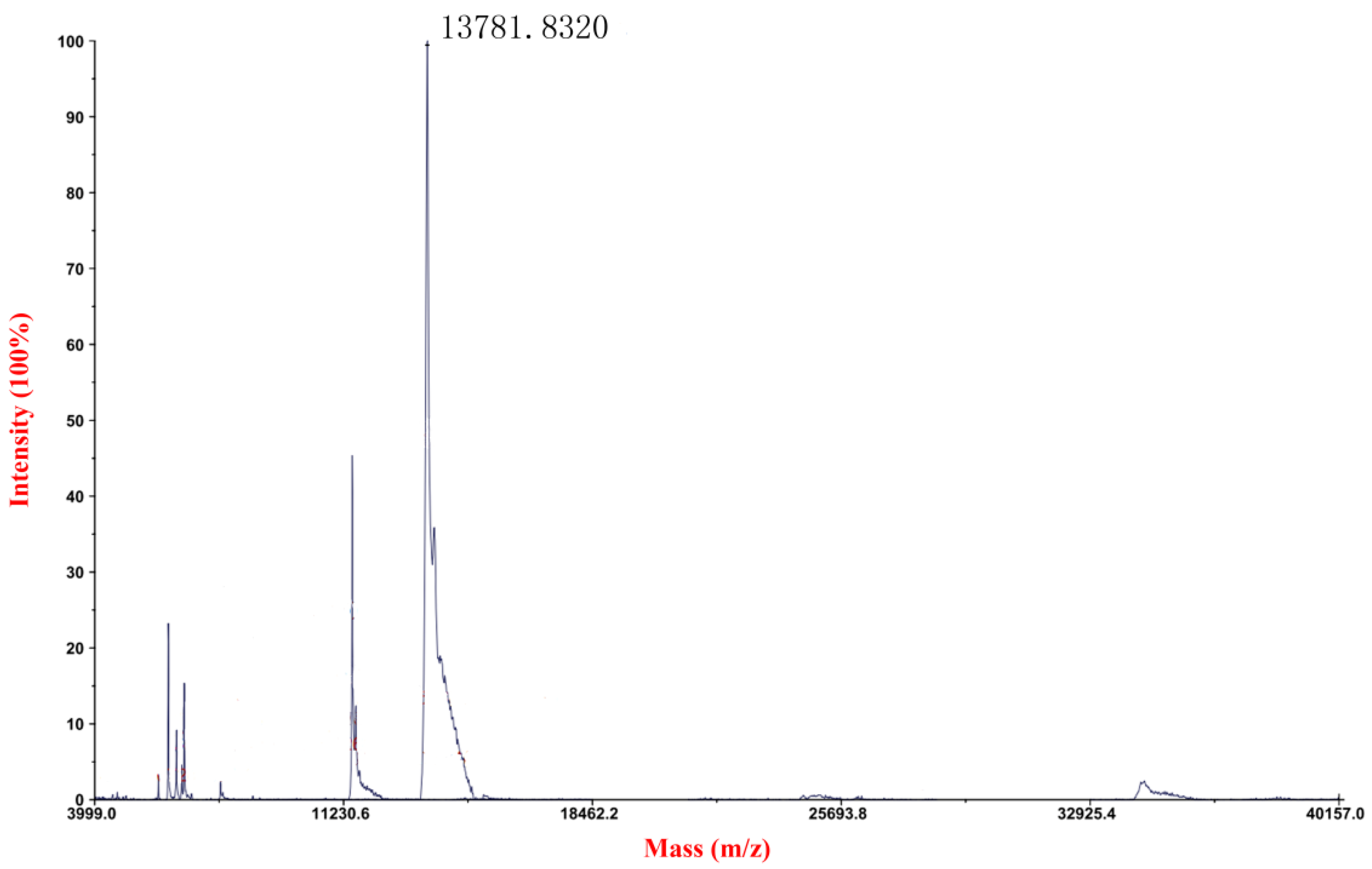
2.1. Expression, Purification and Detection of mFcALF2 Protein
2.2. Binding Assay of mFcALF2 to Bacteria
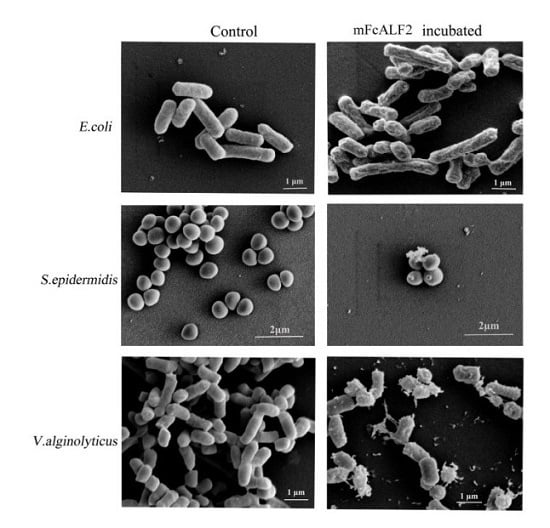
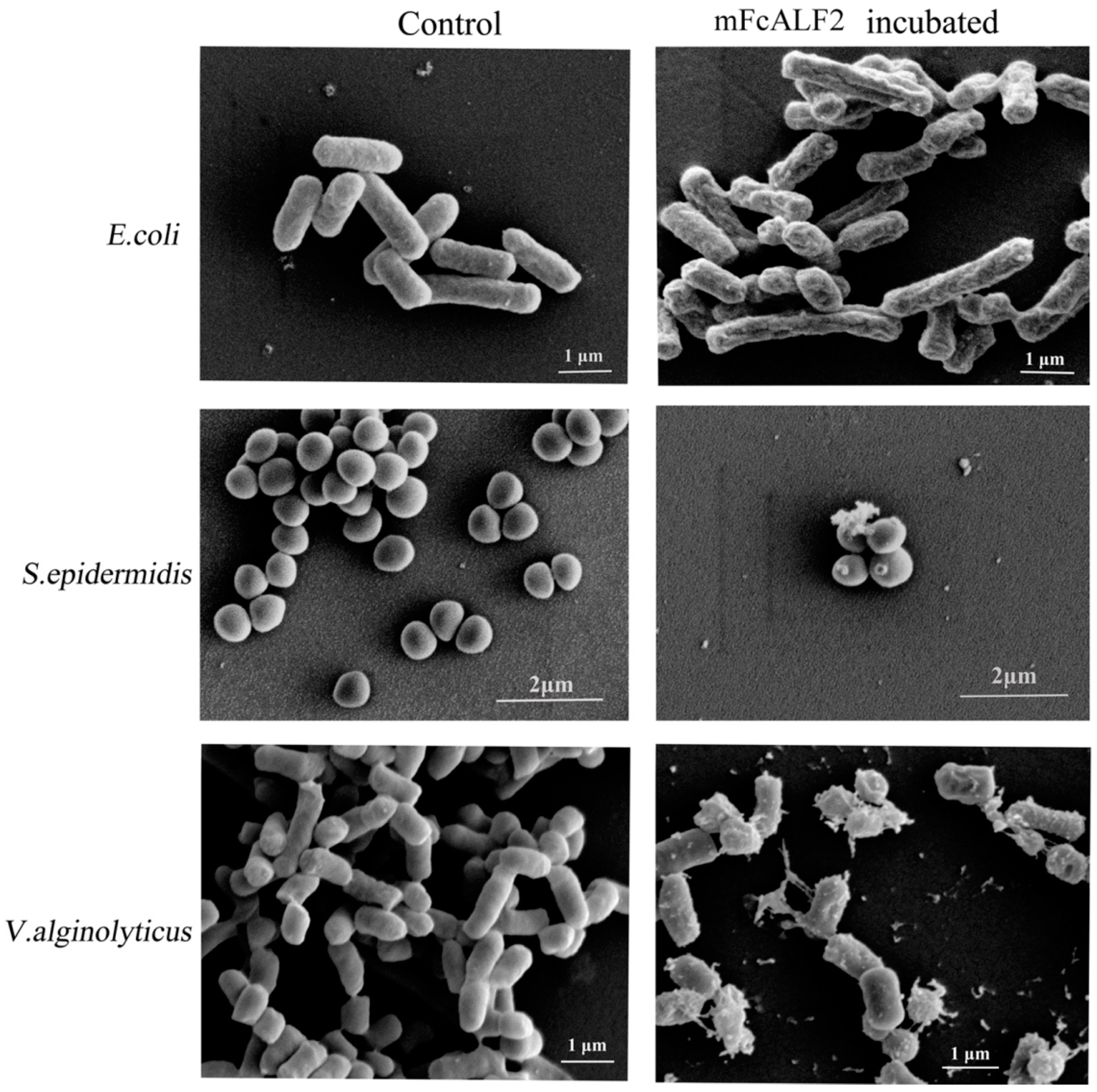
2.3. Observation on the Morphology of Bacterial Cells after Incubation with mFcALF2
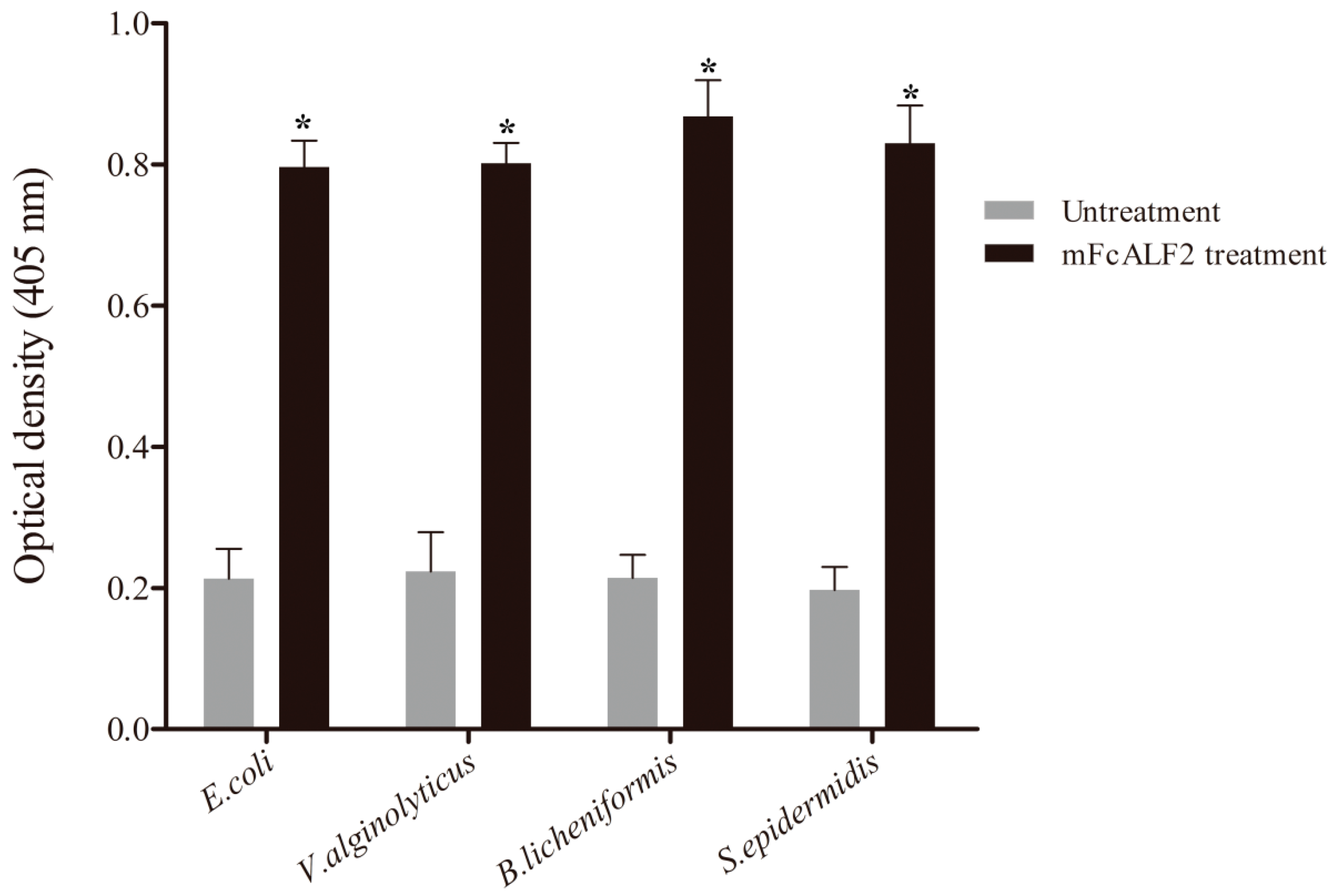
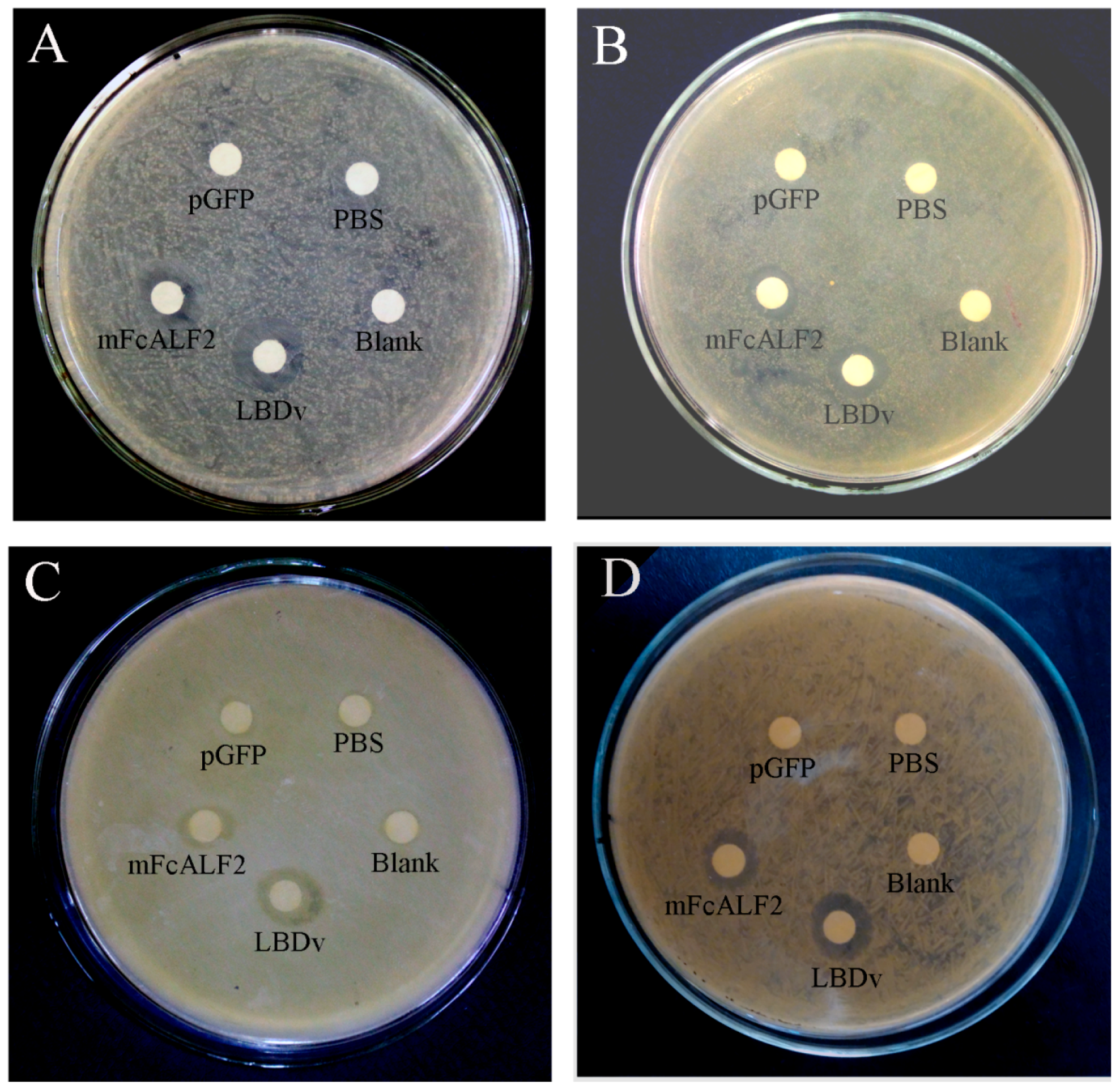
2.4. The Antibacterial Activity of Recombinant mFcALF2 Protein
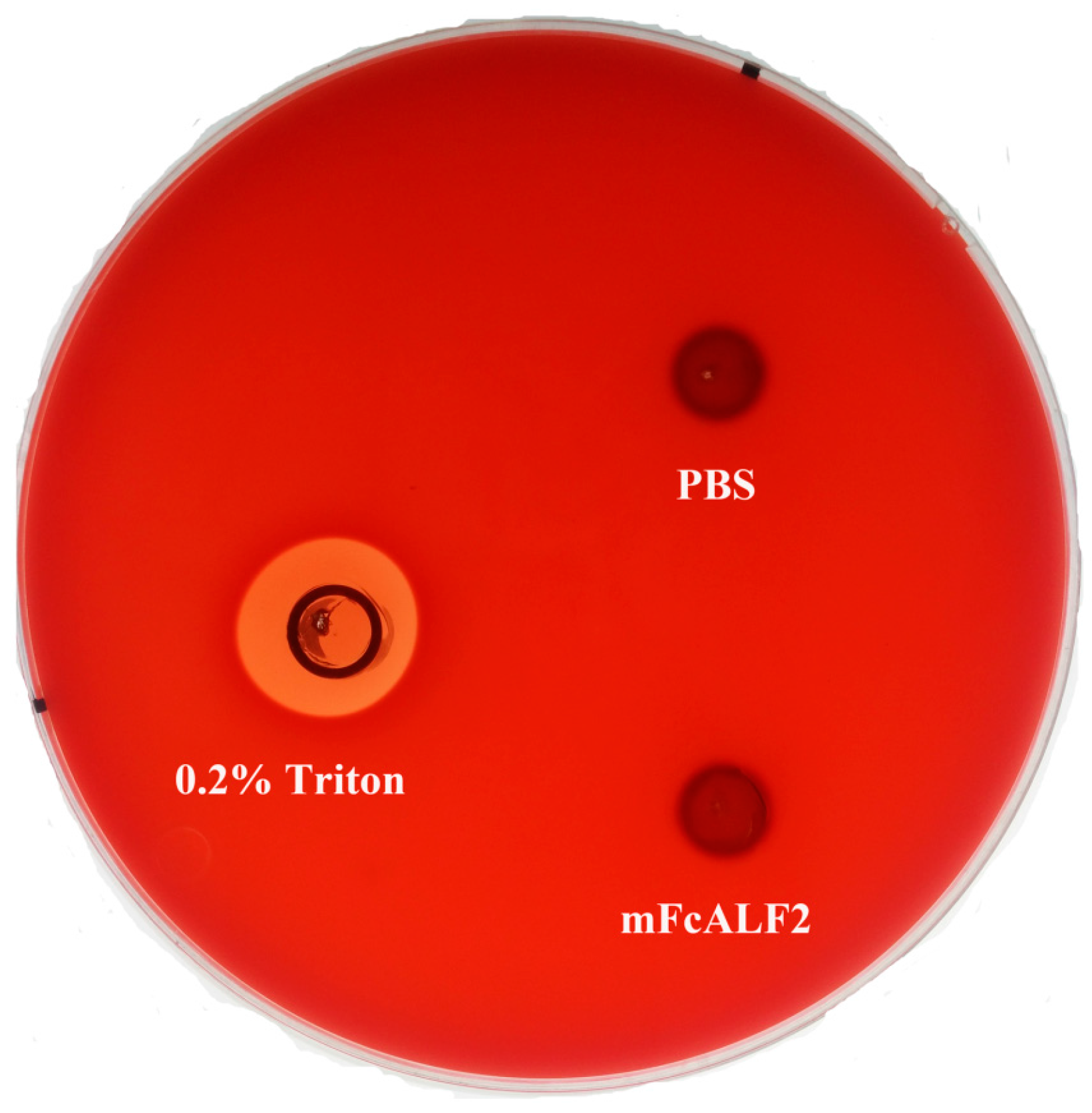
2.5. The Hemolytic Activities of mFcALF2
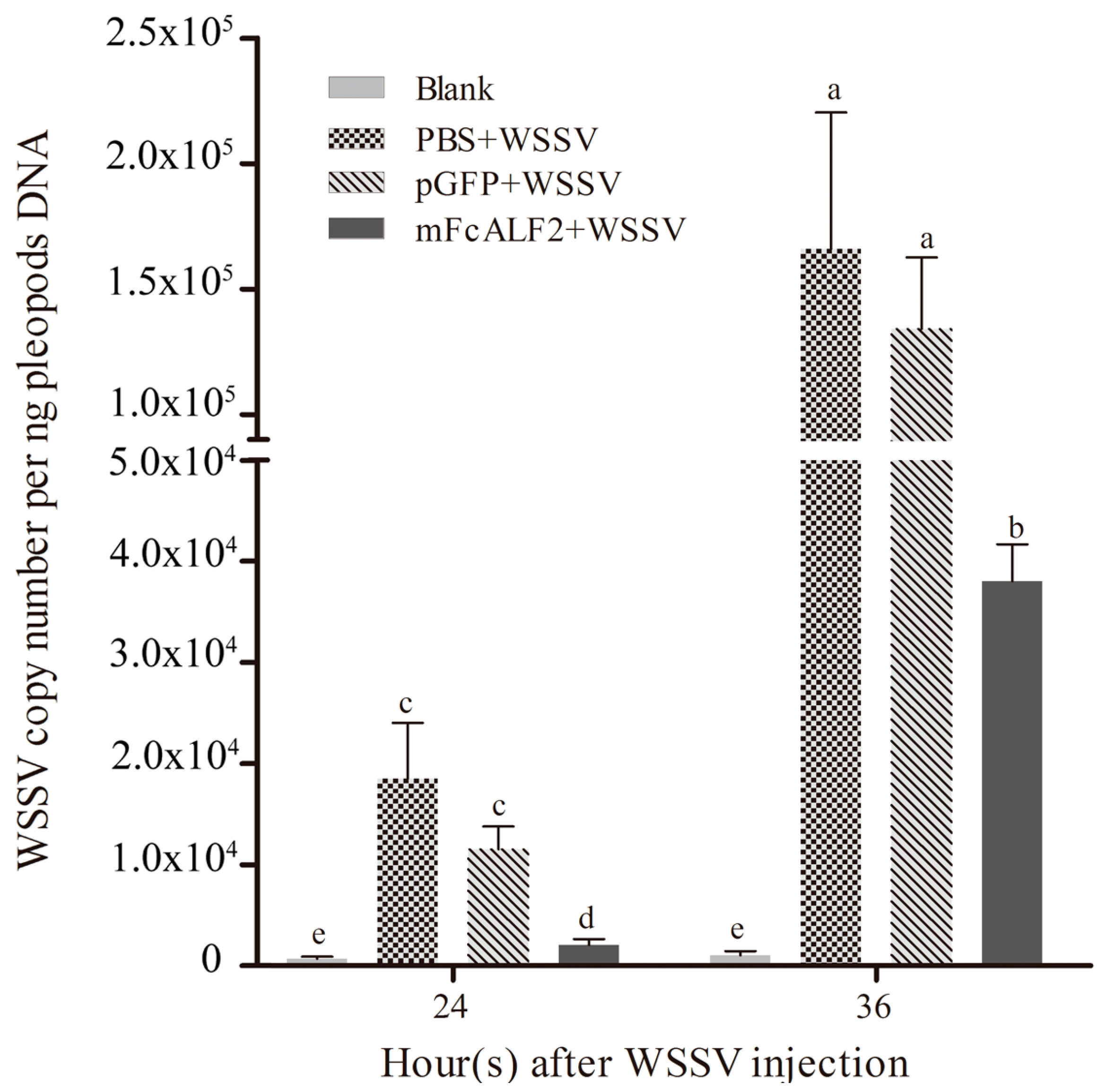
2.6. Inhibition of WSSV Replication by mFcALF2 in Litopenaeus vannamei
3. Discussion
4. Materials and Methods
4.1. Synthesis of the Modified Sequence of FcLAF2 (mFcALF2)
4.2. Construction of the Expression Plasmid, Transformation and Selection of Recombinant Clones
4.3. Production and Purification of the Recombinant Protein
4.4. Western Blot Detection and Mass Spectrometry Analysis
4.5. Bacteria Binding Assay
4.6. Scanning Electron Microscopy (SEM) Detection
4.7. Antimicrobial Activity Assays
4.8. The Hemolytic Activity Test of mFcALF2
4.9. Detection on the Antiviral Activity of mFcALF2
4.10. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Merrifield, R.; Juvvadi, P.; Andreu, D.; Ubach, J.; Boman, A.; Boman, H.G. Retro and retroenantio analogs of cecropin-melittin hybrids. Proc. Natl. Acad. Sci. USA 1995, 92, 3449–3453. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Boman, H.G. Peptide antibiotics and their role in innate immunity. Annu. Rev. Immunol. 1995, 13, 61–92. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, P. Multifunctional host defense peptides: Intracellular-targeting antimicrobial peptides. FEBS J. 2009, 276, 6483–6496. [Google Scholar] [CrossRef] [PubMed]
- Boman, H.G. Antibacterial peptides: Basic facts and emerging concepts. J. Int. Med. 2003, 254, 197–215. [Google Scholar] [CrossRef]
- Hancock, R.E.; Sahl, H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Antibiotic peptides as mediators of innate immunity. Curr. Opin. Immunol. 1992, 4, 3–7. [Google Scholar] [CrossRef]
- Ahmad, A.; Yadav, S.P.; Asthana, N.; Mitra, K.; Srivastava, S.P.; Ghosh, J.K. Utilization of an amphipathic leucine zipper sequence to design antibacterial peptides with simultaneous modulation of toxic activity against human red blood cells. J. Biol. Chem. 2006, 281, 22029–22038. [Google Scholar] [CrossRef] [PubMed]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Anne Pereira, H. Novel therapies based on cationic antimicrobial peptides. Curr. Pharm. Biotechnol. 2006, 7, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Strempel, N.; Strehmel, J.; Overhage, J. Potential Application of Antimicrobial Peptides in the Treatment of Bacterial Biofilm Infections. Curr. Pharm. Des. 2015, 21, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Nakamura, T.; Morita, T.; Iwanaga, S. Limulus Anti-Lps Factor—An Anticoagulant Which Inhibits the Endotoxin-Mediated Activation of Limulus Coagulation System. Biochem. Biophys. Res. Commun. 1982, 105, 717–723. [Google Scholar] [CrossRef]
- Chia, T.J.; Wu, Y.C.; Chen, J.Y.; Chi, S.C. Antimicrobial peptides (AMP) with antiviral activity against fish nodavirus. Fish Shellfish Immunol. 2010, 28, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Krepstakies, M.; Lucifora, J.; Nagel, C.H.; Zeisel, M.B.; Holstermann, B.; Hohenberg, H.; Kowalski, I.; Gutsmann, T.; Baumert, T.F.; Brandenburg, K.; et al. A new class of synthetic peptide inhibitors blocks attachment and entry of human pathogenic viruses. J. Infect. Dis. 2012, 205, 1654–1664. [Google Scholar] [CrossRef] [PubMed]
- Nagoshi, H.; Inagawa, H.; Morii, K.; Harada, H.; Kohchi, C.; Nishizawa, T.; Taniguchi, Y.; Uenobe, M.; Honda, T.; Kondoh, M.; et al. Cloning and characterization of a LPS-regulatory gene having an LPS binding domain in kuruma prawn Marsupenaeus japonicus. Mol. Immunol. 2006, 43, 2061–2069. [Google Scholar] [CrossRef] [PubMed]
- Hoess, A.; Watson, S.; Siber, G.R.; Liddington, R. Crystal structure of an endotoxin-neutralizing protein from the horseshoe crab, Limulus anti-LPS factor, at 1.5 A resolution. EMBO J. 1993, 12, 3351–3356. [Google Scholar] [PubMed]
- Supungul, P.; Klinbunga, S.; Pichyangkura, R.; Hirono, I.; Aoki, T.; Tassanakajon, A. Antimicrobial peptides discovered in the black tiger shrimp Penaeus monodon using the EST approach. Dis. Aquat. Organ. 2004, 61, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.Y.; Li, S.H.; Li, F.H.; Zhang, X.J.; Xiang, J.H. Modification of a synthetic LPS-binding domain of anti-lipopolysaccharide factor from shrimp reveals strong structure-activity relationship in their antimicrobial characteristics. Dev. Comp. Immunol. 2014, 45, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Tharntada, S.; Ponprateep, S.; Somboonwiwat, K.; Liu, H.P.; Soderhall, I.; Soderhall, K.; Tassanakajon, A. Role of anti-lipopolysaccharide factor from the black tiger shrimp, Penaeus monodon, in protection from white spot syndrome virus infection. J. Gen. Virol. 2009, 90, 1491–1498. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H.; Zhang, X.J.; Sun, Z.; Li, F.H.; Xiang, J.H. Transcriptome Analysis on Chinese Shrimp Fenneropenaeus chinensis during WSSV Acute Infection. PLoS ONE 2013, 8, e58627. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.S.; Liu, Y.C.; Li, F.H.; Dong, B.; Xiang, J.H. Molecular cloning and expression profile of putative antilipopolysaccharide factor in Chinese shrimp (Fenneropenaeus chinensis). Mar. Biotechnol. 2005, 7, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H.; Guo, S.Y.; Li, F.H.; Xiang, J.H. Characterization and function analysis of an anti-lipopolysaccharide factor (ALF) from the Chinese shrimp Fenneropenaeus chinensis. Dev. Comp. Immunol. 2014, 46, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Javadpour, M.M.; Juban, M.M.; Lo, W.-C.J.; Bishop, S.M.; Alberty, J.B.; Cowell, S.M.; Becker, C.L.; McLaughlin, M.L. De novo antimicrobial peptides with low mammalian cell toxicity. J. Biol. Chem. 1996, 39, 3107–3113. [Google Scholar] [CrossRef] [PubMed]
- Maloy, W.L.; Kari, U.P. Structure-activity studies on magainins and other host defense peptides. Biopolymers 1995, 37, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, M.L.; Shai, Y. Short native antimicrobial peptides and engineered ultrashort lipopeptides: Similarities and differences in cell specificities and modes of action. Cell Mol. Life Sci. 2011, 68, 2267–2280. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, S.H.; Li, F.H.; Xiang, J.H. Structure and bioactivity of a modified peptide derived from the LPS-binding domain of an anti-lipopolysaccharide factor (ALF) of Shrimp. Mar. Drugs 2016, 14, 96. [Google Scholar] [CrossRef] [PubMed]
- Cregg, J.M.; Cereghino, J.L.; Shi, J.; Higgins, D.R. Recombinant protein expression in Pichia pastoris. Mol. Biotechnol. 2000, 16, 23–52. [Google Scholar] [CrossRef]
- Liu, C.C.; Chung, C.P.; Lin, C.Y.; Sung, H.H. Function of an anti-lipopolysaccharide factor (ALF) isoform isolated from the hemocytes of the giant freshwater prawn Macrobrachium rosenbergii in protecting against bacterial infection. J. Invertebr. Pathol. 2014, 116, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H.; Guo, S.Y.; Li, F.H.; Xiang, J.H. Functional diversity of anti-lipopolysaccharide factor isoforms in shrimp and their characters related to antiviral activity. Mar. Drugs 2015, 13, 2602–2616. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E. Peptide antibiotics. Lancet 1997, 349, 418–422. [Google Scholar] [CrossRef]
- Otero-González, A.J.; Magalhães, B.S.; Garcia-Villarino, M.; López-Abarrategui, C.; Sousa, D.A.; Dias, S.C.; Franco, O.L. Antimicrobial peptides from marine invertebrates as a new frontier for microbial infection control. FASEB. J. 2010, 24, 1320–1334. [Google Scholar] [CrossRef] [PubMed]
- Arbulu, S.; Jiménez, J.J.; Gútiez, L.; Cintas, L.M.; Herranz, C.; Hernández, P.E. Cloning and expression of synthetic genes encoding the broad antimicrobial spectrum bacteriocins SRCAM 602, OR-7, E-760, and L-1077, by recombinant Pichia pastoris. Biomed. Res. Int. 2015, 2015, e767183. [Google Scholar] [CrossRef] [PubMed]
- Ponprateep, S.; Somboonwiwat, K.; Tassanakajon, A. Recombinant anti-lipopolysaccharide factor isoform 3 and the prevention of vibriosis in the black tiger shrimp, Penaeus monodon. Aquaculture 2009, 289, 219–224. [Google Scholar] [CrossRef]
- Cereghino, J.L.; Cregg, J.M. Heterologous protein expression in the methylotrophic yeast Pichia pastoris. Microbiol. Lett. 2000, 24, 45–66. [Google Scholar]
- Jiménez, J.J.; Borrero, J.; Gútiez, L.; Arbulu, S.; Herranz, C.; Cintas, L.M.; Hernández, P.E. Use of synthetic genes for cloning, production and functional expression of the bacteriocins enterocin A and bacteriocin E 50-52 by Pichia pastoris and Kluyveromyces lactis. Mol. Biotechnol. 2014, 56, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Malanovic, N.; Lohner, K. Gram-positive bacterial cell envelopes: The impact on the activity of antimicrobial peptides. Biochim. Biophys. Acta (BBA) Biomembr. 2015, 1858, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Devine, D.A.; Hancock, R.E. Cationic peptides: Distribution and mechanisms of resistance. Curr. Pharm. Des. 2002, 8, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Somboonwiwat, K.; Bachère, E.; Rimphanitchayakit, V.; Tassanakajon, A. Localization of anti-lipopolysaccharide factor (ALFPm3) in tissues of the black tiger shrimp, Penaeus monodon, and characterization of its binding properties. Dev. Comp. Immunol. 2008, 32, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Jaree, P.; Tassanakajon, A.; Somboonwiwat, K. Effect of the anti-lipopolysaccharide factor isoform 3 (ALFPm3) from Penaeus monodon on Vibrio harveyi cells. Dev. Comp. Immunol. 2012, 38, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, D.; Wang, Q.; Yuan, Z.; Wu, H.; Pei, D.; Cong, M.; Li, F.; Ji, C.; Zhao, J. A defensin from clam Venerupis philippinarum: Molecular characterization, localization, antibacterial activity, and mechanism of action. Dev. Comp. Immunol. 2015, 51, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, J.; Zheng, X.; Yang, X.; Ma, P.; Cai, Y.; Zhang, B.; Chen, Y. Design of novel analogues of short antimicrobial peptide anoplin with improved antimicrobial activity. J. Pept. Sci. 2014, 20, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cui, Z.; Li, X.; Song, C.; Li, Q.; Wang, S. A new anti-lipopolysaccharide factor isoform (PtALF4) from the swimming crab Portunus trituberculatus exhibited structural and functional diversity of ALFs. Fish Shellfish Immunol. 2012, 32, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, S.H.; Li, F.H.; Lv, X.J.; Xiang, J.H. Recombinant expression and functional analysis of an isoform of anti-lipopolysaccharide factors (FcALF5) from Chinese shrimp Fenneropenaeus chinensis. Dev. Comp. Immunol. 2015, 53, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.F.; Ho, C.H.; Chen, C.H.; Liu, K.F.; Chiu, Y.L.; Yeh, P.Y.; Peng, S.E.; Hsu, H.C.; Liu, H.C.; Chang, C.F.; et al. Detection and tissue tropism of white spot syndrome baculovirus (WSBV) in captured brooders of Penaeus monodon with a special emphasis on reproductive organs. Dis. Aquat. Organ. 1997, 30, 53–72. [Google Scholar] [CrossRef]
- Liu, H.P.; Jiravanichpaisal, P.; Soderhall, I.; Cerenius, L.; Soderhall, K. Antilipopolysaccharide factor interferes with white spot syndrome virus replication in vitro and in vivo in the crayfish Pacifastacus leniusculus. J. Virol. 2006, 80, 10365–10371. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Liu, G.X.; Li, F.H. Characterization of two pathogenic Photobacterium strains isolated from Exopalaemon carinicauda causing mortality of shrimp. Aquaculture 2016, 464, 129–135. [Google Scholar] [CrossRef]
- Bicca, F.C.; Fleck, L.C.; Ayub, M.Z. Production of biosurfactant by hydrocarbon degrading Rhodococcus ruber and Rhodococcus erythropolis. Rev. Microbiol. 1999, 30, 231–236. [Google Scholar] [CrossRef]
- Sun, Y.M.; Li, F.H.; Chi, Y.H.; Xiang, J.H. Enhanced resistance of marine shrimp Exopalamon carincauda Holthuis to WSSV by injecting live VP28-recombinant bacteria. Acta Oceanol. Sin. 2013, 32, 52–58. [Google Scholar] [CrossRef]
- Sun, Y.M.; Li, F.H.; Xiang, J.H. Analysis on the dynamic changes of the amount of WSSV in Chinese shrimp Fenneropenaeus chinensis during infection. Aquaculture 2013, 376, 124–132. [Google Scholar] [CrossRef]

| Microorganisms | mFcALF2 MIC a (μM) |
|---|---|
| Gram negative bacteria: | - |
| Vibrio alginolyticus | 8–16 |
| Escherichia coli | 4–8 |
| Vibrio harveyi | 8–16 |
| Vibrio parahaemolyticus | 8–16 |
| Gram positive bacteria: | - |
| Bacillus licheniformis | 8–16 |
| Staphylococcus epidermidis | 8–16 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Li, S.; Li, F.; Yu, K.; Yang, F.; Xiang, J. Recombinant Expression of a Modified Shrimp Anti-Lipopolysaccharide Factor Gene in Pichia pastoris GS115 and Its Characteristic Analysis. Mar. Drugs 2016, 14, 152. https://doi.org/10.3390/md14080152
Yang H, Li S, Li F, Yu K, Yang F, Xiang J. Recombinant Expression of a Modified Shrimp Anti-Lipopolysaccharide Factor Gene in Pichia pastoris GS115 and Its Characteristic Analysis. Marine Drugs. 2016; 14(8):152. https://doi.org/10.3390/md14080152
Chicago/Turabian StyleYang, Hui, Shihao Li, Fuhua Li, Kuijie Yu, Fusheng Yang, and Jianhai Xiang. 2016. "Recombinant Expression of a Modified Shrimp Anti-Lipopolysaccharide Factor Gene in Pichia pastoris GS115 and Its Characteristic Analysis" Marine Drugs 14, no. 8: 152. https://doi.org/10.3390/md14080152
APA StyleYang, H., Li, S., Li, F., Yu, K., Yang, F., & Xiang, J. (2016). Recombinant Expression of a Modified Shrimp Anti-Lipopolysaccharide Factor Gene in Pichia pastoris GS115 and Its Characteristic Analysis. Marine Drugs, 14(8), 152. https://doi.org/10.3390/md14080152